Bismuth Vanadate (BiVO4) Nanostructures: Eco-Friendly Synthesis and Their Photocatalytic Applications
Abstract
:1. Introduction
2. Eco-Friendly Synthesis of BiVO4 NPs
2.1. Biosynthesis Techniques
2.2. Microwave- and Ultrasonic-Assisted Synthesis
3. Photocatalytic Applications
- ➢
- Degradation of organic pollutants in industrial effluents,
- ➢
- Water treatment (the removal of stable organic compounds and microorganisms from municipal and laboratory wastewater),
- ➢
- Air purifier,
- ➢
- Paper industry,
- ➢
- Disinfection of surgical instruments, and
- ➢
- The removal of fingerprints from electrically and optically sensitive components.
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohan, R. Green bismuth. Nat. Chem. 2010, 2, 336. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.; Figueiredo, S.A.; Pinto, R.M.; Silvestre, S.M. Bismuth compounds in medicinal chemistry. Future Med. Chem. 2012, 4, 1495–1523. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Ruiz, E.; Zambrano-Robledo, P.; Vazquez-Arenas, J.; Cruz-Ortiz, B.; Peral, J.; García-Pérez, U.M. Photoactivity of nanostructured spheres of BiVO4 synthesized by ultrasonic spray pyrolysis at low temperature. Mater. Res. Bull. 2021, 143, 111447. [Google Scholar] [CrossRef]
- Koventhan, C.; Pandiyarajan, S.; Chen, S.-M. Simple sonochemical synthesis of flake-ball shaped bismuth vanadate for voltammetric detection of furazolidone. J. Alloys Compd. 2022, 895, 162315. [Google Scholar] [CrossRef]
- Packiaraj, R.; Devendran, P.; Asath Bahadur, S.; Nallamuthu, N. Structural and electrochemical studies of Scheelite type BiVO4 nanoparticles: Synthesis by simple hydrothermal method. J. Mater. Sci. Mater. Electron. 2018, 29, 13265–13276. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, M.; Xu, L.; Miao, Y.; Ouyang, R. Characteristics, applications and determination of bismuth. J. Nanosci. Nanotechnol. 2016, 16, 6679–6689. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J. Hydrothermal processing for obtaining of BiVO4 nanoparticles. Mater. Lett. 2009, 63, 1939–1942. [Google Scholar] [CrossRef]
- Trinh, D.T.T.; Khanitchaidecha, W.; Channei, D.; Nakaruk, A. Synthesis, characterization and environmental applications of bismuth vanadate. Res. Chem. Intermed. 2019, 45, 5217–5259. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Zou, Z. Electronic structure and optical properties of monoclinic clinobisvanite BiVO4. Phys. Chem. Chem. Phys. 2011, 13, 4746–4753. [Google Scholar] [CrossRef]
