Lanthanide Oxides in Ammonia Synthesis Catalysts: A Comprehensive Review
Abstract
:1. Introduction
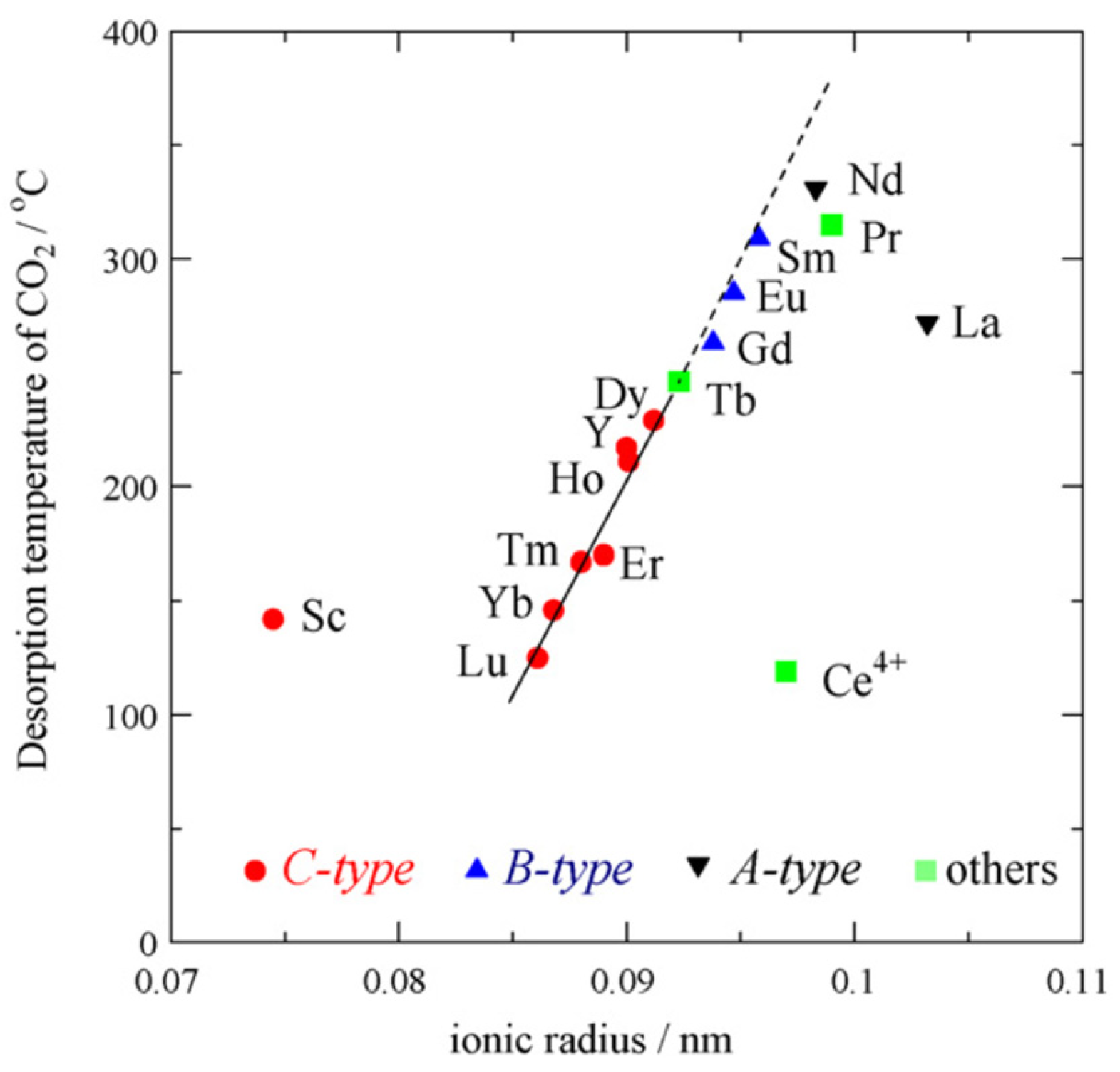
2. Lanthanides, Lanthanide Oxides, and Their Properties
3. Iron-Based Catalysts
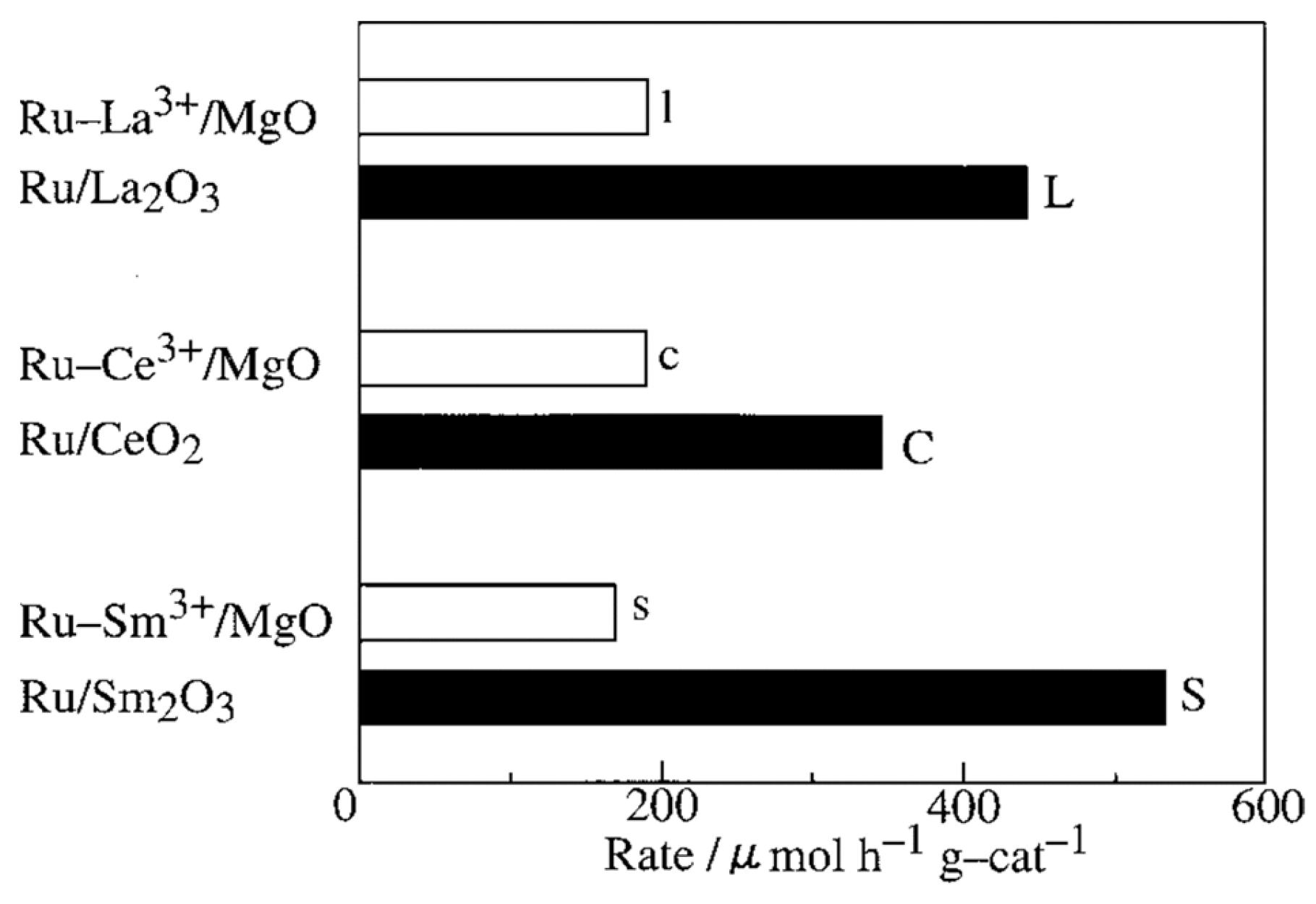
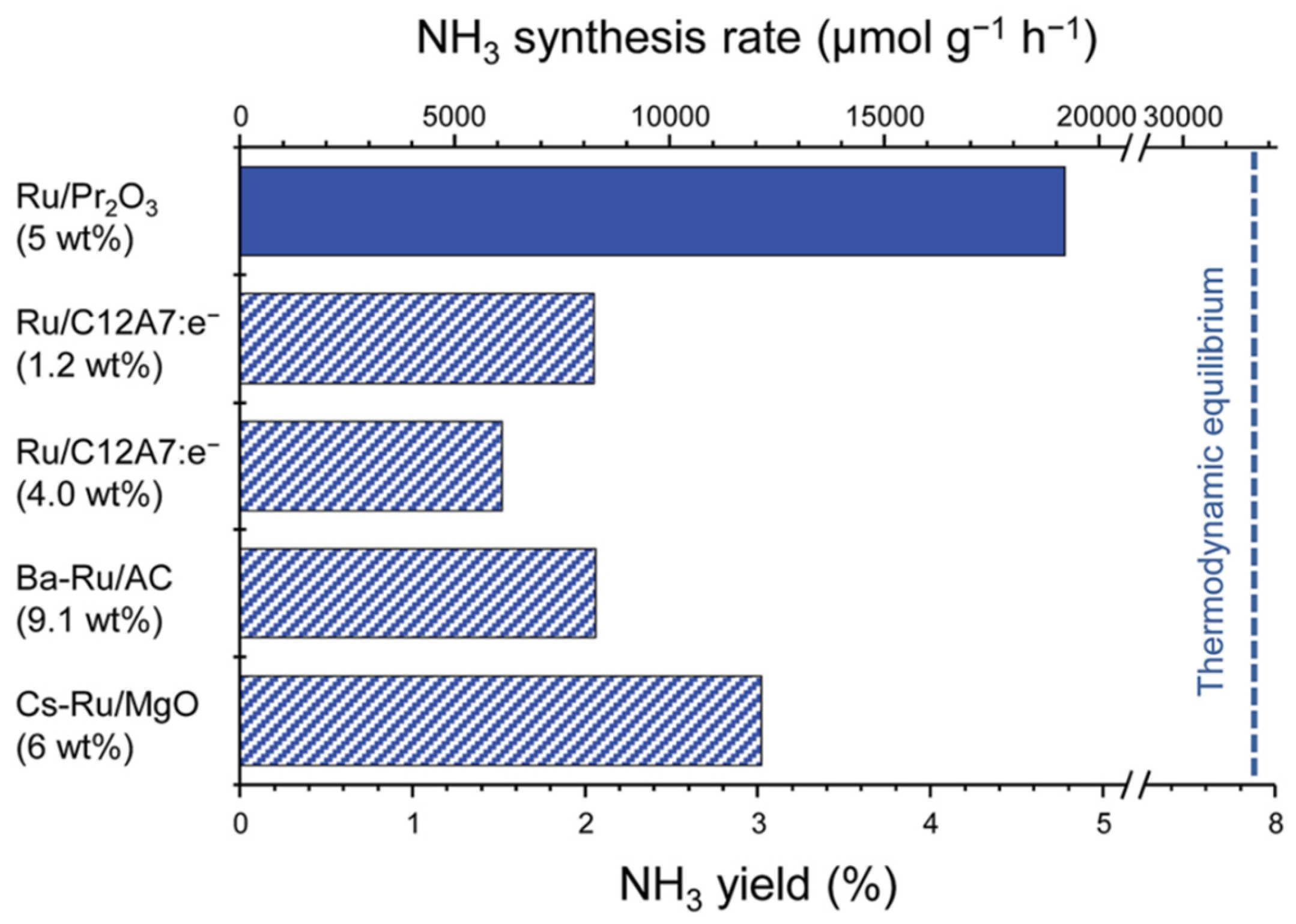
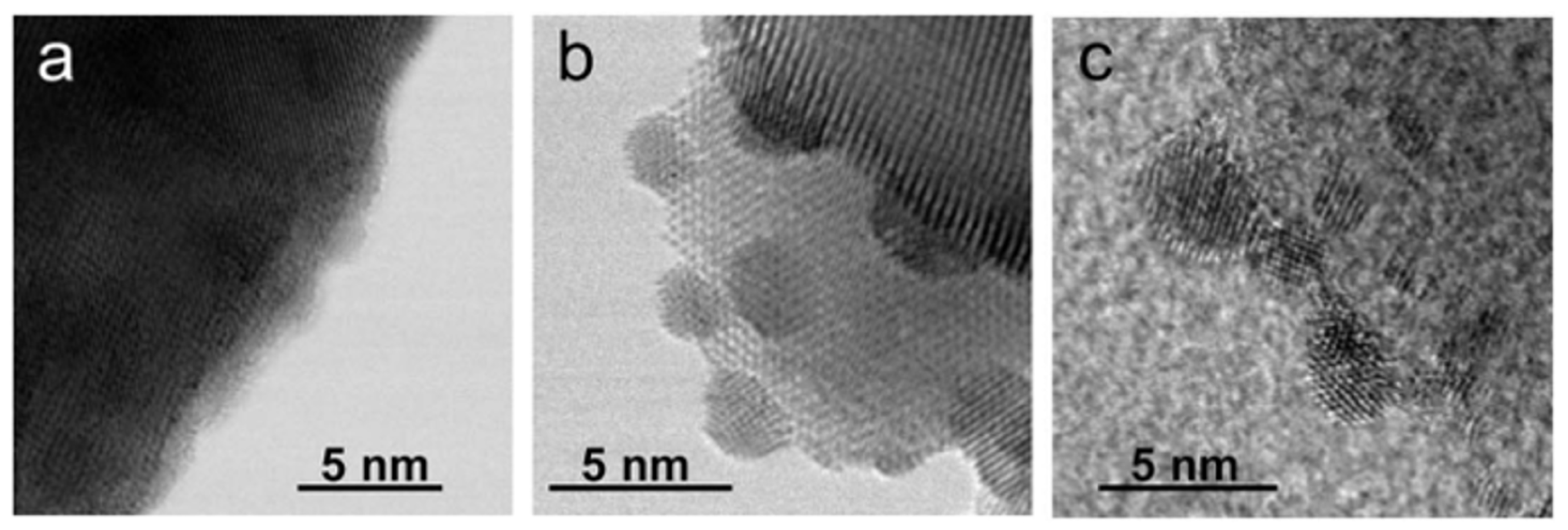
4. Ruthenium-Based Catalysts
5. Cobalt-Based Catalysts
6. Conclusions and Prospectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References and Notes
- Liu, H. Ammonia Synthesis Catalysts; World Scientific: Singapore, 2013. [Google Scholar]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Kubota, J.; Aika, K.I. Infrared studies of adsorbed dinitrogen on supported ruthenium catalysts for ammonia synthesis. Effects of the alumina and magnesia supports and the cesium compound promoter. J. Phys. Chem. 1994, 98, 11293–11300. [Google Scholar] [CrossRef]
- Faria, J.A. Renaissance of ammonia synthesis for sustainable production of energy and fertilizers. Curr. Opin. Green Sustain. Chem. 2021, 29, 100466. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, P.; Ellis, P.; French, S.; Kelly, G.; Lok, C.M. Brønsted-Evans-Polanyi relation of multistep reactions and volcano curve in heterogeneous catalysis. J. Phys. Chem. C 2008, 112, 1308–1311. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Gaigneaux, E.M. Recent Advances in Heterogeneous Catalysis for Ammonia Synthesis. ChemCatChem 2020, 12, 5838–5857. [Google Scholar] [CrossRef]
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber-Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Humphreys, J.; Tao, S. Advancements in Green Ammonia Production and Utilisation Technologies. Johns. Matthey Technol. Rev. 2023, 68, 2000043. