An Approach towards the Conversion of Biomass Feedstocks into Biofuel Using a Zeolite Socony Mobil-5-Based Catalysts via the Hydrothermal Liquefaction Process: A Review
Abstract
:1. Introduction
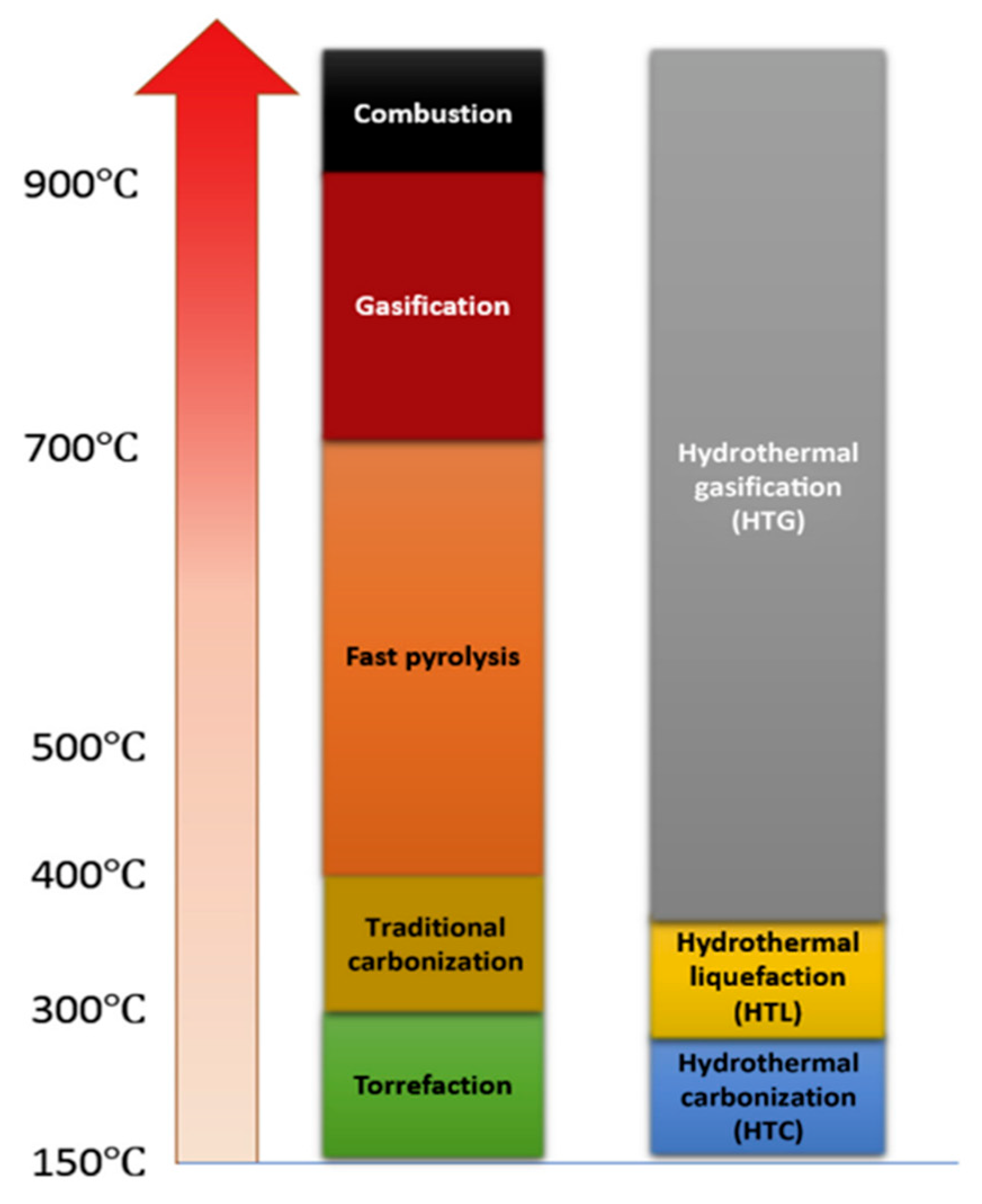
2. Thermochemical Processes
2.1. Direct Combustion
2.2. Gasification
2.3. Pyrolysis
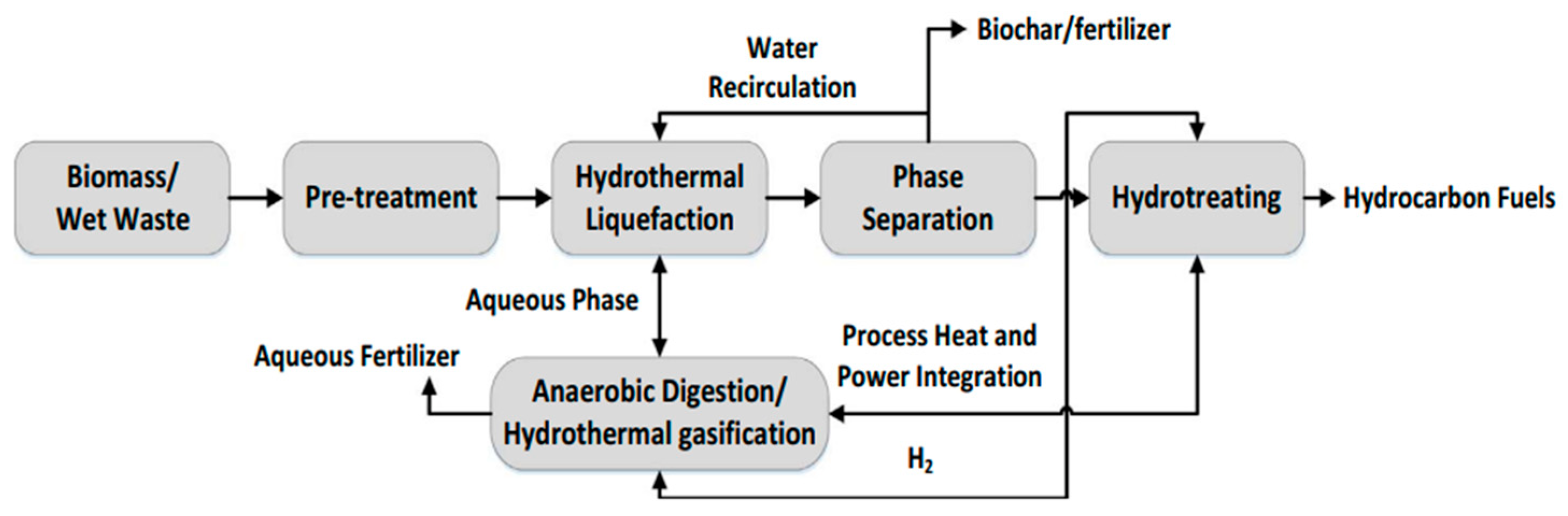
2.4. Hydrothermal Liquefaction
Zeolite Catalysts in HTL Conversion of Biomass
3. Effects of Operating Parameters
3.1. Temperature
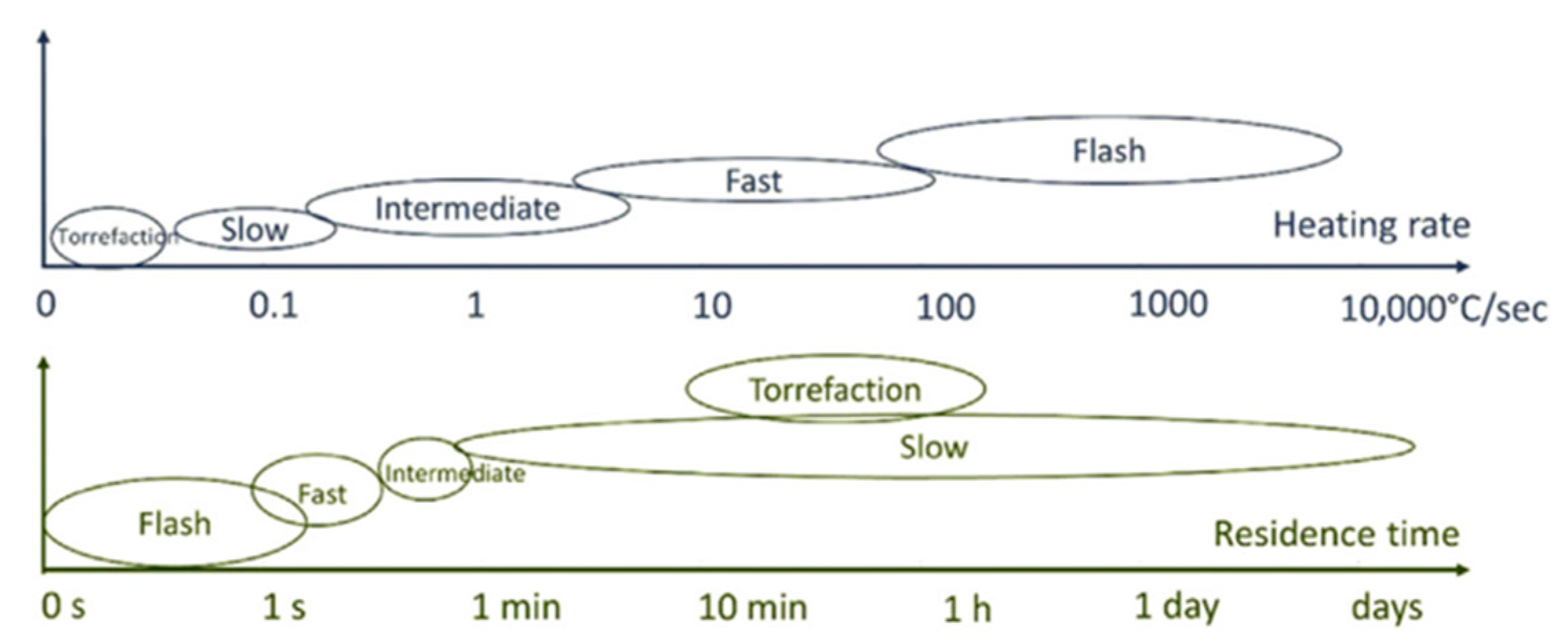
3.2. Residence Time
3.3. Heating Rates
3.4. Type and Composition of Biomass
4. Conclusions and Future Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forero, J.A.J.; Tran, T.H.T.; Tana, T.; Baker, A.; Beltramini, J.; Doherty, W.O.S.; Moghaddam, L. Hydrothermal liquefaction of sugarcane bagasse to bio-oils: Effect of liquefaction solvents on bio-oil stability. Fuel 2022, 312, 122793. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Srirangan, K.; Akawi, L.; Moo-Young, M.; Chou, C.P. Towards sustainable production of clean energy carriers from biomass resources. Appl. Energy 2012, 100, 172–186. [Google Scholar] [CrossRef]
- Qureshi, M.I.; Rasli, A.M.; Zaman, K. Energy crisis, greenhouse gas emissions and sectoral growth reforms: Repairing the fabricated mosaic. J. Clean. Prod. 2016, 112, 3657–3666. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Cao, M.; Long, C.; Sun, S.; Zhao, Y.; Luo, J.; Wu, D. Catalytic hydrothermal liquefaction of peanut shell for the production aromatic rich monomer compounds. J. Energy Inst. 2021, 96, 90–96. [Google Scholar] [CrossRef]
- Deublein, D.; Angelika, S. Biogas from Waste and Renewable Resources; Wiley-VCH Verlag GmmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Biomass Conversion Technologies. In Greenhouse Gases Balances of Bioenergy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–139. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X. The thermochemical conversion of biomass into biofuels. In Biomass, Biopolymer-Based Materials, and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–368. [Google Scholar] [CrossRef]