- Pookmanee, P.; Kojinok, S.; Puntharod, R.; Sangsrichan, S.; Phanichphant, S. Preparation and characterization of BiVO4 powder by the sol-gel method. Ferroelectrics 2013, 456, 45–54. [Google Scholar] [CrossRef]
- Josephine, A.J.; Dhas, C.R.; Venkatesh, R.; Arivukarasan, D.; Christy, A.J.; Monica, S.E.S.; Keerthana, S. Effect of pH on visible-light-driven photocatalytic degradation of facile synthesized bismuth vanadate nanoparticles. Mater. Res. Express 2020, 7, 015036. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Cao, L.; Su, G.; Liu, H.; Wang, X.; Zhang, L. Ultrasound assisted synthesis of monoclinic structured spindle BiVO4 particles with hollow structure and its photocatalytic property. Ultrason. Sonochem. 2010, 17, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Hong, S.-S. Facile solvothermal synthesis of monoclinic-tetragonal heterostructured BiVO4 for photodegradation of rhodamine B. Catal. Commun. 2020, 136, 105920. [Google Scholar] [CrossRef]
- Venkatesan, R.; Velumani, S.; Ordon, K.; Makowska-Janusik, M.; Corbel, G.; Kassiba, A. Nanostructured bismuth vanadate (BiVO4) thin films for efficient visible light photocatalysis. Mater. Chem. Phys. 2018, 205, 325–333. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Biofactories: Engineered nanoparticles via genetically engineered organisms. Green Chem. 2019, 21, 4583–4603. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020, 22, 2643–2661. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and Characterization of Efficient ZnO/g-C3N4 Nanocomposites Photocatalyst for Photocatalytic Degradation of Methylene Blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Trimetallic Nanoparticles: Greener Synthesis and Their Applications. Nanomaterials 2020, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Iravani, S. Green and eco-friendly synthesis of nanophotocatalysts: An overview. Comments Inorg. Chem. 2021, 41, 133–187. [Google Scholar] [CrossRef]
- Tammina, S.K.; Mandal, B.K.; Kadiyala, N.K. Photocatalytic degradation of methylene blue dye by nonconventional synthesized SnO2 nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 10, 339–350. [Google Scholar] [CrossRef]
- Mahanthappa, M.; Kottam, N.; Yellappa, S. Enhanced photocatalytic degradation of methylene blue dye using CuSCdS nanocomposite under visible light irradiation. Appl. Surf. Sci. 2019, 475, 828–838. [Google Scholar] [CrossRef]
