Facile Hydrothermal Synthesis of Cu2MoS4 and FeMoS4 for Efficient Adsorption of Chlortetracycline
Abstract
:1. Introduction
2. Results
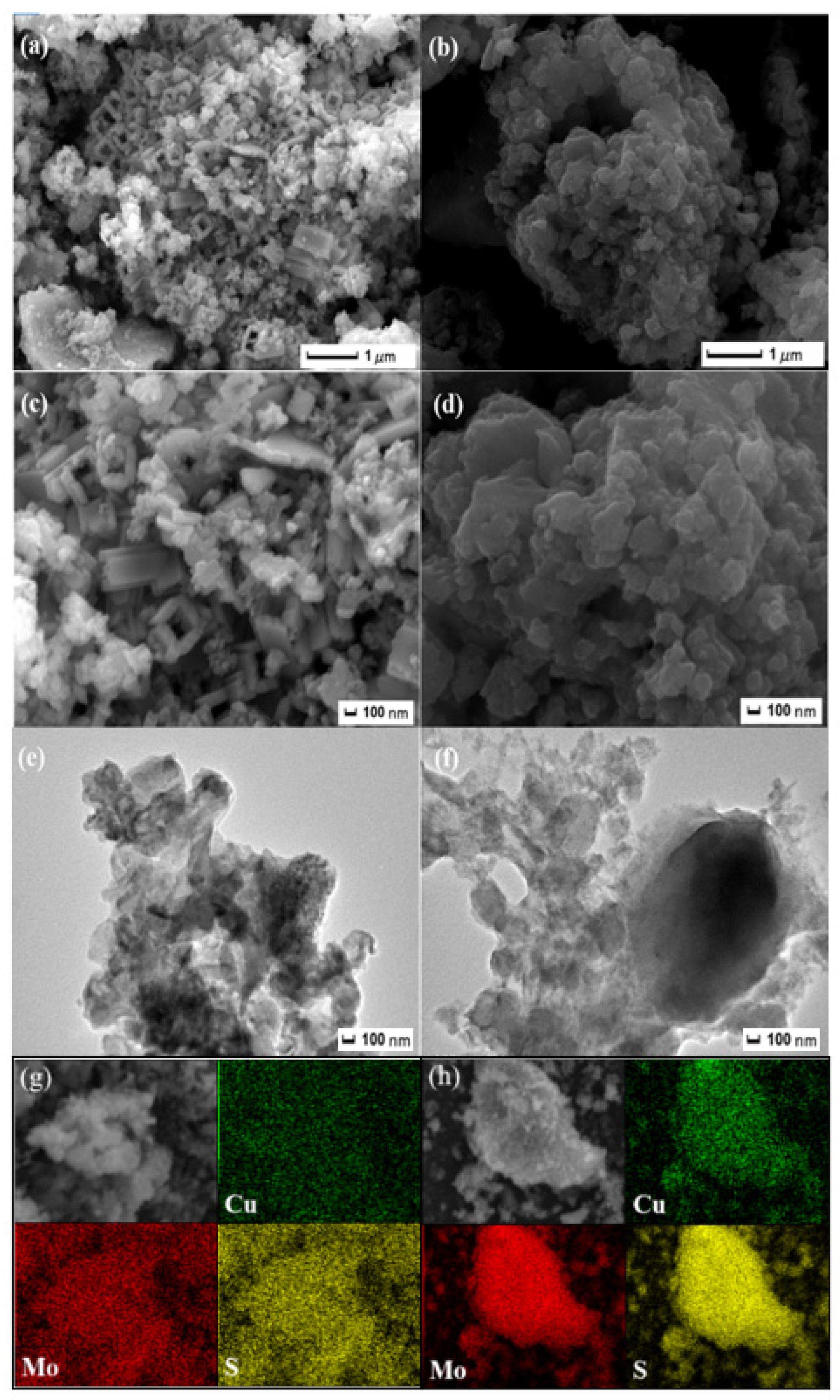
2.1. Characterization and Analysis
2.2. Adsorption Performance of Cu2MoS4 and FeMoS4
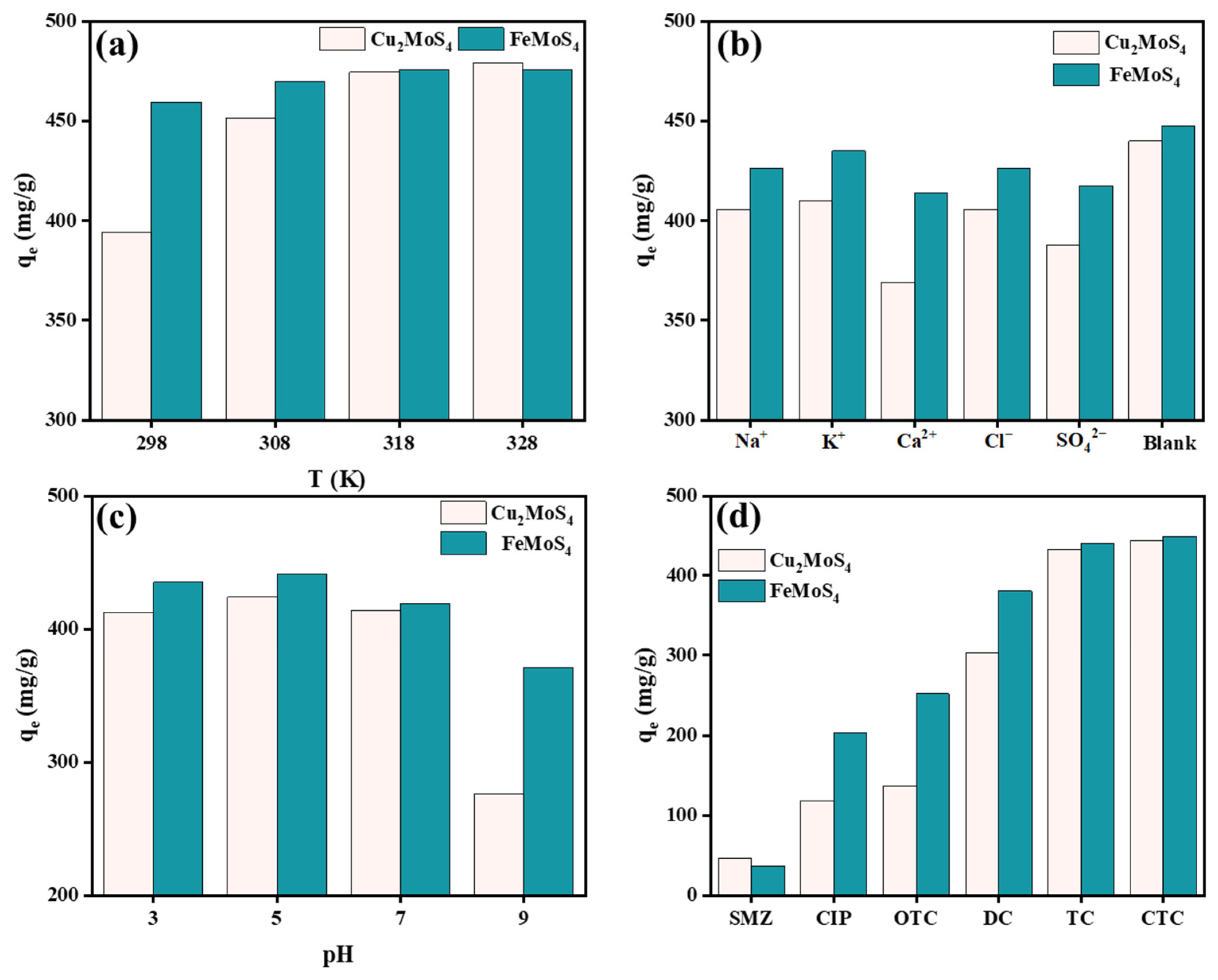
2.2.1. Effect of Synthesis Conditions for Adsorption Performance
2.2.2. Adsorption Kinetics of Cu2MoS4 and FeMoS4 to CTC
2.2.3. Adsorption Isotherms of Cu2MoS4 and FeMoS4 to CTC
2.2.4. Adsorption Thermodynamics
2.2.5. Effect of Solution Coexisting Ions
2.2.6. Effect of Solution pH
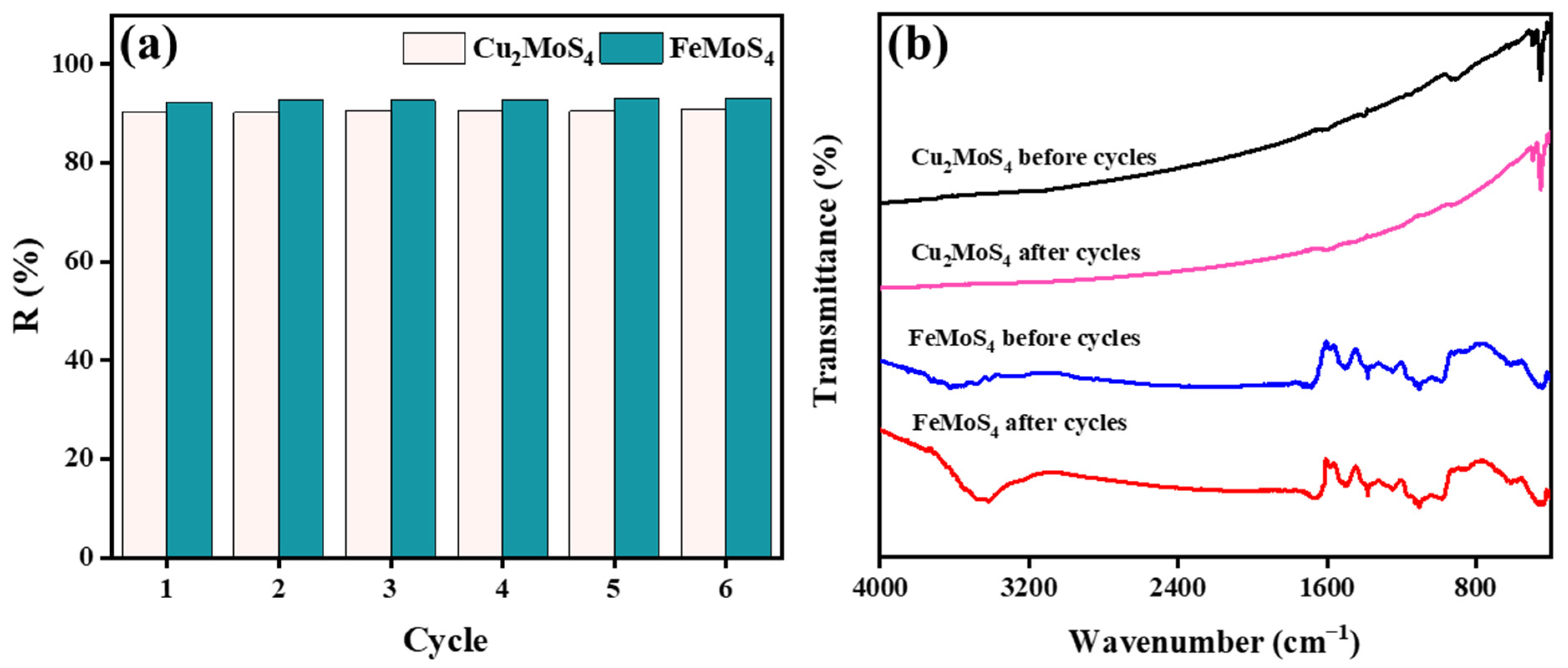
2.2.7. Antibiotic Contamination and Regeneration Study
3. Methods
3.1. Synthesis of Samples
3.1.1. Synthesis of (NH4)2MoS4
3.1.2. Synthesis of Cu2MoS4
3.1.3. Synthesis of FeMoS4
3.2. Adsorption Experiment Set-Up
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Jagdale, P.; Medha, I.; Tiwari, A.K.; Bartoli, M.; Nino, A.; Olivito, F. Biochar-Supported TiO2-Based Nanocomposites for the Photocatalytic Degradation of Sulfamethoxazole in Water-A Review. Toxics 2021, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Shurbaji, S.; Huong, P.T.; Altahtamouni, T.M. Review on the Visible Light Photocatalysis for the Decomposition of Ciprofloxacin, Norfloxacin, Tetracyclines, and Sulfonamides Antibiotics in Wastewater. Catalysts 2021, 11, 437. [Google Scholar] [CrossRef]
- Mourid, E.H.; Lakraimi, M.; Benaziz, L.; Elkhattabi, E.H.; Legrouri, A. Wastewater treatment test by removal of the sulfamethoxazole antibiotic by a calcined layered double hydroxide. Appl. Clay Sci. 2019, 168, 87–95. [Google Scholar] [CrossRef]
- Kong, Y.; Han, K.; Zhuang, Y.; Shi, B. Facile Synthesis of MOFs-Templated Carbon Aerogels with Enhanced Tetracycline Adsorption Performance. Water 2022, 14, 504. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- Mangla, D.; Annu; Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef]
- Wang, H.; Lou, X.; Hu, Q.; Sun, T. Adsorption of antibiotics from water by using Chinese herbal medicine residues derived biochar: Preparation and properties studies. J. Mol. Liq. 2021, 325, 114967. [Google Scholar] [CrossRef]