- Baloch, H.A.; Siddiqui, M.T.; Nizamuddin, S.; Mubarak, N.M.; Khalid, M.; Srinivasan, M.; Griffin, G. Catalytic co-liquefaction of sugarcane bagasse and polyethylene for bio-oil production under supercritical conditions: Effect of catalysts. J. Anal. Appl. Pyrolysis 2021, 153, 104944. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Rehman, A.; Noor, T.; Hussain, A.; Iqbal, N.; Jahan, Z. Role of Catalysis in Biofuels Production Process–A Review. ChemBioEng Rev. 2021, 8, 417–438. [Google Scholar] [CrossRef]
- Lee, A.F.; Wilson, K. Recent developments in heterogeneous catalysis for the sustainable production of biodiesel. Catal. Today 2015, 242, 3–18. [Google Scholar] [CrossRef]
- Saoud, K. Nanocatalyst for Biofuel Production: A Review. In Green Nanotechnology for Biofuel Production, Biofuel and Biorefinery Technologies; Srivastava, N., Srivastava, M., Pandey, H., Mishra, P.K., Ramteke, P.W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 5, pp. 39–62. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Mustafa, A. Synthesis and characterization of new bifunctional SnZrSi oxide catalysts for biodiesel production. J. Mol. Liq. 2022, 354, 118811. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Stefanidis, S.D.; Kalogiannis, K.G.; Delimitis, A.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]
- Mahadevan, R.; Adhikari, S.; Shakya, R.; Wang, K.; Dayton, D.; Lehrich, M.; Taylor, S.E. Effect of Alkali and Alkaline Earth Metals on in-Situ Catalytic Fast Pyrolysis of Lignocellulosic Biomass: A Microreactor Study. Energy Fuels 2016, 30, 3045–3056. [Google Scholar] [CrossRef]
- Aznar, M.P.; Caballero, M.A.; Gil, J.; Martín, J.A.; Corella, J. Commercial steam reforming catalysts to improve biomass gasification with steam-oxygen mixtures. 2. Catalytic tar removal. Ind. Eng. Chem. Res. 1998, 37, 2668–2680. [Google Scholar] [CrossRef]
- Caballero, M.A.; Aznar, P.; Gil, J.; Martı, J.A.; France, E. 98/03884 Commercial steam reforming catalysts to improve biomass gasification with steam-oxygen mixtures 1. Hot gas upgrading by the catalytic reactor. Fuel Energy Abstr. 1998, 39, 363. [Google Scholar] [CrossRef]
- Kouwenhoven, H.W.; De Kroes, B. Preparation of zeolite catalysts. Stud. Surf. Sci. Catal. 2001, 137, 673–706. [Google Scholar] [CrossRef]
