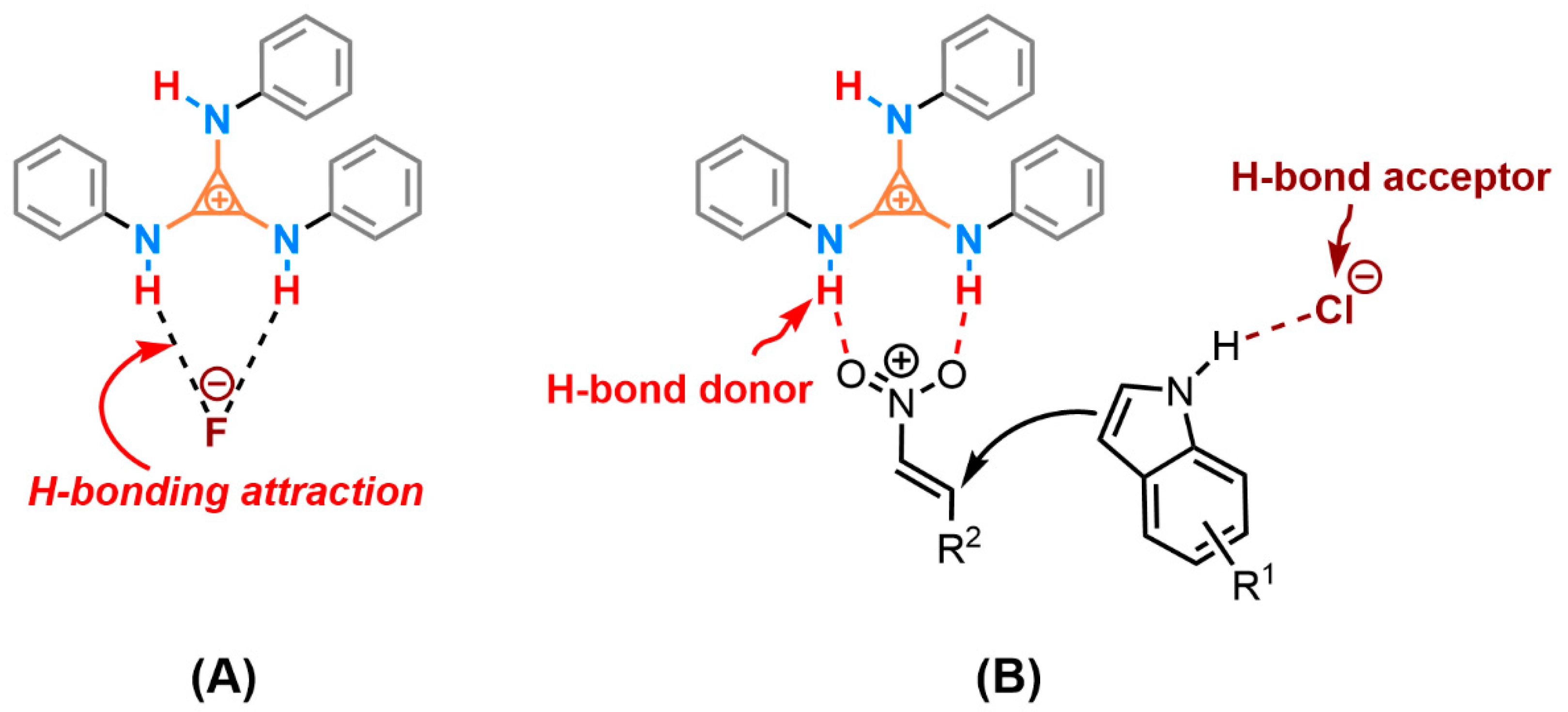
Abstract
H-bonding has achieved massive advancements by utilizing an H-bond donor (HBD) to interact with the electron-rich site of the substrate, and an H-bond acceptor (HBA) to coordinate with the electron-deficient site. Rapid transformation is often correlated with the acidity of HBD, namely the degree of charge deficiency of the hydrogen proton. In addition, the positive cations were employed to enhance the HBD; the electron-withdrawing groups were also a dissimilar approach for increasing the capability of the H-bond donor. We first introduced the H-bonding organic ion pair tris(phenylamino)cyclopropenium (TPAC·Cl) into the Friedel–Crafts alkylation of indoles with nitroalkenes, which was implemented via vicinal positive charges on the cyclopropenium core. The counter ion chloride anion became a potential HBA to activate the electron-deficient part of the substrate. X-ray analyses of a single crystal of TPAC·Cl described the 3D architecture and the delocalized cationic charge in the solid state. The aromatic cyclopropenium endowed the N–H moieties with the ability of the H-bond donor to activate the nitroalkene; meanwhile, the chloride anion acted as the H-bond acceptor to activate the indole. The amino-cyclopropenium-offered HBD and HBA displayed cooperative organocatalysis in the Friedel–Crafts alkylation of indole with nitroalkene. A new class of hydrogen bonding catalysis and a working mechanism were proposed.
1. Introduction
The Friedel–Crafts alkylation is one of the important strategies to facilitate the coupling of the C–C bond [1,2,3]. Indoles were an important intermediate with pharmaceutical activity, and it was interesting to promote the chemical transformation of indole derivatives. In the traditional F–C alkylation of indole, especially with nitroalkene, most of the catalysts utilized were metal catalysts, such as metal–ligand complexes, metal–organic frameworks (MOFs), and metal salts [4,5,6,7,8,9,10,11,12]. The Friedel–Crafts alkylation of indole and nitroalkene catalyzed via organocatalysts has only a few examples and remained underdeveloped [13,14,15].