- Olivito, F.; Jagdale, P. New Technologies to Decontaminate Pollutants in Water: A Report about the State of the Art. Toxics 2022, 10, 128. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, C.; Wang, P.; Rao, L.; Wang, C. Doping of carbon into boron nitride to get the increased adsorption ability for tetracycline from water by changing the pH of solution. Chem. Eng. J. 2020, 387, 124136. [Google Scholar] [CrossRef]
- Yadav, A.; Dindorkar, S.S.; Ramisetti, S.B. Adsorption behaviour of boron nitride nanosheets towards the positive, negative and the neutral antibiotics: Insights from first principle studies. J. Water Process Eng. 2022, 46, 102555. [Google Scholar] [CrossRef]
- Alothman, Z.A.; AlMasoud, N.; Mbianda, X.Y.; Ali, I. Synthesis and characterization of γ-cyclodextrin-graphene oxide nanocomposite: Sorption, kinetics, thermodynamics and simulation studies of tetracycline and chlortetracycline antibiotics removal in water. J. Mol. Liq. 2022, 345, 116993. [Google Scholar] [CrossRef]
- Wu, H.; Chao, Y.; Xia, G.; Luo, J.; Tao, D.; Zhu, L.; Luo, G.; Huang, Y.; Hua, M.; Zhu, W. Enhanced adsorption performance for antibiotics by alcohol-solvent mediated boron nitride nanosheets. Rare Metals 2021, 41, 342–352. [Google Scholar] [CrossRef]
- Sengupta, A.; Jebur, M.; Kamaz, M.; Wickramasinghe, S.R. Removal of Emerging Contaminants from Wastewater Streams Using Membrane Bioreactors: A Review. Membranes 2021, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Arefi-Oskoui, S.; Khataee, A.; Jabbarvand Behrouz, S.; Vatanpour, V.; Haddadi Gharamaleki, S.; Orooji, Y.; Safarpour, M. Development of MoS2/O-MWCNTs/PES blended membrane for efficient removal of dyes, antibiotic, and protein. Sep. Purif. Technol. 2022, 280, 119822. [Google Scholar] [CrossRef]
- Yao, J.; Yang, L.; Huang, L.; Wang, C.; Liu, J.; Huang, L.; Song, Y. Construction of a n-p type Bi12O15Cl6@BiOI-CQDs junction with core-shell structure for boosting photocatalytic degradation and antibacterial performance. Appl. Surf. Sci. 2022, 578, 151913. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, C.; Guo, P.; Gan, S.; Dong, L.; Bai, G.; Guo, Q. A Novel Reduced Graphene Oxide-Attapulgite (RGO-ATP) Supported Fe2O3 Catalyst for Heterogeneous Fenton-like Oxidation of Ciprofloxacin: Degradation Mechanism and Pathway. Catalysts 2020, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Yue, X.; Chang, Y.; Wang, K.; Zhang, J.; Sun, J.; Wei, Z.; Kowalska, E. Photocatalytic Degradation of Tetracycline under Visible Light Irradiation on BiVO4 Microballs Modified with Noble Metals. Catalysts 2022, 12, 1293. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef]
- Al-Mohaimeed, A.M.; Abbasi, A.M.; Ali, M.A.; Dhas, D.S.D. Reduction of multiple antibiotics from the waste water using coated glutathione S-transferase producing biocatalyst. Environ. Res. 2022, 206, 112262. [Google Scholar] [CrossRef] [PubMed]
- Aseman-Bashiz, E.; Rezaee, A.; Moussavi, G. Ciprofloxacin removal from aqueous solutions using modified electrochemical Fenton processes with iron green catalysts. J. Mol. Liq. 2021, 324, 114694. [Google Scholar] [CrossRef]
- Ouyang, Z.; Lei, F.; Hu, E.; Li, S.; Yao, Q.; Guo, X. New insight into transformation of tetracycline in presence of Mn(II): Oxidation versus photolysis. Environ. Pollut. 2022, 300, 118998. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Zheng, B.; Wang, W.; Li, Y.; Yang, Y.; Yang, Z. Magnetic Nitrogen–Doped biochar for adsorptive and oxidative removal of antibiotics in aqueous solutions. Sep. Purif. Technol. 2022, 297, 121508. [Google Scholar] [CrossRef]
- Kouvelis, K.; Kampioti, A.A.; Petala, A.; Frontistis, Z. Degradation of Sulfamethoxazole Using a Hybrid CuOx–BiVO4/SPS/Solar System. Catalysts 2022, 12, 882. [Google Scholar] [CrossRef]
- Juela, D.M. Promising adsorptive materials derived from agricultural and industrial wastes for antibiotic removal: A comprehensive review. Sep. Purif. Technol. 2022, 284, 120286. [Google Scholar] [CrossRef]
- Kern, M.; Skulj, S.; Rozman, M. Adsorption of a wide variety of antibiotics on graphene-based nanomaterials: A modelling study. Chemosphere 2022, 296, 134010. [Google Scholar] [CrossRef]