- Chai, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Confinement in a Zeolite and Zeolite Catalysis. Acc. Chem. Res. 2021, 54, 2894–2904. [Google Scholar] [CrossRef]
- Nature Materials Zeolite catalysts come into focus. Nat. Mater. 2020, 19, 1037. [CrossRef]
- Wang, S.; Wang, Y.; Cai, Q.; Guo, Z. Production of Bio-gasoline by Co-cracking of Acetic Acid in Bio-oil. Chin. J. Chem. Eng. 2014, 22, 98–103. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Chagas, B.M.E.; Dorado, C.; Serapiglia, M.J.; Mullen, C.A.; Boateng, A.A.; Melo, M.A.F.; Ataíde, C.H. Catalytic pyrolysis-GC/MS of Spirulina: Evaluation of a highly proteinaceous biomass source for production of fuels and chemicals q. Fuel 2016, 179, 124–134. [Google Scholar] [CrossRef]
- Horne, A. Analysis of aromatic hydrocarbons in pyrolytic oil derived from biomass. J. Analyrical Appl. Pyrolysis 1995, 31, 15–37. [Google Scholar]
- Lappas, A.A.; Bezergianni, S.; Vasalos, I.A. Production of biofuels via co-processing in conventional refining processes. Catal. Today 2009, 145, 55–62. [Google Scholar] [CrossRef]
- Grande, L.; Pedroarena, I.; Korili, S.A.; Gil, A. Hydrothermal liquefaction of biomass as one of the most promising alternatives for the synthesis of advanced liquid biofuels: A review. Materials 2021, 14, 5286. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Alam, T.; Krishna, B.B.; Bhaskar, T.; Perkins, G. Bioresource Technology A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Bioresour. Technol. 2020, 312, 123596. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Chen, W.; Lin, B.; Huang, M.; Chang, J. Bioresource Technology Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2014, 184, 314–327. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Serio, M.A.; Kroo, E.; Wójtowicz, M.A. Biomass Pyrolysis for Distributed Energy Generation. ACS Div. Fuel Chem. 2003, 48, 589. [Google Scholar]
- Nanda, S.; Kozinski, J.A.; Dalai, A.K. Lignocellulosic Biomass: A Review of Conversion Technologies and Fuel Products. Curr. Biochem. Eng. 2016, 3, 24–36. [Google Scholar] [CrossRef]