- Paul, A.; Dhar, S.S. Construction of hierarchical MnMoO4/NiFe2O4 nanocomposite: Highly efficient visible light driven photocatalyst in the degradation of different polluting dyes in aqueous medium. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124090. [Google Scholar] [CrossRef]
- Fatima, U.; Khalid, N.R.; Nawaz, T.; Tahir, M.B.; Fatima, N.; Kebaili, I.; Alrobei, H.; Alzaid, M.; Shahzad, K.; Ali, A.M. Synthesis of BiVO4/NiFe2O4 composite for photocatalytic degradation of methylene blue. Appl. Nanosci. 2021, 11, 2793–2800. [Google Scholar] [CrossRef]
- Lee, G.-J.; Lee, X.-Y.; Lyu, C.; Liu, N.; Andandan, S.; Wu, J.J. Sonochemical Synthesis of Copper-doped BiVO4/g-C3N4 Nanocomposite Materials for Photocatalytic Degradation of Bisphenol A under Simulated Sunlight Irradiation. Nanomaterials 2020, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Letchumanan, D.; Sok, S.P.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-Based Biosynthesis of Copper/Copper Oxide Nanoparticles: An Update on Their Applications in Biomedicine, Mechanisms, and Toxicity. Biomolecules 2021, 11, 564. [Google Scholar] [CrossRef]
- Pasula, R.R.; Lim, S. Engineering nanoparticle synthesis using microbial factories. Eng. Biol. 2017, 1, 12–17. [Google Scholar] [CrossRef]
- Luo, C.-H.; Shanmugam, V.; Yeh, C.-S. Nanoparticle biosynthesis using unicellular and subcellular supports. NPG Asia Mater. 2015, 7, e209. [Google Scholar] [CrossRef] [Green Version]
- Varma, R.S. Greener approach to nanomaterials and their sustainable applications. Curr. Opin. Chem. Eng. 2012, 1, 123–128. [Google Scholar] [CrossRef]
- Varma, R.S. Journey on greener pathways: From the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chem. 2014, 16, 2027–2041. [Google Scholar] [CrossRef]
- Varma, R.S. Greener and sustainable chemistry. Appl. Sci. 2014, 4, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Varma, R.S. Greener and Sustainable Trends in Synthesis of Organics and Nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Aydin, G.; Altinsoy, B.; Altinkaynak, C.; Koşar, M.; Ocsoy, I. The Effect of Pelargonium endlicherianum Fenzl. root extracts on formation of nanoparticles and their antimicrobial activities. Enzym. Microb. Technol. 2017, 97, 21–26. [Google Scholar] [CrossRef]
- Pramila, S.; Nagaraju, G.; Mallikarjunaswamy, C.; Latha, K.; Chandan, S.; Ramu, R.; Rashmi, V.; Lakshmi Ranganatha, V. Green Synthesis of BiVO4 nanoparticles by microwave method using Aegle marmelos juice as a fuel: Photocatalytic and antimicrobial study. Anal. Chem. Lett. 2020, 10, 298–306. [Google Scholar] [CrossRef]