- Song, Q.; Liang, J.; Fang, Y.; Guo, Z.; Du, Z.; Zhang, L.; Liu, Z.; Huang, Y.; Lin, J.; Tang, C. Nickel (II) modified porous boron nitride: An effective adsorbent for tetracycline removal from aqueous solution. Chem. Eng. J. 2020, 394, 124985. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, J.; Li, H.; Wu, P.; Li, X.; Chang, H.; He, J.; Wu, H.; Li, H.; Zhu, W. Synthesis of boron nitride nanosheets with N-defects for efficient tetracycline antibiotics adsorptive removal. Chem. Eng. J. 2020, 387, 124138. [Google Scholar] [CrossRef]
- Zaher, A.; Taha, M.; Mahmoud, R.K. Possible adsorption mechanisms of the removal of tetracycline from water by La-doped Zn-Fe-layered double hydroxide. J. Mol. Liq. 2021, 322, 114546. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, G.; Kumar, A.; Dhiman, P.; AlGarni, T.S.; Naushad, M.; Alothman, Z.A.; Stadler, F.J. Controlled synthesis of porous Zn/Fe based layered double hydroxides: Synthesis mechanism, and ciprofloxacin adsorption. Sep. Purif. Technol. 2021, 278, 119481. [Google Scholar] [CrossRef]
- Al-Soihi, A.S.; Alsulami, Q.A.; Mostafa, M.M.M. Amalgamated Titanium Oxide-Carbon Hollow Sphere/Nickel-Layered Double Hydroxide as an Efficient Photocatalyst for the Degradation of Methyl Orange. Catalysts 2022, 12, 1200. [Google Scholar] [CrossRef]
- Chao, Y.; Zhu, W.; Wu, X.; Hou, F.; Xun, S.; Wu, P.; Ji, H.; Xu, H.; Li, H. Application of graphene-like layered molybdenum disulfide and its excellent adsorption behavior for doxycycline antibiotic. Chem. Eng. J. 2014, 243, 60–67. [Google Scholar] [CrossRef]
- Liu, J.; Lin, H.; He, Y.; Dong, Y.; Gueret Yadiberet Menzembere, E.R. Novel CoS2/MoS2@Zeolite with excellent adsorption and photocatalytic performance for tetracycline removal in simulated wastewater. J. Clean. Prod. 2020, 260, 121047. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Zheng, Z.; Deng, D. The rise of two-dimensional MoS2 for catalysis. Front. Phys. 2018, 13, 138118. [Google Scholar] [CrossRef]
- Zeng, Z.; Ye, S.; Wu, H.; Xiao, R.; Zeng, G.; Liang, J.; Zhang, C.; Yu, J.; Fang, Y.; Song, B. Research on the sustainable efficacy of g-MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution. Sci. Total Environ. 2019, 648, 206–217. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, S.; Zhang, K.; Song, L. Recent Advances of Ternary Layered Cu2MX4 (M = Mo, W.; X = S, Se) Nanomaterials for Photocatalysis. Solar RRL 2019, 3, 1800320. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Q.; Li, L.; Zhou, X.; Li, L.; Li, H.; Zhai, T. 2D Ternary Chalcogenides. Adv. Opt. Mater. 2018, 6, 18000085. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Pattengale, B.; Yin, J.; An, L.; Cheng, F.; Li, Y.; Huang, J.; Xi, P. High-index faceted CuFeS2 nanosheets with enhanced behavior for boosting hydrogen evolution reaction. Nanoscale 2017, 9, 9230–9237. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Lang, Z.; Sun, H.; Du, Z.; Tan, H.; Qiu, T.; Ho, W.; Zhao, Z.; Wang, Y. Reasonable design of Cu2MoS4 heterophase junction for highly efficient photocatalysis. J. Alloys Compd. 2020, 826, 154076. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, X.-Q.; Jia, Y.; Hou, D.; Li, D.-S. Amorphous CoMoS4 Nanostructure for Photocatalytic H2 Generation, Nitrophenol Reduction, and Methylene Blue Adsorption. ACS Appl. Nano Mater. 2019, 3, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Wang, X.; Gao, J.; Gao, C.; Zhao, R.; Zhai, X.; Wu, Y.; Hao, C.; Yang, J.; Mei, S.; et al. Hydrothermal synthesis of flower-like Cu2MoS4/g-C3N4 composite and its adsorption performances for Rhodamine B. Mater. Chem. Phys. 2019, 223, 648–658. [Google Scholar] [CrossRef]
- Sahoo, S.; Krishnamoorthy, K.; Pazhamalai, P.; Mariappan, V.K.; Kim, S.-J. Copper molybdenum sulfide: A novel pseudocapacitive electrode material for electrochemical energy storage device. Int. J. Hydrogen Energy 2018, 43, 12222–12232. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, Q.; Pan, M.; Dai, L. Interaction between chlortetracycline and calcium-rich biochar: Enhanced removal by adsorption coupled with flocculation. Chem. Eng. J. 2020, 382, 122705. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Feng, P.; Chai, H.; Huang, Y. ZIF-67 derived hollow cobalt sulfide as superior adsorbent for effective adsorption removal of ciprofloxacin antibiotics. Chem. Eng. J. 2018, 344, 95–104. [Google Scholar] [CrossRef]
