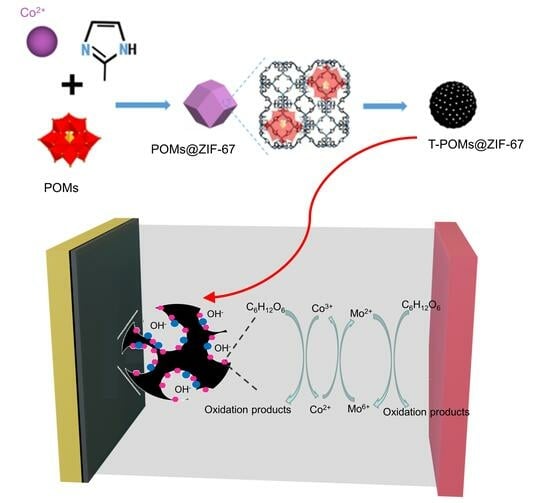
Pyrolyzed POMs@ZIF-67 Exhibiting High Performance as Direct Glucose Fuel Cell Anode Catalysts
Abstract
:1. Introduction
2. Results and Discussion
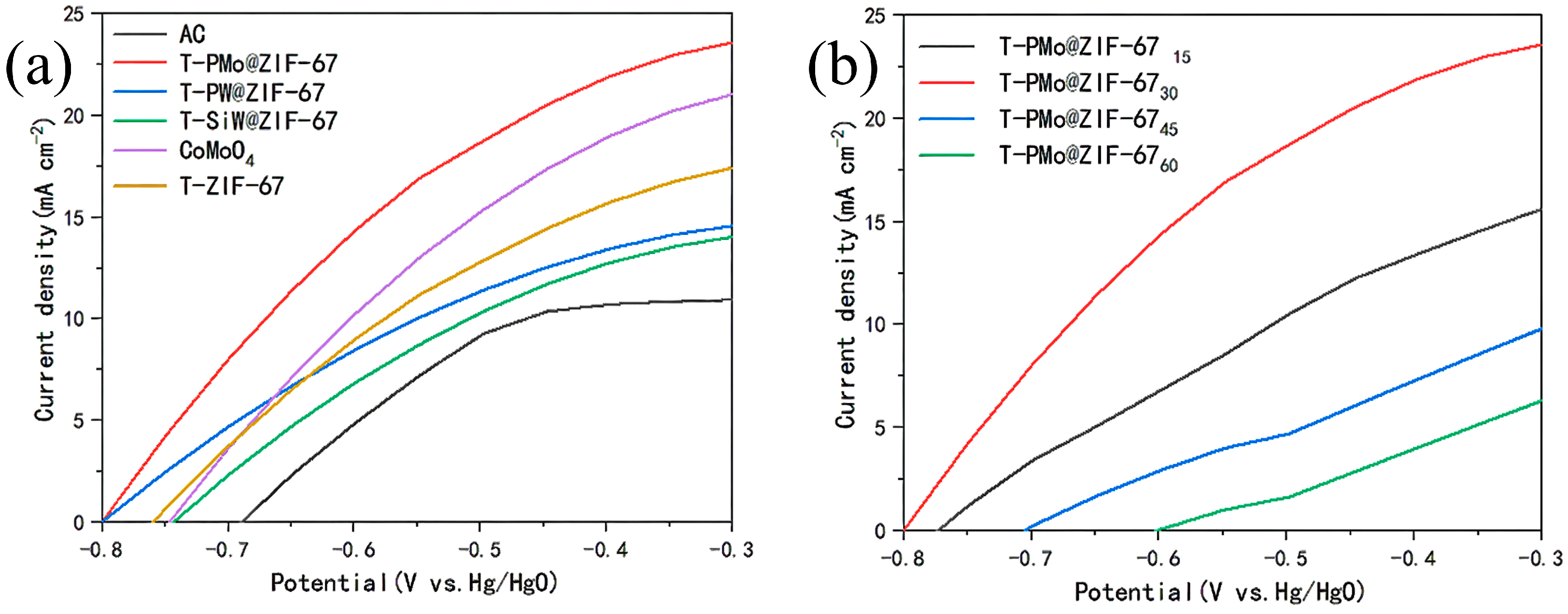
2.1. Electrochemical Performance Test of Catalyst Anodes
2.1.1. Linear Sweep Voltammetry Test for Anodes
2.1.2. Electrochemical Impedance Testing of Anodes
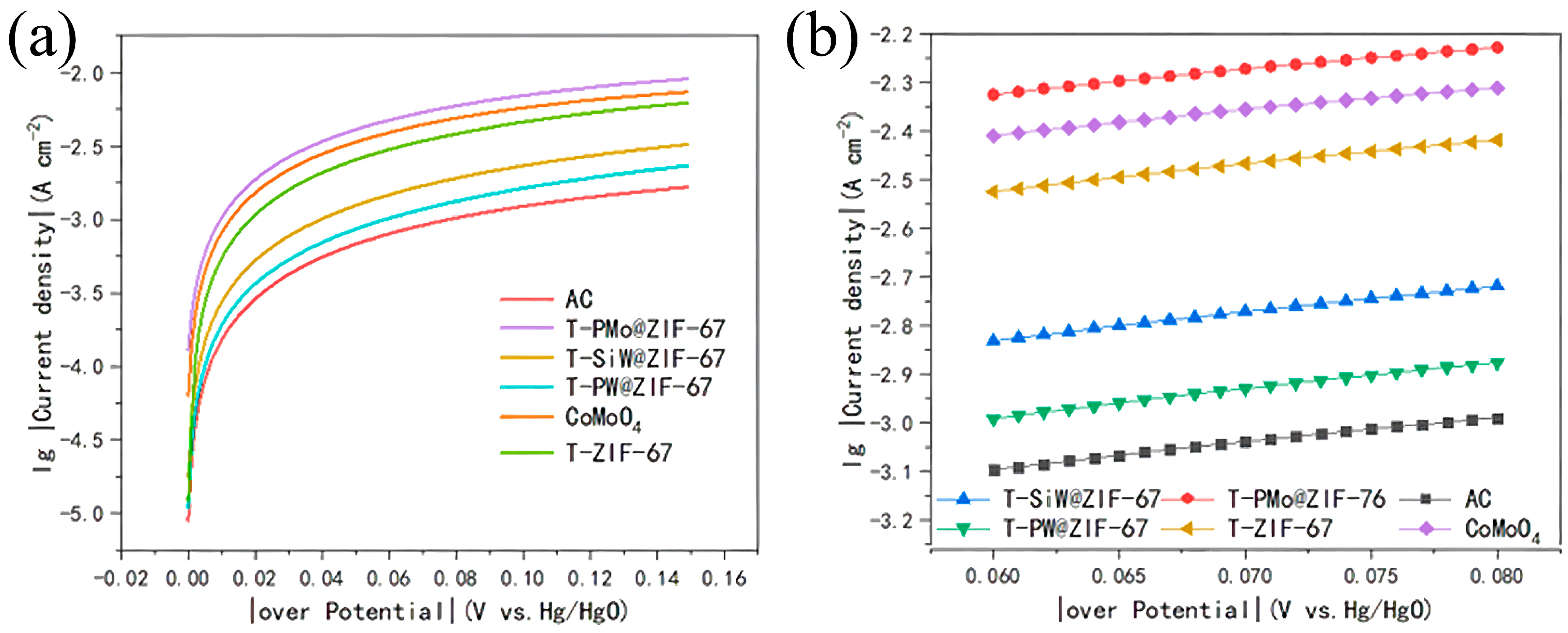
2.1.3. The Tafel Curve of Anodes
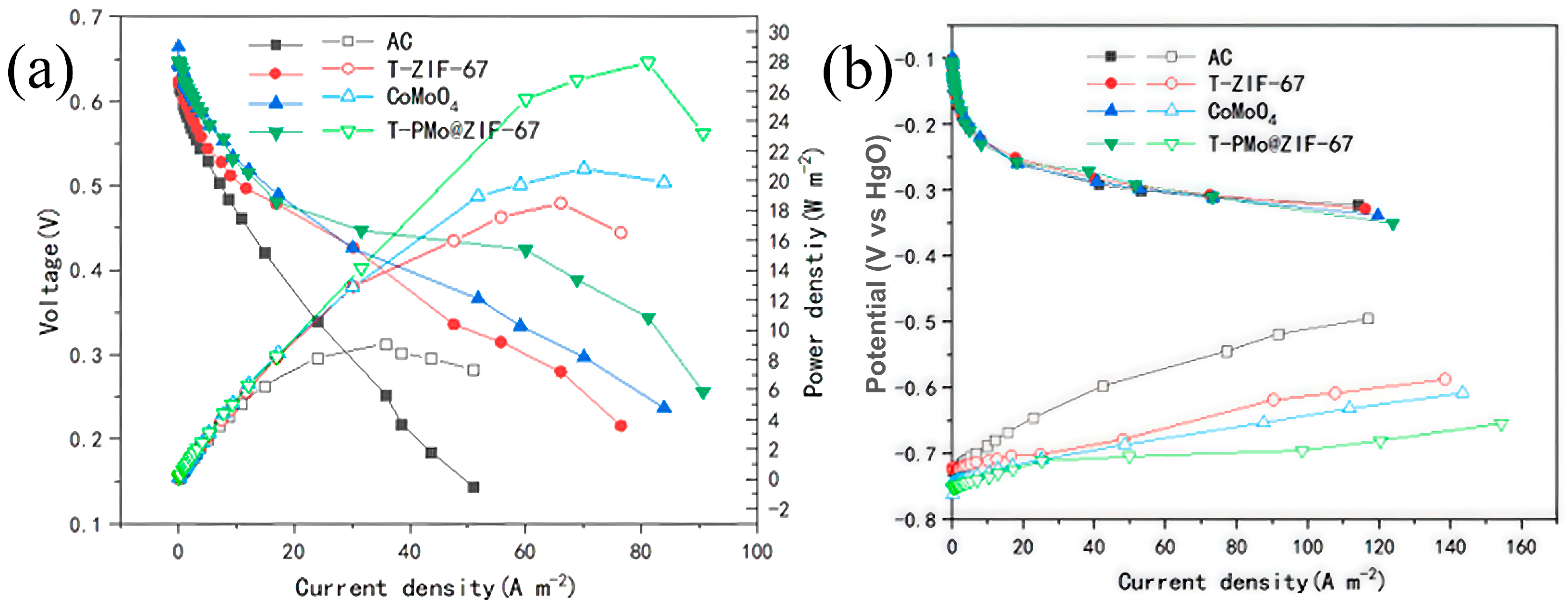
2.1.4. Power Density and Polarization Curves of Different Anodes in Fuel Cells
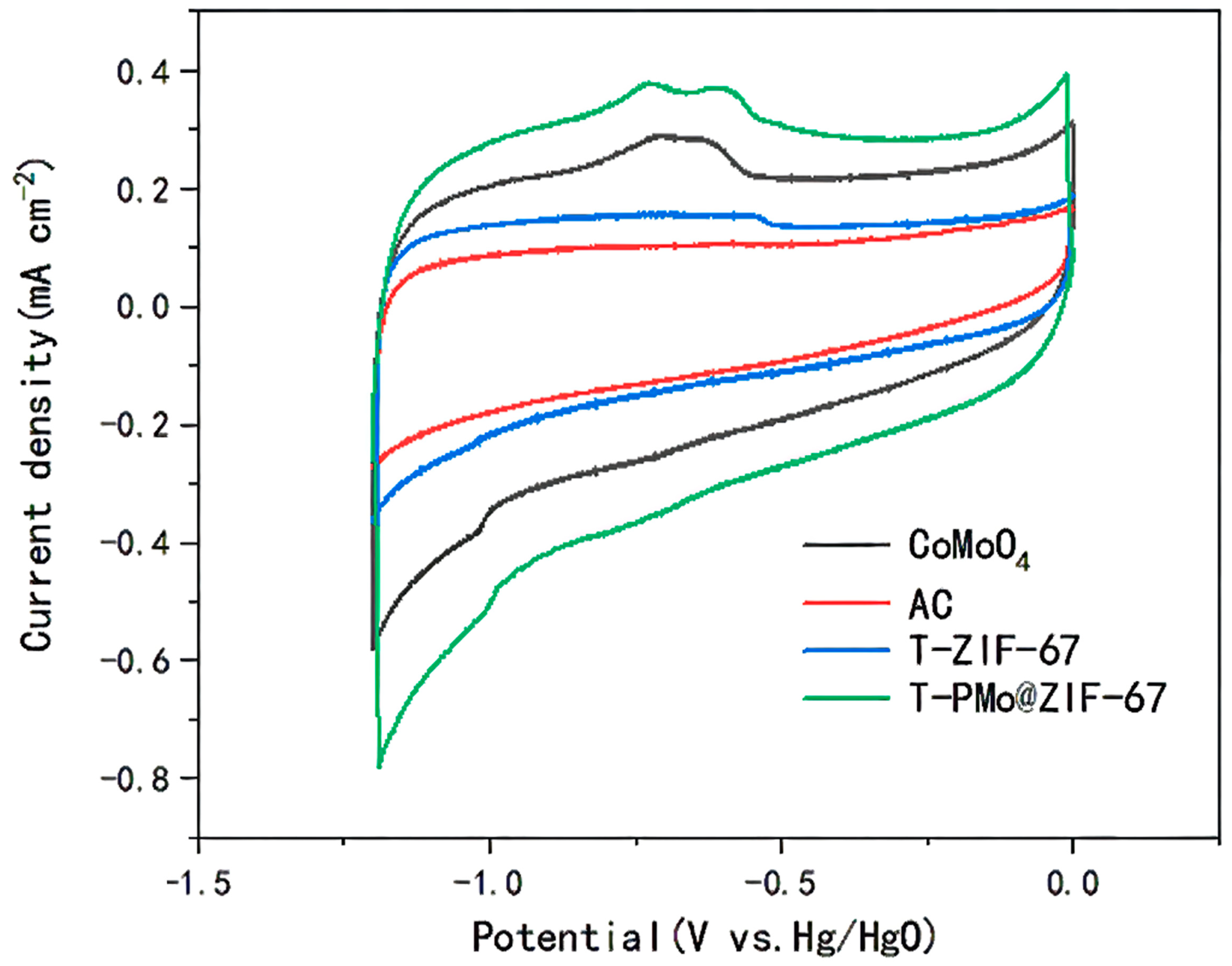
2.1.5. Cyclic Voltammetry Test of Anodes
2.2. Characterization of T-PMo@ZIF-67
2.2.1. Scanning Electron Microscope Analysis
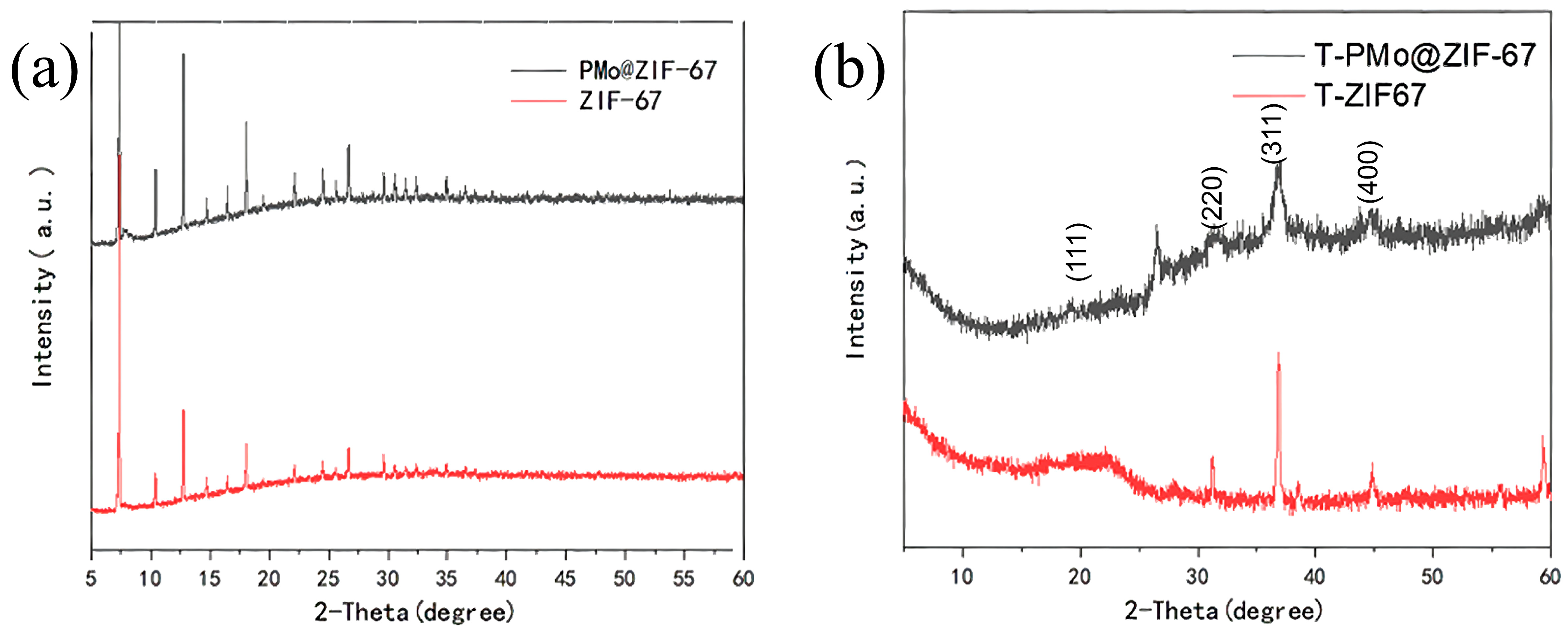
2.2.2. X-ray Diffraction Analysis
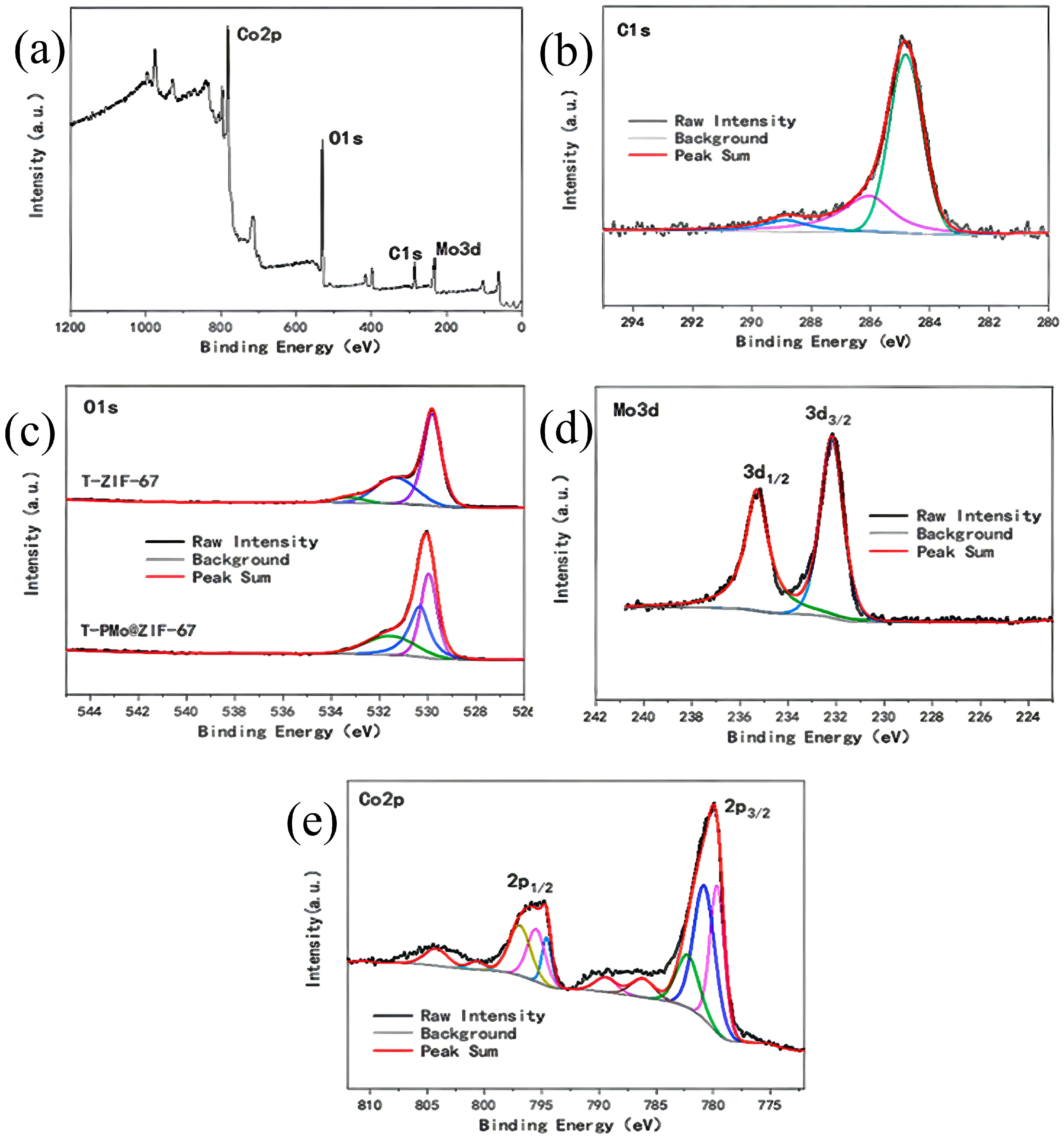
2.2.3. X-ray Photoelectron Spectroscopy Analysis
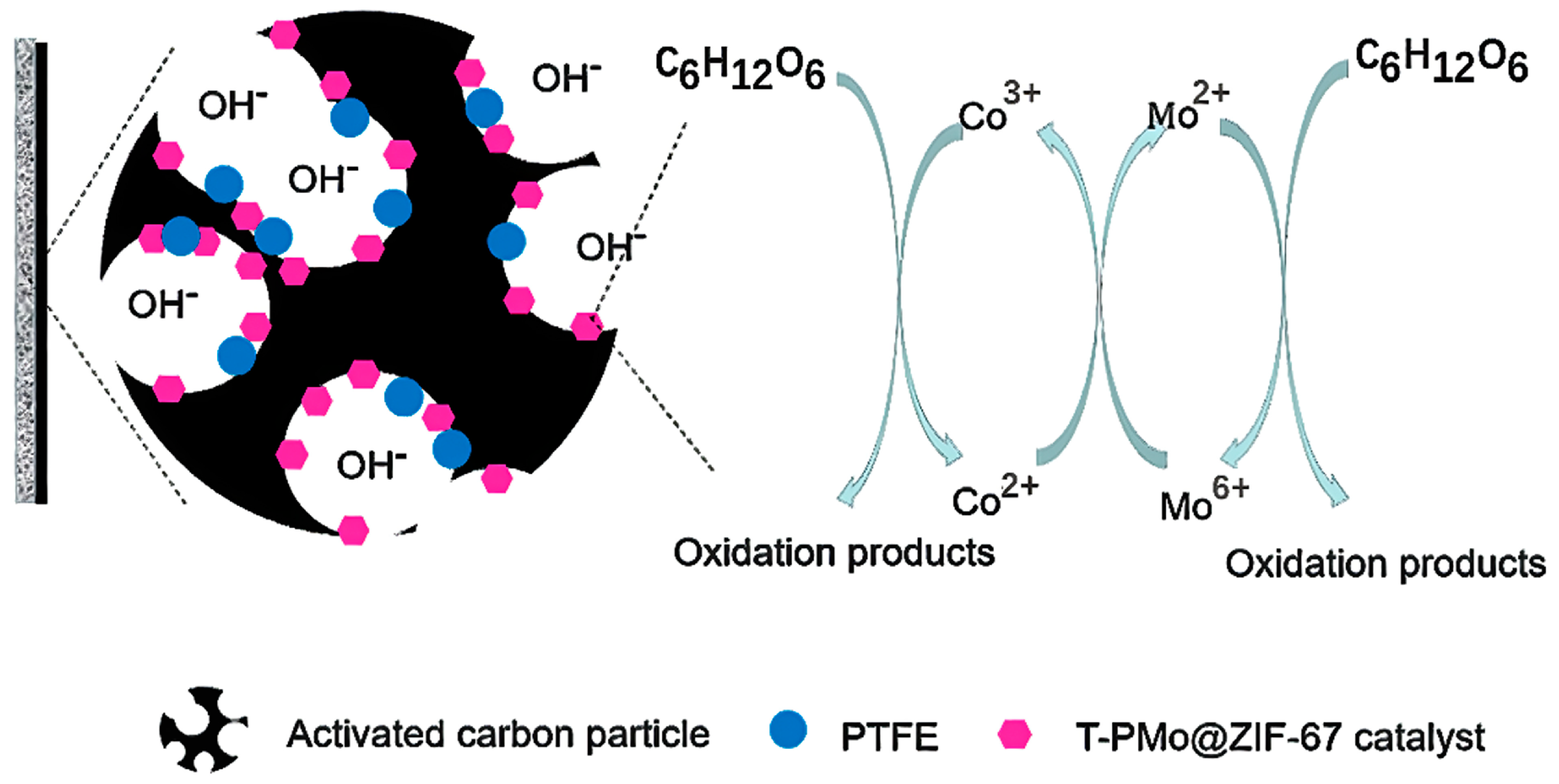
2.3. Discussion of Catalytic Mechanism
3. Materials and Methods
3.1. Characterization
3.2. Preparation of Electrodes
3.2.1. Preparation of POMs@ZIF-67
3.2.2. Preparation of T-POMs@ZIF-67