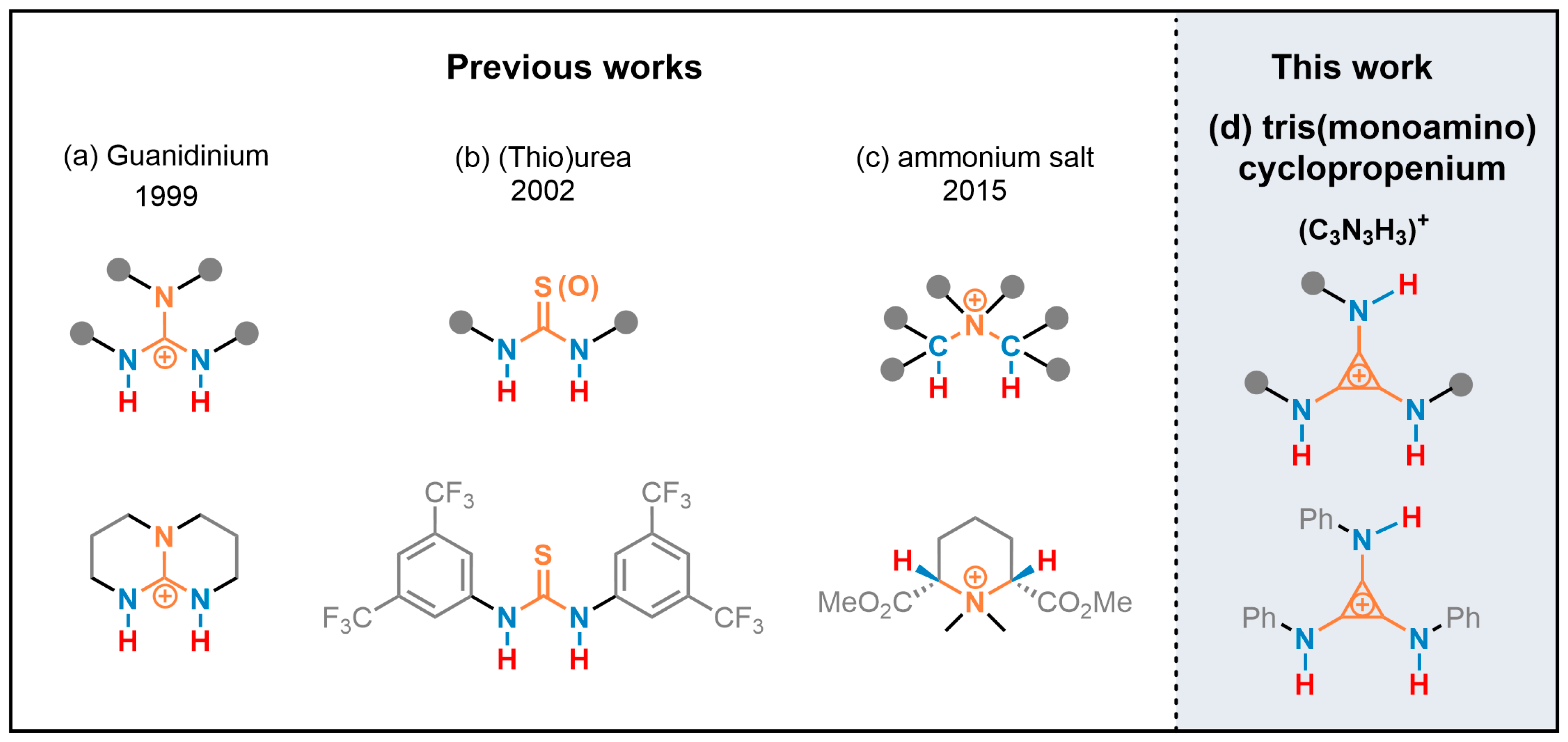
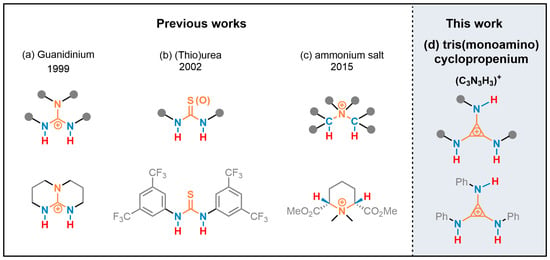
Hydrogen bonding catalysis has become a thriving and vital domain area in the past two decades [16,17,18,19,20,21,22,23,24] and is an important form of organocatalysis. Its behavioral mode was the coordination of an H-bond donor (HBD) with the electron-rich site of a substrate and/or the coordination of an H-bond acceptor (HBA) with the electron-deficient site. The control and selection of the reaction was realized via HBD and HBA co-catalysis [17,25,26,27]. The rate of reaction was often related to the acidity HBD; that is, the degree of the charge deficiency of the H-bond donor proton [28,29,30]. One category of charged-HBD derived from the protonated strong Brønsted base showing a strong H-bond donor capacity due to π-delocalization of the positive charge is guanidiniums [31,32,33,34,35] (Scheme 1a). Neutral HBD, such as the urea and thiourea, is enhanced via the substitution of the electron-withdrawing group and has been developed as one mature direction [24,25,27,36,37,38] (Scheme 1b). Another strategy to increase the ability of HB was electron-withdrawing via σ-bond to the vicinal positively charged atom(s), such as quaternary ammonium [39,40,41,42] (Scheme 1c).
Scheme 1.
(a) H-bond donor (HBD) was enhanced via π-delocalization of the positive charge; (b) HBD was enhanced by electron-withdrawing groups; (c) HBD was enhanced by vicinal positively charged atom; and (d) HBD was implemented via vicinal positive charges on the cyclopropenium core, such as tris(monoamino)cyclopropenium.
The minimal Hückel aromatic compound cyclopropenium has been utilized as organocatalysts for organic transformation in the past two decades [43,44,45]. Cyclopropenium ions were mainly used in asymmetric synthesis [46,47], phase transfer catalysis [48,49], and electrophotocatalysis [50], but had not been well studied in hydrogen bonding catalysis. We suggested that the cyclopropenium [51,52,53] be substituted with amino groups of NHR, and the N–H moieties of tris(monoamino)cyclopropenium would behave as the H-bond donor (Scheme 1d). The capability of the N–H moieties as a new type of H-bond donor was implemented via vicinal positive charges on the cyclopropenium core [54]. The N–H moiety of the unprotected indole was readily activated via hydrogen bonding with the halide anion. Herein, we demonstrated the tris(monoamino)cyclopropenium as a bifunctional H-bonding catalyst to promote the Friedel–Crafts alkylation of indoles with nitroalkenes for the first time.
2. Results and Discussion
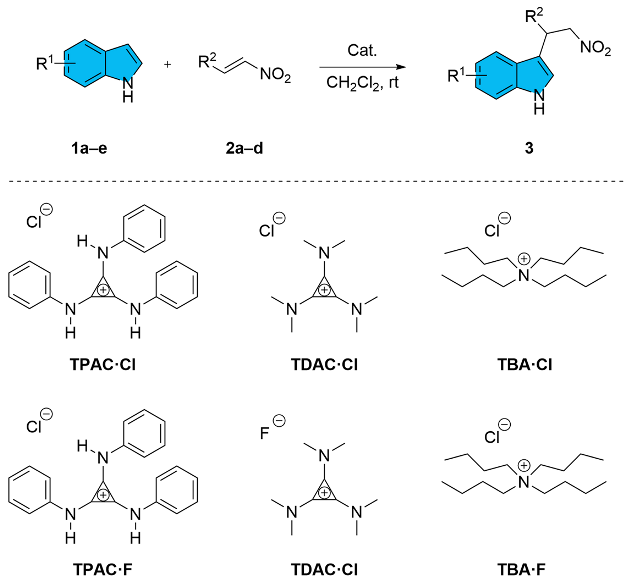
In the context of the ordinary H-bond [2,15,55] and the metal-enhanced H-bond catalysis [56], we expanded the exploration of the novel H-bond donor implemented via the π-conjugate system of the cyclopropenium to apply for the F–C alkylation of indole 1 with trans-β-nitroalkene 2. Tri(phenylamino)cyclopropenium chloride (TPAC·Cl) was selected as the typical catalyst (Table 1). The TPAC·Cl was able to promote the F–C alkylation of 1a with 2a at 25 °C in dichloromethane efficiently (Table 1, entry 2). In contrast, the background reaction without catalysts was negligible (Table 1, entry 1). By switching the chloride to the fluoride anion, The TPAC·F performed no activity in the same reaction (Table 1, entry 3). The poor catalytic performance implied that the chloride anion may be necessary for the F–C alkylation. A possible explanation was that the fluoride anion coordinated with the HBD of the cationic TPAC [23,57,58], which indicated that TPAC·F formed a tight ion pair [59] and the cationic TPAC shown a preference for pairing with the fluoride anion rather than activating the substrate.

