PAN/TiO2 Ultrafiltration Membrane for Enhanced BSA Removal and Antifouling Performance
Abstract
:1. Introduction
2. Results and Discussion
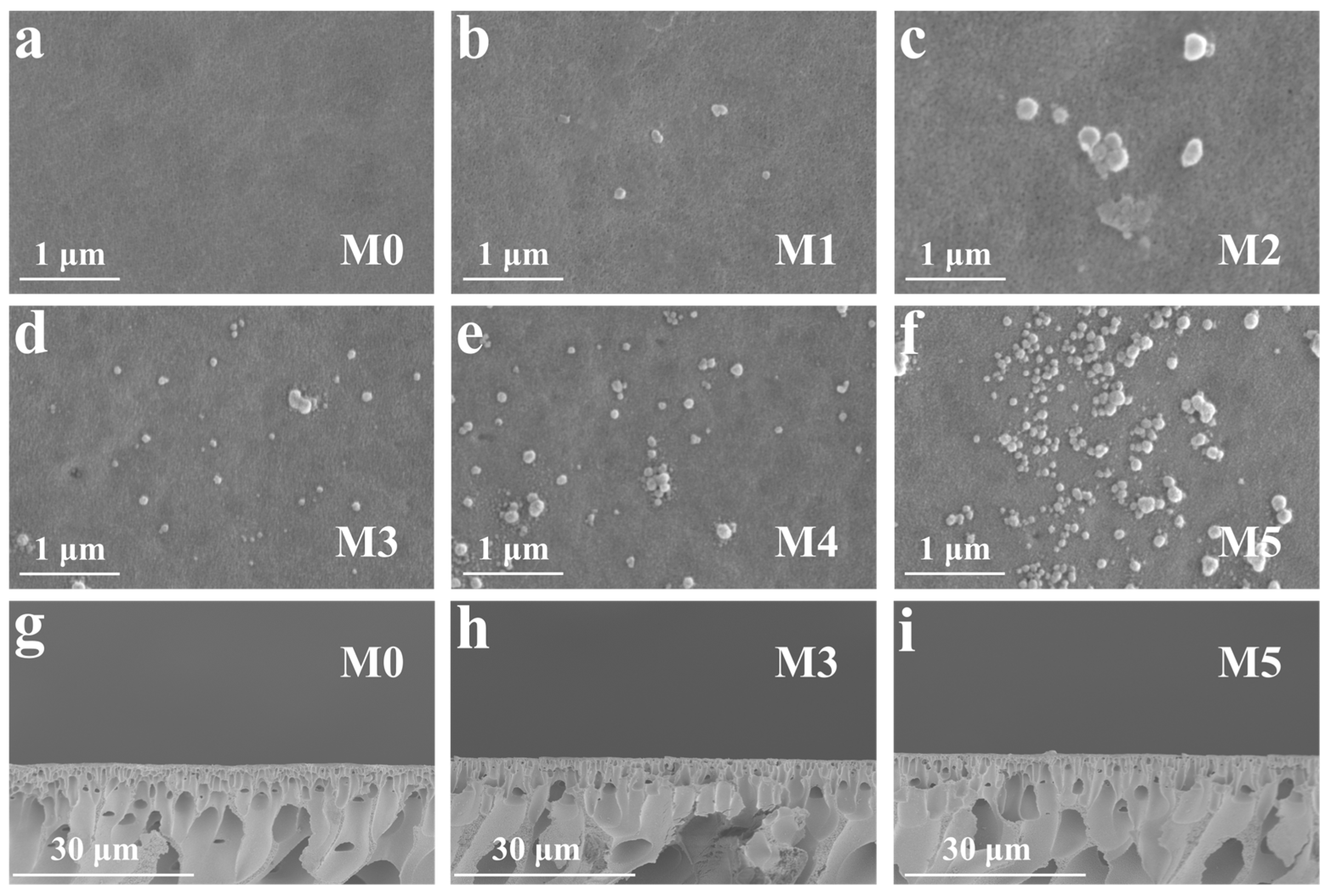
2.1. Morphologies of Membranes
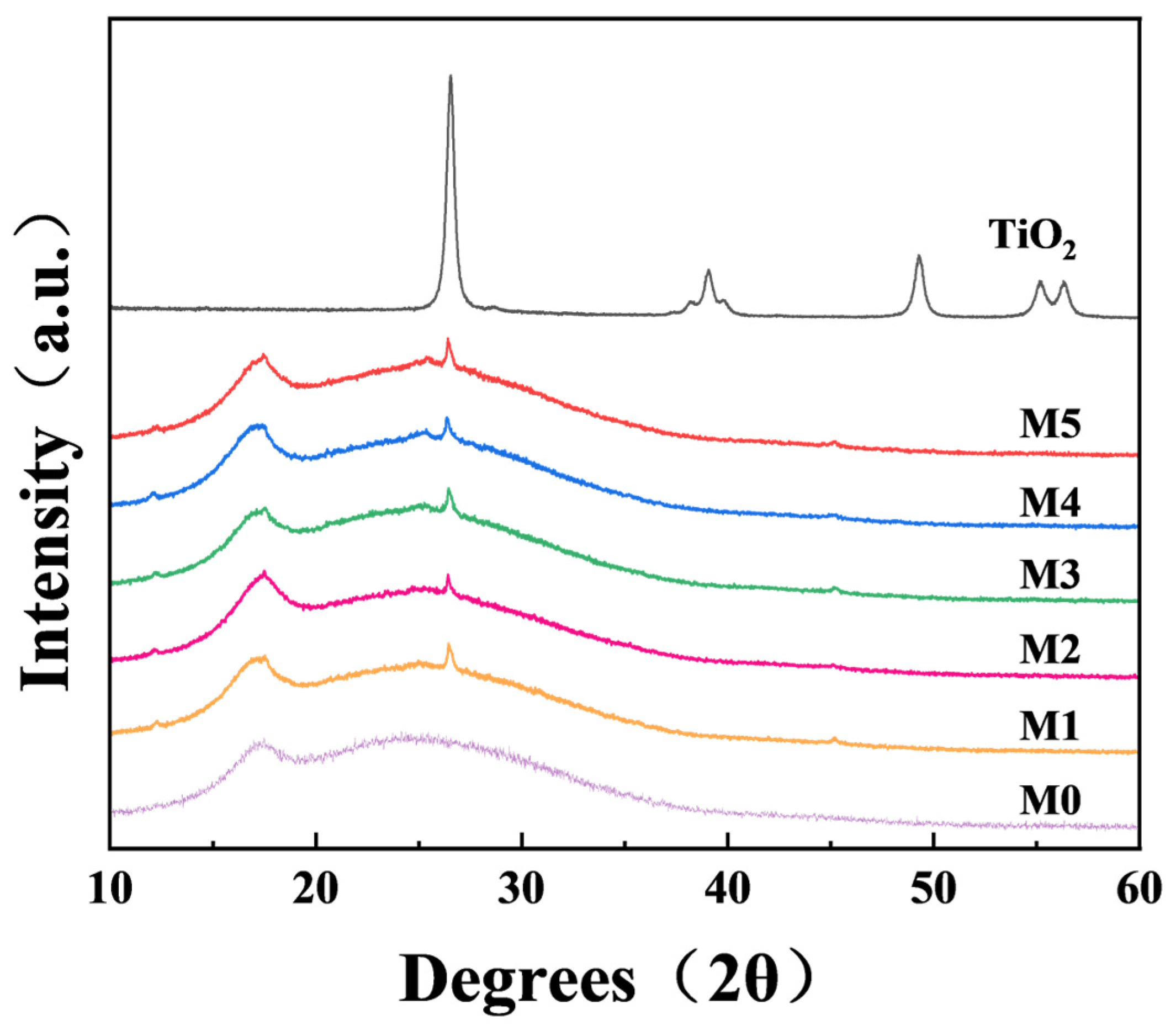
2.2. Chemical Properties of the Membranes
2.3. Separation Performance of the Membranes
2.4. Self-Cleaning Performance of the Membranes
3. Materials and Methods
3.1. Materials
3.2. Preparation of PAN/TiO2 Membranes
3.3. Membrane Characterization
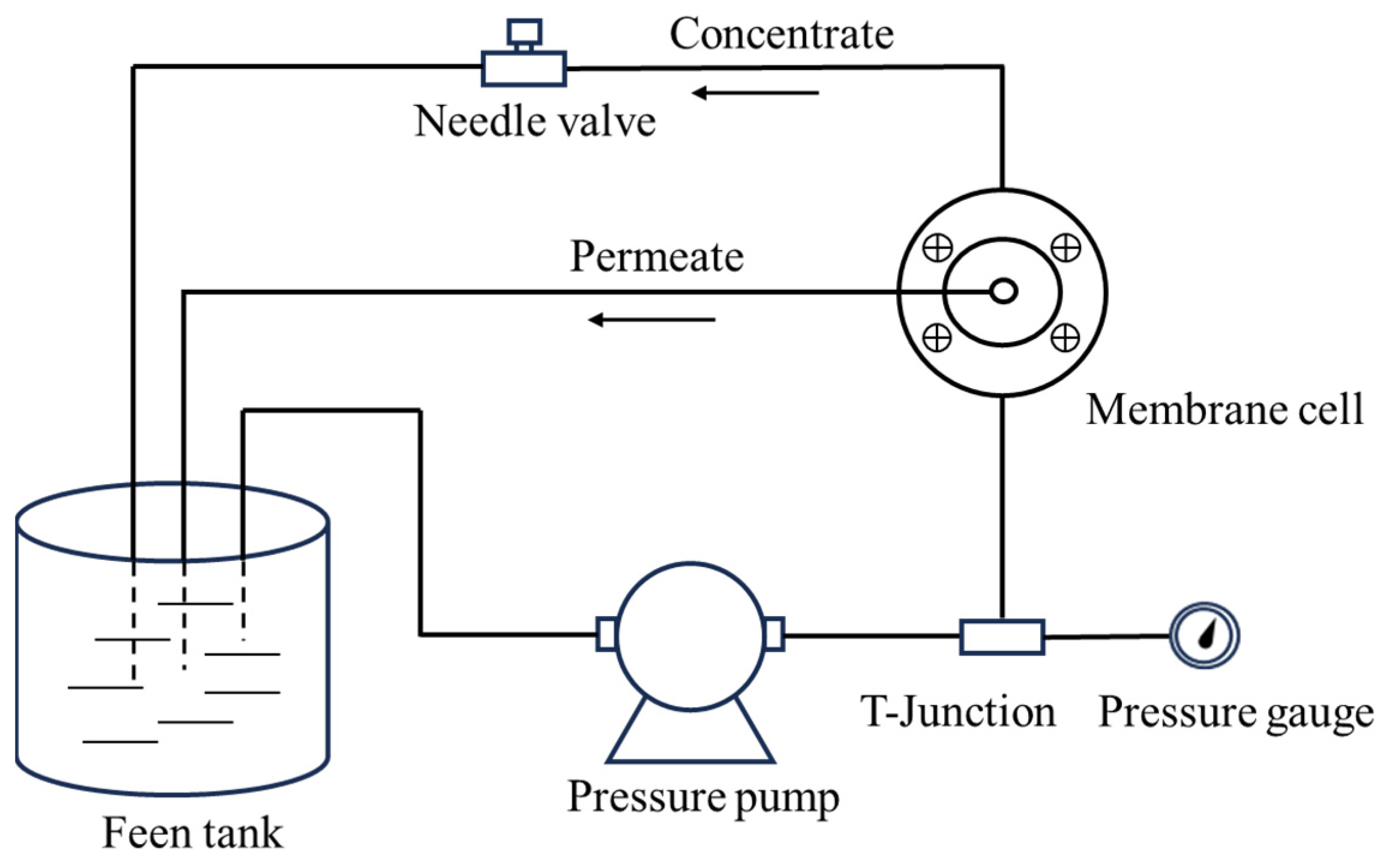
3.4. Ultrafiltration and Antifouling Performance of Membranes
3.5. Self-Cleaning Performance of Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, R.; Khitab, F.; Gul, H.; Khattak, R.; Ihsan, J.; Khan, M.; Khan, A.; Vincevica-Gaile, Z.; Aouissi, H.A. Superparamagnetic zinc ferrite nanoparticles as visible-light active photocatalyst for efficient degradation of selected textile dye in Water. Catalysts 2023, 13, 1061. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Hou, Y.; Li, P.; Sun, H.; Niu, Q.J. Photo-Fenton assisted self-cleaning hybrid ultrafiltration membranes with high-efficient flux recovery for wastewater remediation. Sep. Purif. Technol. 2020, 249, 117159. [Google Scholar] [CrossRef]
- Yabalak, E.; Ozay, Y.; Gizir, A.M.; Dizge, N. Water recovery from textile bath wastewater using combined subcritical water oxidation and nanofiltration. J. Clean. Prod. 2021, 290, 125207. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Zhao, Y.; Wang, C.; Ma, W.; Wong, M.S.; Elimelech, M. In situ electrochemical generation of reactive chlorine species for efficient ultrafiltration membrane self-cleaning. Environ. Sci. Technol. 2020, 54, 6997–7007. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Raisi, B.; Rahmatinejad, J.; Ye, Z.; Barbeau, B.; Rahaman, M.S. A photo-Fenton nanocomposite ultrafiltration membrane for enhanced dye removal with self-cleaning properties. J. Colloid Interface Sci. 2021, 604, 458–468. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, Z.; Li, L.; Xiao, P.; Ma, Y.; Liu, F.; Li, J. PVDF/MOFs mixed matrix ultrafiltration membrane for efficient water treatment. Front. Chem. 2022, 10, 985750. [Google Scholar] [CrossRef]