3.2.3. Synthesis of CoMoO4 Nanomaterials
3.2.4. Preparation of T-POMs@ZIF-67-Modified Anode
3.3. Experimental Methods
3.3.1. Linear Sweep Voltammetry (LSV)
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3.3. Tafel Test (Tafel)
3.3.4. Cyclic Volt Ampere Curve (CV)
3.3.5. Power Density Curve and Polarization Curve
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-organic frameworks for energy storage devices: Fuel cells and supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Jacobs, R.; Patki, P.; Lynch, M.J.; Chen, S.; Morgan, D.; Field, K.G. Materials swelling revealed through automated semantic segmentation of cavities in electron microscopy images. Sci. Rep. 2023, 13, 5178. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.J. An introduction to the virtual issue on Coordination polymers. Acta Crystallogr. Sect. C Struct. Chem. 2014, 70, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W. Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef]
- Sun, J.-K.; Xu, Q. Functional materials derived from open framework templates/precursors: Synthesis and applications. Energy Environ. Sci. 2014, 7, 2071–2100. [Google Scholar] [CrossRef]
- Cao, X.; Tan, C.; Sindoro, M.; Zhang, H. Hybrid micro-/nano-structures derived from metal-organic frameworks: Preparation and applications in energy storage and conversion. Chem. Soc. Rev. 2018, 47, 5997. [Google Scholar] [CrossRef]
- Ma, P.; Hu, F.; Wang, J.; Niu, J. Carboxylate covalently modified polyoxometalates: From synthesis, structural diversity to applications. Coord. Chem. Rev. 2019, 378, 281–309. [Google Scholar] [CrossRef]
- Liu, J.-X.; Zhang, X.-B.; Li, Y.-L.; Huang, S.-L.; Yang, G.-Y. Polyoxometalate functionalized architectures. Coord. Chem. Rev. 2020, 414, 213260. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Jiang, J.; Chen, L.; Zhao, J. Polyoxometalate-based composite materials in electrochemistry: State-of-the-art progress and future outlook. Nanoscale 2020, 12, 5705–5718. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef]
- Zhong, J.; Perez-Ramirez, J.; Yan, N. Biomass valorisation over polyoxometalate-based catalysts. Green Chem. 2021, 23, 18–36. [Google Scholar] [CrossRef]