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, X.; Wang, J.; Ju, X.; Liu, L.; Chen, P. Applications of rare earth oxides in catalytic ammonia synthesis and decomposition. Catal. Sci. Technol. 2021, 11, 6330–6343. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Li, C.; Bao, X.; Hosono, H.; Wang, J. Insight into rare-earth-incorporated catalysts: The chance for a more efficient ammonia synthesis. J. Adv. Ceram. 2022, 11, 1499–1529. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Zhou, Y.; Wang, X.; Au, C.; Jiang, L. Review on catalytic roles of rare earth elements in ammonia synthesis: Development and perspective. J. Rare Earths 2022, 40, 1–10. [Google Scholar] [CrossRef]
- Barbieri, M. Industrial Synthesis of Ammonia: A Patent Landscape. SSRN J. 2022. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive metallurgy of rare earths. Int. Mater. Rev. 1992, 37, 197–248. [Google Scholar] [CrossRef]
- Habashi, F. Extractive metallurgy of rare earths. Can. Metall. Q. 2013, 52, 224–233. [Google Scholar] [CrossRef]
- Kilbourn, B.T. Lanthanide Oxides. In Concise Encyclopedia of Advanced Ceramic Materials; Brooks, R.J., Ed.; Pergamon Press: Oxford, UK, 1991; pp. 276–278. [Google Scholar] [CrossRef]
- Eyring, L. The Binary Lanthanide Oxides: Synthesis and Identification. In Synthesis of Lanthanide and Actinide Compounds; Meyer, G., Morss, L.R., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 187–224. [Google Scholar]
- Huheey, J.E.; Keiter, E.A.; Keiter, R.L. Inorganic Chemistry: Principles of Structure and Reactivity; HarperCollins College Publishers: New York, NY, USA, 1993. [Google Scholar]
- Seitz, M.; Oliver, A.G.; Raymond, K.N. The lanthanide contraction revisited. J. Am. Chem. Soc. 2007, 129, 11153–11160. [Google Scholar] [CrossRef] [PubMed]
