Alternating Ring-Opening Metathesis Polymerization Promoted by Ruthenium Catalysts Bearing Unsymmetrical NHC Ligands
Abstract
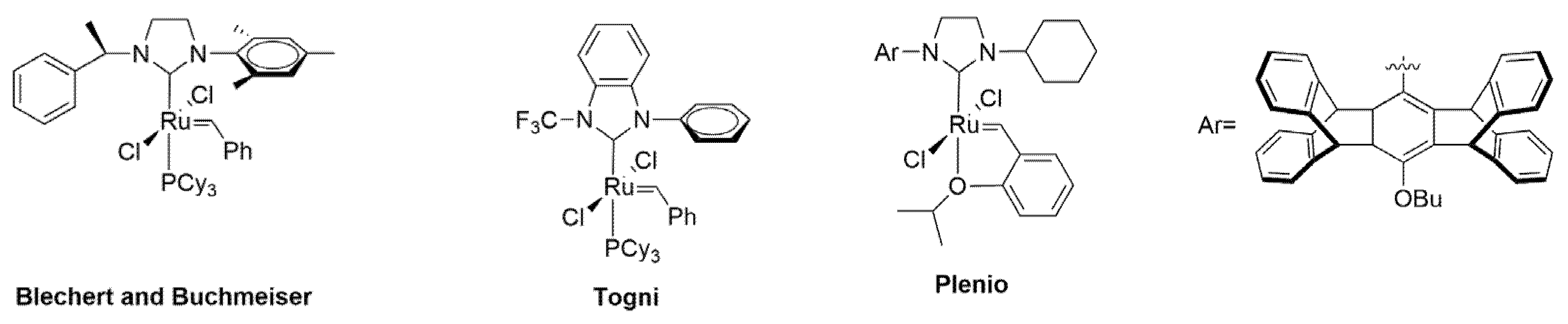
1. Introduction
2. Results and Discussion
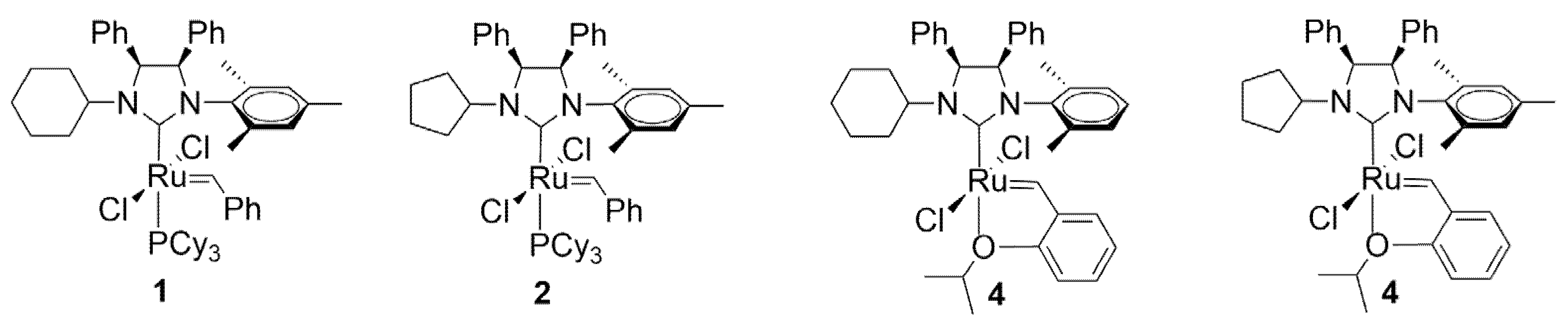
2.1. Synthesis of Complexes
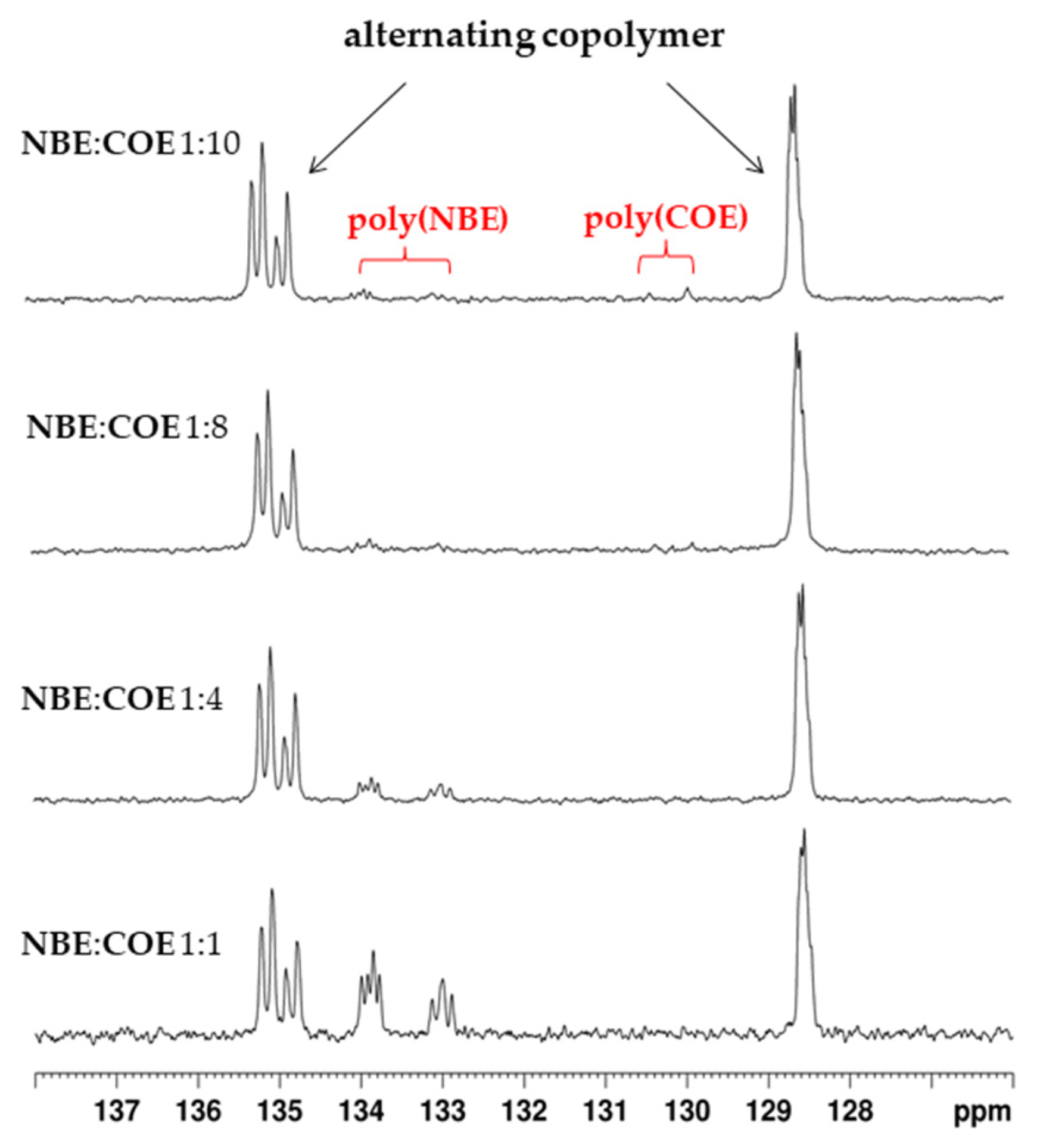
2.2. Alternating ROMP of Norbornene and Cyclooctene
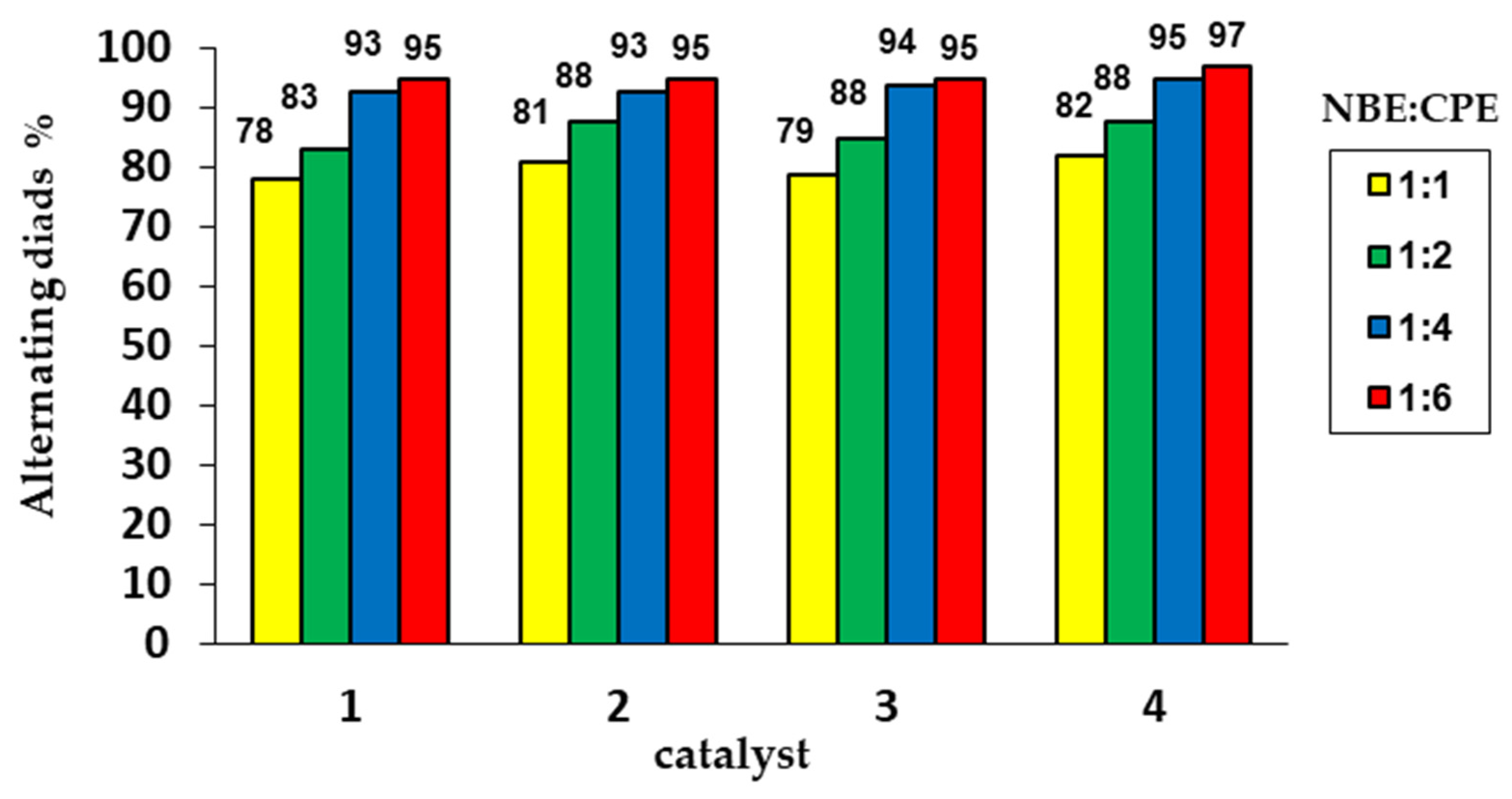
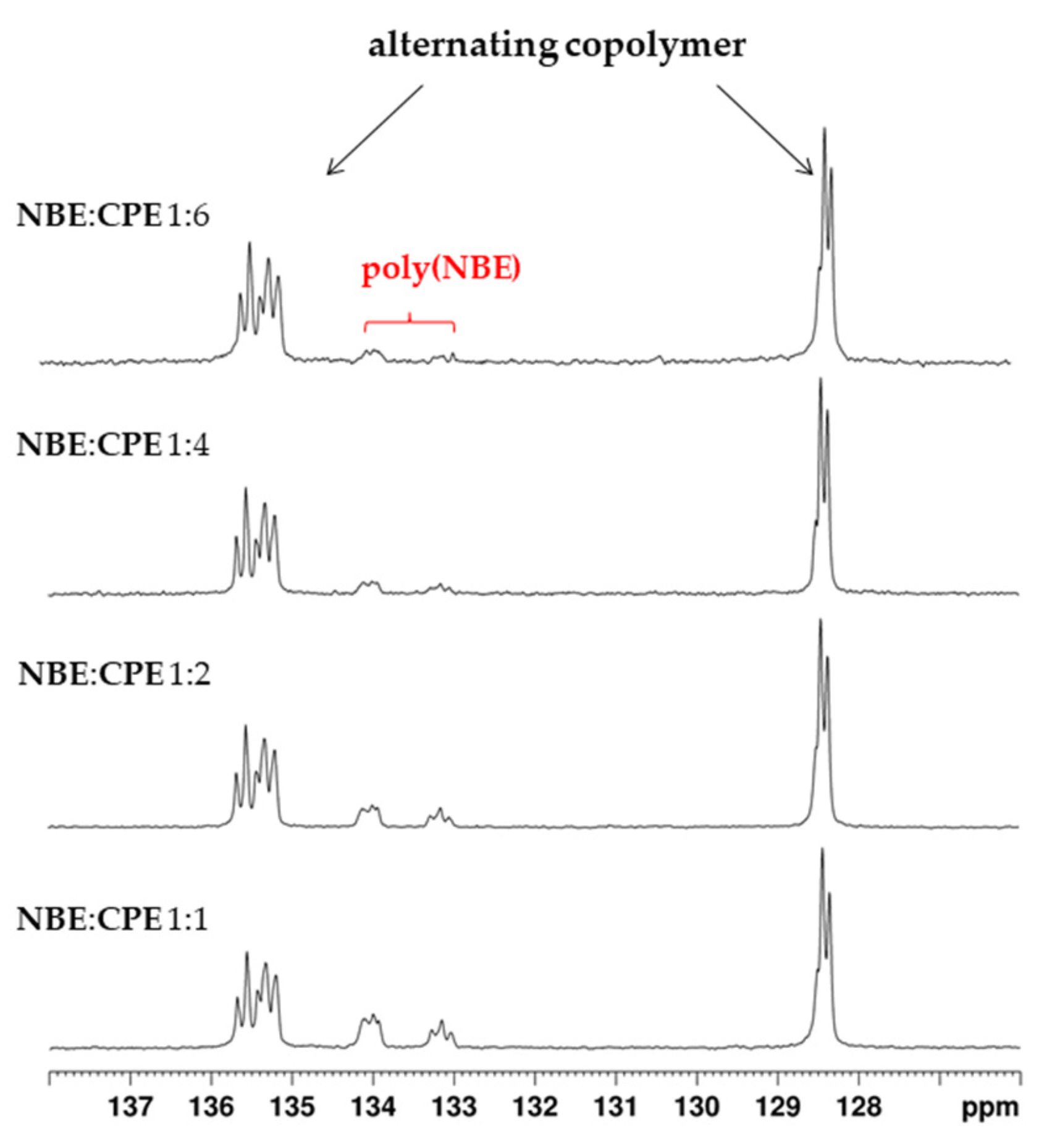
2.3. Alternating ROMP of Norbornene and Cyclopentene
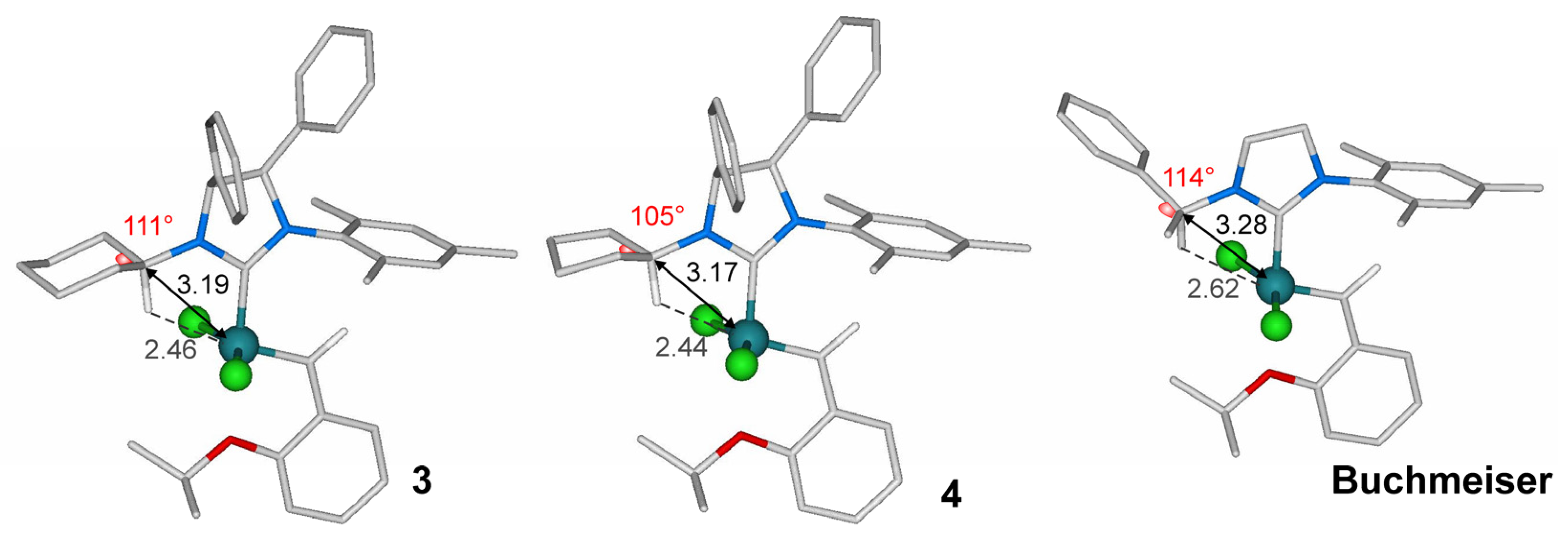
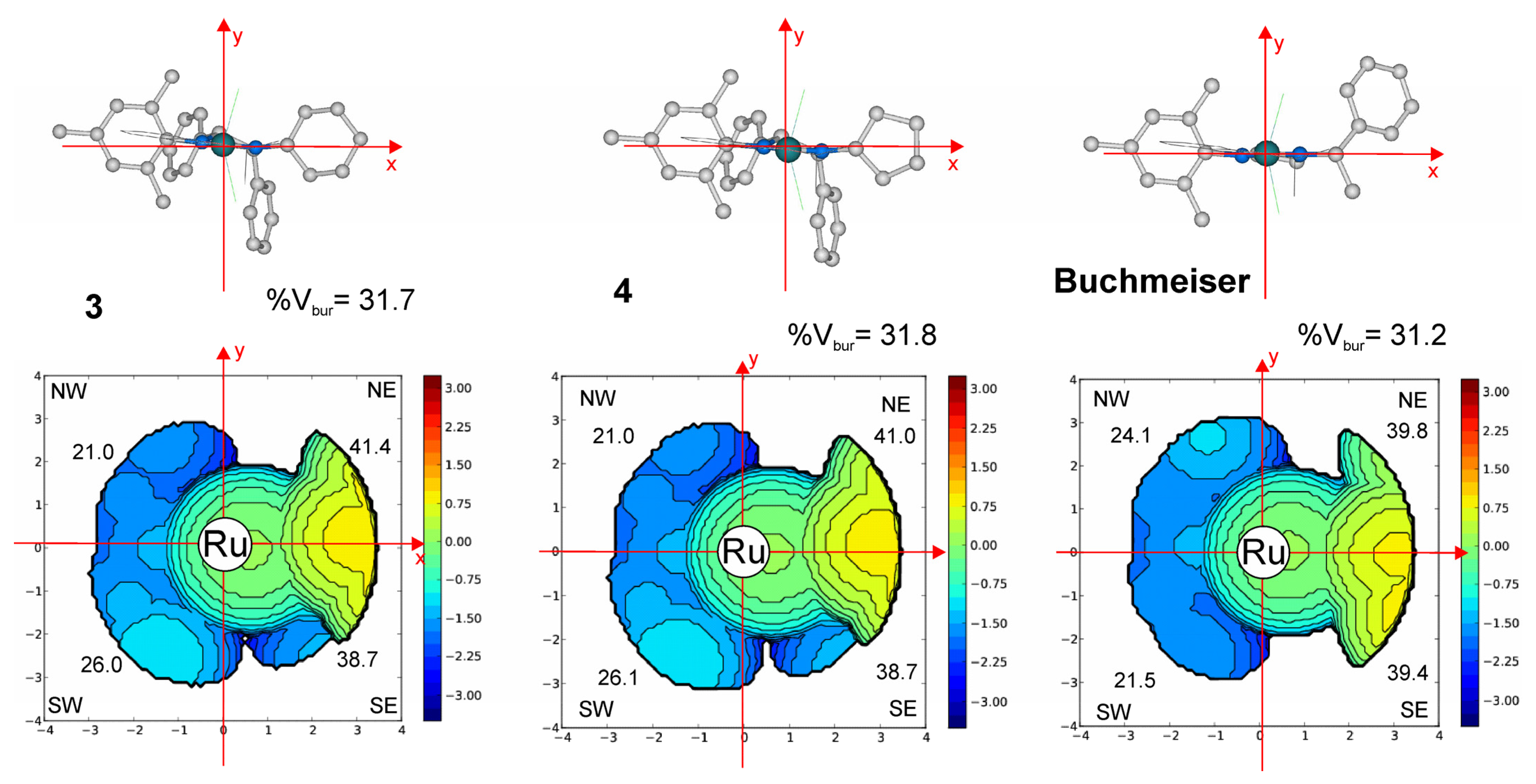
2.4. Molecular Modeling Studies
3. Materials and Methods
3.1. General Information
3.2. Synthesis of N1-Cyclopentyl-N2-Mesityl-1,2-Diphenylethane-1,2-Diamine (B)
3.3. Synthesis of 1-Cyclohexyl-3-Mesityl-4,5-Diphenyl-4,5-Dihydro-1H-Imidazol-3-Ium Tetrafluoroborate (C)
3.4. Synthesis of [1-Cyclopentyl-3-Mesityl-4,5-Diphenyl-2-Imidazolidinylidene](Dichloro) (Benzilydene)(Tricyclohexylphosphine)Ruthenium (2)
3.5. Synthesis of 1-Cyclopentyl-3-Mesityl-4,5-Diphenyl-2-Imidazolidinylidene](Dichloro) (Benzily-Dene)(2-Isopropoxyphenylmethylene)Ruthenium (4)
3.6. General Polymerization Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Nolan, S.P. (Ed.) N-Heterocyclic Carbenes in Synthesis, 1st ed.; WileyWILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; ISBN 978-3-527-31400-3. [Google Scholar]
- Glorius, F. N-Heterocyclic Carbenes in Transition Metal Catalysis; Topics in Organometallic Chemistry; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2007; Volume 21, ISBN 978-3-540-36929-5. [Google Scholar]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-Heterocyclic Carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.J.; Nolan, S.P. Quantifying and Understanding the Electronic Properties of N-Heterocyclic Carbenes. Chem. Soc. Rev. 2013, 42, 6723. [Google Scholar] [CrossRef] [PubMed]
- Dröge, T.; Glorius, F. The Measure of All Rings-N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2010, 49, 6940–6952. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.; Correa, A.; Poater, A.; Costabile, C.; Cavallo, L. Understanding the M-(NHC) (NHC = N-Heterocyclic Carbene) Bond” Coordin. Chem. Rev. 2009, 253, 687–703. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 978-3-527-67124-3. [Google Scholar]