- Orudzhev, F.; Alikhanov, N.; Amirov, A.; Rabadanova, A.; Selimov, D.; Shuaibov, A.; Gulakhmedov, R.; Abdurakhmanov, M.; Magomedova, A.; Ramazanov, S.; et al. Porous hybrid PVDF/BiFeO3 smart composite with magnetic, piezophotocatalytic, and light-emission properties. Catalysts 2023, 13, 874. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, C.; Jin, J.; Xu, W.; Wu, Q.; Gu, J.; Ou, M.; Xu, X. Development of a novel mixed titanium, silver oxide polyacrylonitrile nanofiber as a superior adsorbent and its application for MB removal in wastewater treatment. J. Braz. Chem. Soc. 2018, 29, 560–571. [Google Scholar] [CrossRef]
- Swaminathan, S.; Muthumanickkam, A.; Imayathamizhan, N. An effective removal of methylene blue dye using polyacrylonitrile yarn waste/graphene oxide nanofibrous composite. Int. J. Environ. Sci. Technol. 2015, 12, 3499–3508. [Google Scholar] [CrossRef]
- Fernando, T.L.D.; Ray, S.; Simpson, C.M.; Gommans, L.; Morrison, S. Remediation of fouling on painted steel roofing via solar energy assisted photocatalytic self-cleaning technology: Recent developments and future perspectives. Adv. Eng. Mater. 2022, 24, 2101486. [Google Scholar] [CrossRef]
- Ren, G.; Li, R.; Zhao, M.; Hou, Q.; Rao, T.; Zhou, M.; Ma, X. Membrane electrodes for electrochemical advanced oxidation processes: Preparation, self-cleaning mechanisms and prospects. Chem. Eng. J. 2023, 451, 138907. [Google Scholar] [CrossRef]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Cao, X.-L.; Zhou, F.-Y.; Cai, J.; Zhao, Y.; Liu, M.-L.; Xu, L.; Sun, S.-P. High-permeability and anti-fouling nanofiltration membranes decorated by asymmetric organic phosphate. J. Membr. Sci. 2021, 617, 118667. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, X.; Boo, C.; Zhu, S.; Lu, Y.; Fan, W.; Huo, M.; Elimelech, M.; Yang, X. Self-cleaning anti-fouling hybrid ultrafiltration membranes via side chain grafting of poly(aryl ether sulfone) and titanium dioxide. J. Membr. Sci. 2017, 529, 1–10. [Google Scholar] [CrossRef]