- Rudra, S.; Jayathilake, M. Hydrothermal Liquefaction of Biomass for Biofuel Production. In Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 165–186. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Li, H.; Riisager, A.; Saravanamurugan, S.; Pandey, A.; Sangwan, R.S.; Yang, S.; Luque, R. Carbon-Increasing Catalytic Strategies for Upgrading Biomass into Energy-Intensive Fuels and Chemicals. ACS Catal. 2018, 8, 148–187. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wang, L.; Wang, L.; Xiao, F. Zeolite Fixed Metal Nanoparticles: New Perspective in Catalysis. Acc. Chem. Res. 2021, 54, 2579–2590. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Zhang, H.; Wei, L. Synthesis, Types, and Applications of Zeolite Capsule Catalysts. Cryst. Res. Technol. 2023, 58, 2200287. [Google Scholar] [CrossRef]
- Nagappan, S.; Bhosale, R.R.; Duc, D.; Thuy, N.; Chi, L. Catalytic hydrothermal liquefaction of biomass into bio-oils and other value- added products–A review. Fuel 2021, 285, 119053. [Google Scholar] [CrossRef]
- Perego, C.; Bianchi, D. Biomass upgrading through acid–base catalysis. Chem. Eng. J. 2010, 161, 314–322. [Google Scholar] [CrossRef]
- Scarsella, M.; de Caprariis, B.; Damizia, M.; De Filippis, P. Heterogeneous catalysts for hydrothermal liquefaction of lignocellulosic biomass: A review. Biomass Bioenergy 2020, 140, 105662. [Google Scholar] [CrossRef]
- Grams, J.; Ruppert, A. Development of Heterogeneous Catalysts for Thermo-Chemical Conversion of Lignocellulosic Biomass. Energies 2017, 10, 545. [Google Scholar] [CrossRef]
- Balagurumurthy, B.; Singh, R.; Bhaskar, T. Catalysts for Thermochemical Conversion of Biomass; Elsevier B.V.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Centi, G.; Perez-pariente, J.; Roth, W.J. Zeolite-based materials for novel catalytic applications: Opportunities, perspectives and open problems. Catal. Today 2012, 179, 2–15. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Alsowij, M.; Corbin, F.; Boakye, E.; Gu, Z.; Raynie, D. Catalytic hydrothermal liquefaction (HTL) of biomass for bio-crude production using Ni/HZSM-5 catalysts. AIMS Environ. Sci. 2017, 4, 417–430. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Rabnawaz, M. Catalytic liquefaction of pine sawdust and in-situ hydrogenation of bio- crude over bifunctional Co-Zn/HZSM-5 catalysts. Fuel 2018, 223, 252–260. [Google Scholar] [CrossRef]