- Fu, Y.; Peng, L.; Zeng, Q.; Yang, Y.; Song, H.; Shao, J.; Liu, S.; Gu, J. High efficient removal of tetracycline from solution by degradation and flocculation with nanoscale zerovalent iron. Chem. Eng. J. 2015, 270, 631–640. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C.; et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.; Chen, Y.; Luo, Y. Effective removal of tetracycline antibiotics from wastewater using practically applicable iron(III)-loaded cellulose nanofibres. R. Soc. Open Sci. 2021, 8, 210336. [Google Scholar] [CrossRef]
- Yu, L.L.; Luo, Z.F.; Zhang, Y.Y.; Wu, S.C.; Yang, C.; Cheng, J.H. Contrastive removal of oxytetracycline and chlortetracycline from aqueous solution on Al-MOF/GO granules. Environ. Sci. Pollut. Res. Int. 2019, 26, 3685–3696. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zong, L.; Wang, A. One-step synthesis of magnetic attapulgite/carbon supported NiFe-LDHs by hydrothermal process of spent bleaching earth for pollutants removal. J. Clean. Prod. 2018, 172, 673–685. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wen, S.; Xiang, R.; Han, Y.; Tang, W.; Yue, T.; Li, Z. Interface engineering of zeolite imidazolate framework-8 on two-dimensional Al-metal-organic framework nanoplates enhancing performance for simultaneous capture and sensing tetracyclines. J. Hazard. Mater. 2020, 395, 122615. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bai, W.; Wang, G.; Guo, K.; Duan, H.; Xue, Y.; Tang, C. CoO modified porous boron nitride fibers for the adsorption and removal of chlortetracycline from aqueous solution. Colloid Surf. A 2022, 632, 127749. [Google Scholar] [CrossRef]
- Wu, H.; Chao, Y.; Jin, Y.; Tao, D.; Li, X.; Luo, J.; Xia, G.; Zhu, L.; Zhu, W. Sustainable preparation of graphene-analogue boron nitride by ball-milling for adsorption of organic pollutants. Chin. J. Chem. Eng. 2022, 42, 73–81. [Google Scholar] [CrossRef]





| Model | Parameter | Cu2MoS4 | FeMoS4 |
|---|---|---|---|
| qe,exp (mg/g) | 470.81 | 477.79 | |
| Pseudo-first-order | qe,cal (mg/g) | 170.54 | 293.82 |
| R2 | 0.947 | 0.980 | |
| k1 × 10−3 (min−1) | 1.11 | 2.27 | |
| Pseudo-second-order | qe,cal (mg/g) | 467.29 | 490.19 |
| R2 | 0.998 | 0.998 | |
| k2 × 10−5 (g/mg/min) | 3.94 | 2.31 | |
| t1/2 (min) | 54.32 | 88.47 | |