- Mohamed, H.; Sone, B.; Khamlich, S.; Coetsee-Hugo, E.; Swart, H.; Thema, T.; Sbiaa, R.; Dhlamini, M. Biosynthesis of BiVO4 nanorods using Callistemon viminalis extracts: Photocatalytic degradation of methylene blue. Mater. Today Proc. 2021, 36, 328–335. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Haniadka, R.; Dsouza, J.; Bhat, H.P. Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam (jackfruit): A review. Food Res. Int. 2011, 44, 1800–1811. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Pramila, S.; Nagaraju, G.; Ramu, R.; Ranganatha, V.L. Green synthesis and evaluation of antiangiogenic, photocatalytic, and electrochemical activities of BiVO4 nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 14028–14046. [Google Scholar] [CrossRef]
- Manjunatha, A.S.; Pavithra, N.S.; Marappa, S.; Prashanth, S.A.; Nagaraju, G. Green synthesis of flower-like BiVO4 nanoparticles by solution combustion method using lemon (Citrus limon) juice as a fuel: Photocatalytic and electrochemical study. ChemistrySelect 2018, 3, 13456–13463. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Paré, P.W. Phytochemical analysis and anti-inflammatory potential of Hyphaene thebaica L. fruit. J. Food Sci. 2013, 78, C1503–C1508. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Zohra, T.; Alam, M.M.; Ikram, A.; Shinwari, Z.K.; Maaza, M. Phytosynthesis of BiVO4 nanorods using Hyphaene thebaica for diverse biomedical applications. AMB Express 2019, 9, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, R.; Nandal, U. Nutritional and therapeutic potential of bael (Aegle marmelos Corr.) fruit juice: A review. Nutr. Food Sci. 2015, 45, 895–919. [Google Scholar] [CrossRef]
- Burakova, E.A.; Dyachkova, T.P.; Rukhov, A.V.; Tugolukov, E.N.; Galunin, E.V.; Tkachev, A.G.; Basheer, A.A.; Ali, I. Novel and economic method of carbon nanotubes synthesis on a nickel magnesium oxide catalyst using microwave radiation. J. Mol. Liq. 2018, 253, 340–346. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: A review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C.; Shi, S.; Chen, C. Low-temperature synthesis multiwalled carbon nanotubes by microwave plasma chemical vapor deposition using CH4–CO2 gas mixture. Jpn. J. Appl. Phys. 2003, 42, 614–619. [Google Scholar] [CrossRef]
- Daniel Abraham, S.; Theodore David, S.; Biju Bennie, R.; Joel, C.; Sanjay Kumar, D. Eco-friendly and green synthesis of BiVO4 nanoparticle using microwave irradiation as photocatalayst for the degradation of Alizarin Red S. J. Mol. Struct. 2016, 1113, 174–181. [Google Scholar] [CrossRef]