- Raymond, K.N.; Wellman, D.L.; Sgarlata, C.; Hill, A.P. Curvature of the lanthanide contraction: An explanation. Comptes Rendus Chim. 2010, 13, 849–852. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Gatica, J.M.; Pérez-Omil, J.A.; Pintado, J.M.; Vidal, H. Chemical Reactivity of Binary Rare Earth Oxides. In Binary Rare Earth Oxides; Adachi, G., Imanaka, N., Kang, Z.C., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 9–55. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Jia, Y.Q. Crystal radii and effective ionic radii of the rare earth ions. J. Solid State Chem. 1991, 95, 184–187. [Google Scholar] [CrossRef]
- Schweda, E. Rare Earth Oxides. Key Eng. Mater. 1992, 68, 187–216. [Google Scholar] [CrossRef]
- Haire, R.G.; Eyring, L. Chapter 125 Comparisons of the binary oxides. Handb. Phys. Chem. Rare Earths 1994, 18, 413–505. [Google Scholar] [CrossRef]
- Borchert, Y.; Sonström, P.; Wilhelm, M.; Borchert, H.; Bäumer, M. Nanostructured praseodymium oxide: Preparation, structure, and catalytic properties. J. Phys. Chem. C 2008, 112, 3054–3063. [Google Scholar] [CrossRef]
- Sonström, P.; Birkenstock, J.; Borchert, Y.; Schilinsky, L.; Behrend, P.; Gries, K.; Müller, K.; Rosenauer, A.; Bäumer, M. Nanostructured praseodymium oxide: Correlation between phase transitions and catalytic activity. ChemCatChem 2010, 2, 694–704. [Google Scholar] [CrossRef]
- Antic-Fidancev, E.; Hölsä, J.; Lastusaari, M. Crystal field strength in C-type cubic rare earth oxides. J. Alloys Compd. 2002, 341, 82–86. [Google Scholar] [CrossRef]
- Kang, Z.; Eyring, L. The Structural Basis of the Fluorite-Related Rare Earth Higher Oxides. Aust. J. Chem. 1996, 49, 981. [Google Scholar] [CrossRef]
- Adachi, G.; Imanaka, N. The Binary Rare Earth Oxides. Chem. Rev. 1998, 98, 1479–1514. [Google Scholar] [CrossRef]
- Rosynek, M.P. Catalytic Properties of Rare Earth Oxides. Catal. Rev.-Sci. Eng. 1977, 16, 111–154. [Google Scholar] [CrossRef]