- Lin, J.C.Y.; Huang, R.T.W.; Lee, C.S.; Bhattacharyya, A.; Hwang, W.S.; Lin, I.J.B. Coinage Metal-N-Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3561–3598. [Google Scholar] [CrossRef]
- Visbal, R.; Gimeno, M.C. N-Heterocyclic Carbene Metal Complexes: Photoluminescence and Applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef]
- Zhang, D.; Zi, G. N-Heterocyclic Carbene (NHC) Complexes of Group 4 Transition Metals. Chem. Soc. Rev. 2015, 44, 1898–1921. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S.; Dagorne, S. Group 1 and 2 and Early Transition Metal Complexes Bearing N-Heterocyclic Carbene Ligands: Coordination Chemistry, Reactivity, and Applications. Chem. Rev. 2014, 114, 8747–8774. [Google Scholar] [CrossRef]
- Lee, J.; Hahm, H.; Kwak, J.; Kim, M. New Aspects of Recently Developed Rhodium(N-Heterocyclic Carbene)-Catalyzed Organic Transformations. Adv. Synth. Catal. 2019, 361, 1479–1499. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-Heterocyclic Carbene Complexes in C–H Activation Reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Jiang, X.; So, Y.-M.; To, C.T.; He, G. Recent Advances in Rare Earth Complexes Containing N-Heterocyclic Carbenes: Synthesis, Reactivity, and Applications in Polymerization. Catalysts 2020, 10, 71. [Google Scholar] [CrossRef]
- César, V.; Bellemin-Laponnaz, S.; Gade, L.H. Chiral N-Heterocyclic Carbenes as Stereodirecting Ligands in Asymmetric Catalysis. Chem. Soc. Rev. 2004, 33, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Müller, D.; Schlepphorst, C.; Glorius, F. Privileged Chiral N-Heterocyclic Carbene Ligands for Asymmetric Transition-Metal Catalysis. Chem. Soc. Rev. 2017, 46, 4845–4854. [Google Scholar] [CrossRef]
- Foster, D.; Borhanuddin, S.M.; Dorta, R. Designing Successful Monodentate N-Heterocyclic Carbene Ligands for Asymmetric Metal Catalysis. Dalton Trans. 2021, 50, 17467–17477. [Google Scholar] [CrossRef]