- Claudino, C.H.; Kuznetsova, M.; Rodrigues, B.S.; Chen, C.; Wang, Z.; Sardela, M.; Souza, J.S. Facile one-pot microwave-assisted synthesis of tungsten-doped BiVO4/WO3 heterojunctions with enhanced photocatalytic activity. Mater. Res. Bull. 2020, 125, 110783. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Yang, X.; Yang, H.; Lu, Z.; Chen, R. Monoclinic BiVO4 micro-/nanostructures: Microwave and ultrasonic wave combined synthesis and their visible-light photocatalytic activities. J. Alloys Compd. 2013, 551, 544–550. [Google Scholar] [CrossRef]
- Souza, J.S.; Hirata, F.T.H.; Corio, P. Microwave-assisted synthesis of bismuth vanadate nanoflowers decorated with gold nanoparticles with enhanced photocatalytic activity. J. Nanopart. Res. 2019, 21, 35. [Google Scholar] [CrossRef]
- Pingmuang, K.; Nattestad, A.; Kangwansupamonkon, W.; Wallace, G.G.; Phanichphant, S.; Chen, J. Phase-controlled microwave synthesis of pure monoclinic BiVO4 nanoparticles for photocatalytic dye degradation. Appl. Mater. Today 2015, 1, 67–73. [Google Scholar] [CrossRef]
- Rodrigues, B.S.; Branco, C.M.; Corio, P.; Souza, J.S. Controlling Bismuth Vanadate Morphology and Crystalline Structure through Optimization of Microwave-Assisted Synthesis Conditions. Cryst. Growth Des. 2020, 20, 3673–3685. [Google Scholar] [CrossRef]
- Kansaard, T.; Bangbai, C.; Jayasankar, C.K.; Pecharapa, W. Effect of Ultrasonic Irradiation Time on Physical Properties and Photocatalytic Performance of BiVO4 Nanoparticles Prepared via Sonochemical Process. Integr. Ferroelectr. 2021, 214, 123–132. [Google Scholar] [CrossRef]
- Tahir, M.B.; Iqbal, T.; Kiran, H.; Hasan, A. Insighting role of reduced graphene oxide in BiVO4 nanoparticles for improved photocatalytic hydrogen evolution and dyes degradation. Int. J. Energy Res. 2019, 43, 2410–2417. [Google Scholar] [CrossRef]
- Walsh, A.; Yan, Y.; Huda, M.N.; Al-Jassim, M.M.; Wei, S.-H. Band Edge Electronic Structure of BiVO4: Elucidating the Role of the Bi s and V d Orbitals. Chem. Mater. 2009, 21, 547–551. [Google Scholar] [CrossRef]
- Lai, B.-R.; Lin, L.-Y.; Xiao, B.-C.; Chen, Y.-S. Facile synthesis of bismuth vanadate/bismuth oxide heterojunction for enhancing visible light-responsive photoelectrochemical performance. J. Taiwan Inst. Chem. Eng. 2019, 100, 178–185. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, I.N.; Koutavarapu, R.; Reddy, K.R.; Kim, D.; Shim, J. Novel BiVO4 nanostructures for environmental remediation, enhanced photoelectrocatalytic water oxidation and electrochemical energy storage performance. Sol. Energy 2020, 207, 441–449. [Google Scholar] [CrossRef]
