Ni-BaMnO3 Perovskite Catalysts for NOx-Assisted Soot Oxidation: Analyzing the Effect of the Nickel Addition Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. BaMn1−xNixO3 Catalysts: Effect of Nickel Content
2.1.1. Chemical and Morphological Properties
2.1.2. Crystalline Structure
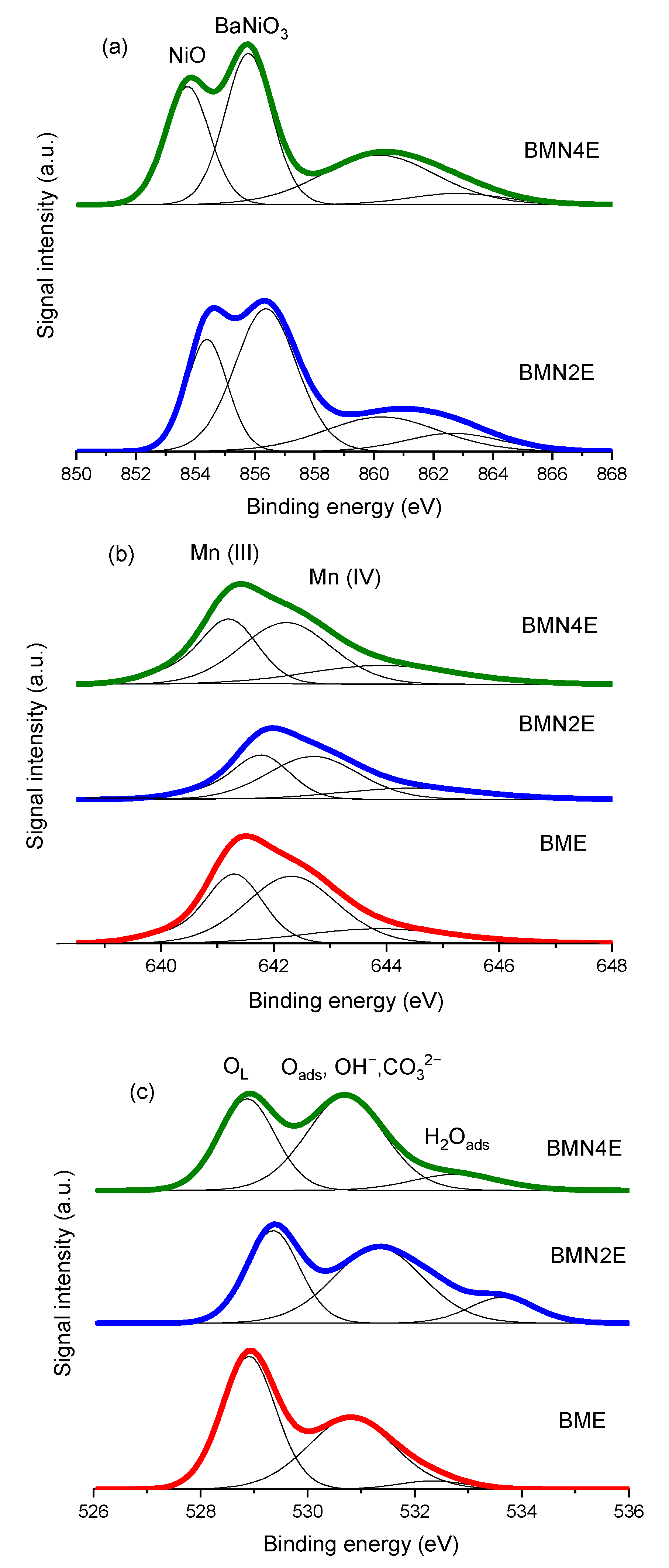
2.1.3. Surface Properties
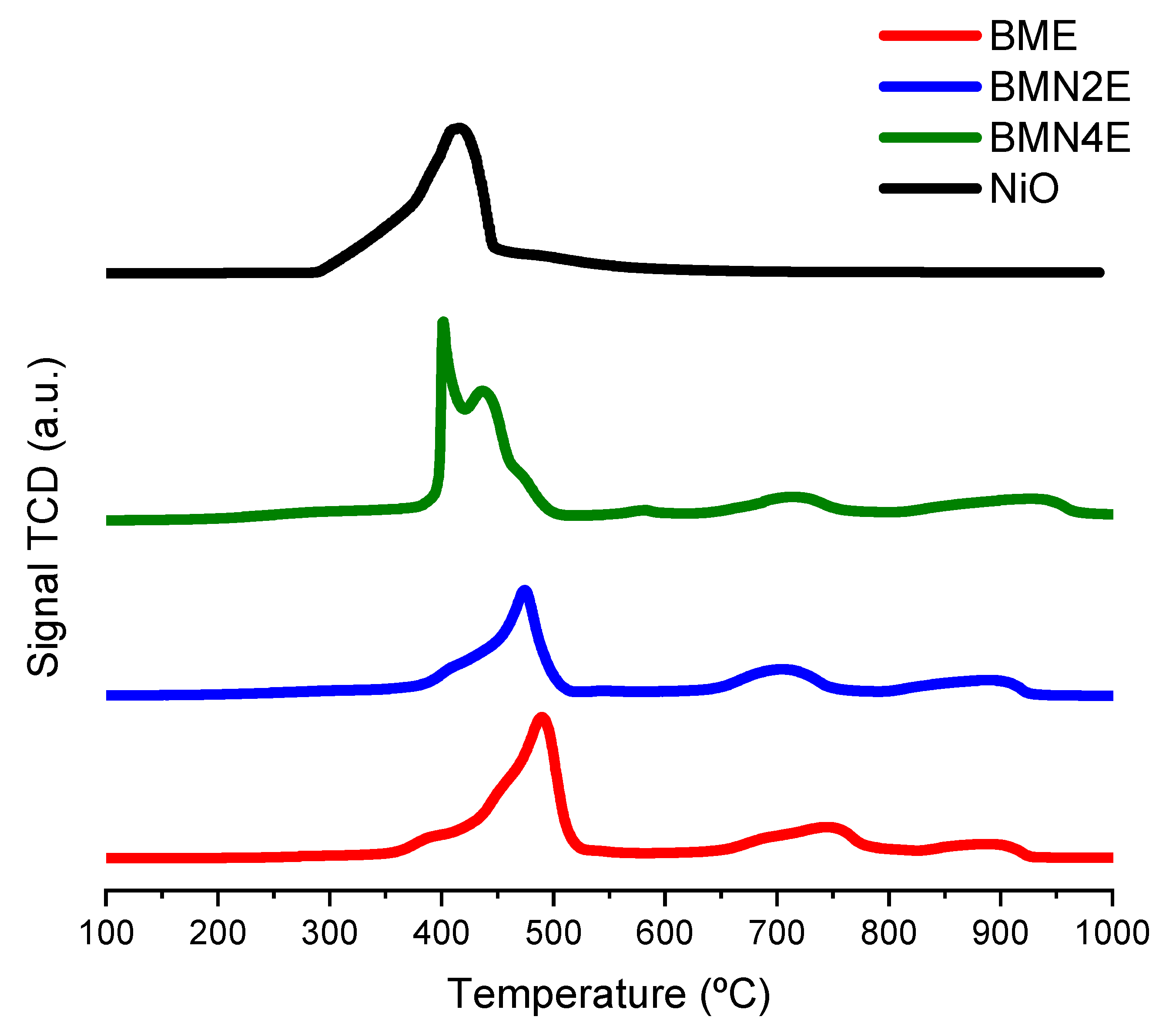
2.1.4. Reducibility
2.1.5. Catalytic Activity Tests
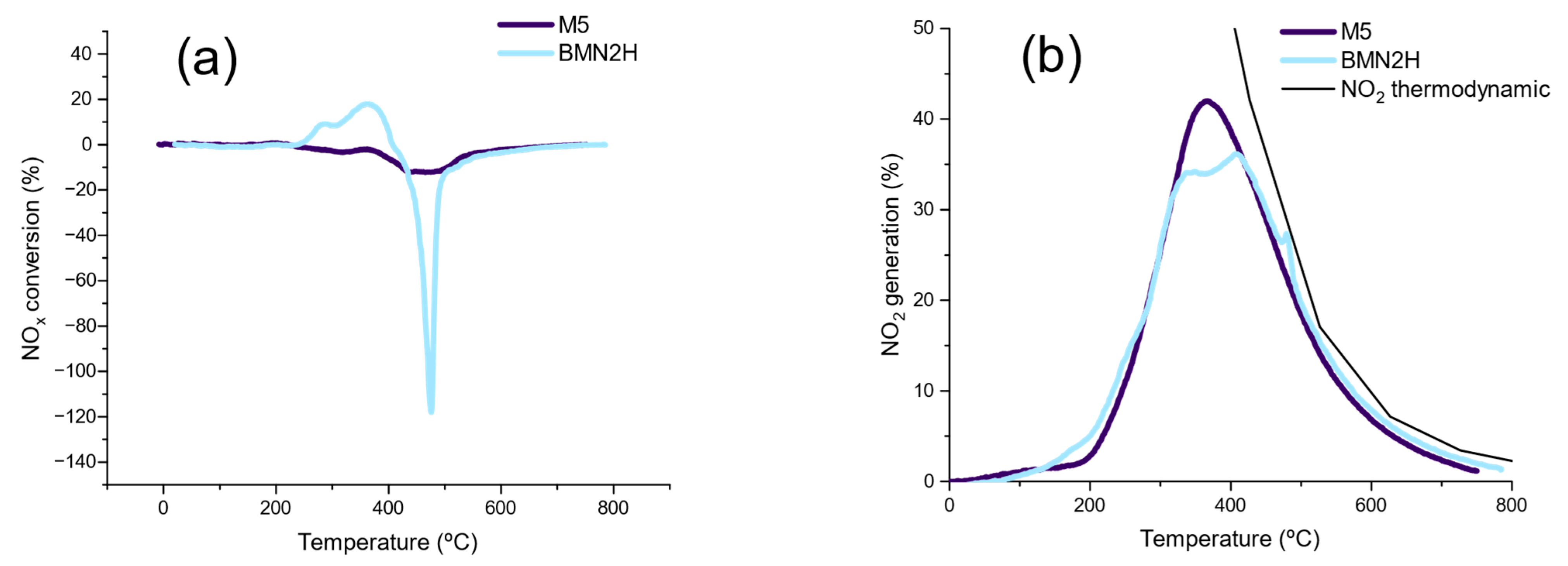
NO to NO2 Oxidation (NOx–TPR)
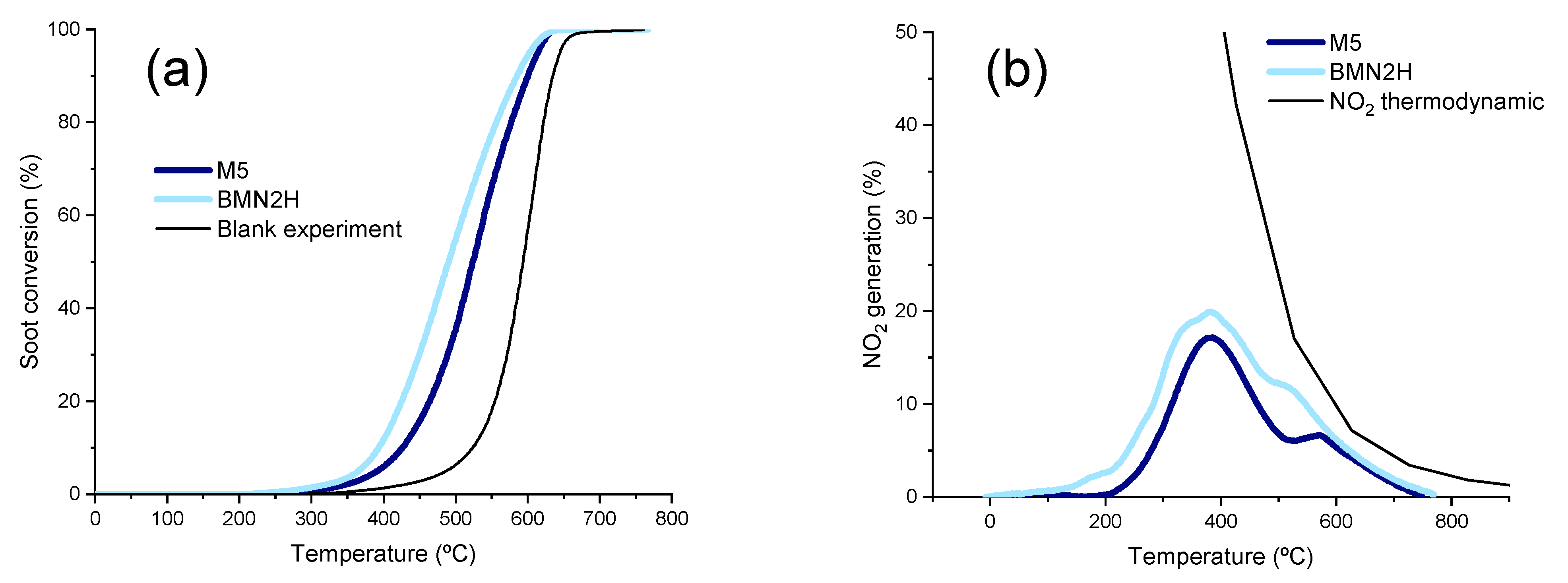
Soot Oxidation (Soot–NOx–TPR)