- Liu, W.; Mu, W.; Liu, M.; Zhang, X.; Cai, H.; Deng, Y. Solar-induced direct biomass-to-electricity hybrid fuel cell using polyoxometalates as photocatalyst and charge carrier. Nat. Commun. 2014, 5, 3208. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, H.; Liu, Y.; Jing, Q. Advanced redox flow fuel cell using ferric chloride as main catalyst for complete conversion from carbohydrates to electricity. Sci. Rep. 2017, 7, 5142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, F.; Jiao, S.; Wang, J.; Dai, Y.; Irfan, M.; Liu, X. Co2+-P(W3O10)43− modified activated carbon as an efficient anode catalyst for direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2022, 47, 22952–22962. [Google Scholar] [CrossRef]
- Xia, K.; Yamaguchi, K.; Suzuki, K. Recent Advances in Hybrid Materials of Metal Nanoparticles and Polyoxometalates. Angew. Chem. Int. Ed. 2023, 62, e202214506. [Google Scholar] [CrossRef]
- Samaniyan, M.; Mirzaei, M.; Khajavian, R.; Eshtiagh-Hosseini, H.; Streb, C. Heterogeneous Catalysis by Polyoxometalates in Metal-Organic Frameworks. ACS Catal. 2019, 9, 10174–10191. [Google Scholar] [CrossRef]