- Costabile, C.; Pragliola, S.; Grisi, F. C2-Symmetric N-Heterocyclic Carbenes in Asymmetric Transition-Metal Catalysis. Symmetry 2022, 14, 1615. [Google Scholar] [CrossRef]
- Samojłowicz, C.; Bieniek, M.; Grela, K. Ruthenium-Based Olefin Metathesis Catalysts Bearing N -Heterocyclic Carbene Ligands. Chem. Rev. 2009, 109, 3708–3742. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef]
- Bieniek, M.; Michrowska, A.; Usanov, D.L.; Grela, K. In an Attempt to Provide a User’s Guide to the Galaxy of Benzylidene, Alkoxybenzylidene, and Indenylidene Ruthenium Olefin Metathesis Catalysts. Chem. Eur. J. 2008, 14, 806–818. [Google Scholar] [CrossRef]
- Ogba, O.M.; Warner, N.C.; O’Leary, D.J.; Grubbs, R.H. Recent Advances in Ruthenium-Based Olefin Metathesis. Chem. Soc. Rev. 2018, 47, 4510–4544. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.; Costabile, C.; Grisi, F. NHC Backbone Configuration in Ruthenium-Catalyzed Olefin Metathesis. Molecules 2016, 21, 117. [Google Scholar] [CrossRef]
- Paradiso, V.; Costabile, C.; Grisi, F. Ruthenium-Based Olefin Metathesis Catalysts with Monodentate Unsymmetrical NHC Ligands. Beilstein J. Org. Chem. 2018, 14, 3122–3149. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, T.P.; Johns, A.M.; Grubbs, R.H. Recent Advancements in Stereoselective Olefin Metathesis Using Ruthenium Catalysts. Catalysts 2017, 7, 87. [Google Scholar] [CrossRef]
- Hamad, F.B.; Sun, T.; Xiao, S.; Verpoort, F. Olefin Metathesis Ruthenium Catalysts Bearing Unsymmetrical Heterocylic Carbenes. Coord. Chem. Rev. 2013, 257, 2274–2292. [Google Scholar] [CrossRef]
- Tornatzky, J.; Kannenberg, A.; Blechert, S. New Catalysts with Unsymmetrical N-Heterocyclic Carbene Ligands. Dalton Trans. 2012, 41, 8215. [Google Scholar] [CrossRef]
- Monsigny, L.; Kajetanowicz, A.; Grela, K. Ruthenium Complexes Featuring Unsymmetrical N-Heterocyclic Carbene Ligands–Useful Olefin Metathesis Catalysts for Special Tasks. Chem. Rec. 2021, 21, 3648–3661. [Google Scholar] [CrossRef]
- Al Samak, B.; Carvill, A.G.; Rooney, J.J.; Thompson, J.M. Alternating Ring-Opening Metathesis Copolymerization of Bicyclo[2.2.1]Hept-2-Ene and Cyclopentene. Chem. Commun. 1997, 21, 2057–2058. [Google Scholar] [CrossRef]
