Nanosized Silica-Supported 12-Tungstophosphoric Acid: A Highly Active and Stable Catalyst for the Alkylation of p-Cresol with tert-Butanol
Abstract
:1. Introduction
2. Results and Discussion
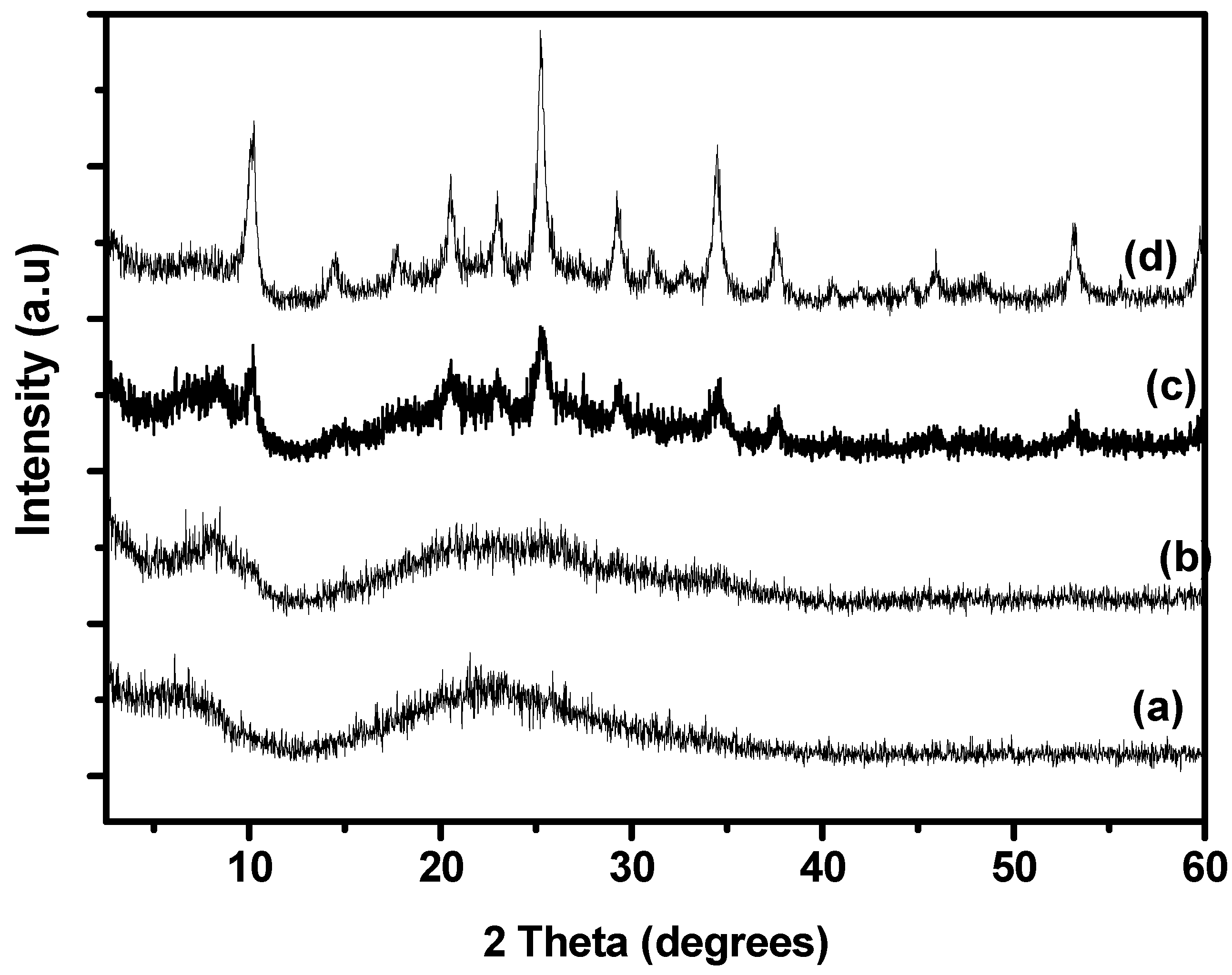
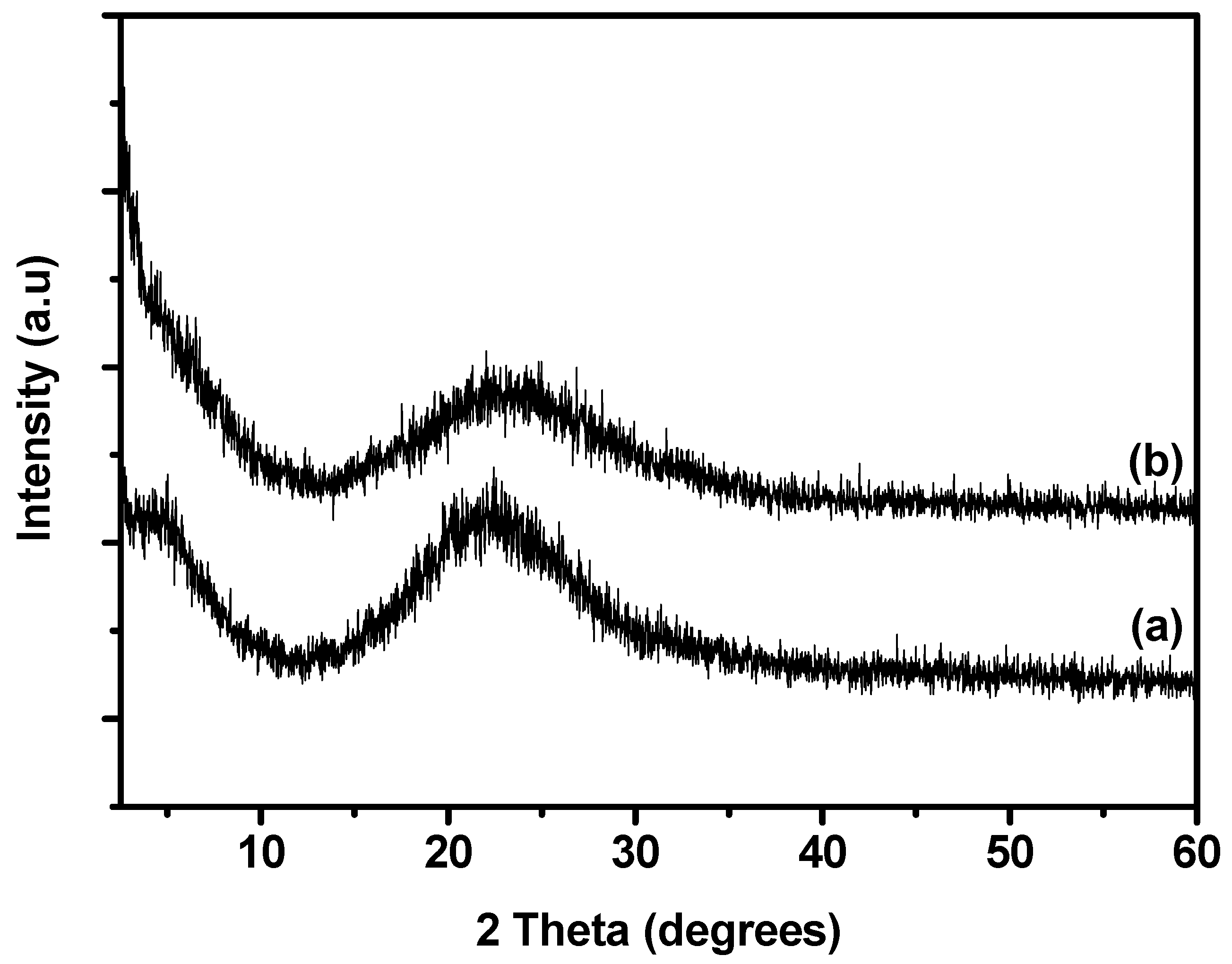
2.1. XRD Analysis
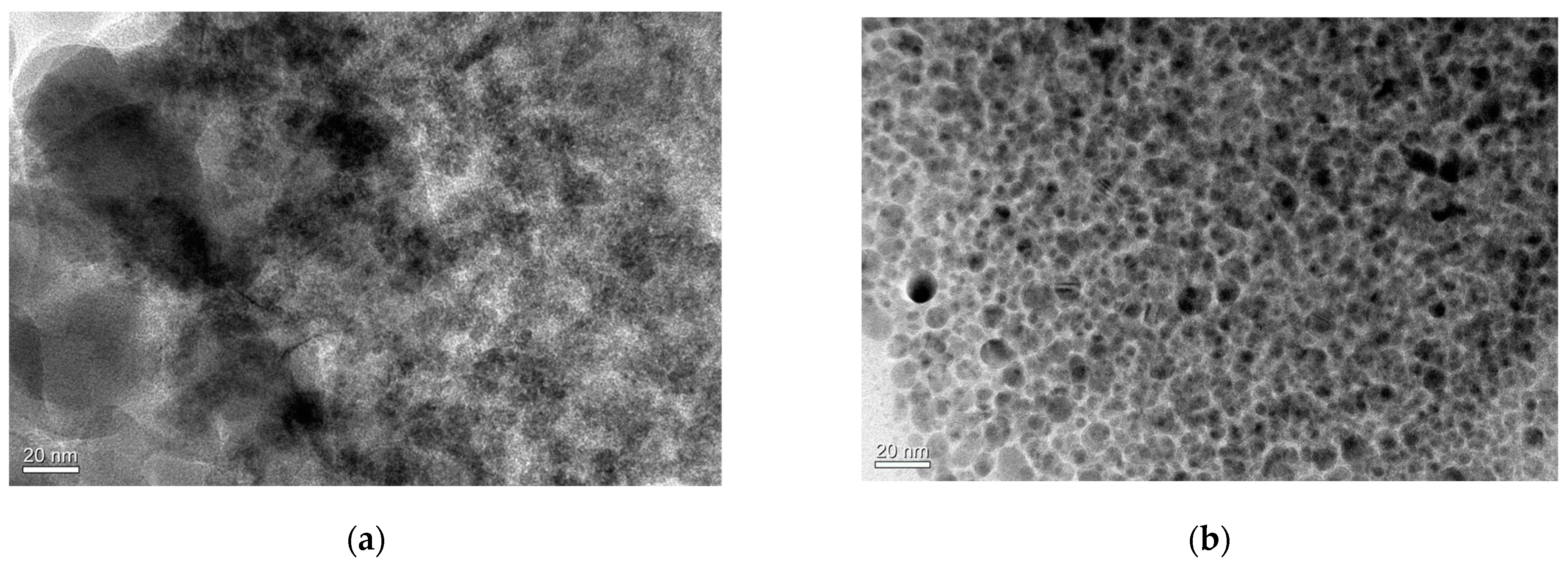
2.2. TEM Analysis
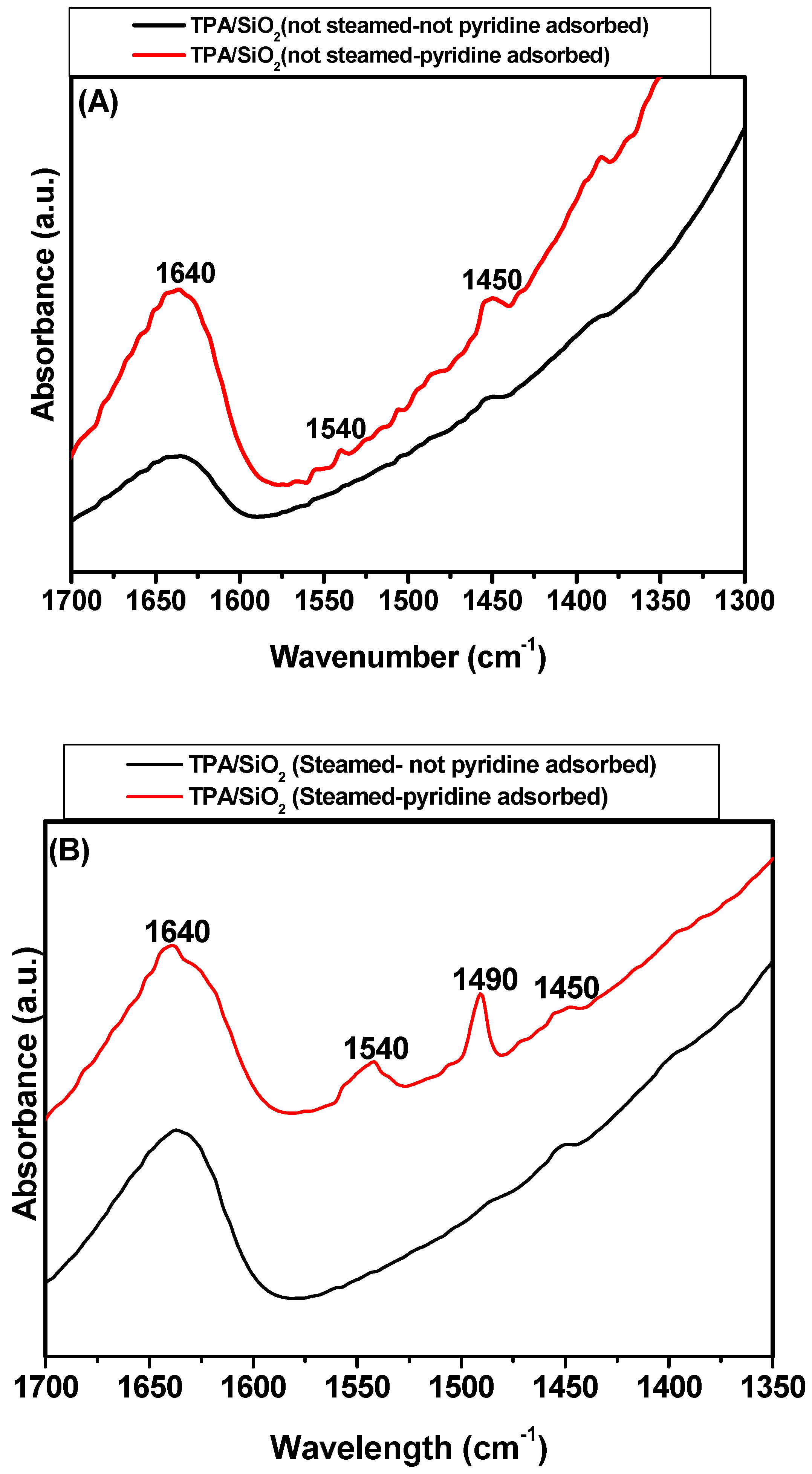
2.3. Surface Acidity of the TPA/SiO2 Catalysts
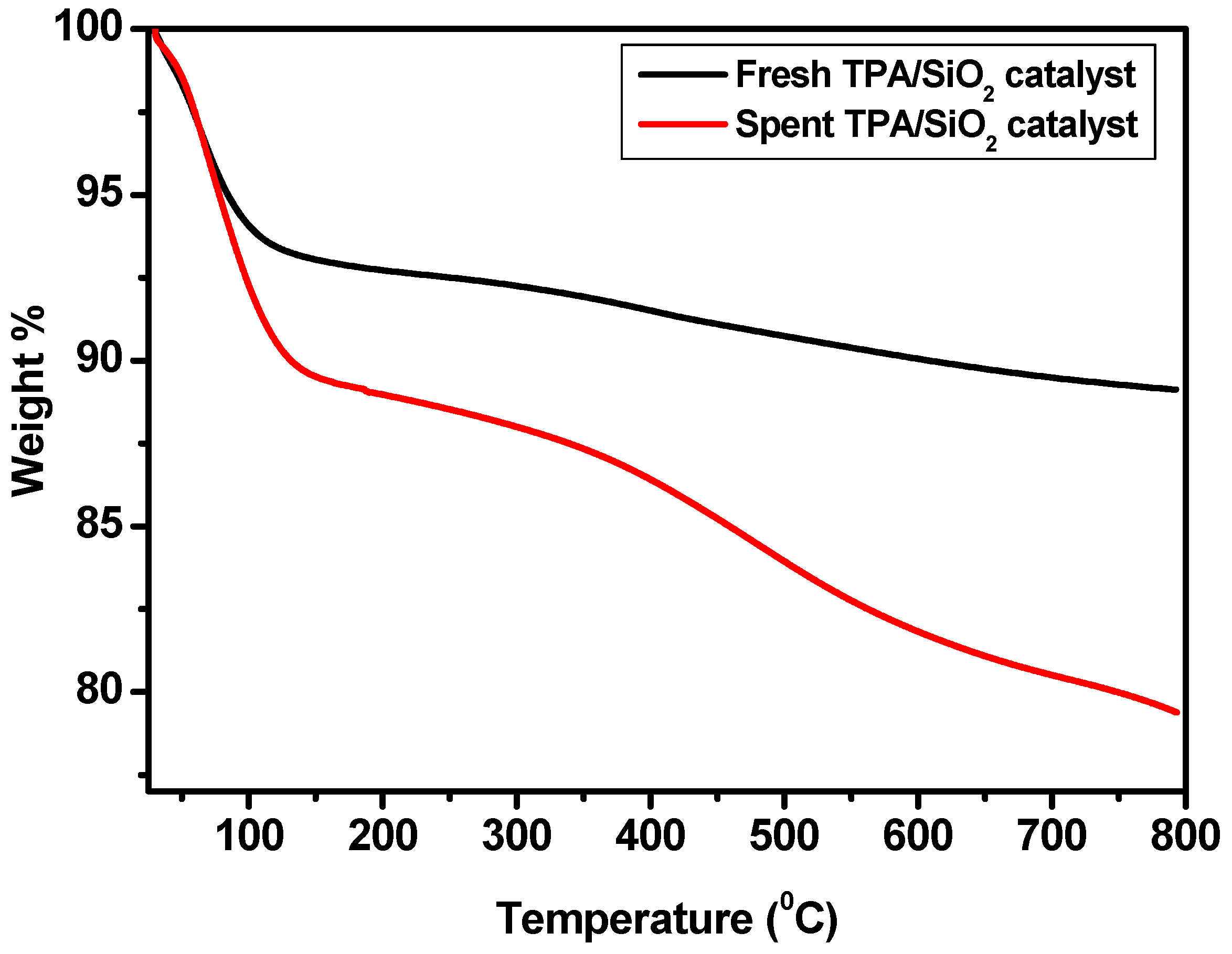
2.4. Thermogravimetric Analysis (TGA) of Fresh and Used Catalyst
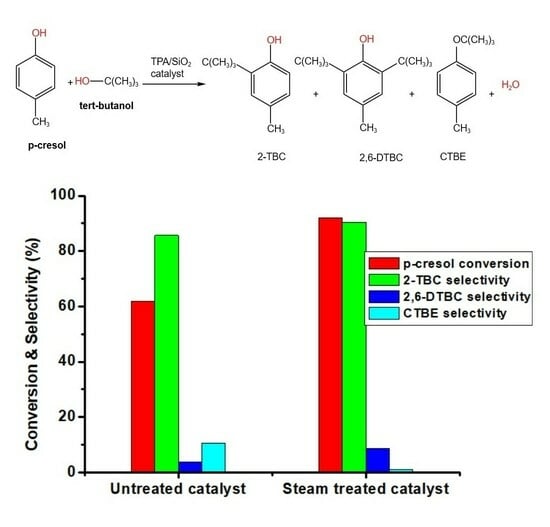
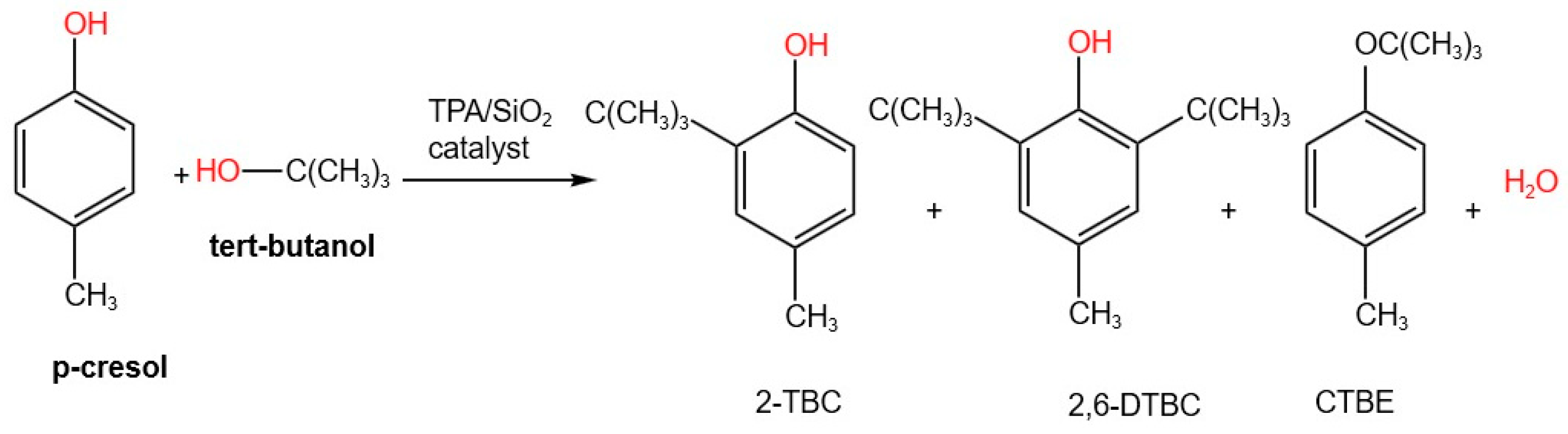
2.5. Catalytic Studies
2.5.1. Effect of Steam Treatment
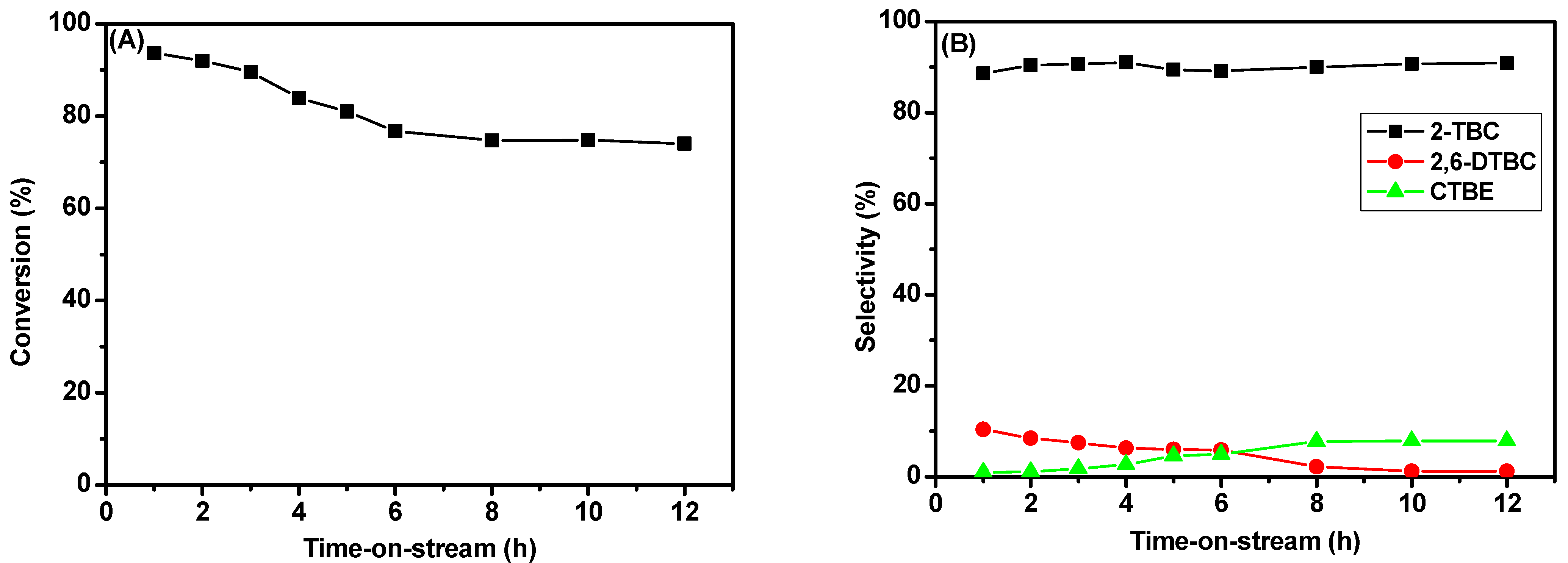
2.5.2. Effect of Time-on-Stream
2.5.3. Effect of Reactant Feed Rate
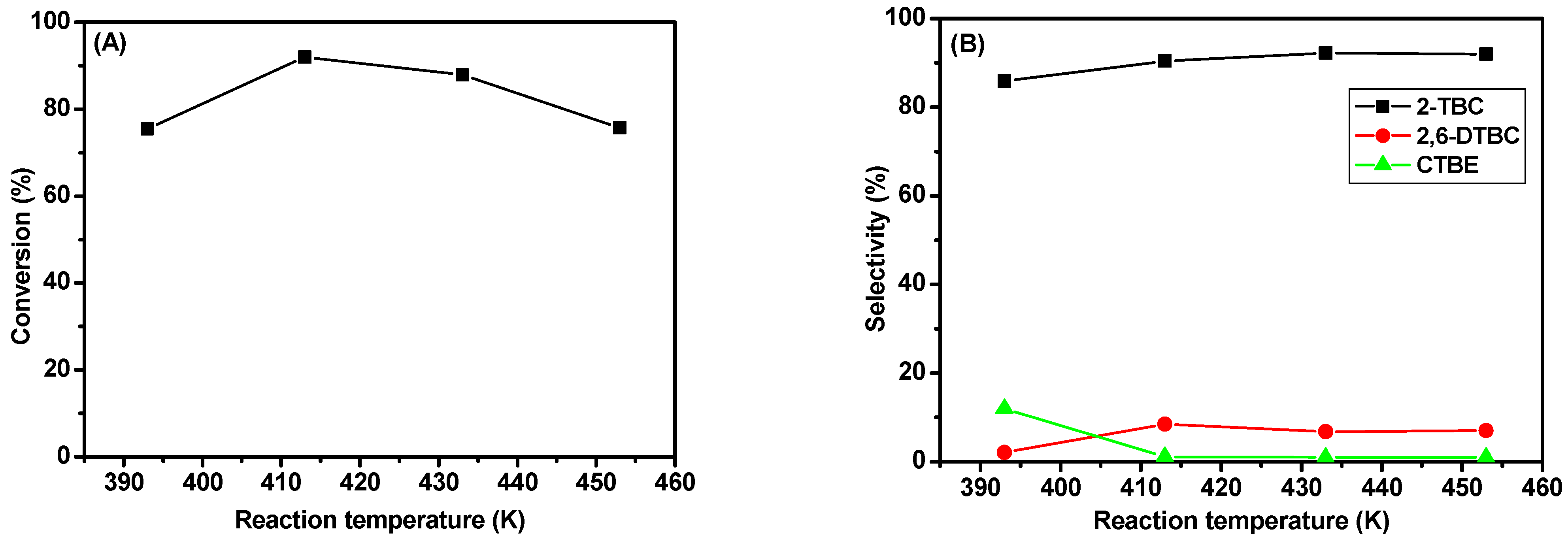
2.5.4. Effect of Reaction Temperature
2.5.5. Effect of Molar Ratio of tert-Butyl Alcohol to p-Cresols
2.5.6. Reusability of the Catalyst
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of 12-Tungstophosphoric Acid Supported on Silica Catalysts
3.3. Characterization Methods
3.4. Catalytic Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pospíšil, J. Mechanistic action of phenolic antioxidants in polymers—A review. Polym. Degrad. Stab. 1988, 20, 181–202. [Google Scholar] [CrossRef]
- Huston, R.C.; Lewis, W.C. Action of aromatic alcohols on aromatic compounds in the presence of aluminum chloride. VII condensation of benzyl alcohol with para-cresol. J. Am. Chem. Soc. 1931, 53, 2379–2382. [Google Scholar] [CrossRef]
- Murphy, J. The Additives for Plastics Handbook; Elsevier Advanced Technology: Oxford, UK, 1996. [Google Scholar]
- Shinde, A.B.; Shrigadi, N.B.; Samant, S.D. tert-Butylation of phenols using tert-butyl alcohol in the presence of FeCl3-modified montmorillonite K10. Appl. Catal. A Gen. 2004, 276, 5–8. [Google Scholar] [CrossRef]
- Lebedev, N.N. Chemistry and Technology of Basic Organic and Petrochemical Synthesis. MIR Publishers. Moscow 1 and 2. J. Polym. Sci. Part C Polym. Lett. 1984, 24, 305. [Google Scholar] [CrossRef]
- Melnikov, N.N.; Baskahov, Y.A.; Bokrev, K.S. Chemistry of herbicides and plant growth regulators. Gkhi. Moscow 1954, 38-42, 38–42. [Google Scholar]