2.2. Effect of Nickel Addition Method
2.2.1. Characterization of BMN2H and M5 Catalysts
2.2.2. Catalytic Activity Tests
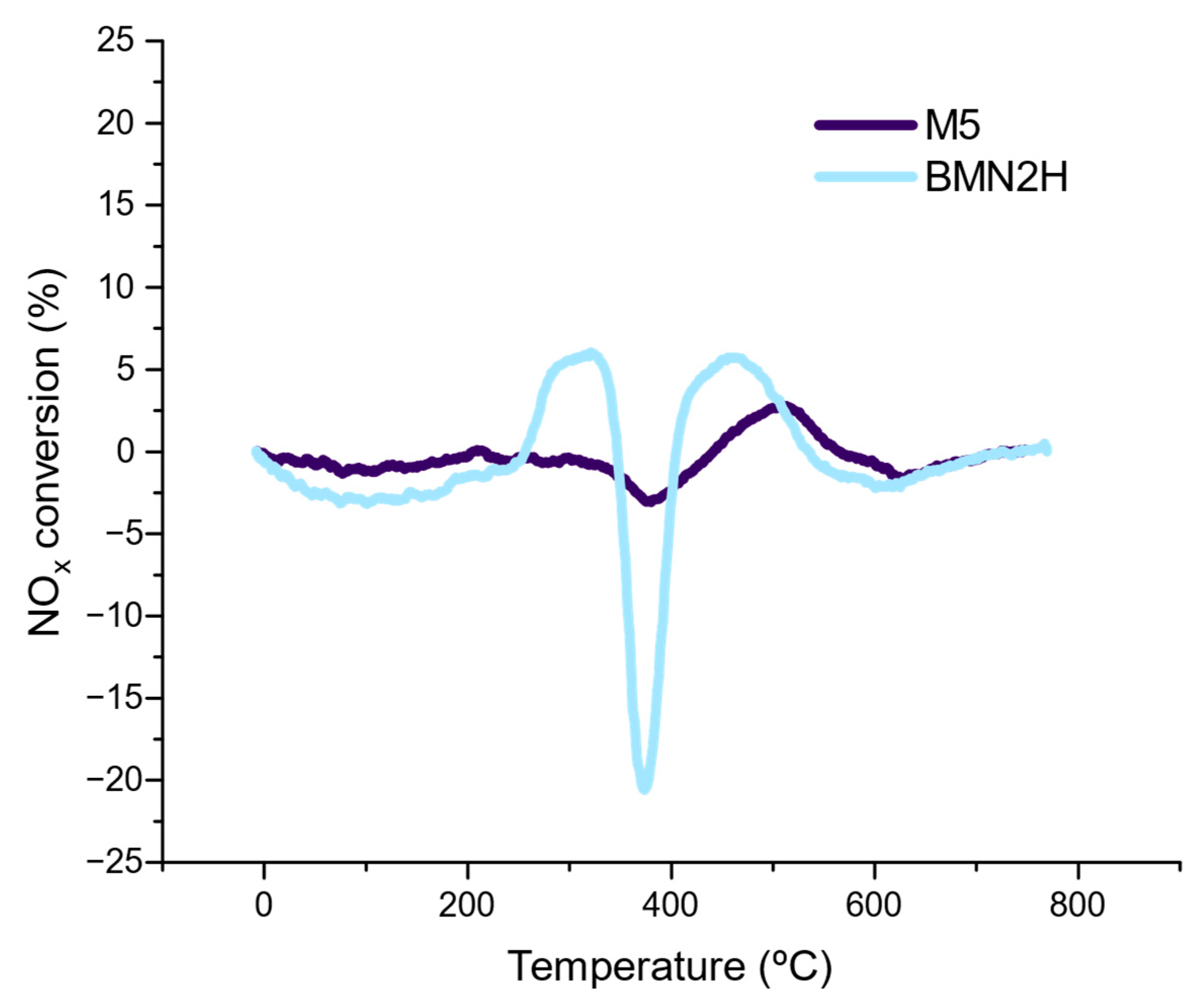
NO to NO2 Oxidation (NOx–TPR)
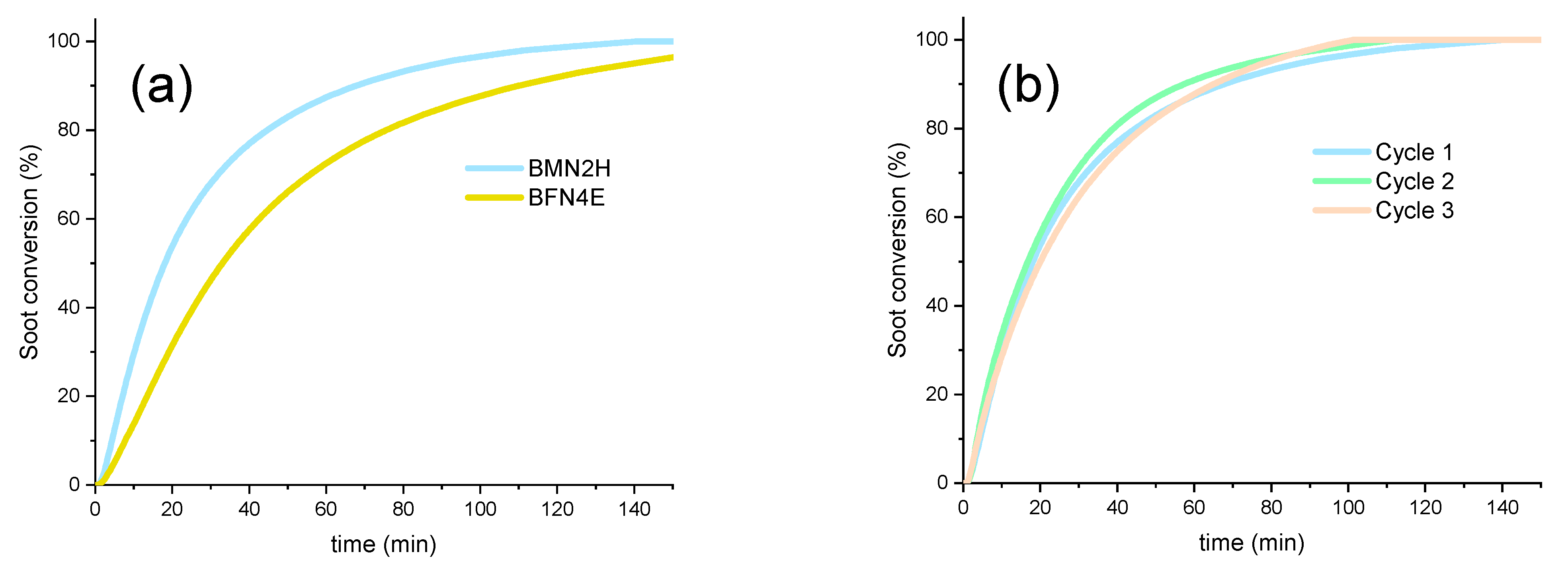
Stability Tests
3. Experimental Section
3.1. Synthesis and Characterization of Catalysts
3.2. Activity Tests
4. Conclusions
- -
- Nickel is not incorporated into the lattice of the BaMnO3 perovskite as it forms a BaNiO3 perovskite. If the sol–gel or hydrothermal method is used for nickel addition, an oxygen-deficient perovskite structure (BaNiO2.55) is also detected.