- Ilker, M.F.; Coughlin, E.B. Alternating Copolymerizations of Polar and Nonpolar Cyclic Olefins by Ring-Opening Metathesis Polymerization. Macromolecules 2002, 35, 54–58. [Google Scholar] [CrossRef]
- Bornand, M.; Chen, P. Mechanism-Based Design of a ROMP Catalyst for Sequence-Selective Copolymerization. Angew. Chem. Int. Ed. 2005, 44, 7909–7911. [Google Scholar] [CrossRef]
- Bornand, M.; Torker, S.; Chen, P. Mechanistically Designed Dual-Site Catalysts for the Alternating ROMP of Norbornene and Cyclooctene. Organometallics 2007, 26, 3585–3596. [Google Scholar] [CrossRef]
- Torker, S.; Müller, A.; Sigrist, R.; Chen, P. Tuning the Steric Properties of a Metathesis Catalyst for Copolymerization of Norbornene and Cyclooctene toward Complete Alternation. Organometallics 2010, 29, 2735–2751. [Google Scholar] [CrossRef]
- Torker, S.; Müller, A.; Chen, P. Building Stereoselectivity into a Chemoselective Ring-Opening Metathesis Polymerization Catalyst for Alternating Copolymerization. Angew. Chem. 2010, 122, 3850–3854. [Google Scholar] [CrossRef]
- Vehlow, K.; Wang, D.; Buchmeiser, M.R.; Blechert, S. Alternating Copolymerizations Using a Grubbs-Type Initiator with an Unsymmetrical, Chiral N-Heterocyclic Carbene Ligand. Angew. Chem. Int. Ed. 2008, 47, 2615–2618. [Google Scholar] [CrossRef]
- Lichtenheldt, M.; Wang, D.; Vehlow, K.; Reinhardt, I.; Kühnel, C.; Decker, U.; Blechert, S.; Buchmeiser, M. Alternating Ring-Opening Metathesis Copolymerization by Grubbs-Type Initiators with Unsymmetrical N-Heterocyclic Carbenes. Chem. Eur. J. 2009, 15, 9451–9457. [Google Scholar] [CrossRef] [PubMed]
- Buchmeiser, M.R.; Ahmad, I.; Gurram, V.; Kumar, P.S. Pseudo-Halide and Nitrate Derivatives of Grubbs and Grubbs–Hoveyda Initiators: Some Structural Features Related to the Alternating Ring-Opening Metathesis Copolymerization of Norborn-2-Ene with Cyclic Olefins. Macromolecules 2011, 44, 4098–4106. [Google Scholar] [CrossRef]