- Shreve, R.N.; Brink, J.A. Chemical Process Industries, 4th ed.; McGraw-Hill International Book Company: London, UK, 1977; pp. 812–814. [Google Scholar]
- Dimitriev, S.A.; Korener, K.D.; Tsvetkov, O.N. Synthesis of detergents of o,p–type based on phenols derived from peat oils. Torfyanaya. Prom. 1961, 32, 24–27. [Google Scholar]
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalysed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Clark, J.H.; Macquarrie, D.J. Environmentally friendly catalytic methods. Chem. Soc. Rev. 1996, 25, 303–310. [Google Scholar] [CrossRef]
- Clark, J.H.; Macquarrie, D.J. Catalysis of liquid phase organic reactions using chemically modified mesoporous inorganic solids. Chem. Commun. 1998, 8, 853–860. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heteropolyacid catalysts. Curr. Opin. Solid State Mater. Sci. 1997, 2, 84–89. [Google Scholar] [CrossRef]
- Shubhashish, S.; Wijenayake, S.; Huang, X.; Posada, L.F.; Samantha Joy, B.R.; Khanna, H.S.; Dziengiel, D.; Mansour, A.; Suib, S.L. Highly Mesoporous MoO3 Catalysts for Electrophilic Aromatic Substitution. Appl. Mater. Interfaces 2002, 14, 51041–51052. [Google Scholar] [CrossRef] [PubMed]
- Hartmut, Die Alkylierung von p-Kresol mitIsobuten an polymerenFestsäuren. Chem.-Ing.-Tech. 1980, 52, 825. [CrossRef]
- Su, Z.-R.; Wang, T.-J. 12-Tungstophosphoric acid immobilized on modified macroporous phenol-furfural sulfonic acid resin for tert-butylation of p-cresol. React. Funct. Polym. 1995, 28, 97–102. [Google Scholar] [CrossRef]
- Santacessaria, E.; Silvani, R.; Wilkinson, P.; Carra, S. Alkylation of p-Cresol with Isobutene Catalysed by Cation-Exchange Resins: A Kinetic Study. Ind. Eng. Chem. Res. 1988, 27, 541–548. [Google Scholar] [CrossRef]
- Harmer, M.A.; Sun, Q. Solid acid catalysis using ion-exchange resins. Appl. Catal. A Gen. 2001, 221, 45–62. [Google Scholar] [CrossRef]
- Chandra, K.G.; Sharma, M.M. Alkylation of phenol with MTBE and other tert-butyl ethers: Cation exchange resins as catalysts. Catal. Lett. 1993, 19, 309–317. [Google Scholar] [CrossRef]