- -
- Mn(III) and Mn(IV) oxidation states coexist on the surface of samples, with Mn(IV) being the main one in most of them. BaMn1−xNixO3 catalysts present oxygen vacancies to compensate for the positive charge imbalance caused by the presence of Mn(III) and Ni(II).
- -
- For nickel samples, a Ni–Mn synergistic effect exists which decreases the manganese reduction temperature during H2–TPR.
- -
- All samples increase the activity for the oxidation of NO and soot at T < 400 °C, with the nickel-containing samples showing the highest activity. BMN2E and BMN4E also present a higher selectivity to CO2 than BME, so it seems that nickel catalysts have more active sites for total soot oxidation. However, no effect of nickel amount in the catalytic performance was observed.
- -
- The method used for nickel addition to BaMnO3 perovskite seems to be relevant as BMN2H, synthetized by hydrothermal procedure, is more active than M5 (obtained by incipient wetness impregnation of BaMnO3) and than BMN2E (obtained by the sol–gel method) as it presents: (i) more surface oxygen vacancies which are active sites for oxidation reactions, (ii) improved redox properties, and (iii) a lower NiO average crystal size which enables a higher amount of nickel active sites for NO and soot oxidation. As a consequence of these properties, BMN2H features a high soot oxidation rate, which minimizes the accumulation of soot and, thus, the deactivation of the catalyst during the reaction.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cunanan, C.; Tran, M.-K.; Lee, Y.; Kwok, S.; Leung, V.; Fowler, M.A. Review of Heavy-Duty Vehicle Powertrain Technologies: Diesel Engine Vehicles, Battery Electric Vehicles, and Hydrogen Fuel Cell Electric Vehicles. Clean. Technol. 2021, 3, 474–489. [Google Scholar] [CrossRef]
- Ahmadi, P.; Khoshnevisan, A. Dynamic simulation and lifecycle assessment of hydrogen fuel cell electric vehicles considering various hydrogen production methods. Int. J. Hydrogen Energy 2022, 47, 26758–26769. [Google Scholar] [CrossRef]
- Joyn, N.; Balan, K.N.; Nagappan, B.; Abraham-baby, S.J. Emission analysis of diesel and butanol blends in research diesel engine. Pet. Sci. Technol. 2020, 38, 289–296. [Google Scholar] [CrossRef]
- Leach, F.; Kalghatgi, G.; Stone, R.; Miles, P. The scope for improving the efficiency and environmental impact of internal combustion engines. Trans. Eng. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Ayodhya, A.S.; Narayanappa, K.G. An overview of after-treatment systems for diesel engines. Environ. Sci. Pollut. Res. 2018, 25, 35034–35047. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, K.; Wu, Q.; Yao, P.; Ke, J.; Wang, B.; Wang, Y. Diesel Engine Emission Aftertreatment Device Aging Mechanism and Durability Assessment Methods: A Review. Atmosphere 2023, 14, 314. [Google Scholar] [CrossRef]
- Legutko, P.; Stelmachowski, P.; Yu, X.; Zhao, Z.; Sojka, Z.; Kotarba, A. Catalytic Soot Combustion-General Concepts and Alkali Promotion. ACS Catal. 2023, 13, 3395–3418. [Google Scholar] [CrossRef]
- Dey, S.; Mehta, N.S. Automobile pollution control using catalysis. Resour. Environ. Sustain. 2020, 2, 100006. [Google Scholar] [CrossRef]
- Sanchez, A. The Contribution of Transport to Air Quality TERM 2012: Transport Indicators Tracking Progress towards Environmental Targets in Europe; European Environment Agency: Copenhagen, Denmark, 2012.
- DieselNet. Available online: https://www.dieselnet.com (accessed on 24 September 2023).