- Qian, Y.; Liang, S.; Wang, T.; Wang, Z.; Xie, W.; Xu, X. Enhancement of pyrolysis gasoline hydrogenation over Zn- and Mo-promoted Ni/γ -Al2O3 catalysts. Catcom 2011, 12, 851–853. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, T.; Ma, L.; Zhang, Q.; Liang, W. Upgrading of the liquid fuel from fast pyrolysis of biomass over. Appl. Energy 2010, 87, 2886–2891. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support. Appl. Catal. B Environ. 2012, 117–118, 105–117. [Google Scholar] [CrossRef]
- Wei, Y.; Fakudze, S.; Zhang, Y.; Song, M.; Xue, T.; Xie, R.; Chen, J. Low-temperature hydrothermal liquefaction of pomelo peel for production of 5-hydroxymethylfurfural-rich bio-oil using ionic liquid loaded ZSM-5. Bioresour. Technol. 2022, 352, 127050. [Google Scholar] [CrossRef]
- Liu, C.; Kong, L.; Wang, Y.; Dai, L. Catalytic hydrothermal liquefaction of spirulina to bio-oil in the presence of formic acid over palladium-based catalysts. Algal Res. 2018, 33, 156–164. [Google Scholar] [CrossRef]
- Karagoz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Hydrothermal upgrading of biomass: Effect of KCO concentration and biomass/water ratio on products distribution. Bioresour. Technol. 2006, 97, 90–98. [Google Scholar] [CrossRef]
- Song, C.; Hu, H.; Zhu, S.; Wang, G.; Chen, G. Nonisothermal Catalytic Liquefaction of Corn Stalk in Subcritical and Supercritical Water. Energy Fuels 2004, 18, 90–96. [Google Scholar] [CrossRef]
- Minowa, T.; Yokoyama, S.; Kishimoto, M. Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel 1995, 74, 1735–1738. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y.; Uddin, M.A. Low-temperature hydrothermal treatment of biomass: Effect of reaction parameters on products and boiling point distributions. Energy Fuels 2004, 18, 234–241. [Google Scholar] [CrossRef]
- Seehar, T.H.; Toor, S.S.; Shah, A.A.; Pedersen, T.H.; Rosendahl, L.A. Biocrude Production from Wheat Straw at Sub and Supercritical Hydrothermal Liquefaction. Energies 2020, 13, 3114. [Google Scholar] [CrossRef]
- Makhado, T. Hydrothermal Conversion of Agricultural and Food Waste. 2022. Available online: http://hdl.handle.net/11394/9087 (accessed on 6 November 2023).