- Devassy, B.M.; Shanbhag, G.V.; Lefebvre, F.; Halligudi, S.B. Alkylation of p-cresol with tert-butanol catalyzed by heteropoly acid supported on zirconia catalyst. J. Mol. Catal. A Chem. 2004, 210, 125–130. [Google Scholar] [CrossRef]
- Yadav, G.D.; Thorat, T.S. Kinetics of Alkylation of p-Cresol with Isobutylene Catalyzed by Sulfated Zirconia. Ind. Eng. Chem. Res. 1996, 35, 721–731. [Google Scholar] [CrossRef]
- DeCastro, C.; Sauvage, E.; Valkenberg, M.H.; Hölderich, W.F. Immobilised Ionic Liquids as Lewis Acid Catalysts for the Alkylation of Aromatic Compounds with Dodecene. J. Catal. 2000, 196, 86–94. [Google Scholar] [CrossRef]
- Ono, Y. Role of Basic Sites in Catalysis by Zeolites. Stud. Surf. Sci. Catal. 1980, 5, 19–27. [Google Scholar]
- Yadav, G.D.; Nair, J.J. Sulfated zirconia and its modified versions as promising catalysts for industrial processes. Microporous Mesoporous Mater. 1999, 33, 1–48. [Google Scholar] [CrossRef]
- Sreenivasan, R.; Keogh, R.A.; Milburn, D.R.; Davis, B.H. Sulfated Zirconia Catalysts: Characterization by TGA/DTA Mass Spectrometry. J. Catal. 1995, 153, 123–130. [Google Scholar] [CrossRef]
- Rahman, N.J.A.; Ramli, A.; Jumbri, K.; Uemura, Y. Tailoring the surface area and the acid–base properties of ZrO2 for biodiesel production from Nannochloropsis sp. Sci. Rep. 2019, 9, 16223. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Fujitani, T. Catalytic conversion of levulinic acid and its esters to γ-valerolactone over silica-supported zirconia catalysts. Bull. Chem. Soc. Jpn. 2014, 87, 1252–1254. [Google Scholar] [CrossRef]
- Ward, A.J.; Pujari, A.A.; Costanzo, L.; Masters, A.F.; Maschmeyer, T. Ionic liquid-templated preparation of mesoporous silica embedded with nanocrystalline sulfated zirconia. Nanoscale Res. Lett. 2011, 6, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Abdel Salam, M.S.; Betiha, M.A.; Shaban, S.A.; Elsabagh, A.M.; Abd El-Aal, R.M.; El Kady, F.Y. Synthesis and characterization of MCM-41-supported nano zirconia catalysts. Egypt. J. Pet. 2015, 24, 49–57. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Sakthinathan, S.; Santhana Krishnan, P.; Ramesh, A.; Mangesh, V.L.; Abilarasu, A.; Narayanan, S.; Shanthi, K.; Chiu, T.W. Catalytic activity of ratio-dependent SBA-15 supported zirconia catalysts for highly selective oxidation of benzyl alcohol to benzaldehyde and environmental pollutant heavy metal ions detection. J. Mol. Struct. 2019, 1176, 650–661. [Google Scholar] [CrossRef]