- Koehler, W.C.; Wollan, E.O. Neutron-diffraction study of the structure of the A-form of the rare earth sesquioxides. Acta Crystallogr. 1953, 6, 741–742. [Google Scholar] [CrossRef]
- Douglass, R.M.; Staritzky, E. Crystallographic Data. 112. Samarium Sesquioxide, Sm2O3, Form B. Anal. Chem. 1956, 28, 552. [Google Scholar] [CrossRef]
- Cromer, D.T. The Crystal Structure of Monoclinic Sm2O2. J. Phys. Chem. 1957, 61, 753–755. [Google Scholar] [CrossRef]
- Pauling, L.; Shappell, M.D. The Crystal Structure of Bixbyite and the C-Modification of the Sesquioxides. Zeitschrift für Krist.-Cryst. Mater. 1930, 75, 128–142. [Google Scholar] [CrossRef]
- Templeton, D.H.; Dauben, C.H. Lattice Parameters of Some Rare Earth Compounds and a Set of Crystal Radii. J. Am. Chem. Soc. 1954, 76, 5237–5239. [Google Scholar] [CrossRef]
- Zhang, G.; Hattori, H.; Tanabe, K. Aldol Addition of Acetone, Catalyzed by Solid Base Catalysts: Magnesium Oxide, Calcium Oxide, Strontium Oxide, Barium Oxide, Lanthanum (III) Oxide and Zirconium Oxide. Appl. Catal. 1988, 36, 189–197. [Google Scholar] [CrossRef]
- Busca, G. Acid and Basic Catalysts: Fundamentals. In Heterogeneous Catalytic Materials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 57–101. [Google Scholar]
- Sato, S.; Takahashi, R.; Kobune, M.; Gotoh, H. Basic properties of rare earth oxides. Appl. Catal. A Gen. 2009, 356, 57–63. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Kobune, M.; Inoue, H.; Izawa, Y.; Ohno, H.; Takahashi, K. Dehydration of 1,4-butanediol over rare earth oxides. Appl. Catal. A Gen. 2009, 356, 64–71. [Google Scholar] [CrossRef]
- Maitra, A.M. Determination of solid state basicity of rare earth oxides by thermal analysis of their carbonates. J. Therm. Anal. 1990, 36, 657–675. [Google Scholar] [CrossRef]