- Engl, P.S.; Fedorov, A.; Copéret, C.; Togni, A. N-Trifluoromethyl NHC Ligands Provide Selective Ruthenium Metathesis Catalysts. Organometallics 2016, 35, 887–893. [Google Scholar] [CrossRef]
- Vasiuta, R.; Stockert, A.; Plenio, H. Alternating Ring-Opening Metathesis Polymerization by Grubbs-Type Catalysts with N -Pentiptycenyl, N -Alkyl-NHC Ligands. Chem. Commun. 2018, 54, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.; Bertolasi, V.; Grisi, F. Novel Olefin Metathesis Ruthenium Catalysts Bearing Backbone-Substituted Unsymmetrical NHC Ligands. Organometallics 2014, 33, 5932–5935. [Google Scholar] [CrossRef]
- Paradiso, V.; Bertolasi, V.; Costabile, C.; Grisi, F. Ruthenium Olefin Metathesis Catalysts Featuring Unsymmetrical N-Heterocyclic Carbenes. Dalton Trans. 2016, 45, 561–571. [Google Scholar] [CrossRef]
- Paradiso, V.; Grisi, F. Ruthenium-Catalyzed Alternating Ring-Opening Metathesis Copolymerization of Norborn-2-ene with Cyclic Olefins. Adv. Synth. Catal. 2019, 361, 4133–4139. [Google Scholar] [CrossRef]
- Paradiso, V.; Bertolasi, V.; Costabile, C.; Caruso, T.; Dąbrowski, M.; Grela, K.; Grisi, F. Expanding the Family of Hoveyda–Grubbs Catalysts Containing Unsymmetrical NHC Ligands. Organometallics 2017, 36, 3692–3708. [Google Scholar] [CrossRef]
- Sanford, M.S.; Ulman, M.; Grubbs, R.H. New Insights into the Mechanism of Ruthenium-Catalyzed Olefin Metathesis Reactions. J. Am. Chem. Soc. 2001, 123, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Sanford, M.S.; Love, J.A.; Grubbs, R.H. Mechanism and Activity of Ruthenium Olefin Metathesis Catalysts. J. Am. Chem. Soc. 2001, 123, 6543–6554. [Google Scholar] [CrossRef] [PubMed]
- Love, J.A.; Sanford, M.S.; Day, M.W.; Grubbs, R.H. Synthesis, Structure, and Activity of Enhanced Initiators for Olefin Metathesis. J. Am. Chem. Soc. 2003, 125, 10103–10109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Olefin Metathesis Catalysts Coordinated with Unsymmetrical N-Heterocyclic Carbene Ligands: Synthesis, Structure, and Catalytic Activity. Chem. Eur. J. 2008, 14, 7545–7556. [Google Scholar] [CrossRef] [PubMed]