- Kim, K.D.; Kim, J.; Teoh, W.Y.; Kim, J.C.; Huang, J.; Ryoo, R. Cascade reaction engineering on zirconia-supported mesoporous MFI zeolites with tuneable Lewis-Brønsted acid sites: A case of the one-pot conversion of furfural to γ-valerolactone. RSC Adv. 2020, 10, 35318–35328. [Google Scholar] [CrossRef]
- Bikmetova, L.I.; Kazantsev, K.V.; Zatolokina, E.V.; Dzhikiya, O.V.; Molikov, S.M.D.; Belyi, A.S. Sulfated zirconia catalysts supported on alumina for hexane isomerization. AIP Conf. Proc. 2020, 2301, 030002. [Google Scholar] [CrossRef]
- Saris, S.; Devassy, B.M.; Halligudi, S.B. tert-Butylation of p-cresol over WOx/ZrO2 solid acid catalysts. J. Mol. Catal. A Chem. 2005, 235, 44–51. [Google Scholar] [CrossRef]
- Malpani, S.K.; Goyal, D.; Chinnam, S.; Sharma, S.K.; Katara, S.; Rani, A. Vapor Phase Alkylation of Isomeric Cresols with Tert-Butyl Alcohol over Perlite Supported Sulfated Zirconia Catalyst. Sustainability 2000, 14, 5149–5170. [Google Scholar] [CrossRef]
- Chumbhale, V.R.; Gore, K.U.; Hegde, S.G.; Kim, J.-S.; Lee, S.-B.; Choi, M.-J. Tertiary Butylation of Phenol over HY and Dealuminated HY Zeolites. J. Ind. Eng. Chem. 2003, 9, 748–752. [Google Scholar]
- Dumitriu, E.; Hulea, V. Effects of channel structures and acid properties of large-pore zeolites in the liquid-phase tert-butylation of phenol. J. Catal. 2003, 218, 249–257. [Google Scholar] [CrossRef]
- Anand, R.; Maheswari, R.; Gore, K.; Tope, B. Tertiary butylation of phenol over HY and dealuminated HY zeolites. J. Mol. Catal. A Chem. 2003, 193, 251–257. [Google Scholar] [CrossRef]
- Padmasri, A.H.; Kumari, V.D.; Rao, P.K. Supported 12-tungstophosphoric acid: A recoverable solid acid catalyst for liquid phase Friedel–Crafts alkylation of phenol. Stud. Surf. Sci. Catal. 1998, 113, 561–563. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Selvaraj, M.; Jeon, S.H.; Han, J.; Sinha, P.K.; Lee, T.G. A novel route to produce 4-t-butyltoluene by t-butylation of toluene with t-butylalcohol over mesoporous Al-MCM-41 molecular sieves. Appl. Catal. A Gen. 2005, 286, 44–51. [Google Scholar] [CrossRef]