- Rao, G.V.S.; Ramdas, S.; Mehrotra, P.N.; Rao, C.N.R. Electrical transport in rare-earth oxides. J. Solid State Chem. 1970, 2, 377–384. [Google Scholar] [CrossRef]
- Imanaka, N. Physical and chemical properties of rare earth oxides. In Binary Rare Earth Oxides; Adachi, G., Imanaka, N., Kang, Z.C., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 111–133. [Google Scholar]
- Chandrashekhar, G.V.; Mehrotra, P.N.; Rao, G.V.S.; Subbarao, E.C.; Rao, C.N.R. Semiconduction in non-stoichiometric rare earth oxides. Trans. Faraday Soc. 1967, 63, 1295. [Google Scholar] [CrossRef]
- Aleksic, B.; Jovanovic, N.; Terlecki-Baricevic, A. In Proceedings of the Geterog. Katal. Tr. Mezhdunar. Simp. 3rd, 1978. p. 147.
- Aleksic, B.; Bogdanov, S. Thermal analysis of the effect of samarium oxide on the reduction of precipitated ammonia synthesis catalyst. Thermochim Acta 1985, 93, 693. [Google Scholar] [CrossRef]
- Mitov, I.; Klisurski, D.; Aleksic, B.; Gyurova, D.; Nikolov, G. Effect of samarium oxide (Sm2O3) on the reduction kinetics of precipitated iron catalysts for ammonia synthesis. Coprecipitated catalysts. Izu Khim 1984, 17, 305. [Google Scholar]
- Berengarten, M.; Rudnitskii, L.; Zubova, I.; Alekseev, A.; Dmitrienko, L.; Mishchenko, S. Electron work function and specific catalytic activity of coprecipitated iron catalysts for ammonia synthesis promoted by rare earth element oxides. Kinet. Katal. 1974, 15, 250. [Google Scholar]
- Dimitrov, M.; Rusev, R. God. Viss. Khim.-Tekhnol. Inst. Sofia 1980, 26, 103.
- Rozin, A.; Komarov, V.; Efros, M.; Lemeshonok, G.; Eremenko, S. In Proceedings of the Vestsi Akad. Navuk BSSR Ser. Khim. Navuk, 1980. p. 35.
- Rozin, A.; Komarov, V.; Efros, M. In Proceedings of the Vestsi Akad. Navuk BSSR Ser. Khim. Navuk, 1980. p. 27.
- Dimitrov, M.; Rusev, R. God. Viss. Khim.-Tekhnol. Inst. Sofia 1980, 26, 111.