- Huang, S.; Ras, R.H.A.; Tian, X. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Rosman, N.; Wan Salleh, W.N.; Jaafar, J.; Harun, Z.; Aziz, F.; Ismail, A.F. Photocatalytic filtration of zinc oxide-based membrane with enhanced visible light responsiveness for ibuprofen removal. Catalysts 2022, 12, 209. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Li, K.; Chen, S.; Yue, D. Photocatalytic reactor as a bridge to Link the commercialization of photocatalyst in water and air purification. Catalysts 2022, 12, 724. [Google Scholar] [CrossRef]
- Lee, J.-P.; Choi, S.; Cho, S.; Song, W.-J.; Park, S. Fabrication of carbon nanofibers decorated with various kinds of metal oxides for battery applications. Energies 2021, 14, 1353. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Yu, S.; Chen, B.; Chen, C.; Shen, L.; Li, B.; Lin, H. The coupling of persulfate activation and membrane separation for the effective pollutant degradation and membrane fouling alleviation. Chem. Eng. J. 2023, 451, 139009. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Sisay, E.J.; Kertész, S.; Fazekas, Á.; Jákói, Z.; Kedves, E.Z.; Gyulavári, T.; Ágoston, Á.; Veréb, G.; László, Z. Application of BiVO4/TiO2/CNT composite photocatalysts for membrane fouling control and photocatalytic membrane regeneration during dairy wastewater treatment. Catalysts 2023, 13, 315. [Google Scholar] [CrossRef]
- Zhang, H.; Mane, A.U.; Yang, X.; Xia, Z.; Barry, E.F.; Luo, J.; Wan, Y.; Elam, J.W.; Darling, S.B. Visible-light-activated photocatalytic films toward self-cleaning membranes. Adv. Funct. Mater. 2020, 30, 2002847. [Google Scholar] [CrossRef]
- Deemter, D.; Coelho, F.E.B.; Oller, I.; Malato, S.; Amat, A.M. Assessment of a Novel Photocatalytic TiO2-Zirconia Ultrafiltration Membrane and Combination with Solar Photo-Fenton Tertiary Treatment of Urban Wastewater. Catalysts 2022, 12, 552. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Polyvinylidene fluoride ultrafiltration membrane blended with nano-ZnO particle for photo-catalysis self-cleaning. Desalination 2014, 332, 67–75. [Google Scholar] [CrossRef]
- Meng, M.; Li, B.; Zhu, Y.; Yan, Y.; Feng, Y. A novel mixed matrix polysulfone membrane for enhanced ultrafiltration and photocatalytic self-cleaning performance. J. Colloid Interface Sci. 2021, 599, 178–189. [Google Scholar] [CrossRef]
- Bao, X.; Liu, Q.; Yang, J.; Wang, F.; Yu, F.; Yu, J.; Yang, Y. Cascading in-situ generation of H2O2 and Fenton-like reaction in photocatalytic composite ultrafiltration membrane for high self-cleaning performance in wastewater treatment. J. Membr. Sci. 2022, 660, 120866. [Google Scholar] [CrossRef]