Table 1.
F–C alkylation of indoles 1a–e with nitroalkenes 2a–d a.
Tri(alkylamino)cyclopropenium was tested on the F–C alkylation to verify the assumption that the N–H moieties of the TPAC as HBD were essential (Table 1, entries 4 and 5). No products were determined with the tris(dimethylamino)cyclopropenium chloride (TDAC·Cl) as the catalyst (Table 1, entry 4). The lack of the N–H moieties on the cationic core of TDAC may be the possible reason for its diminished capability to function as an H-bond donor. Although fluoride anion was considered an excellent H-bond acceptor and strong nucleophilic, the TDAC·F as the catalyst was not workable on the F–C alkylation (Table 1, entry 5). These experimental results supported the vital role of N–H HBD on TPAC in facilitating this transformation.
The tetrabutylammonium chloride (TBA·Cl) along with tetrabutylammonium fluoride (TBA·F), are counterparts to TPAC·Cl and TPAC·F, respectively, and performed inactive in the benchmark F–C alkylations (Table 1, entries 6 and 7). The discrepancy between TPAC·Cl and TBA·Cl suggested the necessity of the structure of cyclopropenium cation. We suggested that the strong N–H H-bond donor enhanced by the vicinal cyclopropenium exhibited a better catalytic performance.
With the optimal catalyst in hand, the substrate scopes of indoles and nitroalkenes in the alkylation reaction were investigated (Table 1, entries 8 to 15). Indoles 1a–e bearing different substituents on both the benzene ring and the pyrrole ring were conductive in their reactions with nitroalkene 2a. Whereas the reactions of unsubstituted indole 1a and the indoles with electron-donating groups (1b and 1c) afforded the corresponding products 3aa, 3ba, and 3ca in good yields (Table 1, entries 8–9), the electron-withdrawing chloride on the 5-position caused 1d to convert into 3da in a moderate yield (Table 1, entry 10). Steric hindrance at the 7-position inhibited the reaction (Table 1, entry 11), which could be suffering from the unfavorable interference to the chloride anion. The applicability of the catalyst was further supported by the variation in the nitroalkene. Nitroalkenes with various substitutions on the benzene ring (2b–d), both electron-donating and -withdrawing, decreased the yields (Table 1, entries 12–14) as compared with a non-substituted one. Nevertheless, thienyl nitroalkene 2d reacted stably with indole 1a to obtain the corresponding products 3ad in good yield (Table 1, entry 14).
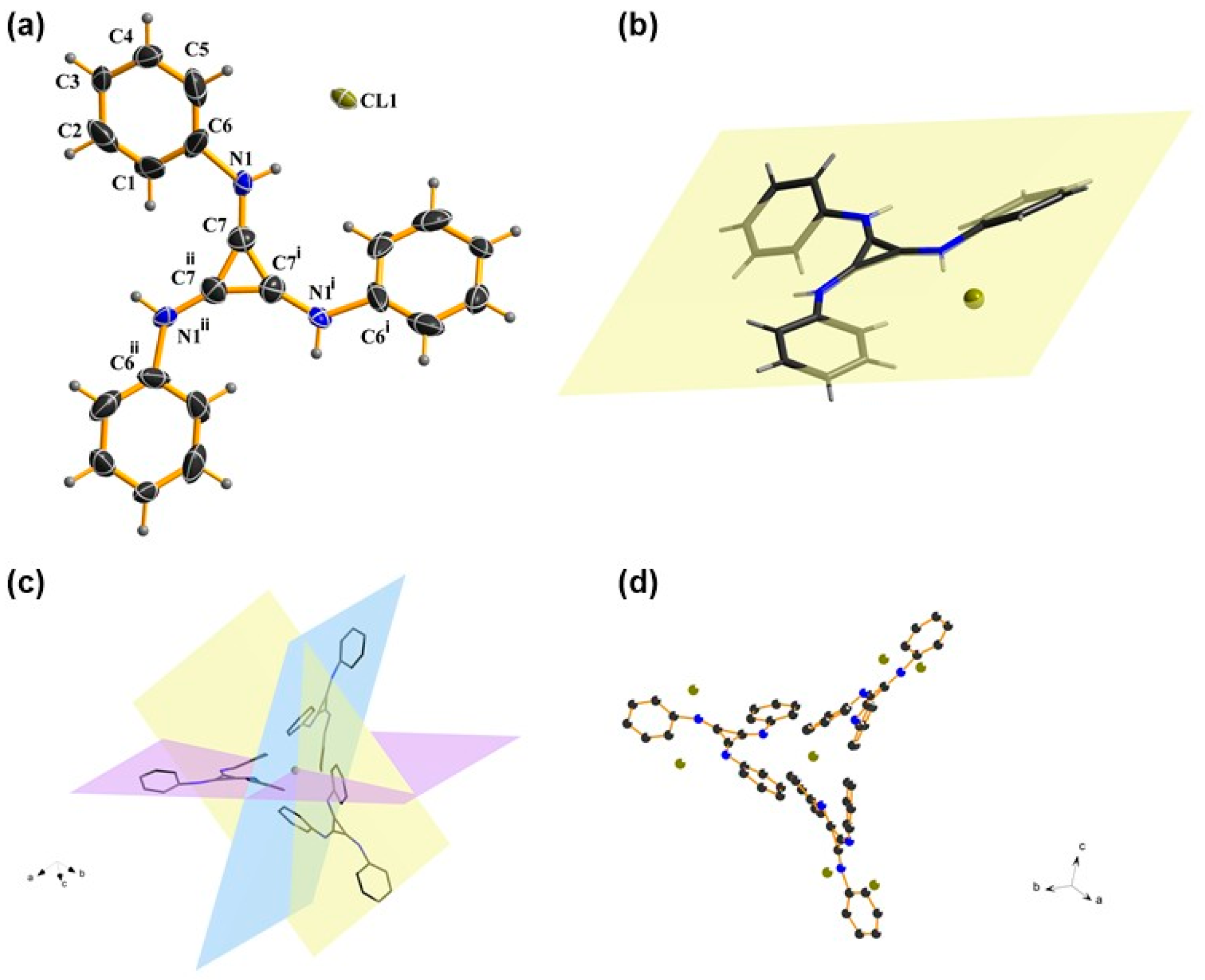
The single crystal of TPAC·Cl was prepared to show the visual view of the construction in the solid, including the interionic distances. A cubic system was confirmed via an X-ray diffraction analysis of the catalyst TPAC·Cl. The chloride anion was closer to the benzene ring than the formally cationic core (C3N3)+ (Figure 1a). The chloride anion is coplanar with a cyclopropenium core (Figure 1b), but each phenyl group is slightly skewed out of the plane. Three TPACs coordinate to one Cl (Figure 1c), while each TPAC aligns in one of the three orthogonal planes of x, y, and z (Figure 1d). The structure of TPAC·Cl is C3v symmetry. The distances between the chloride anion and the positive core (C3)+ of the three carbons showed distinctly larger distances than the normal ones, viz. 4.1086 Å, 4.8124 Å, and 5.3485 Å, respectively [60]. These data described the 3D architecture of TPAC·Cl in the solid state.