| h (mg/g/min) | 8.61 | 5.54 | |
| Intra-particle | kd1 (mg/g/min) | 21.16 | 16.17 |
| diffusion | I1 | 163.94 | 96.31 |
| R2 | 0.779 | 0.963 | |
| Elovich | α (mg/g/min) | 72.061 | 89.859 |
| β (g/mg) | 0.00697 | 0.0147 | |
| R2 | 0.971 | 0.966 |
| Models | Parameters | Samples | |
|---|---|---|---|
| Cu2MoS4 | FeMoS4 | ||
| Langmuir | KL (L/mg) | 0.276 | 0.108 |
| qm (mg/g) | 1203.81 | 2169.19 | |
| RL | 0.007–0.034 | 0.018–0.084 | |
| R2 | 0.992 | 0.993 | |
| APE% | 2.85 | 1.93 | |
| Freundlich | KF (mg/g) | 499.79 | 425.01 |
| 1/n | 0.183 | 0.369 | |
| R2 | 0.903 | 0.916 | |
| APE% | 8.08 | 9.16 | |
| Temkin | KT (L/mg) | 11.378 | 1.345 |
| B | 167.33 | 422.29 | |
| R2 | 0.962 | 0.986 | |
| APE% | 5.57 | 4.36 | |
| Adsorbent | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| MWCNT/MIL-53(Fe) | 180.68 | [48] |
| Fe(III)@CNFs | 232.56 | [49] |
| Al-MOF/GO | 240.13 | [50] |
| APT/C@NiFe-LDHs | 308.21 | [51] |
| Fe3O4/ZIF-8-G | 608.06 | [52] |
| CoO-c/P-BNFs | 655.47 | [53] |
| Cu2MoS4 | 1203.81 | This work |
| FeMoS4 | 2169.19 | This work |
| ΔH0 (kJ/mol) | ΔS0 (kJ/mol/K) | ΔG0 (kJ/mol) | ||||
|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | |||
| FeMoS4 | 19.45 | 0.101 | −10.44 | −11.81 | −12.97 | −13.38 |
| Cu2MoS4 | 59.61 | 0.225 | −7.41 | −10.21 | −12.81 | −14.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhu, K.; Wang, Y.; Cui, P.; Zhu, L.; Wu, H.; Hua, M.; Huang, Y.; Luo, G.; Chao, Y.; et al. Facile Hydrothermal Synthesis of Cu2MoS4 and FeMoS4 for Efficient Adsorption of Chlortetracycline. Catalysts 2023, 13, 61. https://doi.org/10.3390/catal13010061
Zhou J, Zhu K, Wang Y, Cui P, Zhu L, Wu H, Hua M, Huang Y, Luo G, Chao Y, et al. Facile Hydrothermal Synthesis of Cu2MoS4 and FeMoS4 for Efficient Adsorption of Chlortetracycline. Catalysts. 2023; 13(1):61. https://doi.org/10.3390/catal13010061
Chicago/Turabian StyleZhou, Junhui, Keyu Zhu, Yong Wang, Peng Cui, Linhua Zhu, Haofeng Wu, Mingqing Hua, Yan Huang, Guiling Luo, Yanhong Chao, and et al. 2023. "Facile Hydrothermal Synthesis of Cu2MoS4 and FeMoS4 for Efficient Adsorption of Chlortetracycline" Catalysts 13, no. 1: 61. https://doi.org/10.3390/catal13010061