- Wu, J.; Yi, S.; Wang, Y.; Yao, J.; Gao, W. Polymer-based TiO2 nanocomposite membrane: Synthesis and organic pollutant removal. Int. J. Smart Nano Mater. 2021, 12, 129–145. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, G.; Han, K.; Ye, H.; Wei, S.; Zhou, Y. One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: Crossflow filtration operation and membrane fouling analysis. Chem. Eng. Process.-Process Intensif. 2017, 120, 20–26. [Google Scholar] [CrossRef]
- Pan, Z.; Cao, S.; Li, J.; Du, Z.; Cheng, F. Anti-fouling TiO2 nanowires membrane for oil/water separation: Synergetic effects of wettability and pore size. J. Membr. Sci. 2019, 572, 596–606. [Google Scholar] [CrossRef]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with ionic liquid modified nano-TiO2 particles. J. Membr. Sci. 2013, 427, 259–269. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J. Macroporous PVDF/TiO2 membranes with three-dimensionally interconnected pore structures produced by directional melt crystallization. Chem. Eng. J. 2016, 301, 158–165. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Y.; Shen, L.; Xu, Y.; Li, R.; Huang, L.; Lin, H. Magnetic field assisted arrangement of photocatalytic TiO2 particles on membrane surface to enhance membrane antifouling performance for water treatment. J. Colloid Interface Sci. 2020, 570, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Ma, W.; Pan, J.; Ren, W.; Chen, L.; Huang, L.; Xu, S.; Jiang, Z. Fabrication of antibacterial and self-cleaning CuxP@g-C3N4/PVDF-CTFE mixed matrix membranes with enhanced properties for efficient ultrafiltration. J. Membr. Sci. 2022, 659, 120792. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Loginova, E.; Plisko, T.; Burts, K.; Bildyukevich, A.; Penkova, A. Modification strategies of polyacrylonitrile ultrafiltration membrane using TiO2 for enhanced antifouling performance in water treatment. Sep. Purif. Technol. 2022, 286, 120500. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Gao, Q.; Chen, M.; Wang, Y.; Yao, Z. A new perspective on the internal structure of polyacrylonitrile-based preoxidized fibers through ultrathin sections. Polym. Degrad. Stab. 2019, 167, 269–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Li, L.; Wang, H.; Dong, X.; Pan, Y.; Wang, T. Insight into the influences of thermal crosslinking on the transition from polyacrylonitrile based ultrafiltration membrane to organic solvent nanofiltration membrane. J. Membr. Sci. 2023, 679, 121694. [Google Scholar] [CrossRef]
- Akhter, P.; Nawaz, S.; Shafiq, I.; Nazir, A.; Shafique, S.; Jamil, F.; Park, Y.-K.; Hussain, M. Efficient visible light assisted photocatalysis using ZnO/TiO2 nanocomposites. Mol. Catal. 2023, 535, 112896. [Google Scholar] [CrossRef]
- Xiu, L.; Wang, Z.; Yu, M.; Wu, X.; Qiu, J. Aggregation-Resistant 3D MXene-Based Architecture as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Nano 2018, 12, 8017–8028. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Li, X.; Dong, L.; Wang, Z.; Shen, J.; Van der Bruggen, B. Membranes with ZIF-8 regulated MXene nanosheet stacks for efficient molecular sieving. Desalination 2023, 546, 116184. [Google Scholar] [CrossRef]
- Chi, M.; Sun, X.; Lozano-Blanco, G.; Tatarchuk, B.J. XPS and FTIR investigations of the transient photocatalytic decomposition of surface carbon contaminants from anatase TiO2 in UHV starved water/oxygen environments. Appl. Surf. Sci. 2021, 570, 151147. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, L.; Yang, Z.; Zhang, R.; Liu, Y.-N.; He, M.; Su, Y.; Jiang, Z. Loose nanofiltration membrane for dye/salt separation through interfacial polymerization with in-situ generated TiO2 nanoparticles. Appl. Surf. Sci. 2017, 410, 494–504. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Roh, I.J.; Greenberg, A.R.; Khare, V.P. Synthesis and characterization of interfacially polymerized polyamide thin films. Desalination 2006, 191, 279–290. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dinh, T.T.L.; Ahn, Y.-H. Enhanced antifouling performance of PVDF ultrafiltration membrane by blending zinc oxide with support of graphene oxide nanoparticle. Chemosphere 2020, 241, 125068. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P. Fabrication of cellulose acetate–zirconia hybrid membranes for ultrafiltration applications: Performance, structure and fouling analysis. Sep. Purif. Technol. 2010, 74, 230–235. [Google Scholar] [CrossRef]