- Sajid, M.M.; Amin, N.; Shad, N.A.; Bashir khan, S.; Javed, Y.; Zhang, Z. Hydrothermal fabrication of monoclinic bismuth vanadate (m-BiVO4) nanoparticles for photocatalytic degradation of toxic organic dyes. Mater. Sci. Eng. B 2019, 242, 83–89. [Google Scholar] [CrossRef]
- Ganeshbabu, M.; Kannan, N.; Sundara Venkatesh, P.; Paulraj, G.; Jeganathan, K.; MubarakAli, D. Synthesis and characterization of BiVO4 nanoparticles for environmental applications. RSC Adv. 2020, 10, 18315–18322. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Uma; Singh, S.; Verma, A.; Khanuja, M. Visible light induced bactericidal and photocatalytic activity of hydrothermally synthesized BiVO4 nano-octahedrals. J. Photochem. Photobiol. B Biol. 2016, 162, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lopes, O.F.; Carvalho, K.T.; Macedo, G.K.; de Mendonca, V.R.; Avansi, W.; Ribeiro, C. Synthesis of BiVO 4 via oxidant peroxo-method: Insights into the photocatalytic performance and degradation mechanism of pollutants. New J. Chem. 2015, 39, 6231–6237. [Google Scholar] [CrossRef]
- Dowla Biswas, M.R.U.; Ho, B.S.; Oh, W.-C. Eco-friendly conductive polymer-based nanocomposites, BiVO4/graphene oxide/polyaniline for excellent photocatalytic performance. Polym. Bull. 2020, 77, 4381–4400. [Google Scholar] [CrossRef]
- Tahir, M.B.; Riaz, K.N.; Asiri, A.M. Boosting the performance of visible light-driven WO3/g-C3N4 anchored with BiVO4 nanoparticles for photocatalytic hydrogen evolution. Int. J. Energy Res. 2019, 43, 5747–5758. [Google Scholar] [CrossRef]
- Dhabarde, N.; Carrillo-Ceja, O.; Tian, S.; Xiong, G.; Raja, K.; Subramanian, V.R. Bismuth Vanadate Encapsulated with Reduced Graphene Oxide: A Nanocomposite for Optimized Photocatalytic Hydrogen Peroxide Generation. J. Phys. Chem. C 2021, 125, 23669–23679. [Google Scholar] [CrossRef]
- Kansaard, T.; Pecharapa, W. Characterization of BiVO4 nanoparticles prepared by sonochemical process. Ferroelectrics 2019, 552, 140–147. [Google Scholar] [CrossRef]
- Tian, H.; Wu, H.; Fang, Y.; Li, R.; Huang, Y. Hydrothermal synthesis of m-BiVO4/t-BiVO4 heterostructure for organic pollutants degradation: Insight into the photocatalytic mechanism of exposed facets from crystalline phase controlling. J. Hazard. Mater. 2020, 399, 123159. [Google Scholar] [CrossRef]