- Xue, Y.; Chen, H.; Zhao, W.; Yang, C.; Ma, P.; Han, S. A review on the operating conditions of producing bio-oil from hydrothermal liquefaction of biomass. Int. J. Energy Res. 2016, 40, 865–877. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2004, 71, 803–815. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Bioresource Technology Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Singh, R.; Prakash, A.; Balagurumurthy, B.; Bhaskar, T. Hydrothermal Liquefaction of Biomass. Recent Adv. Thermochem. Convers. Biomass 2015, 269–291. [Google Scholar] [CrossRef]
- Panisko, E.; Wietsma, T.; Lemmon, T.; Albrecht, K.; Howe, D. ScienceDirect Characterization of the aqueous fractions from hydrotreatment and hydrothermal liquefaction of lignocellulosic feedstocks. Biomass Bioenergy 2015, 74, 162–171. [Google Scholar] [CrossRef]
| Property | Combustion | Gasification | Pyrolysis | Hydrothermal Liquefaction |
|---|---|---|---|---|
| Process type | Oxidative reaction with oxygen | Partial oxidation without complete combustion | Thermochemical decomposition in the absence of oxygen | Liquefaction in the presence of water under high pressure and temperature |
| Products | CO2, H2O, heat, and ash | Syngas (CO, H2), ash | Biochar, bio-oil, syngas, and gases | Biocrude oil, gaseous products, and solid residues |
| Temperature range | Above 700 °C | 500 °C–1000 °C | 350 °C–800 °C | 250 °C–450 °C |
| Oxygen availability | Oxygen-rich environment | Controlled oxygen supply | Oxygen-limited | Oxygen present in the form of water |
| Energy efficiency | High | Moderate to high | Moderate | Moderate |
| Feedstock sustainability | Broad range of organic materials | Biomass, coal, and waste materials | Biomass and waste materials | Biomass, algae, and organic waste |
| Syngas composition | CO2, CO, H2, and water vapour | CO, H2, CH4, tar, and ash | H2, CO, CH4, and other hydrocarbons | CO2, CO, H2, and other hydrocarbons |
| End-Products Use | Heat generation, electricity, or direct heat use | Syngas for electricity, heat, or biofuels | Biochar for soil improvement, bio-oil for bioenergy | Biocrude for biofuels, gaseous fuel, and by-products |
| Residue/Char quality | Ash residue | High-quality biochar | Solid biochar | Solid residues with potential applications |
| Environmental impact | CO2 emissions, air pollutants | Lower CO2 emissions compared to combustion | Moderate emissions, biochar sequestration potential | Lower CO2 emissions, potential carbon capture |
| Application focus | Traditional power generation, heat | Bioenergy synthetic fuels | Bioenergy, soil improvement | Biofuels, wastewater treatment, biomass conversion |
| Feedstock | Catalyst | Catalyst Preparation | Reaction Conditions | Yield (wt%) | Reference | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | |||||
| Pine sawdust | HZSM-5, Ni/HZSM-5, and K2CO3 | Ni/HZSM-5 catalysts with different Ni loading (6 wt% and 12 wt%) were impregnated with aqueous nickel (II) nitrate hexahydrate solutions. The prepared Ni/HZSM-5 and HZSM-5 catalysts were dried at 120 °C for 4 h, and then calcined at 550 °C for 6 h. | 300 | 60 | 61–68 | [48] |