- Vetrivel, S.; Rana, D.; Sri Abirami Saraswathi, M.S.; Divya, K.; Kaleekkal, N.J.; Nagendran, A. Cellulose acetate nanocomposite ultrafiltration membranes tailored with hydrous manganese dioxide nanoparticles for water treatment applications. Polym. Adv. Technol. 2019, 30, 1943–1950. [Google Scholar] [CrossRef]
- Jafar Mazumder, M.A.; Raja, P.H.; Isloor, A.M.; Usman, M.; Chowdhury, S.H.; Ali, S.A.; Inamuddin; Al-Ahmed, A. Assessment of sulfonated homo and co-polyimides incorporated polysulfone ultrafiltration blend membranes for effective removal of heavy metals and proteins. Sci. Rep. 2020, 10, 7049. [Google Scholar] [CrossRef]
- Rameesha, L.; Rana, D.; Kaleekkal, N.J.; Nagendran, A. Efficacy of MOF-199 in improvement of permeation, morphological, antifouling and antibacterial characteristics of polyvinylidene fluoride membranes. New J. Chem. 2022, 46, 7638–7649. [Google Scholar] [CrossRef]
- Rong, G.; Zhou, D.; Pang, J. Preparation of high-performance antifouling polyphenylsulfone ultrafiltration membrane by the addition of sulfonated polyaniline. J. Polym. Res. 2018, 25, 66. [Google Scholar] [CrossRef]
- Sri Abirami Saraswathi, M.; Kausalya, R.; Kaleekkal, N.J.; Rana, D.; Nagendran, A. BSA and humic acid separation from aqueous stream using polydopamine coated PVDF ultrafiltration membranes. J. Environ. Chem. Eng. 2017, 5, 2937–2943. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Bao, C.; Xu, X.; Li, D.; Chen, J.; Hong, M.; Peng, B.; Zhang, Q. Self-cleaning catalytic membrane for water treatment via an integration of Heterogeneous Fenton and membrane process. J. Membr. Sci. 2021, 624, 119121. [Google Scholar] [CrossRef]
- Ademola Bode-Aluko, C.; Pereao, O.; Kyaw, H.H.; Al-Naamani, L.; Al-Abri, M.Z.; Tay Zar Myint, M.; Rossouw, A.; Fatoba, O.; Petrik, L.; Dobretsov, S. Photocatalytic and antifouling properties of electrospun TiO2 polyacrylonitrile composite nanofibers under visible light. Mater. Sci. Eng. B 2021, 264, 114913. [Google Scholar] [CrossRef]
- Khalid, A.; Abdel-Karim, A.; Ali Atieh, M.; Javed, S.; McKay, G. PEG-CNTs nanocomposite PSU membranes for wastewater treatment by membrane bioreactor. Sep. Purif. Technol. 2018, 190, 165–176. [Google Scholar] [CrossRef]