- Dimitrov, M.; Rusev, R. God. Viss. Khim.-Tekhnol. Inst. Sofia 1980, 26, 126.
- Karaslavova, K.; Anastasov, M.D. In Proceedings of Geterog. Katal. Tr. Mezhdunar. Simp. 3rd, 1978. p. 297.
- Zakieva, K.; Rabina, P.; Zubova, I.; Khaustova, L.; Pavlova, N.; Kuznetsov, L. Effect of the oxides of rare (scandium, yttrium) and rare earth (lanthanum, praseodymium, erbium) elements on the kinetics of reduction of iron catalysts. Tr. Mosk. Khim. Tekhnol. Inst. 1973, 73, 120. [Google Scholar]
- Yu, X.; Lin, B.; Lin, J.; Wang, R.; Wei, K. A novel fused iron catalyst for ammonia synthesis promoted with rare earth gangue. J. Rare Earths 2008, 26, 711–716. [Google Scholar] [CrossRef]
- Aika, K.-I.; Hori, H.; Ozaki, A. Activation of nitrogen by alkali metal promoted transition metal I. Ammonia synthesis over ruthenium promoted by alkali metal. J. Catal. 1972, 27, 424–431. [Google Scholar] [CrossRef]
- Rambeau, G.; Amariglio, H. Ammonia synthesis on ruthenium powder from 100 to 500 °C and hydrogenation of preadsorbed nitrogen down to −70 °C. J. Catal. 1981, 72, 1–11. [Google Scholar] [CrossRef]
- Aika, K. Preparation and characterization of chlorine-free ruthenium catalysts and the promoter effect in ammonia synthesis 3. A magnesia-supported ruthenium catalyst. J. Catal. 1992, 136, 126–140. [Google Scholar] [CrossRef]
- Kadowaki, Y.; Aika, K. Promoter Effect of Sm2O3 on Ru/Al2O3 in Ammonia Synthesis. J. Catal. 1996, 161, 178–185. [Google Scholar] [CrossRef]
- Ni, J.; Lin, J.; Wang, X.; Lin, B.; Lin, J.; Jiang, L. Promoting Effects of Lanthan on Ru/AC for Ammonia Synthesis: Tuning Catalytic Efficiency and Stability Simultaneously. ChemistrySelect 2017, 2, 6040–6046. [Google Scholar] [CrossRef]
- Murata, S. Preparation and characterization of chlorine-free ruthenium catalysts and the promoter effect in ammonia synthesis 2. A lanthanide oxide-promoted Ru/Al2O3 catalyst. J. Catal. 1992, 136, 118–125. [Google Scholar] [CrossRef]
- Murata, S.; Aika, K.; Onishi, T. Lanthanide Nitrates as Effective Promoters of a Ru/Al2O3 Catalyst for Ammonia Synthesis. Chem. Lett. 1990, 19, 1067–1068. [Google Scholar] [CrossRef]
- Niwa, Y.; Aika, K. The Effect of Lanthanide Oxides as a Support for Ruthenium Catalysts in Ammonia Synthesis. J. Catal. 1996, 162, 138–142. [Google Scholar] [CrossRef]
- Niwa, Y.; Aika, K. Ruthenium Catalyst Supported on CeO2 for Ammonia Synthesis. Chem. Lett. 1996, 25, 3–4. [Google Scholar] [CrossRef]
- van Ommen, J.G.; Bolink, W.J.; Prasad, J.; Mars, P. The nature of the potassium compound acting as a promoter in iron-alumina catalysts for ammonia synthesis. J. Catal. 1975, 38, 120–127. [Google Scholar] [CrossRef]
- Sato, K.; Imamura, K.; Kawano, Y.; Miyahara, S.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem. Sci. 2017, 8, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Imamura, K.; Sato, K. Ammonia Synthesis Catalyst and Method for Producing Same. WO2016133213A1, 25 August 2016. [Google Scholar]
- Song, Z.; Cai, T.; Hanson, J.C.; Rodriguez, J.A.; Hrbek, J. Structure and Reactivity of Ru Nanoparticles Supported on Modified Graphite Surfaces: A Study of the Model Catalysts for Ammonia Synthesis. J. Am. Chem. Soc. 2004, 126, 8576–8584. [Google Scholar] [CrossRef]
- Imamura, K.; Miyahara, S.-I.; Kawano, Y.; Sato, K.; Nakasaka, Y.; Nagaoka, K. Kinetics of ammonia synthesis over Ru/Pr2O3. J. Taiwan Inst. Chem. Eng. 2019, 105, 50–56. [Google Scholar] [CrossRef]
- Rosowski, F.; Hornung, A.; Hinrichsen, O.; Herein, D.; Muhler, M.; Ertl, G. Ruthenium catalysts for ammonia synthesis at high pressures: Preparation, characterization, and power-law kinetics. Appl. Catal. A Gen. 1997, 151, 443–460. [Google Scholar] [CrossRef]
- Fernández, C.; Bion, N.; Gaigneaux, E.M.; Duprez, D.; Ruiz, P. Kinetics of hydrogen adsorption and mobility on Ru nanoparticles supported on alumina: Effects on the catalytic mechanism of ammonia synthesis. J. Catal. 2016, 344, 16–28. [Google Scholar] [CrossRef]