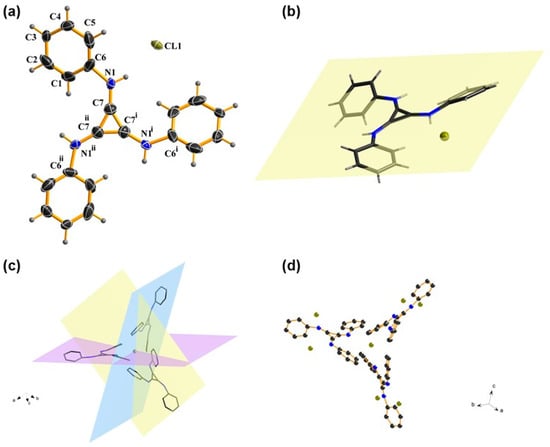
Figure 1.
(a) Crystal structure of TPAC·Cl. (b) Coplanar TPAC·Cl. (c) Side-on view of the three orthogonal planes that the cations aligned around one chloride anion. (d) Side-on view of the coordination between chloride anion and cation.
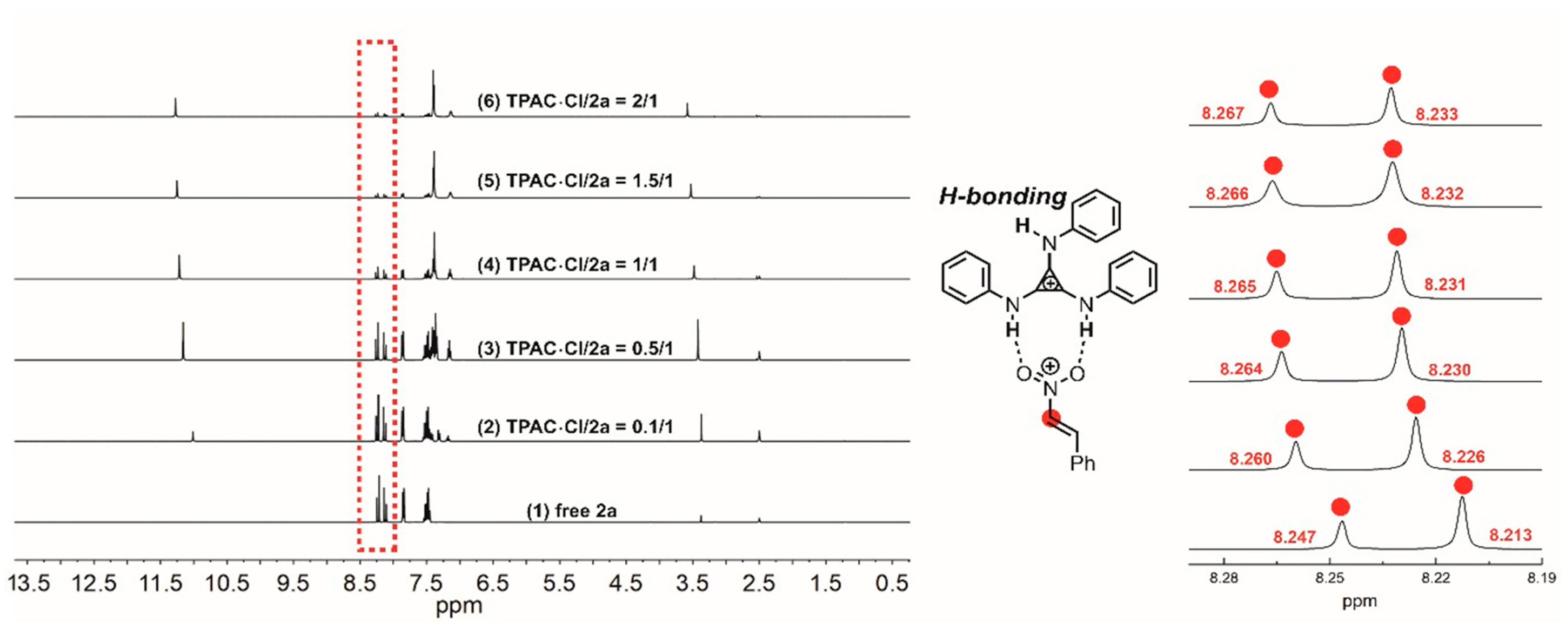
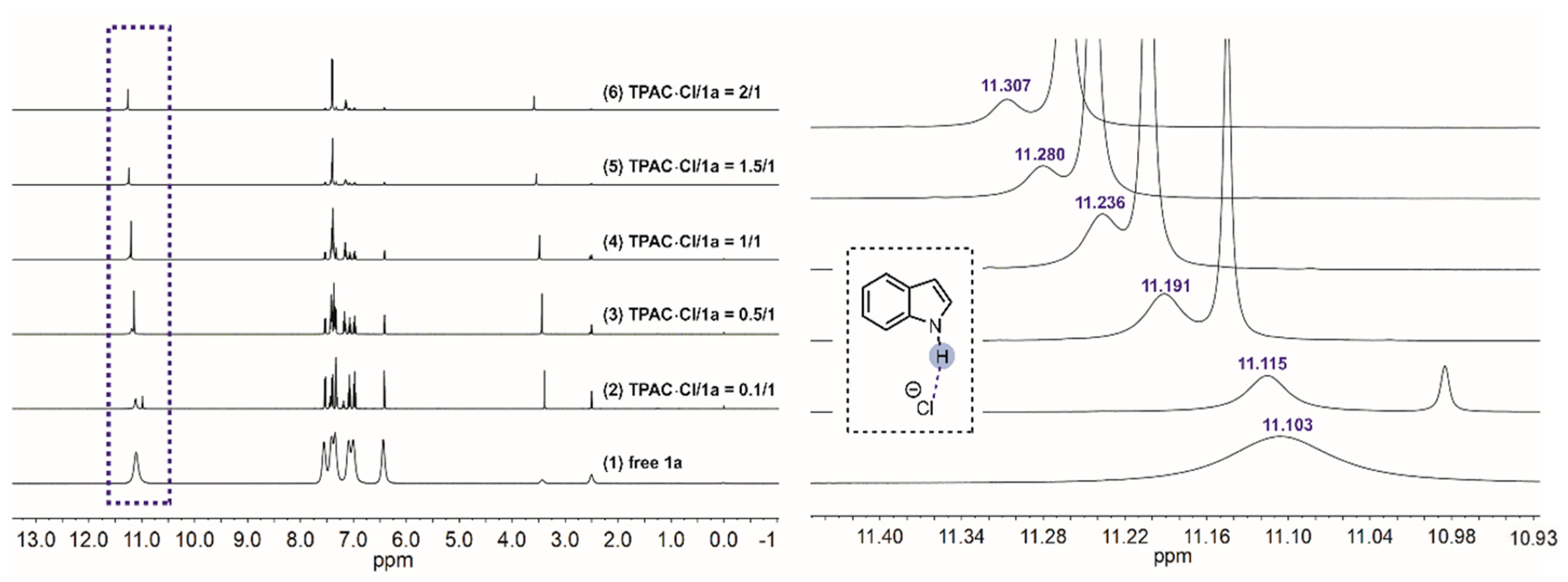
NMR titration experiments were performed to verify the H-bonding interaction between the N–Hs of TPAC·Cl and the substrate of nitroalkene 2a (Figure 2). The chemical shifts of the methine of 2a exhibited downfield shifts from 8.247 to 8.267 ppm by increasing the ratio of [TPAC·Cl]/[2a]0 from 0 to 2 (Figure 2). The two different methines were due to the geometric isomerism of the 2a by C=C. These shifts were important evidence that the catalyst cation of TPAC·Cl as HBD could activate the nitro compounds of 2a via H-bonding. To validate the chloride anion as a potential H-bond acceptor (HBA) with N–H of indole 1a, NMR titration experiments were performed (Figure 3). The chemical shifts of the H-bonding N–H of the indole exhibited downfield shifts from 11.103 to 11.307 ppm by increasing the ratio of [TPAC·Cl]/[1a]0 from 0 to 2 (Figure 3). These were important evidence that the counter anion of catalyst TPAC·Cl as HBA could activate the indole 1a via H-bonding.