- Jung, Y.; Pyo, Y.D.; Jang, J.; Kim, G.C.; Cho, C.P.; Yang, C. NO, NO2 and N2O emissions over a SCR using DOC and DPF systems with Pt reduction. Chem. Eng. J. 2019, 369, 1059–1067. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, D.Y.; Choung, J.W.; Kim, C.H.; Ham, H.C.; Lee, K.Y. Roles of noble metals (M = Ag, Au, Pd, Pt and Rh) on CeO2 in enhancing activity toward soot oxidation: Active oxygen species and DFT calculations. J. Hazard. Mater. 2021, 403, 124085. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, R.; Lan, G.; Yuan, T.; Tan, D. Diesel particulate filter regeneration mechanism of modern automobile engines and methods of reducing PM emissions: A review. Environ. Sci. Pollut. Res. 2023, 30, 39338–39376. [Google Scholar] [CrossRef] [PubMed]
- Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. A Review on the Preparation, Characterization and Potential Application of Perovskites as Adsorbents for Wastewater Treatment. Chemosphere 2020, 244, 125474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Zhu, T.; Li, D.; Wang, H.; Zhu, X. Oxygen Storage Characteristics and Redox Behaviors of Lanthanum Perovskite Oxides with Transition Metals in the B-Sites. Energy Fuels 2023, 37, 9419–9433. [Google Scholar] [CrossRef]
- Wu, X.; Fischer, M.; Nolte, A.; Lenßen, P.; Wang, B.; Ohlerth, T.; Wöll, D.; Heufer, K.A.; Pischinger, S.; Simon, U. Perovskite Catalyst for In-Cylinder Coating to Reduce Raw Pollutant Emissions of Internal Combustion Engines. ACS Omega 2022, 7, 5340–5349. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; Urrutxua, M.; De La Torre, U.; González-Velasco, J.R. Boosting NOx Removal by Perovskite-Based Catalyst in NSR–SCR Diesel Aftertreatment Systems. Ind. Eng. Chem. Res. 2021, 60, 6525–6537. [Google Scholar] [CrossRef]
- Díaz Verde, A.; Montilla Verdú, S.; Torregrosa Rivero, V.; Illán Gómez, M.J. Tailoring the Composition of BaxBO3 (B = Fe, Mn) Mixed Oxides as CO or Soot Oxidation Catalysts in Simulated GDI Engine Exhaust Conditions. Molecules 2023, 28, 3327. [Google Scholar] [CrossRef]
- Torregrosa Rivero, V.; Sánchez-Adsuar, M.S.; Illán Gómez, M.J. BaFe1−xCuxO3 Perovskites as Active Phase for Diesel (DPF) and Gasoline Particle Filters (GPF). Nanomaterials 2019, 9, 1551. [Google Scholar] [CrossRef]
- Torregrosa Rivero, V.; Sánchez-Adsuar, M.S.; Illán Gómez, M.J. Analyzing the role of copper in the soot oxidation performance of BaMnO3-perovskite-based catalyst obtained by modified sol-gel synthesis. Fuel 2022, 328, 125258. [Google Scholar] [CrossRef]
- Torregrosa Rivero, V.; Sánchez-Adsuar, M.S.; Illán Gómez, M.J. Improving the Performance of BaMnO3 Perovskite as Soot Oxidation Catalyst Using Carbon Black during Sol-Gel Synthesis. Nanomaterials 2022, 12, 219. [Google Scholar] [CrossRef]
- Montilla Verdú, S.; Torregrosa Rivero, V.; Díaz Verde, A.; Illán Gómez, M.J. BaFe1−xNixO3 Catalysts for NOx-Assisted Diesel Soot Oxidation. Top. Catal. 2023, 66, 839–849. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa Rivero, V.; Sánchez-Adsuar, M.S.; Illán Gómez, M.J. Exploring the effect of using carbon black in the sol-gel synthesis of BaMnO3 and BaMn0.7Cu0.3O3 perovskite catalysts for CO oxidation. Catal. Today 2023, 423, 114028. [Google Scholar] [CrossRef]
- Atkins, P.W.; Overton, T.; Rourke, J.; Weller, M.; Armstrong, F.; Hagerman, M. Shriver & Atkins’ Inorganic Chemistry, 5th ed.; W.H. Freeman and Company, Ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Venezia, A.M. X-ray photoelectron spectroscopy (XPS) for catalysts characterization. Catal. Today 2003, 77, 359–370. [Google Scholar] [CrossRef]
- Ao, R.; Ma, L.; Dai, Q.; Guo, Z.; Liu, H.; Xiong, X.; Pan, Q. Simultaneous catalytic oxidation of NO and Hg0 over LaBO3 (B = Co, Mn, Ni, and Cu) perovskites. J. Environ. Chem. Eng. 2021, 9, 106612. [Google Scholar] [CrossRef]