- Ho, W.H.; Chen, T.-Y.; Otake, K.-I.; Chen, Y.-C.; Wang, Y.-S.; Li, J.-H.; Chen, H.-Y.; Kung, C.-W. Polyoxometalate adsorbed in a metal-organic framework for electrocatalytic dopamine oxidation. Chem. Commun. 2020, 56, 11763–11766. [Google Scholar] [CrossRef]
- Sajid, A.; Pervaiz, E.; Ali, H.; Noor, T.; Baig, M.M. A perspective on development of fuel cell materials: Electrodes and electrolyte. Int. J. Energy Res. 2022, 46, 6953–6988. [Google Scholar] [CrossRef]
- Pei, P.; Chen, H. Main factors affecting the lifetime of Proton Exchange Membrane fuel cells in vehicle applications: A review. Appl. Energy 2014, 125, 60–75. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Lee, A.S.; Atanasov, V.; Kerres, J.; Park, E.J.; Adhikari, S.; Maurya, S.; Manriquez, L.D.; Jung, J.; Fujimoto, C.; et al. Protonated phosphonic acid electrodes for high power heavy-duty vehicle fuel cells. Nat. Energy 2022, 7, 248–259. [Google Scholar] [CrossRef]
- Mano, N.; de Poulpiquet, A. O2 Reduction in Enzymatic Biofuel Cells. Chem. Rev. 2018, 118, 2392–2468. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. External abiotic glucose fuel cells. Sustain. Energy Fuels 2021, 5, 5038–5060. [Google Scholar] [CrossRef]