- Ma, T.; Su, Y.; Li, Y.; Zhang, R.; Liu, Y.; He, M.; Li, Y.; Dong, N.; Wu, H.; Jiang, Z. Fabrication of electro-neutral nanofiltration membranes at neutral pH with antifouling surface via interfacial polymerization from a novel zwitterionic amine monomer. J. Membr. Sci. 2016, 503, 101–109. [Google Scholar] [CrossRef]







| Membrane | Operating Pressure (bar) | Permeability (L m−2 h−1 bar−1) | BSA Concentrations (g/L) | BSA Rejection (%) | Ref. |
|---|---|---|---|---|---|
| CA-1 | 3.5 | 41.6 ± 0.4 | 1.0 | 95.9 ± 0.6 | [49] |
| PSf-sPI5 | 5.0 | 72.1 ± 0.8 | 0.8 | 98.0 ± 0.5 | [50] |
| PVDF/MOF-199 | 3.5 | 54.2 ± 0.4 | 1.0 | 96.2 ± 0.2 | [51] |
| PPSU/SPANI | 1.5 | 173.3 ± 2.6 | 1.0 | 95.5 ± 0.6 | [52] |
| PVDF/PEG/PD | 4.1 | 28.8 ± 0.6 | 1.0 | 90.5 ± 0.5 | [53] |
| MIL53(Al)/LiCl@PVDF | 1.0 | 43.6 ± 1.0 | 1.0 | 82.1 ± 0.9 | [7] |
| M3 | 2.0 | 207.0 ± 10.4 | 1.0 | 99.0 ± 0.3 | This work |
| Membrane ID | Compositions | ||
|---|---|---|---|
| DMF (mL) | PAN (wt%) | TiO2 (wt%) | |
| PAN13 | 20.0 | 13.0 | - |
| PAN14 | 20.0 | 14.0 | - |
| PAN15/M0 | 20.0 | 15.0 | - |
| PAN16 | 20.0 | 16.0 | - |
| PAN17 | 20.0 | 17.0 | - |
| M1 | 20.0 | 15.0 | 0.2 |
| M2 | 20.0 | 15.0 | 0.4 |
| M3 | 20.0 | 15.0 | 0.6 |
| M4 | 20.0 | 15.0 | 0.8 |
| M5 | 20.0 | 15.0 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Wang, X.; Li, H.; Wang, T.; Feng, W.; Li, J. PAN/TiO2 Ultrafiltration Membrane for Enhanced BSA Removal and Antifouling Performance. Catalysts 2023, 13, 1320. https://doi.org/10.3390/catal13101320
Xie Y, Wang X, Li H, Wang T, Feng W, Li J. PAN/TiO2 Ultrafiltration Membrane for Enhanced BSA Removal and Antifouling Performance. Catalysts. 2023; 13(10):1320. https://doi.org/10.3390/catal13101320
Chicago/Turabian StyleXie, Yinshan, Xinning Wang, Hulin Li, Tao Wang, Wei Feng, and Jian Li. 2023. "PAN/TiO2 Ultrafiltration Membrane for Enhanced BSA Removal and Antifouling Performance" Catalysts 13, no. 10: 1320. https://doi.org/10.3390/catal13101320
APA StyleXie, Y., Wang, X., Li, H., Wang, T., Feng, W., & Li, J. (2023). PAN/TiO2 Ultrafiltration Membrane for Enhanced BSA Removal and Antifouling Performance. Catalysts, 13(10), 1320. https://doi.org/10.3390/catal13101320