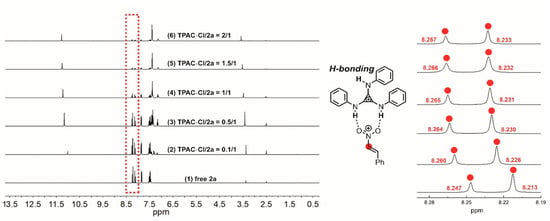
Figure 2.
The chemical shifts of the methine of 2a in the 1H NMR spectrum (DMSO-d6) observed via titration of TPAC·Cl with 2a: (1) free 2a, (2) TPAC·Cl/2a = 0.1/1, (3) TPAC·Cl/2a = 0.5/1, (4) TPAC·Cl/2a = 1/1, (5) TPAC·Cl/2a = 1.5/1, and (6) TPAC·Cl/2a = 2/1.
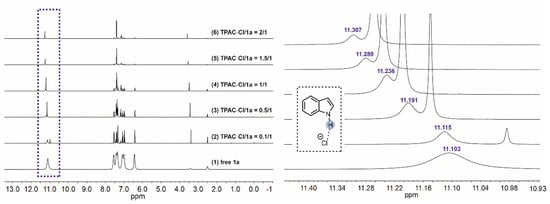
Figure 3.
The chemical shifts of the H-bonding N–Hs of 1a in the 1H NMR spectrum (DMSO-d6) observed via titration of TPAC·Cl with 1a: (1) free 1a, (2) TPAC·Cl/1a = 0.1/1, (3) TPAC·Cl/1a = 0.5/1, (4) TPAC·Cl/1a = 1/1, (5) TPAC·Cl/1a = 1.5/1, and (6) TPAC·Cl/1a = 2/1.
Two plausible mechanisms were proposed (Scheme 2) based on the experiments and XRD analysis. One possible competitive interaction was the H-bonding attraction between the cationic TPAC and the counter anion, which decreased the activation of cationic HBD to the substrate, especially the fluoride anion (Scheme 2A). Thus, the changes of the anion from the fluoride to chloride anion promoting the catalytic performance may be the reason for the weak coordination between the TPAC and the chloride anion. The high-lying closed-shell HOMO of the amino-cyclopropenium cation contending against the closed-shell HOMO of chloride anion will counteract the ionic electrostatic attractions [61]. The phenomenon is called “ion pair strain” [62,63]. The cationic TPAC and the anionic Cl− will keep away from each other due to the repulsion and reach obviously larger interionic distances under the readjusted dynamic equilibrium. The counter-chloride anion was the potential for HBA to activate the substrate via H-bonding. The TPAC acted as an H-bond donor to coordinate with the two oxygen of the triangle planar nitro group (Scheme 2B). The counter-chloride anion Cl−, cooperatively, possibly coordinates to hydrogen of the N–H on the indole ring, played the role of an H-bond acceptor (HBA). Different from the prevailing viewpoint of HBD and the Lewis base as co-catalysis, we preferred to suggest tris(monoamino)cyclopropenium as HBD and the counter-chloride anion as HBA in cooperative catalysis.
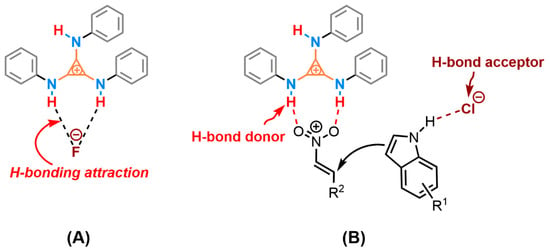
Scheme 2.
Two possible cooperative activations of the catalyst TPAC·Cl in Friedel–Crafts alkylation. (A) the cationic TPAC shown a preference for pairing with the fluoride anion. (B) the N–H on cation activated the nitroalkene and the chloride anion as HBA activated the indole.
3. Materials and Methods
The NMR spectra were recorded at room temperature on a Bruker AVANCE 400 spectrometer in deuterated solvents as noted. Chemical shifts (δ) are reported in parts per million (ppm) relative to a residual solvent resonance as the internal standard (1H δ 7.26 for CDCl3, δ 2.50 for DMSO-d6; 13C δ 77.16 for CDCl3, δ 39.52 for DMSO-d6). NMR peak multiplicities are abbreviated as follows: brs = broad signal, s = singlet, d = doublet, t = triplet, q = quartet, sept = septet, and m = multiplet. The substrates of indoles and nitroalkenes were purchased from Sigma Aldrich (Saint Louis, MO, USA) without additional purification. All experiments were executed via standard Schlenk reaction techniques under an argon atmosphere. Dichloromethane was stirred with CaH2 for 10 h and distilled under an argon atmosphere. The purified dichloromethane was stored in 3 Å molecular sieve pellets. Toluene, sodium, and diphenyl ketone were heated and stirred until a dark purple color came flooding out. The purified toluene was deposited in 3 Å molecular sieve pellets.
- Preparation of N-trimethylsilylaniline [64,65]
Argon airflow was employed to protect all operations progressing under the standard Schlenk techniques. Freshly distilled aniline (3 mL, 33 mmol, 2.5 equiv.) was mixed with chlorotrimethylsilane (1.7 mL, 13 mmol, 1 equiv.) in 20.0 mL of dry benzene at a reflux for 1 h. Aniline hydrochloride was separated out of this system and removed via filtration. The filtrate was dried using a rotary evaporator to obtain N-trimethylsilylaniline as a yellow oil: 2.46 g, 76% yield. 1H NMR (400 MHz, CDCl3) δ 7.17 (dd, J = 8.5, 7.5 Hz, 2H), 6.73 (t, J = 7.5 Hz, 1H), 6.68 (d, J = 8.5 Hz, 2H), and 3.45 (brs, 1H), 0.30 (s, 9H).