- Tripodi, A.; Compagnoni, M.; Bahadori, E.; Rossetti, I. Process simulation of ammonia synthesis over optimized Ru/C catalyst and multibed Fe + Ru configurations. J. Ind. Eng. Chem. 2018, 66, 176–186. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitano, M.; Kishida, K.; Abe, H.; Niwa, Y.; Sasase, M.; Fujita, Y.; Ishikawa, H.; Yokoyama, T.; Hara, M.; et al. Efficient and Stable Ammonia Synthesis by Self-Organized Flat Ru Nanoparticles on Calcium Amide. ACS Catal. 2016, 6, 7577–7584. [Google Scholar] [CrossRef]
- Miyahara, S.-I.; Sato, K.; Kawano, Y.; Imamura, K.; Ogura, Y.; Tsujimaru, K.; Nagaoka, K. Ammonia synthesis over lanthanoid oxide-supported ruthenium catalysts. Catal. Today 2021, 376, 36–40. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Ni, J.; Lin, J.; Lin, B.; Xu, X.; Wei, K. Effect of La2O3 on Ru/CeO2-La2O3 catalyst for ammonia synthesis. Catal. Letters 2009, 133, 382–387. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Zhang, Z.; Ni, J.; Wang, X.; Lin, J.; Lin, B.; Jiang, L. Improving the ammonia synthesis activity of Ru/CeO2 through enhancement of the metal-support interaction. J. Energy Chem. 2021, 60, 403–409. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, S.; Pei, X.; Xiong, X.; Hu, B. New insights into the support morphology-dependent ammonia synthesis activity of Ru/CeO2 catalysts. Catal. Sci. Technol. 2017, 7, 191–199. [Google Scholar] [CrossRef]
- Liu, P.; Niu, R.; Li, W.; Wang, S.; Li, J. The effect of barium-promoted for microsphere Ru/CeO2 catalysts in ammonia synthesis. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 41, 689–699. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, L.; Wang, Z.; Ni, J.; Wang, R.; Wei, K. The effect of Ag as a promoter for Ru/CeO2 catalysts in ammonia synthesis. J. Mol. Catal. A Chem. 2013, 366, 375–379. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Heng, L.; Wang, X.; Ni, J.; Lin, J.; Jiang, L. Morphology Effect of Ceria on the Catalytic Performances of Ru/CeO2 Catalysts for Ammonia Synthesis. Ind. Eng. Chem. Res. 2018, 57, 9127–9135. [Google Scholar] [CrossRef]
- Wang, X.; Ni, J.; Lin, B.; Wang, R.; Lin, J.; Wei, K. Highly efficient Ru/MgO–CeO2 catalyst for ammonia synthesis. Catal. Commun. 2010, 12, 251–254. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Zhang, Y.; Ni, J.; Au, C.T.; Jiang, L. Efficient ammonia synthesis over a core-shell Ru/CeO2 catalyst with a tunable CeO2 size: DFT calculations and XAS spectroscopy studies. Inorg. Chem. Front. 2019, 6, 396–406. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Niu, R.; Li, J.; Wang, S. Influence of CeO2 supports prepared with different precipitants over Ru/CeO2 catalysts for ammonia synthesis. Solid State Sci. 2020, 99, 105983. [Google Scholar] [CrossRef]
- Lin, B.; Wu, Y.; Ni, J.; Lin, J.; Jiang, L. Ruthenium-Based Ammonia Synthesis Catalyst with Cerium Oxide as Carrier. CN110252295A, 20 September 2019. [Google Scholar]
- Lin, B.; Wu, Y.; Ni, J.; Lin, J.; Jiang, L. Ruthenium-Based Ammonia Synthesis Catalyst Using Cerium Oxide as Carrier. CN109277100A, 29 January 2019. [Google Scholar]
- Manaka, Y.; Nagata, Y.; Kobayashi, K.; Kobayashi, D.; Nanba, T. The effect of a ruthenium precursor on the low-temperature ammonia synthesis activity over Ru/CeO2. Dalt. Trans. 2020, 49, 17143–17146. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, Z.; Liu, H. La2Ce2O7 supported ruthenium as a robust catalyst for ammonia synthesis. J. Rare Earths 2019, 37, 492–499. [Google Scholar] [CrossRef]