- Kerzenmacher, S.; Ducree, J.; Zengerle, R.; von Stetten, F. Energy harvesting by implantable abiotically catalyzed glucose fuel cells. J. Power Sources 2008, 182, 1–17. [Google Scholar] [CrossRef]
- Liu, T. Glucose Fuel Cells and Membranes: A Brief Overview and Literature Analysis. Sustainability 2022, 14, 8376. [Google Scholar] [CrossRef]
- Zhao, C.-E.; Gai, P.; Song, R.; Chen, Y.; Zhang, J.; Zhu, J.-J. Nanostructured material-based biofuel cells: Recent advances and future prospects. Chem. Soc. Rev. 2017, 46, 1545–1564. [Google Scholar] [CrossRef]
- Maru, K.; Kalla, S.; Jangir, R. MOF/POM hybrids as catalysts for organic transformations. Dalton Trans. 2022, 51, 11952–11986. [Google Scholar] [CrossRef]
- Freire, C.; Fernandes, D.M.; Nunes, M.; Abdelkader, V.K. POM & MOF-based Electrocatalysts for Energy-related Reactions. ChemCatChem 2018, 10, 1703–1730. [Google Scholar]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Lin, H.; Gupta, S.; Wang, C.; Tao, Z.; Fu, H.; Wang, T.; Zheng, J.; Wu, G.; et al. Directly converting Fe-doped metal organic frameworks into highly active and stable Fe-N-C catalysts for oxygen reduction in acid. Nano Energy 2016, 25, 110–119. [Google Scholar] [CrossRef]