- Gottschall, R.; Schöllhorn, R.; Muhler, M.; Jansen, N.; Walcher, D.; Gütlich, P. Electronic state of nickel in barium nickel oxide, BaNiO3. Inorg. Chem. 1998, 37, 1513–1518. [Google Scholar] [CrossRef]
- Lee, J.G.; Hwang, H.J.; Kwon, O.; Jeon, O.K.; Jang, J.; Shul, Y.-G. Synthesis and application of hexagonal perovskite BaNiO3 with quadrivalent nickel under atmospheric and low-temperature conditions. Chem. Commun. 2016, 52, 10731. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Relationship between Catalytic Deactivation and Physicochemical Properties of LaMnO3 Perovskite Catalyst during Catalytic Oxidation of Vinyl Chloride. Appl. Catal. B Environ. 2016, 186, 173–183. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; De Rossi, S.; Faticanti, M.; Porta, P. Methane combustion and CO oxidation on LaAl1-xMnxO3 perovskite-type oxide solid solutions. Appl. Catal. B Environ. 2003, 43, 397–406. [Google Scholar] [CrossRef]
- Teraoka, Y.; Nii, H.; Kagawa, S.; Jansson, K.; Nygren, M. Influence of the simultaneous substitution of Cu and Ru in the perovskite-type (La,Sr)MO3 (M = Al, Mn, Fe, Co) on the catalytic activity for CO oxidation and CO-NO reactions. Appl. Catal. A Gen. 2000, 194–195, 35–41. [Google Scholar] [CrossRef]
- Petrolekas, P.D.; Metcalfe, I.S. Solid Electrolyte Potentiometric Study of La(Sr)MnO3 Catalyst During Carbon-Monoxide Oxidation. J. Catal. 1995, 152, 147–163. [Google Scholar] [CrossRef]
- Patcas, F.; Buciuman, F.C.; Zsako, J. Oxygen Non-Stoichiometry and Reducibility of B-Site Substituted Lanthanum Manganites. Thermochim. Acta 2000, 360, 71–76. [Google Scholar] [CrossRef]
- Moreno Marcos, C.; Torregrosa Rivero, V.; Albaladejo Fuentes, V.; Sánchez-Adsuar, M.S.; Illán Gómez, M.J. BaFe1−xCuxO3 Perovskites as Soot Oxidation Catalysts for Gasoline Particulate Filters (GPF): A Preliminary Study. Top. Catal. 2019, 62, 413–418. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Eloy, P.; Cadús, L.E. La1−xCaxCoO3 perovskite-type oxides: Identification of the surface oxygen species by XPS. Appl. Surf. Sci. 2006, 253, 1489–1493. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Ding, J.; Zhang, Y.; Jia, J.; Sun, T. Catalytic oxidation of VOCs over SmMnO3 perovskites: Catalyst synthesis, change mechanism of active species, and degradation path of toluene. Inorg. Chem. 2019, 58, 14275–14283. [Google Scholar] [CrossRef]
- Bai, C.-S.; Soled, S.; Kershaw, R.; Dwight, K.; Wold, A. The Preparation and Characterization of the Phases Formed by the Reactions of Nickel and Lanthanum Nitrates with Magnesium Aluminate. J. Solid State Chem. 1992, 100, 307–312. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Copper Doped BaMnO3 Perovskite Catalysts for NO Oxidation and NO2-Assisted Diesel Soot Removal. RSC Adv. 2017, 7, 35228–35238. [Google Scholar] [CrossRef]
- Díaz-Verde, A.; Torregrosa-Rivero, V.; Illán-Gómez, M.J. Copper Catalysts Supported on Barium Deficient Perovskites for CO Oxidation Reaction. Top. Catal. 2023, 66, 895–907. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Feng, X.; Lin, D.; Qian, Q.; Wang, X.; Zhang, Y.; Chen, Q.; Zhang, X. Controllable P Doping of the LaCoO3 Catalyst for Efficient Propane Oxidation: Optimized Surface Co Distribution and Enhanced Oxygen Vacancies. ACS Appl. Mater. Interfaces 2020, 12, 23789–23799. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Fang, Y.; Hoang, S.; Li, L.; Yang, W.; Liang, Z.; Wu, J.; Hu, J.; Xiao, W.; et al. Oxygen Vacancy Promoted O2 Activation over Perovskite Oxide for Low-Temperature CO Oxidation. ACS Catal. 2019, 9, 9751–9763. [Google Scholar] [CrossRef]