- Perego, C.; Amarilli, S.; Carati, A.; Flego, C.; Pazzuconi, G.; Rizzo, C.; Bellussi, G. Mesoporous silica-aluminas as catalysts for the alkylation of aromatic hydrocarbons with olefins. Microporous Mesoporous Mater. 1999, 27, 345–354. [Google Scholar] [CrossRef]
- Čejka, J.; Krejčí, A.; Žilková, N.; Dědeček, J.; Hanika, J. Alkylation and disproportionation of aromatic hydrocarbons over mesoporous molecular sieves. Microporous Mesoporous Mater. 2001, 44–45, 499–507. [Google Scholar] [CrossRef]
- Selvaraj, M.; Sinha, P.K. Highly selective synthesis of t-butyl-p-cresol (TBC) by t-butylation of p-cresol with t-butyl alcohol over microporous and mesoporous catalysts. J. Mol. Catal. A Chem. 2007, 264, 44–49. [Google Scholar] [CrossRef]
- Kamalakar, G.; Komura, K.; Sugi, Y. The Di-t-butylation of p-cresol with t-butanol in Supercritical CO2 over Tungstophosphoric Acid Supported on Ordered Mesoporous Silica. Catal. Lett. 2006, 108, 31–35. [Google Scholar] [CrossRef]
- Misono, M. Heterogeneous Catalysis by Heteropoly Compounds of Molybdenum and Tungsten. Catal. Rev. 1987, 29, 269–321. [Google Scholar] [CrossRef]
- Molnár, Á.; Keresszegi, C.; Török, B. Heteropoly acids immobilized into a silica matrix: Characterization and catalytic applications. Appl. Catal. A Gen. 1999, 189, 217–224. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Li, C.; Wen, L.; Min, E. Characterization and kinetic investigation of tungstophosphoric supported on SiO2 for alkylation of benzene with 1-dodecene to synthesize linear alkylbenzene. J. Mol. Catal. A Chem. 2003, 198, 359–367. [Google Scholar] [CrossRef]
- Kaur, J.; Griffin, K.; Harrison, B.; Kozhevnikov, I.V. Friedel–Crafts Acylation Catalysed by Heteropoly Acids. J. Catal. 2002, 208, 448–455. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heterogeneous Catalysis. Chem. Rev. 1998, 98, 199–218. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef]