- Vorfalt, T.; Wannowius, K.-J.; Plenio, H. Probing the Mechanism of Olefin Metathesis in Grubbs-Hoveyda and Grela Type Complexes. Angew. Chem. Int. Ed. 2010, 49, 5533–5536. [Google Scholar] [CrossRef]
- Thiel, V.; Hendann, M.; Wannowius, K.-J.; Plenio, H. On the Mechanism of the Initiation Reaction in Grubbs–Hoveyda Complexes. J. Am. Chem. Soc. 2012, 134, 1104–1114. [Google Scholar] [CrossRef]
- Nuñez-Zarur, F.; Solans-Monfort, X.; Rodríguez-Santiago, L.; Sodupe, M. Differences in the Activation Processes of Phosphine-Containing and Grubbs–Hoveyda-Type Alkene Metathesis Catalysts. Organometallics 2012, 31, 4203–4215. [Google Scholar] [CrossRef]
- Piers, W.E. Olefin Metathesis and Metathesis Polymerization By K. J. Ivin (The Queen’s University of Belfast) and J. C. Mol (University of Amsterdam). Academic Press: San Diego. 1997. Xvi + 472 Pp. $70.00. ISBN 0-12-377045-9. J. Am. Chem. Soc. 1997, 119, 8396. [Google Scholar] [CrossRef]
- Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. SambVca 2. A Web Tool for Analyzing Catalytic Pockets with Topographic Steric Maps. Organometallics 2016, 35, 2286–2293. [Google Scholar] [CrossRef]
- Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Towards the Online Computer-Aided Design of Catalytic Pockets. Nat. Chem. 2019, 11, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Dorta, R.; Stevens, E.D.; Scott, N.M.; Costabile, C.; Cavallo, L.; Hoff, C.D.; Nolan, S.P. Steric and Electronic Properties of N-Heterocyclic Carbenes (NHC): A Detailed Study on Their Interaction with Ni(CO)4. J. Am. Chem. Soc. 2005, 127, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, L.; Correa, A.; Costabile, C.; Jacobsen, H. Steric and Electronic Effects in the Bonding of N-Heterocyclic Ligands to Transition Metals. J. Organomet. Chem. 2005, 690, 5407–5413. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J. P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824, Erratum in Phys. Rev. B 1986, 34, 7406–7406. [Google Scholar] [CrossRef]
- Schaefer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Haeusermann, U.; Dolg, M.; Stoll, H.; Preuss, H.; Schwerdtfeger, P.; Pitzer, R.M. Accuracy of energy-adjusted quasirelativistic ab initio pseudopotentials. Mol. Phys. 1993, 78, 1211–1224. [Google Scholar] [CrossRef]