- Preparation of the catalyst TPAC·Cl [66]
Argon airflow was employed to protect all operations progressing under the standard Schlenk techniques. Freshly prepared N-trimethylsilylaniline (2.1 g, 12 mmol, 3 equiv.) was placed in tetrachlorocyclopropene (0.5 mL, 4 mmol, 1 equiv.) in 50.0 mL of dry dichloromethane and stirred for 6 h. The white precipitate was precipitated gradually. The dichloromethane was used to clean up the white solid. Finally, the white solid was recrystallized from methanol: 0.78 g, 52% yield; m.p, 207.3 °C (decomp.); 1H NMR (400 MHz, DMSO-d6) δ 11.11 (s, 3H), 7.41 (t, J = 7.8 Hz, 6H), 7.36 (d, J = 7.6 Hz, 6H), and 7.16 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 138.78, 129.68, 123.94, 118.02, 112.83; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H18N3 312.1495; found 312.1463.
- Preparation of the catalyst TDAC·Cl [67]
Argon airflow was employed to protect all operations progressing under the standard Schlenk techniques. N,N-Dimethyltrimethylsilylamine (0.78 mL, 4.89 mmol, 3 equiv.) was dissolved in 20 mL of dichloromethane and cooled to 0 °C. Tetrachlorocyclopropene (0.2 mL, 1.63 mmol, 1equiv.) was added dropwise, and the solution was allowed to warm to ambient temperature. The solution was removed via rotary evaporation. The residue was dried under vacuum at 60 °C for 12 h and presented the product as a faint yellow solid: 0.31 g, 94% yield; 1H NMR (400 MHz, CDCl3) δ 3.19 (s, 18H). 13C NMR (100 MHz, CDCl3): δ 117.9, 42.8. HRMS (ESI-ToF) m/z [M]+ calcd for C9H18N3 168.1495, found 168.1498.
- The general method for Friedel–Crafts Alkylation catalyzed via TPAC·Cl
Argon airflow was employed to protect all operations progressing under the standard Schlenk techniques. Thin-layer chromatography (TLC), combined with UV light, was used to monitor the reaction process. Purification was performed via flash column chromatography with silica gel 60 N (Kanto Chemical Co., Inc., Tokyo, Japan) or Isolera one with a SNAP Ultra Column. In a 10 mL reaction tube, nitroalkenes 2a–d (1 mmol, 1 equiv.) along with the TPAC·Cl (0.0347 g, 0.1 mmol, 10 mol%) were weighted in 1 mL of dichloromethane, where the indoles 1a–e (1.5 mmol, 1.5 equiv.) were placed. The reaction tube was then placed at room temperature for 24 h, and the product 3 was obtained via column chromatography (n-hexane/EtOAc).
4. Conclusions
In summary, we first observed the tris(monoamino)cyclopropenium cation as an H-bond donor (HBD) and the counter anion as a potential H-bond acceptor (HBA) in cooperative organocatalysis. The capability of the N–H moieties as a new type of H-bond donor was implemented via vicinal positive charges on the cyclopropenium core. Tris(phenylamino)cyclopropenium chloride (TPAC·Cl) as a representative H-bonding catalyst was used in the Friedel–Crafts alkylation of indoles with nitroalkenes. The X-ray analyses verified the 3D architecture of the TPAC·Cl in the solid state. The TPAC exhibited an H-bond-donating ability to the nitroalkene enhanced via vicinal cyclopropenium. The counter-chloride anion acted as a potential HBA to activate the indole via H-bonding. NMR titration, XRD analysis, and the experiment results verified a plausible bifunctional activation mechanism. Taken together, an HBD/HBA as cooperative organocatalysis via TPAC·Cl displayed an initial mode in organic transformations. Investigations on a new class of hydrogen bonding catalysis in the wider scope of catalysis and transformations are currently underway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13101370/s1, References [2,13,14,15,54,68,69,70] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, Z.L.; Funding acquisition, Z.L.; Investigation, X.M., J.X., J.L., J.H., Q.Y., N.L. and D.Q.; Methodology, X.M., J.X. and J.L.; Project administration, Z.L.; Resources, Z.L.; Validation, J.H.; Writing—original draft, X.M., J.X. and T.C.; Writing—review and editing, X.M., T.C. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (22078150), the Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture (XTB2201), and the Top-Notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
Data Availability Statement
Data is contained within the article or Supplementary Material. The data presented in this study are available in Supplementary.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bartoli, G.; Bencivenni, G.; Dalpozzo, R. Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 2010, 39, 4449–4465. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.P.; Sgarzani, V.; Bernardi, L.; Ricci, A. Catalytic enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes by using a simple thiourea organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 6576–6579. [Google Scholar] [CrossRef] [PubMed]
- Narumi, T.; Tsuzuki, S.; Tamamura, H. Imidazolium Salt-Catalyzed Friedel–Crafts-Type Conjugate Addition of Indoles: Analysis of Indole/Imidazolium Complex by High Level ab Initio Calculations. Asian J. Org. Chem. 2014, 3, 497–503. [Google Scholar] [CrossRef]
- Capito, E.; Brown, J.M.; Ricci, A. Directed palladation: Fine tuning permits the catalytic 2-alkenylation of indoles. Chem. Commun. 2005, 14, 1854–1856. [Google Scholar] [CrossRef] [PubMed]
- Fizala, M.B.; Saranya, P.V.; Anilkumar, G. Copper-catalyzed alkylation reactions of indole: An overview. Chem. Pap. 2023, 77, 6425–6457. [Google Scholar] [CrossRef]
- Wan, N.N.; Yang, Y.L.; Wang, W.P.; Xie, Z.F.; Wang, J.D. Friedel–Crafts alkylation of indoles with nitroalkenes catalyzed by Cu(II)–imine complex. Chin. Chem. Lett. 2011, 22, 1155–1158. [Google Scholar] [CrossRef]
- Singh, M.; Neogi, S. Urea-engineering mediated hydrogen-bond donating Friedel–Crafts alkylation of indoles and nitroalkenes in a dual-functionalized microporous metal–organic framework with high recyclability and pore-fitting-induced size-selectivity. Inorg. Chem. Front. 2022, 9, 1897–1911. [Google Scholar] [CrossRef]
- Jia, Y.-X.; Zhu, S.-F.; Yang, Y.; Zhou, Q.-L. Asymmetric Friedel–Crafts Alkylations of Indoles with Nitroalkenes Catalyzed by Zn(II)–Bisoxazoline Complexes. J. Org. Chem. 2006, 71, 75–80. [Google Scholar] [CrossRef]