- Mohana, R.K.; Gobetto, R.; Iannibello, A.; Zecchina, A. Solid state NMR and IR studies of phosphomolybdenum and phosphotungsten heteropoly acids supported on SiO2, γ-Al2O3, and SiO2—Al2O3. J. Catal. 1989, 119, 512–516. [Google Scholar] [CrossRef]
- Kasztelari, S.; Moffat, J.B. The oxidation of methane on heteropolyoxometalates III. Effect of the addition of cesium on silica-supported 12-molybdophosphoric acid, molybdena, vanadia, and iron oxide. J. Catal. 1988, 112, 54–65. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V.; Sinnema, A.; Jansen, R.J.J.; Pamin, K.; Van Bekkum, H. New acid catalyst comprising heteropoly acid on a mesoporous molecular sieve MCM-41. Catal. Lett. 1994, 30, 241–252. [Google Scholar] [CrossRef]
- Devassy, B.M.; Halligudi, S.B.; Hegde, S.G.; Halgeri, A.B.; Lefebvre, F. 12-Tungstophosphoric acid/zirconia—A highly active stable solid acid—Comparison with a tungstate zirconia catalyst. Chem. Commun. 2002, 10, 1074–1075. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.; Patel, A. Liquidphase tert-butylation of cresols catalysed by 12-tungstophosphoric acid and 12-tungstosilicicacid supported onto neutral alumina. Catal. Lett. 2007, 113, 99–103. [Google Scholar] [CrossRef]
- Kumbar, S.M.; Shanbhag, G.V.; Lefebvre, F.; Halligudi, S.B. Heteropoly acid supported on titania as solid acid catalyst in alkylation of p-cresol with tert-butanol. J. Mol. Catal. A Chem. 2006, 256, 324–334. [Google Scholar] [CrossRef]
- Kurhade, A.; Zhu, J.; Hu, Y.; Dalai, A.K. Surface Investigation of Tungstophosphoric Acid Supported on Ordered Mesoporous Aluminosilicates for Biodiesel Synthesis. ACS Omega 2018, 3, 14064–14075. [Google Scholar] [CrossRef]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Mesoporous silica-supported 12-tungstophosphoric acid catalysts for the liquid phase dehydration of d-xylose. Microporous Mesoporous Mater. 2006, 94, 214–225. [Google Scholar] [CrossRef]
- Huang, J.; Fan, Y.; Zhang, G.; Ma, Y. Protective dissolution: Generating secondary pores in zeolite by mechanochemical reaction. RSC Adv. 2020, 10, 13583–13590. [Google Scholar] [CrossRef]
- Aboul-Gheit, A.K.; Al-Hajjaji, M.A.; Summan, A.M. Differential scanning calorimetry probes acidity strength distribution in catalytic materials. Thermochim. Acta 1987, 118, 9–16. [Google Scholar] [CrossRef]