- Xia, W.; Zou, R.; An, L.; Xia, D.; Guo, S. A metal-organic framework route to in situ encapsulation of Co@Co3O4@C core@bishell nanoparticles into a highly ordered porous carbon matrix for oxygen reduction. Energy Environ. Sci. 2015, 8, 568–576. [Google Scholar] [CrossRef]
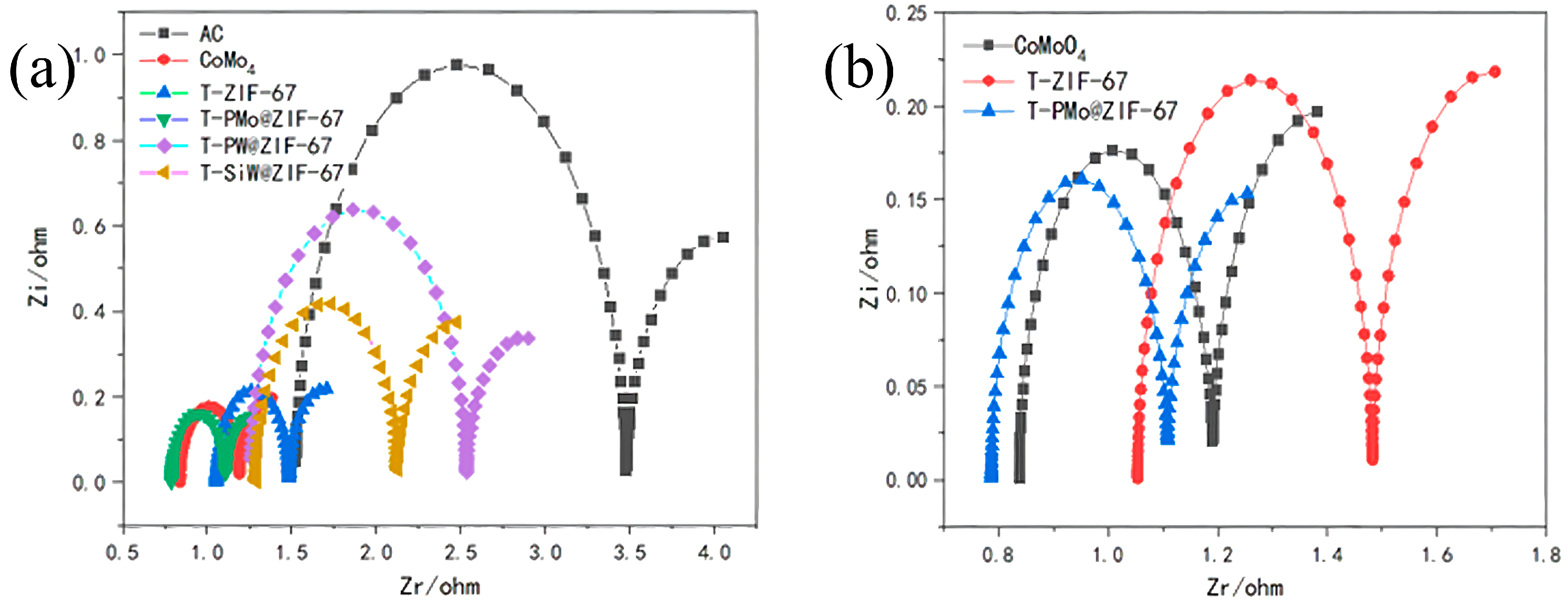

| Blank | CoMoO4 | T-ZIF-67 | T-PMo@ZIF-67 | T-PW@ZIF-67 | T-SiW@ZIF-67 | |
|---|---|---|---|---|---|---|
| Rs (Ω) | 1.518 | 0.8369 | 1.054 | 0.7869 | 1.255 | 1.289 |
| Rct (Ω) | 1.151 | 0.3944 | 0.4368 | 0.3063 | 1.277 | 0.8368 |
| Rd (Ω) | 1.957 | 0.3529 | 0.4284 | 0.3212 | 0.6763 | 0.7524 |
| Rt (Ω) | 4.626 | 1.5842 | 1.9192 | 1.4144 | 3.2083 | 2.8782 |
| Fitting Equation | R2 | Tafel | 10−4 I0 | |
|---|---|---|---|---|
| AC | y = 5.37143 × −3.41673 | 0.9983 | 186.1702 | 3.831 |
| T-ZIF-67 | y = 5.31818 × −2.84043 | 0.99776 | 188.0343 | 14.44 |
| T-PMo@ZIF-67 | y = 4.87273 × −2.66701 | 0.99636 | 205.2238 | 21.527 |
| CoMoO4 | y = 4.96623 × −2.70418 | 0.99684 | 201.36 | 19.762 |
| T-SiW@ZIF-67 | y = 5.61818 × −3.16615 | 0.99829 | 177.9936 | 6.821 |