- Azizi, N.; Arynasab, F.; Saidi, M.R. Efficient Friedel–Crafts alkylation of indoles and pyrrole with enones and nitroalkene in water. Org. Biomol. Chem. 2006, 4, 4275–4277. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Nakamura, A.; Senko, Y.; Nagatsugi, F.; Sasaki, S. Effects of Halogenated WNA Derivatives on Sequence Dependency for Expansion of Recognition Sequences in Non-Natural-Type Triplexes. J. Org. Chem. 2006, 71, 2115–2122. [Google Scholar] [CrossRef]
- Karimi, B.; Jafari, E.; Mansouri, F.; Tavakolian, M. Catalytic asymmetric Friedel–Crafts alkylation of unprotected indoles with nitroalkenes using a novel chiral Yb(OTf)3-pybox complex. Sci. Rep. 2023, 13, 14736. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Al Majid, A.M.A.; Al-Othman, Z.A.; Barakat, A. Highly enantioselective Friedel–Crafts alkylation of indole with electron deficient trans-β-nitroalkenes using Zn(II)–oxazoline–imidazoline catalysts. Tetrahedron Asymmetry 2014, 25, 245–251. [Google Scholar] [CrossRef]
- Suzuki, T.; Chisholm, J.D. Friedel–Crafts Alkylation of Indoles with Trichloroacetimidates. Tetrahedron Lett. 2019, 60, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Dündar, E.; Tanyeli, C. Enantioselective Friedel–Crafts alkylation of indole with nitroalkenes in the presence of bifunctional squaramide organocatalysts. Tetrahedron Lett. 2021, 73, 153153. [Google Scholar] [CrossRef]
- Zhuang, W.; Hazell, R.G.; Jorgensen, K.A. Enantioselective Friedel–Crafts type addition of indoles to nitro-olefins using a chiral hydrogen-bonding catalyst–synthesis of optically active tetrahydro-[small beta]-carbolines. Org. Biomol. Chem. 2005, 3, 2566–2571. [Google Scholar] [CrossRef]
- Schreiner, P.R. Metal-free organocatalysis through explicit hydrogen bonding interactions. Chem. Soc. Rev. 2003, 32, 289–296. [Google Scholar] [CrossRef]
- Doyle, A.G.; Jacobsen, E.N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 2007, 107, 5713–5743. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric catalysis by chiral hydrogen-bond donors. Angew. Chem. Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef]
- Malerich, J.P.; Hagihara, K.; Rawal, V.H. Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts. J. Am. Chem. Soc. 2008, 130, 14416–14417. [Google Scholar] [CrossRef]
- Phipps, R.J.; Hamilton, G.L.; Toste, F.D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 2012, 4, 603–614. [Google Scholar] [CrossRef]
- Auvil, T.J.; Schafer, A.G.; Mattson, A.E. Design Strategies for Enhanced Hydrogen-Bond Donor Catalysts. Eur. J. Org. Chem. 2014, 2014, 2633–2646. [Google Scholar] [CrossRef]
- Min, C.; Seidel, D. Asymmetric Bronsted acid catalysis with chiral carboxylic acids. Chem. Soc. Rev. 2017, 46, 5889–5902. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y. Recent topics in dual hydrogen bonding catalysis. Tetrahedron Lett. 2018, 59, 216–223. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Schreiner, P.R. (Thio)urea organocatalysis—What can be learnt from anion recognition? Chem. Soc. Rev. 2009, 38, 1187–1198. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Cleemann, F.; Mukherjee, S.; Müller, T.N.; Lex, J. Highly Efficient Dynamic Kinetic Resolution of Azlactones by Urea-Based Bifunctional Organocatalysts. Angew. Chem. Int. Ed. 2005, 44, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Connon, S.J. Organocatalysis Mediated by (Thio)urea Derivatives. Chem. Eur. J. 2006, 12, 5418–5427. [Google Scholar] [CrossRef]
- Jensen, K.H.; Sigman, M.S. Systematically Probing the Effect of Catalyst Acidity in a Hydrogen-Bond-Catalyzed Enantioselective Reaction. Angew. Chem. Int. Ed. 2007, 46, 4748–4750. [Google Scholar] [CrossRef]
- Jensen, K.H.; Sigman, M.S. Evaluation of Catalyst Acidity and Substrate Electronic Effects in a Hydrogen Bond-Catalyzed Enantioselective Reaction. J. Org. Chem. 2010, 75, 7194–7201. [Google Scholar] [CrossRef]
- Li, X.; Deng, H.; Zhang, B.; Li, J.; Zhang, L.; Luo, S.; Cheng, J.P. Physical Organic Study of Structure–Activity–Enantioselectivity Relationships in Asymmetric Bifunctional Thiourea Catalysis: Hints for the Design of New Organocatalysts. Chem. Eur. J. 2010, 16, 450–455. [Google Scholar] [CrossRef]
- Corey, E.J.; Grogan, M.J. Enantioselective synthesis of alpha-amino nitriles from N-benzhydryl imines and HCN with a chiral bicyclic guanidine as catalyst. Org. Lett. 1999, 1, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Nakano, M.; Ube, H. Axially chiral guanidine as highly active and enantioselective catalyst for electrophilic amination of unsymmetrically substituted 1,3-dicarbonyl compounds. J. Am. Chem. Soc. 2006, 128, 16044–16045. [Google Scholar] [CrossRef] [PubMed]
- Uyeda, C.; Jacobsen, E.N. Enantioselective Claisen rearrangements with a hydrogen-bond donor catalyst. J. Am. Chem. Soc. 2008, 130, 9228–9229. [Google Scholar] [CrossRef]
- Selig, P. Guanidine Organocatalysis. Synthesis 2013, 45, 703–718. [Google Scholar] [CrossRef]
- Fu, X.; Tan, C.-H. Mechanistic considerations of guanidine-catalyzed reactions. Chem. Commun. 2011, 47, 8210–8222. [Google Scholar] [CrossRef]
- Wittkopp, A.; Schreiner, P.R. Metal-Free, Noncovalent Catalysis of Diels–Alder Reactions by Neutral Hydrogen Bond Donors in Organic Solvents and in Water. Chem. Eur. J. 2003, 9, 407–414. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Highly enantioselective thiourea-catalyzed nitro-Mannich reactions. Angew. Chem. Int. Ed. 2005, 44, 466–468. [Google Scholar] [CrossRef]
- Aleman, J.; Parra, A.; Jiang, H.; Jorgensen, K.A. Squaramides: Bridging from Molecular Recognition to Bifunctional Organocatalysis. Chem. Eur. J. 2011, 17, 6890–6899. [Google Scholar] [CrossRef]
- Reetz, M.T.; Huette, S.; Goddard, R. Tetrabutylammonium salts of CH-acidic carbonyl compounds: Real carbanions or supramolecules? J. Am. Chem. Soc. 1993, 115, 9339–9340. [Google Scholar] [CrossRef]