- Aboul-Gheit, A.K. Desorption of presorbed ammonia, triethylamine and pyridine from the acid sites of mordenites via differential scanning calorimetry. Thermochim. Acta 1988, 132, 257. [Google Scholar] [CrossRef]
- Kim, Y.S.; Wang, F.; Hickner, M.; Zawodzinski, T.A.; McGrath, J.E. Fabrication and characterization of heteropolyacid (H3PW12O40)/directly polymerized sulfonated poly (arylene ether sulfone) copolymer composite membranes for higher temperature fuel cell applications. J. Membr. Sci. 2003, 212, 263–282. [Google Scholar] [CrossRef]
- Abedin, M.A.; Kanitkar, S.; Kumar, N.; Wang, Z.; Ding, K.; Hutchings, G.; Spivey, J.J. Probing the Surface Acidity of Supported Aluminum Bromide Catalysts. Catalysts 2020, 10, 869. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Anderson, J.A. FTIR and reaction studies of the acylation of anisole with acetic anhydride over supported HPA catalysts. J. Catal. 2004, 228, 225–233. [Google Scholar] [CrossRef]
- Asiedu, A.; Davis, R.; Kumar, S. Catalytic transfer hydrogenation and characterization of flash hydrolyzed microalgae into hydrocarbon fuels production (jet fuel). Fuel 2020, 261, 116440. [Google Scholar] [CrossRef]
- Nie, P.; Liu, X.; Zhang, P.; Yuan, X.; Li, X.; Lin, S.; Yin, Z. Quaternary ammonium cellulose promoted synthesis of hollow nano-sized ZSM-5 zeolite as stable catalyst for benzene alkylation with ethanol. J. Mater. Sci. 2021, 56, 8461–8478. [Google Scholar] [CrossRef]
- Yang, E.; Moon, D.J. CO2 Reforming of Methane over Ni0/La2O3 Catalyst Without Reduction Step: Effect of Calcination Atmosphere. Top. Catal. 2017, 60, 697–705. [Google Scholar] [CrossRef]
- Yan, T.; Yang, L.; Dai, W.; Wang, C.; Wu, G.; Guan, N.; Hunger, M.; Li, L. On the deactivation mechanism of zeolite catalyst in ethanol to butadiene conversion. J. Catal. 2018, 367, 7–15. [Google Scholar] [CrossRef]
- Alamdari, R.F.; Hosseinabadi, Z.; Khouzani, M.F. Synthesis, characterization and investigation of catalytic activity of Cu1−xCox Fe2O4nanocatalysts in t-butylation of p-cresol. J. Chem. Sci. 2012, 124, 827–834. [Google Scholar] [CrossRef]