- Pereñiguez, R.; Gonzalez de la Cruz, V.M.; Caballero, A.; Holgado, J.P. LaNiO3 as a precursor of Ni/La2O3 for CO2 reforming of CH4: Effect of the presence of an amorphous NiO phase. Appl. Catal. B Environ. 2012, 123–124, 324–332. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Modified BaMnO3-Based Catalysts for Gasoline Particle Filters (GPF): A Preliminary Study. Catalysts 2022, 12, 1325. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Álvarez, A.; Chavez-Ángel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Kumar Yadav, P.; Das, T. Production of syngas from carbon dioxide reforming of methane by using LaNixFe1−xO3 perovskite type catalysts. Int. J. Hydrogen Energy 2019, 44, 1659–1670. [Google Scholar] [CrossRef]
- Mortazavi-Manesh, A.; Safari, N.; Bahadoran, F.; Khani, Y. Synthesis, characterization, and methanol steam reforming performance of Cu/perovskite-structured catalysts. Heliyon 2023, 9, 13742. [Google Scholar] [CrossRef]






| Sample | Nomenclature | BET Surface Area (m2/g) | Nominal Ni (wt %) | Actual Ni (wt %) | 2θ for Main Peak of BaMnO3 (°) | NiO Average Crystal Size (nm) |
|---|---|---|---|---|---|---|
| BaMnO3 | BME | 7 | - | - | 31.4 | - |
| BaMn0.8Ni0.2O3 | BMN2E | 5 | 4.8 | 4.8 | 31.4 | 30 |
| BaMn0.6Ni0.4O3 | BMN4E | 6 | 9.6 | 9.5 | 31.4 | 35 |
| Sample | t Factor (Ni2+, Fe3+) |
|---|---|
| BaMnO3 | 1.09 |
| BaMn0.8Ni0.2O3 | 1.07 |
| BaMn0.6Ni0.4O3 | 1.06 |
| BaNiO3 | 1.02 |
| Samples | B.E Max Mn(III) (eV) | B.E Max Mn(VI) (eV) | B.E Max OL (eV) | Ni (wt %) ICP-OES | Ni (wt %) XPS | Surface Ni (%) 1 | NiO/ BaNiO3 2 | Mn(IV)/ Mn(III) | OL/ Mn+Ni+Ba |
|---|---|---|---|---|---|---|---|---|---|
| BME | 641.3 | 642.4 | 528.9 | - | - | - | - | 1.4 | 0.9 |
| BMN2E | 641.8 | 642.8 | 529.4 | 4.8 | 3.5 | 73 | 0.5 | 1.4 | 0.8 |
| BMN4E | 641.2 | 642.2 | 529.0 | 9.5 | 8.0 | 84 | 0.7 | 1.3 | 0.7 |
| Sample | T50% (°C) | Selectivity to CO2 (%) |
|---|---|---|
| BME | 563 ± 8 | 75 |
| BMN2E | 548 ± 8 | 84 |
| BMN4E | 546 ± 8 | 83 |
| Blank | 593 ± 8 | 38 |
| Sample | 2θ for Main Peak of BaMnO3 (°) | NiO Average Crystal Size (nm) |
|---|---|---|
| M5 | 31.4 | 24 |
| BMN2H | 31.4 | 19 |
| Catalyst |
B.E Max
Mn(III) (eV) |
B.E Max
Mn(VI) (eV) |
B.E Max
OL (eV) | Ni (wt %) ICP-OES | Ni (wt %) XPS | Surface Ni (%) 1 | NiO/ BaNiO3 2 | Mn(IV)/ Mn(III) | OL/ Mn+Ni+Ba |
|---|---|---|---|---|---|---|---|---|---|
| M5 | 641.5 | 643.1 | 529.0 | 4.9 | 20.2 | 412 | 0.7 | 0.6 | 2.0 |
| BMN2H | 641.2 | 642.3 | 528.9 | 4.9 | 9.8 | 200 | 0.9 | 0.9 | 1.1 |
| Sample | T50% (°C) | Selectivity CO2 (%) |
|---|---|---|
| M5 | 523 ± 8 | 92 |
| BMN2H | 490 ± 8 | 93 |
| Blank | 593 ± 8 | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montilla-Verdú, S.; Díaz-Verde, Á.; Torregrosa-Rivero, V.; Illán-Gómez, M.J. Ni-BaMnO3 Perovskite Catalysts for NOx-Assisted Soot Oxidation: Analyzing the Effect of the Nickel Addition Method. Catalysts 2023, 13, 1453. https://doi.org/10.3390/catal13111453
Montilla-Verdú S, Díaz-Verde Á, Torregrosa-Rivero V, Illán-Gómez MJ. Ni-BaMnO3 Perovskite Catalysts for NOx-Assisted Soot Oxidation: Analyzing the Effect of the Nickel Addition Method. Catalysts. 2023; 13(11):1453. https://doi.org/10.3390/catal13111453
Chicago/Turabian StyleMontilla-Verdú, Salvador, Álvaro Díaz-Verde, Verónica Torregrosa-Rivero, and María José Illán-Gómez. 2023. "Ni-BaMnO3 Perovskite Catalysts for NOx-Assisted Soot Oxidation: Analyzing the Effect of the Nickel Addition Method" Catalysts 13, no. 11: 1453. https://doi.org/10.3390/catal13111453
APA StyleMontilla-Verdú, S., Díaz-Verde, Á., Torregrosa-Rivero, V., & Illán-Gómez, M. J. (2023). Ni-BaMnO3 Perovskite Catalysts for NOx-Assisted Soot Oxidation: Analyzing the Effect of the Nickel Addition Method. Catalysts, 13(11), 1453. https://doi.org/10.3390/catal13111453