- Kuechle, W.; Dolg, M.; Stoll, H.; Preuss, H. Energy—Adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J. Chem. Phys. 1994, 100, 7535–7542. [Google Scholar] [CrossRef]
- Leininger, T.; Nicklass, A.; Stoll, H.; Dolg, M.; Schwerdtfeger, P. The accuracy of the pseudopotential approximation. II. A comparison of various core sizes for indium pseudopotentials in calculations for spectroscopic constants of InH, InF, and InCl. J. Chem. Phys. 1996, 105, 1052–1059. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.v.R. Efficient diffuse function-augmented basis-sets for anion calculations. 3. The 3-21+G basis set for 1st-row elements, Li-F. J. Comp. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]



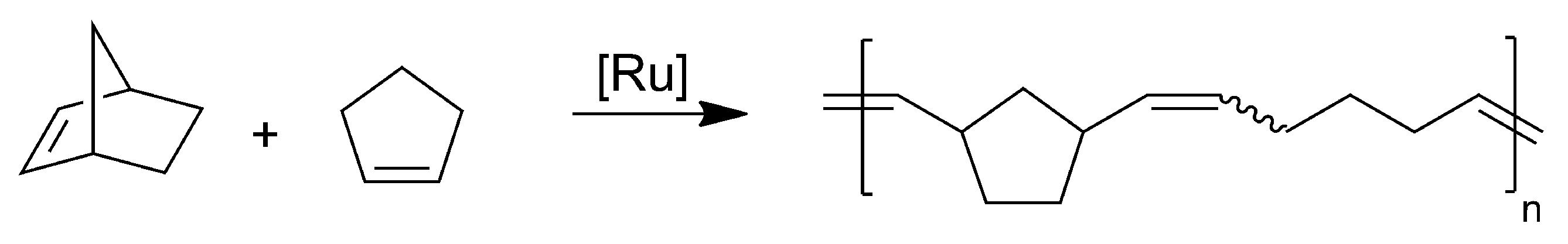

| Entry 1 | Catalyst | NBE/COE | Poly(NBE) [%] 2,3 | Poly(COE) [%] 2,3 | Alternating Diads [%] 3 | Mn4 (g/mol) | Đ4 | Yield (mg) |
|---|---|---|---|---|---|---|---|---|
| 1 5 | 1 | 1:1 | 26 | <1 | 74 (62) | 9.2 × 105 | 1.86 | 167 |
| 2 5 | 1 | 1:4 | 13 | <1 | 87 (63) | 4.2 × 105 | 2.07 | 220 |
| 3 5 | 1 | 1:8 | 5 | 1 | 94 (63) | 6.6 × 105 | 1.93 | 235 |
| 4 5 | 1 | 1:10 | 1 | 1 | 98 (63) | 8.0 × 105 | 1.84 | 244 |
| 5 | 2 | 1:1 | 28 | - | 72 (61) | 2.3 × 105 | 1.92 | 161 |
| 6 | 2 | 1:4 | 9 | - | 90 (60) | 3.6 × 105 | 1.90 | 200 |
| 7 | 2 | 1:8 | 2 | - | 98 (61) | 4.0 × 105 | 1.77 | 238 |
| 8 | 2 | 1:10 | 2 | - | 98 (63) | 2.8 × 105 | 1.86 | 234 |
| 9 | 3 | 1:1 | 22 | - | 78 (62) | 1.1 × 106 | 1.73 | 159 |
| 10 | 3 | 1:4 | 12 | - | 88 (63) | 1.7 × 106 | 1.49 | 228 |
| 11 | 3 | 1:8 | 5 | <1 | 95 (63) | 1.1 × 106 | 1.79 | 230 |
| 12 | 3 | 1:10 | 2 | <1 | 98 (63) | 1.3 × 106 | 1.61 | 244 |
| 13 | 4 | 1:1 | 28 | - | 72 (61) | 1.7 × 106 | 1.60 | 171 |
| 14 | 4 | 1:4 | 7 | - | 93 (62) | 1.3 × 106 | 1.66 | 203 |
| 15 | 4 | 1:8 | 2 | - | 98 (63) | 1.0 × 106 | 1.86 | 196 |
| 16 | 4 | 1:10 | 1 | 1 | 98 (62) | 9.6 × 105 | 1.85 | 224 |
| Entry 1 | Catalyst | NBE/CPE | Poly(NBE) [%] 2,3 | Poly(CPE) [%] 2,3 | Alternating Diads [%] 3 | Mn4 (g/mol) | Đ4 | Yield (mg) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 5 | 1 | 1:1 | 22 | <1 | 78 | 8.7 × 104 | 1.59 | 163 | |