- Shirakawa, S.; Liu, S.; Kaneko, S.; Kumatabara, Y.; Fukuda, A.; Omagari, Y.; Maruoka, K. Tetraalkylammonium Salts as Hydrogen-Bonding Catalysts. Angew. Chem. Int. Ed. 2015, 54, 15767–15770. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumatabara, Y.; Shimizu, S.; Maruoka, K.; Shirakawa, S. Hydrogen-bonding catalysis of sulfonium salts. Chem. Commun. 2017, 53, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Kumatabara, Y.; Kaneko, S.; Nakata, S.; Shirakawa, S.; Maruoka, K. Hydrogen-Bonding Catalysis of Tetraalkylammonium Salts in an Aza-Diels-Alder Reaction. Chem. Asian J. 2016, 11, 2126–2129. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.M.; Lambert, T.H. Cyclopropenium Ions in Catalysis. Acc. Chem. Res. 2022, 55, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.J.; Crocker, R.D.; Blumel, M.; Nguyen, T.V. Promotion of Organic Reactions by Non-Benzenoid Carbocyclic Aromatic Ions. Angew. Chem. Int. Ed. 2017, 56, 1466–1484. [Google Scholar] [CrossRef] [PubMed]
- Bandar, J.S.; Lambert, T.H. Aminocyclopropenium Ions: Synthesis, Properties, and Applications. Synthesis 2013, 45, 2485–2498. [Google Scholar]
- Bandar, J.S.; Lambert, T.H. Cyclopropenimine-catalyzed enantioselective Mannich reactions of tert-butyl glycinates with N-Boc-imines. J. Am. Chem. Soc. 2013, 135, 11799–11802. [Google Scholar] [CrossRef]
- Bandar, J.S.; Lambert, T.H. Enantioselective Bronsted base catalysis with chiral cyclopropenimines. J. Am. Chem. Soc. 2012, 134, 5552–5555. [Google Scholar] [CrossRef]
- Bandar, J.S.; Tanaset, A.; Lambert, T.H. Phase-Transfer and Other Types of Catalysis with Cyclopropenium Ions. Chem. Eur. J. 2015, 21, 7365–7368. [Google Scholar] [CrossRef]
- Mir, R.; Dudding, T. Phase-Transfer Catalyzed O-Silyl Ether Deprotection Mediated by a Cyclopropenium Cation. J. Org. Chem. 2017, 82, 709–714. [Google Scholar] [CrossRef]
- Huang, H.; Strater, Z.M.; Rauch, M.; Shee, J.; Sisto, T.J.; Nuckolls, C.; Lambert, T.H. Electrophotocatalysis with a Trisaminocyclopropenium Radical Dication. Angew. Chem. Int. Ed. 2019, 58, 13318–13322. [Google Scholar] [CrossRef]
- Weiss, R.; Hertel, M. NITROGEN ANALOG OF DELTIC ACID. J. Chem. Soc. Chem. Commun. 1980, 11, 223–224. [Google Scholar] [CrossRef]
- Krebs, A.W. Cyclopropenylium Compounds and Cyclopropenones. Angew. Chem. Int. Ed. 1965, 4, 10–22. [Google Scholar] [CrossRef]
- Breslow, R.; Groves, J.T.; Ryan, G. Cyclopropenyl cation. J. Am. Chem. Soc. 1967, 89, 5048. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, Z.; Xu, S.; Wang, H.; Guo, T.; Gao, Y.; Zhang, L.; Zhang, C.; Guo, K. Opposite-charge repulsive cation and anion pair cooperative organocatalysis in ring-opening polymerization. Polym. Chem. 2018, 9, 2183–2192. [Google Scholar] [CrossRef]
- Lancianesi, S.; Palmieri, A.; Petrini, M. Synthetic Approaches to 3-(2-Nitroalkyl) Indoles and Their Use to Access Tryptamines and Related Bioactive Compounds. Chem. Rev. 2014, 114, 7108–7149. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, D.M.; Mattson, A.E. Transition Metal and Hydrogen Bond Donor Hybrids: Catalysts for the Activation of Alkylidene Malonates. Chem. Eur. J. 2012, 18, 8310–8314. [Google Scholar] [CrossRef]
- Boiocchi, M.; Del Boca, L.; Gómez, D.E.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. Nature of Urea–Fluoride Interaction: Incipient and Definitive Proton Transfer. J. Am. Chem. Soc. 2004, 126, 16507–16514. [Google Scholar] [CrossRef]
- Cametti, M.; Rissanen, K. Recognition and sensing of fluoride anion. Chem. Commun. 2009, 20, 2809–2829. [Google Scholar] [CrossRef]
- Lacour, J.; Moraleda, D. Chiral anion-mediated asymmetric ion pairing chemistry. Chem. Commun. 2009, 46, 7073–7089. [Google Scholar] [CrossRef]
- Komatsu, K.; Kitagawa, T. Cyclopropenylium Cations, Cyclopropenones, and Heteroanalogues Recent Advances. Chem. Rev. 2003, 103, 1371–1428. [Google Scholar] [CrossRef]
- Weiss, R.; Schwab, O.; Hampel, F. Ion-Pair Strain as the Driving Force for Hypervalent Adduct Formation between Iodide Ions and Substituted Iodobenzenes: Structural Alternatives to Meisenheimer Complexes. Chem. Eur. J. 1999, 5, 968–974. [Google Scholar] [CrossRef]
- Weiss, R.; Brenner, T.; Hampel, F.; Wolski, A. The Consequences of an Electrostatic “Forced Marriage” between Two Electron-Rich Particles: Strained Ion Pairs. Angew. Chem. Int. Ed. 1995, 34, 439–441. [Google Scholar] [CrossRef]
- Weiss, R.; Rechinger, M.; Hampel, F.; Wolski, A. Stable 1:1 Adducts from Iodoacetylenes and Iodide Ions: Ion Pair Strain as an Additional Driving Force? Angew. Chem. Int. Ed. 1995, 34, 441–443. [Google Scholar] [CrossRef]
- Fuchter, M.J.; Smith, C.J.; Tsang, M.W.S.; Boyer, A.; Saubern, S.; Ryan, J.H.; Holmes, A.B. Clean and efficient synthesis of O-silylcarbamates and ureas in supercritical carbon dioxide. Chem. Commun. 2008, 18, 2152–2154. [Google Scholar] [CrossRef] [PubMed]
- Gaul, D.A.; Just, O.; Rees, W.S. Synthesis and characterization of a series of zinc bis (alkyl)(trimethylsilyl)amide compounds. Inorg. Chem. 2000, 39, 5648–5654. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.P.; Cumming, W.D. Conformational preferences of the N-trimethylsilyl and O-trimethylsilyl groups. J. Am. Chem. Soc. 1971, 93, 928–932. [Google Scholar] [CrossRef]
- Xu, J.; Xian, A.; Li, Z.; Liu, J.; Zhang, Z.; Yan, R.; Gao, L.; Liu, B.; Zhao, L.; Guo, K. A Strained Ion Pair Permits Carbon Dioxide Fixation at Atmospheric Pressure by C–H H-Bonding Organocatalysis. J. Org. Chem. 2021, 86, 3422–3432. [Google Scholar] [CrossRef]
- Fleming, E.M.; McCabe, T.; Connon, S.J. Novel axially chiral bis-arylthiourea-based organocatalysts for asymmetric Friedel–Crafts type reactions. Tetrahedron Lett. 2006, 47, 7037–7042. [Google Scholar] [CrossRef]
- Itoh, J.; Fuchibe, K.; Akiyama, T. Chiral phosphoric acid catalyzed enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes: Cooperative effect of 3 Å molecular sieves. Angew. Chem. Int. Ed. 2008, 47, 4016–4018. [Google Scholar] [CrossRef]
- Huang, K.; Pei, X.; Yin, X.; Chen, Z. Novel Chiral Secondary Amine-Amide Catalysts Friedel-Craft Alkylation Reaction. Asian J. Chem. 2017, 29, 595–600. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).