- Roselin, L.S.; Selvin, R.; Aneesh, P.; Bououdina, M.; Krishnaswamy, S. Highly Active and Reusable Catalyst for Fries Rearrangement of Phenyl Acetate. Kinet. Catal. 2011, 52, 823–827. [Google Scholar] [CrossRef]
- Chen, D.; Rebo, H.P.; Moljord, K.; Holmen, A. Influence of Coke Deposition on Selectivity in Zeolite Catalysis. Ind. Eng. Chem. Res. 1997, 36, 3473–3479. [Google Scholar] [CrossRef]
- Subramanian, S.; Mitra, A.; Satyanarayana, C.V.V.; Chakrabarty, D.K. Para-selective butylation of phenol over silicoaluminophosphate molecular sieve SAPO-11 catalyst. Appl. Catal. A Gen. 1997, 159, 229–240. [Google Scholar] [CrossRef]




| Name of Sample | Peak Temperature (°C) | ∆H (J/g) | Type of Acid Site |
|---|---|---|---|
| TPA/SiO2 25% | 190 | 632 | LA a |
| 426 | 286 | BA b | |
| TPA/SiO2 25% (After Steaming) | 204, 258 | 1356 | LA a |
| 402 | 445 | BA b |
| Catalyst | Conversion (wt.%) | |||
|---|---|---|---|---|
| p-Cresol | 2-TBC | 2,6-TBC | CTBE | |
| Untreated catalyst | 61.8 | 85.7 | 3.8 | 10.5 |
| Steam-treated catalyst | 92 | 90.4 | 8.5 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, W.; Alharbi, K.H.; Roselin, L.S.; Savidha, R.; Selvin, R. Nanosized Silica-Supported 12-Tungstophosphoric Acid: A Highly Active and Stable Catalyst for the Alkylation of p-Cresol with tert-Butanol. Catalysts 2023, 13, 1432. https://doi.org/10.3390/catal13111432
Alharbi W, Alharbi KH, Roselin LS, Savidha R, Selvin R. Nanosized Silica-Supported 12-Tungstophosphoric Acid: A Highly Active and Stable Catalyst for the Alkylation of p-Cresol with tert-Butanol. Catalysts. 2023; 13(11):1432. https://doi.org/10.3390/catal13111432
Chicago/Turabian StyleAlharbi, Walaa, Khadijah H. Alharbi, L. Selva Roselin, R. Savidha, and Rosilda Selvin. 2023. "Nanosized Silica-Supported 12-Tungstophosphoric Acid: A Highly Active and Stable Catalyst for the Alkylation of p-Cresol with tert-Butanol" Catalysts 13, no. 11: 1432. https://doi.org/10.3390/catal13111432
APA StyleAlharbi, W., Alharbi, K. H., Roselin, L. S., Savidha, R., & Selvin, R. (2023). Nanosized Silica-Supported 12-Tungstophosphoric Acid: A Highly Active and Stable Catalyst for the Alkylation of p-Cresol with tert-Butanol. Catalysts, 13(11), 1432. https://doi.org/10.3390/catal13111432