| Pine sawdust | Co-Zn modified ZSM-5 | Co- or Zn-loaded HZSM-5 catalysts with different Co or Zn mass loading ratios (20 wt% Co/HZSM-5, 10 wt% Co-10 wt% Zn/HZSM-5, and 20 wt% Zn/HZSM-5) were prepared by a wet impregnation method. The catalysts were dried in air at 120 °C for 10 h, and then prepared catalysts were calcined in air at 550 °C for 4 h | 300 | 60 | 65.02–67.38 | [49] |
| Pomelo peel | Ionic liquid loaded ZSM-5, ZSM-5 | ZSM-5 was added to [AMIm]Cl solvent and stirred for 10 h. After stirring, deionised water was added to cool the mixture before extraction. A low-speed centrifuge was used to centrifuge the reaction mixture, and then dried overnight to obtain a solid product. | 200–300 | 60 | Ionic liquid loaded ZSM-5: 29.21 (at 200 °C) 20.83 (at 300 °C) ZSM-5: 18.23 (at 200 °C) 17.06 (at 300 °C | [53] |
| Spirulina | Pd/HZSM-5@MS, Pd/HZSM-5 | Pd(NO3)2 was added in water, and HZSM-5/HZSM-5@MS were added in a beaker with 10 mL water. The mixture was stirred at room temperature for 24 h, and then dried in the oven for 10 h at 120 °C. The powders were washed with water, filtered, and calcine for 4 h at 400 °C | 380 | 120 | Pd/HZSM-5@MS—37.30 Pd/HZSM-5—37.20 | [54] |
| Pine sawdust | HZSM-5, Zn/ZSM-5 | Several Zn loadings (5 wt%, 10 wt%, 15 wt%, and 20 wt%) of Zn/HZSM-5 catalysts were prepared by impregnation with aqueous solutions of zinc nitrate hexahydrate. The prepared Zn/HZSM-5 and HZSM-5 catalysts were dried in air for four hours at 120 °C, and then they were calcined for six hours in air at 550 °C. | 300 | 60 | 15% Zn/ZSM-5—59 wt% Was recorded as the highest yield | [49] |
| Wood biomass | K2CO3 | The catalyst was purchased and used as is. | 280 | 15 | Run 1—8.6 Run 2—6.4 Run 3—8.5 | [55] |
| Corn stalk | 1.0 wt% Na2CO3 | The catalyst was purchased and used as is. | 409.85 | 15 | 47.2 | [56] |
| Microalga (Dunaliella tertiolecta) | 0 and 5.0 wt% Na2CO3 | The catalyst was purchased and used as is. | 250, 300, 340 | 5 and 60 | 31–44 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jideani, T.; Chukwuchendo, E.; Khotseng, L. An Approach towards the Conversion of Biomass Feedstocks into Biofuel Using a Zeolite Socony Mobil-5-Based Catalysts via the Hydrothermal Liquefaction Process: A Review. Catalysts 2023, 13, 1425. https://doi.org/10.3390/catal13111425
Jideani T, Chukwuchendo E, Khotseng L. An Approach towards the Conversion of Biomass Feedstocks into Biofuel Using a Zeolite Socony Mobil-5-Based Catalysts via the Hydrothermal Liquefaction Process: A Review. Catalysts. 2023; 13(11):1425. https://doi.org/10.3390/catal13111425
Chicago/Turabian StyleJideani, Thandiswa, Emmanuel Chukwuchendo, and Lindiwe Khotseng. 2023. "An Approach towards the Conversion of Biomass Feedstocks into Biofuel Using a Zeolite Socony Mobil-5-Based Catalysts via the Hydrothermal Liquefaction Process: A Review" Catalysts 13, no. 11: 1425. https://doi.org/10.3390/catal13111425
APA StyleJideani, T., Chukwuchendo, E., & Khotseng, L. (2023). An Approach towards the Conversion of Biomass Feedstocks into Biofuel Using a Zeolite Socony Mobil-5-Based Catalysts via the Hydrothermal Liquefaction Process: A Review. Catalysts, 13(11), 1425. https://doi.org/10.3390/catal13111425