| T-PW@ZIF-67 | y = 5.76753 × −3.33493 | 0.99828 | 173.3844 | 4.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, S.; Kang, N.; Zhang, Y.; Li, Y.; Maryam, B.; Zhang, X.; Zhang, P.; Liu, X. Pyrolyzed POMs@ZIF-67 Exhibiting High Performance as Direct Glucose Fuel Cell Anode Catalysts. Catalysts 2023, 13, 1339. https://doi.org/10.3390/catal13101339
Jiao S, Kang N, Zhang Y, Li Y, Maryam B, Zhang X, Zhang P, Liu X. Pyrolyzed POMs@ZIF-67 Exhibiting High Performance as Direct Glucose Fuel Cell Anode Catalysts. Catalysts. 2023; 13(10):1339. https://doi.org/10.3390/catal13101339
Chicago/Turabian StyleJiao, Shipu, Ning Kang, Yihao Zhang, Yang Li, Bushra Maryam, Xu Zhang, Pingping Zhang, and Xianhua Liu. 2023. "Pyrolyzed POMs@ZIF-67 Exhibiting High Performance as Direct Glucose Fuel Cell Anode Catalysts" Catalysts 13, no. 10: 1339. https://doi.org/10.3390/catal13101339
APA StyleJiao, S., Kang, N., Zhang, Y., Li, Y., Maryam, B., Zhang, X., Zhang, P., & Liu, X. (2023). Pyrolyzed POMs@ZIF-67 Exhibiting High Performance as Direct Glucose Fuel Cell Anode Catalysts. Catalysts, 13(10), 1339. https://doi.org/10.3390/catal13101339