| 2 5 | 1 | 1:2 | 17 | <1 | 83 | 7.1 × 104 | 1.63 | 115 | |
| 3 5 | 1 | 1:4 | 7 | <1 | 93 | 1.0 × 105 | 1.78 | 135 | |
| 4 5 | 1 | 1:6 | 5 | <1 | 95 | 1.1 × 105 | 1.87 | 165 | |
| 5 | 2 | 1:1 | 19 | - | 81 | 1.8 × 105 | 2.10 | 163 | |
| 6 | 2 | 1:2 | 12 | <1 | 88 | 1.6 × 105 | 1.95 | 172 | |
| 7 | 2 | 1:4 | 6 | 1 | 93 | 1.5 × 105 | 2.18 | 186 | |
| 8 | 2 | 1:6 | 3 | 2 | 95 | 1.5 × 105 | 2.28 | 200 | |
| 9 | 3 | 1:1 | 21 | - | 79 | 3.1 × 105 | 1.98 | 157 | |
| 10 | 3 | 1:2 | 15 | - | 85 | 1.8 × 105 | 1.96 | 165 | |
| 11 | 3 | 1:4 | 6 | <1 | 94 | 2.0 × 105 | 2.01 | 179 | |
| 12 | 3 | 1:6 | 4 | 1 | 95 | 1.3 × 105 | 1.70 | 171 | |
| 13 | 4 | 1:1 | 18 | - | 82 | 3.2 × 105 | 1.64 | 151 | |
| 14 | 4 | 1:2 | 12 | - | 88 | 2.0 × 105 | 1.85 | 176 | |
| 15 | 4 | 1:4 | 5 | - | 95 | 3.3 × 105 | 1.97 | 168 | |
| 16 | 4 | 1:6 | 3 | <1 | 97 | 2.1 × 105 | 1.95 | 172 | |
| Catalyst | %VBur 1 | ΔΔG 2 | Ru Charge 3 |
|---|---|---|---|
| 3 | 31.7 | 0.7 | −0.30214 |
| 4 | 31.8 | 0.7 | −0.30677 |
| Buchmeiser | 31.2 | 2.0 | −0.29546 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troiano, R.; Costabile, C.; Grisi, F. Alternating Ring-Opening Metathesis Polymerization Promoted by Ruthenium Catalysts Bearing Unsymmetrical NHC Ligands. Catalysts 2023, 13, 34. https://doi.org/10.3390/catal13010034
Troiano R, Costabile C, Grisi F. Alternating Ring-Opening Metathesis Polymerization Promoted by Ruthenium Catalysts Bearing Unsymmetrical NHC Ligands. Catalysts. 2023; 13(1):34. https://doi.org/10.3390/catal13010034
Chicago/Turabian StyleTroiano, Rubina, Chiara Costabile, and Fabia Grisi. 2023. "Alternating Ring-Opening Metathesis Polymerization Promoted by Ruthenium Catalysts Bearing Unsymmetrical NHC Ligands" Catalysts 13, no. 1: 34. https://doi.org/10.3390/catal13010034
APA StyleTroiano, R., Costabile, C., & Grisi, F. (2023). Alternating Ring-Opening Metathesis Polymerization Promoted by Ruthenium Catalysts Bearing Unsymmetrical NHC Ligands. Catalysts, 13(1), 34. https://doi.org/10.3390/catal13010034