- Jiang, H.; Dai, H.; Meng, X.; Zhang, L.; Deng, J.; Liu, Y.; Au, C.T. Hydrothermal fabrication and visible-light-driven photocatalytic properties of bismuth vanadate with multiple morphologies and/or porous structures for Methyl Orange degradation. J. Environ. Sci. 2012, 24, 449–457. [Google Scholar] [CrossRef]
- Choe, H.R.; Kim, J.H.; Ma, A.; Jung, H.; Kim, H.Y.; Nam, K.M. Understanding Reaction Kinetics by Tailoring Metal Co-catalysts of the BiVO4 Photocatalyst. ACS Omega 2019, 4, 16597–16602. [Google Scholar] [CrossRef]
- Ma, Y.; Pendlebury, S.R.; Reynal, A.; Formal, F.L.; Durrant, J.R. Dynamics of photogenerated holes in undoped BiVO4 photoanodes for solar water oxidation. Chem. Sci. 2014, 5, 2964–2973. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Sun, S.; Song, Y.; Yan, X.; Guan, W.; Liu, X.; Shi, W. Microwave-assisted in situ synthesis of reduced graphene oxide-BiVO4 composite photocatalysts and their enhanced photocatalytic performance for the degradation of ciprofloxacin. J. Hazard. Mater. 2013, 250–251, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bao, Z.; Yao, F.; Cao, H.; Wang, J.; Qiu, L.; Lv, J.; Sun, X.; Zhang, Y.; Wu, Y. One-dimensional bismuth vanadate nanostructures constructed Z-scheme photocatalyst for highly efficient degradation of antibiotics. J. Water Process Eng. 2022, 46, 102599. [Google Scholar] [CrossRef]
- Hemavibool, K.; Sansenya, T.; Nanan, S. Enhanced Photocatalytic Degradation of Tetracycline and Oxytetracycline Antibiotics by BiVO4 Photocatalyst under Visible Light and Solar Light Irradiation. Antibiotics 2022, 11, 761. [Google Scholar] [CrossRef]
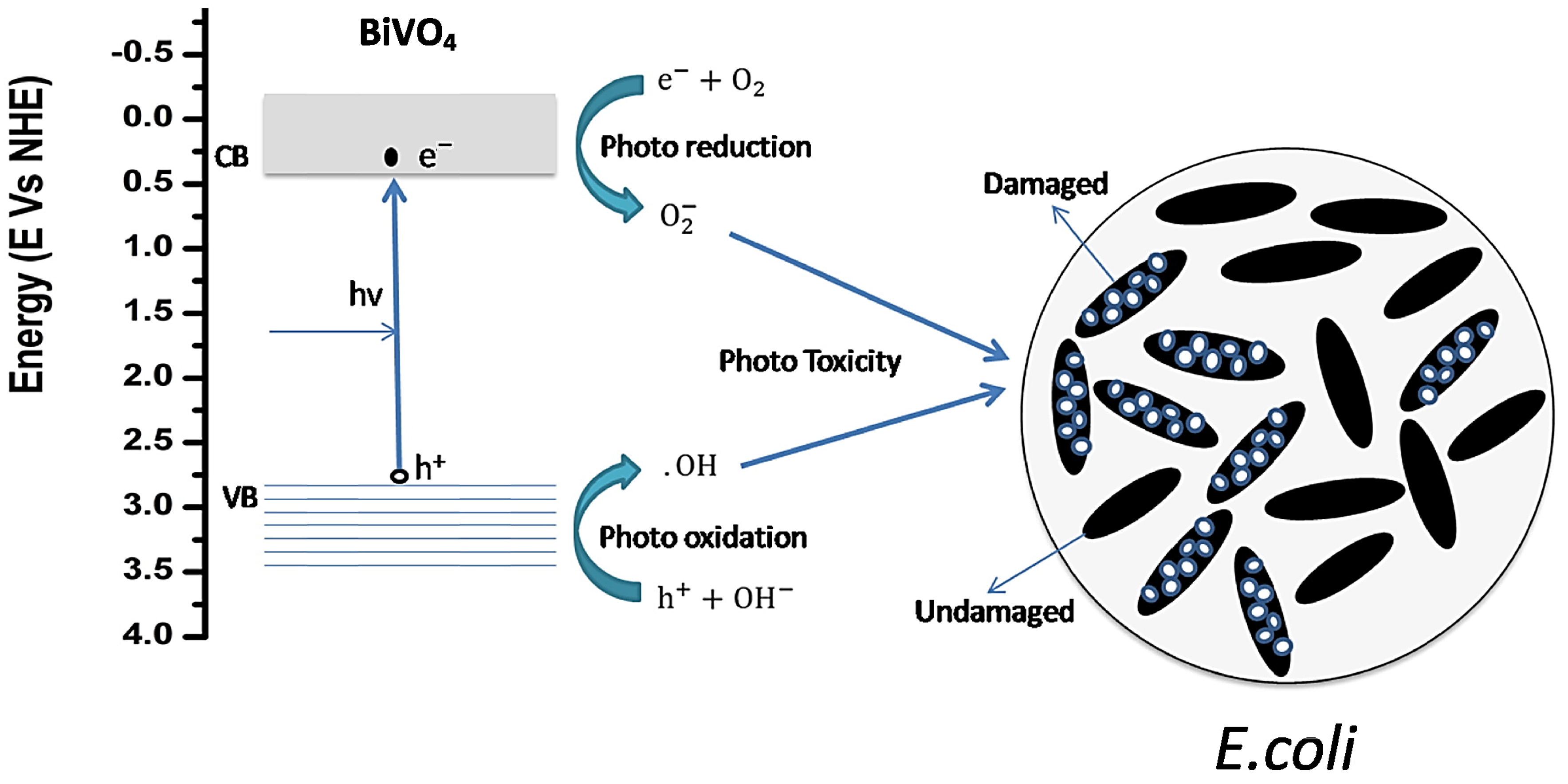
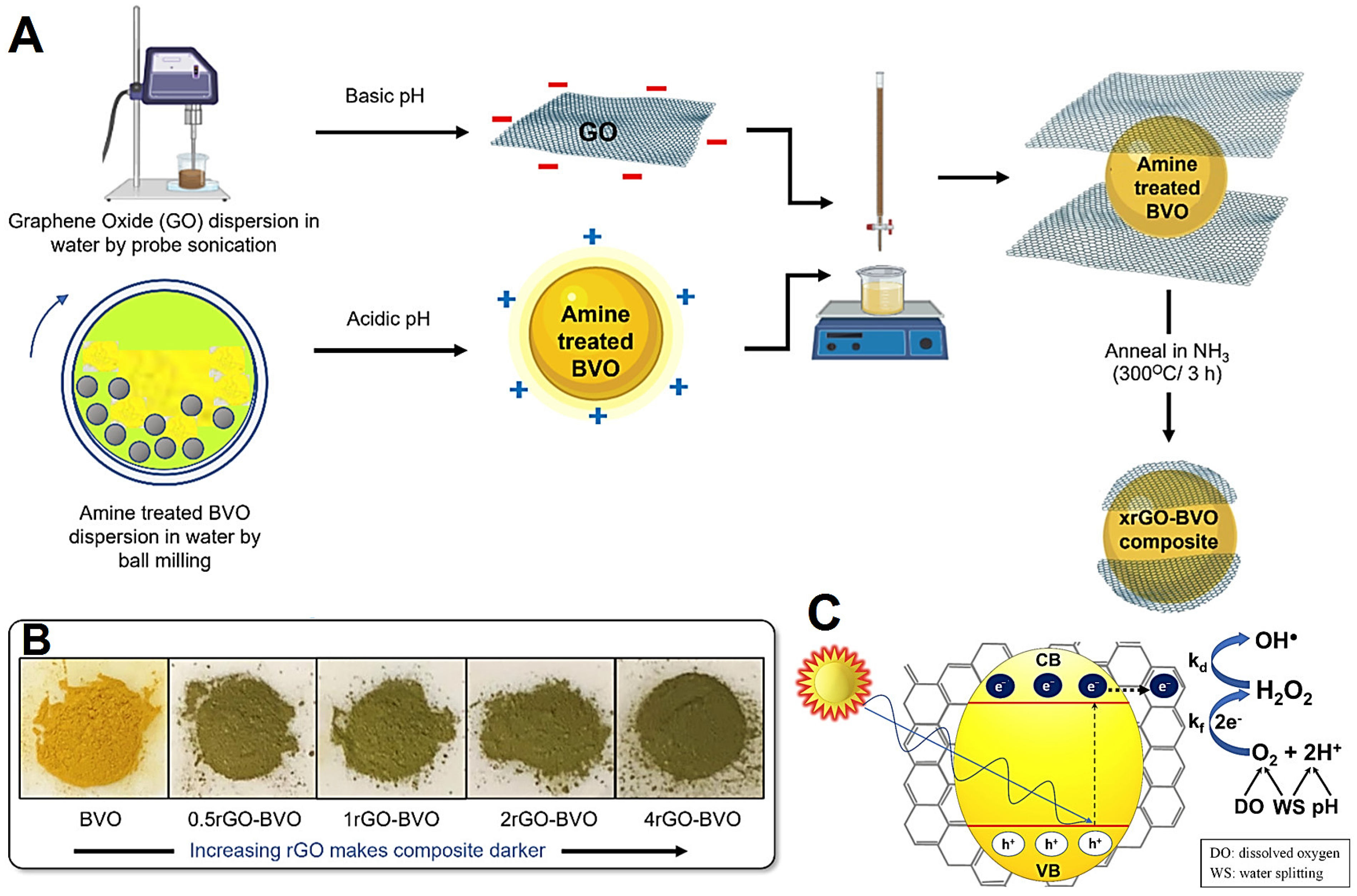
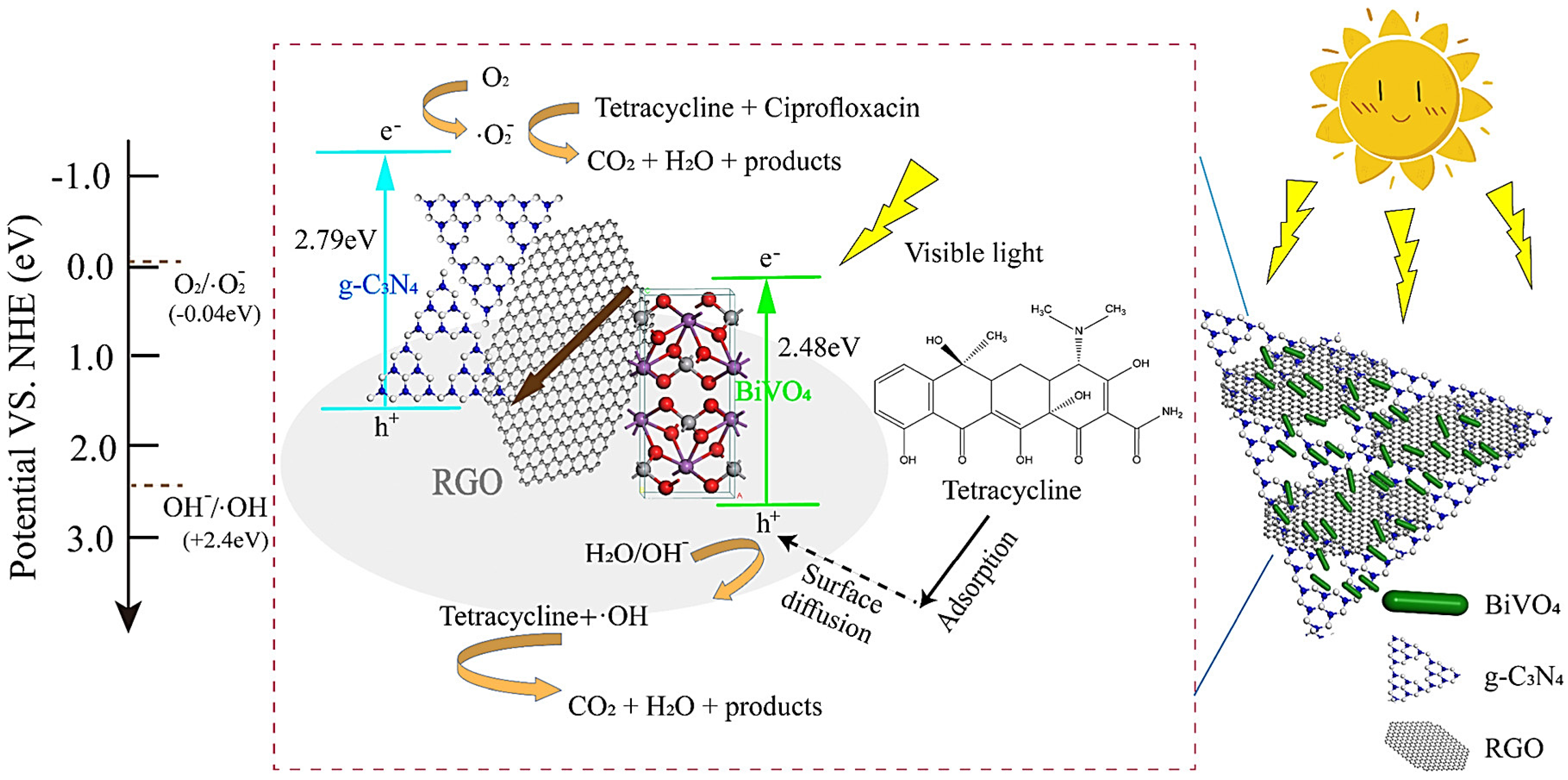
| Salts | Chelating Agents | Crystal Structure | Shape of NPs | Plant Phytochemicals | Applications | Refs. |
|---|---|---|---|---|---|---|
| Bi(NO3)3 and VOSO4 | Flower extract of Callistemon viminalis (bottlebrush) | Monoclinic scheelite | Nanorods (the basal and longitudinal dimensions of the nanorods ranged from 350 to 450 nm and 1.2–2 μm, respectively) | Flavonoids, saponins, alkaloids, steroids and triterpenoids | Photocatalytic activity (methylene blue) | [39] |
| NH4VO3 and Bi(NO3)3 | Fruit extract of Unripe jackfruit | Monoclinic scheelite | The asymmetrically arranged BiVO4 nanostructures (the shapes were nearly spherical and hexagonal); the size being ~90–250 nm | Carotenoids, flavonoids, volatile acids sterols and tannins stilbenoids, and arylbenzofurons | Anticancer (breast cancer cell lines), Photocatalytic activity (Methylene blue) and electrochemical sensor | [40,41] |
| - | Citrus Limon (lemon) | Pure monoclinic | Flower-like structures | Citric acid | Photocatalytic activity (Indigo Carmine) and electrochemical sensor | [42] |
| Bi(NO3)3 and VOSO4 | Fruit extract of Hyphaene thebaica | Clinobisvanite monoclinic | Nanorods (well-aligned rod shaped) | Cinnamic acid, flavonoids, vanillic acid, epicatechin, glycosides, stilbene and sugars composition | Antibacterial, antifungal, and antiviral activity | [43,44] |
| NH4VO3 and Bi(NO3)3 | Fruit extract of Aegle marmelos (bael) | Monoclinic scheelite | - | Flavonoids, polyphenols tannins, alkaloids, coumarins, steroids and natural sugar | Antibacterial, antifungal, and photocatalytic activity (methylene blue) | [38,45] |
| Shape and Crystal Structure of NPs | Plant Extracts | Pollutants | Degradation Efficiency (%) | Absorption Peak (nm) | Gap Band (eV) | Refs. |
|---|---|---|---|---|---|---|
| Monoclinic scheelite and nanorods | Callistemon viminalis | Methylene blue | 82.63 | 661 | 2.59 | [39] |
| Monoclinic scheelite | Aegle marmelos | Methylene blue | 90 | 663 | 2.5 | [38] |
| Monoclinic scheelite and quasispherical-like structure | Unripe jackfruit | Methylene blue | 98.3 | 250–300 | 2.4 | [41] |
| Flower-like and monoclinic scheelite | Citrus Limon | Indigo Carmine | 90.6 | 610 | ∼2.6–2.8 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Q. Alijani, H.; Iravani, S.; Varma, R.S. Bismuth Vanadate (BiVO4) Nanostructures: Eco-Friendly Synthesis and Their Photocatalytic Applications. Catalysts 2023, 13, 59. https://doi.org/10.3390/catal13010059
Q. Alijani H, Iravani S, Varma RS. Bismuth Vanadate (BiVO4) Nanostructures: Eco-Friendly Synthesis and Their Photocatalytic Applications. Catalysts. 2023; 13(1):59. https://doi.org/10.3390/catal13010059
Chicago/Turabian StyleQ. Alijani, Hajar, Siavash Iravani, and Rajender S. Varma. 2023. "Bismuth Vanadate (BiVO4) Nanostructures: Eco-Friendly Synthesis and Their Photocatalytic Applications" Catalysts 13, no. 1: 59. https://doi.org/10.3390/catal13010059
APA StyleQ. Alijani, H., Iravani, S., & Varma, R. S. (2023). Bismuth Vanadate (BiVO4) Nanostructures: Eco-Friendly Synthesis and Their Photocatalytic Applications. Catalysts, 13(1), 59. https://doi.org/10.3390/catal13010059