- Ogura, Y.; Sato, K.; Miyahara, S.I.; Kawano, Y.; Toriyama, T.; Yamamoto, T.; Matsumura, S.; Hosokawa, S.; Nagaoka, K. Efficient ammonia synthesis over a Ru/La0.5Ce0.5O1.75 catalyst pre-reduced at high temperature. Chem. Sci. 2018, 9, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Tsujimaru, K.; Sato, K.; Miyahara, S.I.; Toriyama, T.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. Ru/La0.5Pr0.5O1.75 Catalyst for Low-Temperature Ammonia Synthesis. ACS Sustain. Chem. Eng. 2018, 6, 17258–17266. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, J.; Ni, J.; Wang, R.; Wei, K. Highly efficient Ru/Sm2O3-CeO2 catalyst for ammonia synthesis. Catal. Commun. 2011, 15, 23–26. [Google Scholar] [CrossRef]
- Ma, Z.; Xiong, X.; Song, C.; Hu, B.; Zhang, W. Electronic metal-support interactions enhance the ammonia synthesis activity over ruthenium supported on Zr-modified CeO2 catalysts. RSC Adv. 2016, 6, 51106–51110. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Fang, B.; Wang, X.; Ni, J.; Lin, B.; Lin, J.; Jiang, L. Enhanced ammonia synthesis performance of ceria-supported Ru catalysts via introduction of titanium. Chem. Commun. 2020, 56, 1141–1144. [Google Scholar] [CrossRef]
- Lin, B.; Chi, Z.; Wu, Y.; Ni, J.; Lin, J.; Jiang, L. Ruthenium-Based Ammonia Synthesis Catalyst with Ce-Ti Composite Oxide as Carrier and Preparation Method Thereof. CN110368933A, 25 October 2019. [Google Scholar]
- Saito, M.; Itoh, M.; Iwamoto, J.; Li, C.-Y.; Machida, K. Synergistic Effect of MgO and CeO2 as a Support for Ruthenium Catalysts in Ammonia Synthesis. Catal. Lett. 2006, 106, 107–110. [Google Scholar] [CrossRef]
- Javaid, R.; Aoki, Y.; Nanba, T. Highly efficient Ru/MgO–Er2O3 catalysts for ammonia synthesis. J. Phys. Chem. Solids 2020, 146, 109570. [Google Scholar] [CrossRef]
- Javaid, R.; Nanba, T. Effect of preparation method and reaction parameters on catalytic activity for ammonia synthesis. Int. J. Hydrogen Energy 2021, 46, 35209–35218. [Google Scholar] [CrossRef]
- Javaid, R.; Nanba, T. Effect of texture and physical properties of catalysts on ammonia synthesis. Catal. Today 2022, 397–399, 592–597. [Google Scholar] [CrossRef]
- Rambeau, G.; Jorti, A.; Amariglio, H. Catalytic activity of a cobalt powder in NH3 synthesis in relation with the allotropic transformation of the metal. J. Catal. 1985, 94, 155–165. [Google Scholar] [CrossRef]
- Larson, A.T.; Brooks, A.P. Ammonia Catalysts. Ind. Eng. Chem. 1926, 18, 1305–1307. [Google Scholar] [CrossRef]
- Smith, P.J.; Taylor, D.W.; Dowden, D.A.; Kemball, C.; Taylor, D. Ammonia synthesis and related reactions over iron-cobalt and iron-nickel alloy catalysts. Appl. Catal. 1982, 3, 303–314. [Google Scholar] [CrossRef]
- Taylor, D.W.; Smith, P.J.; Dowden, D.A.; Kemball, C.; Whan, D.A. Ammonia synthesis and related reactions over iron-cobalt and iron-nickel alloy catalysts. Part I. Catalysts reduced at 853 K. Appl. Catal. 1982, 3, 161–176. [Google Scholar] [CrossRef]
- Kaleńczuk, R.J. Effect of cobalt on the morphology and activity of fused iron catalyst for ammonia synthesis. Appl. Catal. A Gen. 1994, 112, 149–160. [Google Scholar] [CrossRef]
- Kaleńczuk, R.J. Cobalt promoted fused iron catalyst for ammonia synthesis. Int. J. Inorg. Mater. 2000, 2, 233–239. [Google Scholar] [CrossRef]
- Hagen, S.; Barfod, R.; Fehrmann, R.; Jacobsen, C.J.H.H.; Teunissen, H.T.; Ståhl, K.; Chorkendorff, I. New efficient catalyst for ammonia synthesis: Barium-promoted cobalt on carbon. Chem. Commun. 2002, 11, 1206–1207. [Google Scholar] [CrossRef] [PubMed]
- Hagen, S.; Barfod, R.; Fehrmann, R.; Jacobsen, C.J.H.; Teunissen, H.T.; Chorkendorff, I. Ammonia synthesis with barium-promoted iron-cobalt alloys supported on carbon. J. Catal. 2003, 214, 327–335. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Kepiński, L.; Kaszkur, Z.; Kielar, K.; Kowalczyk, Z. Ammonia synthesis over barium-promoted cobalt catalysts supported on graphitised carbon. J. Catal. 2007, 249, 24–33. [Google Scholar] [CrossRef]
- Tarka, A.; Zybert, M.; Truszkiewicz, E.; Mierzwa, B.; Kępiński, L.; Moszyński, D.; Raróg-Pilecka, W. Effect of a Barium Promoter on the Stability and Activity of Carbon-Supported Cobalt Catalysts for Ammonia Synthesis. ChemCatChem 2015, 7, 2836–2839. [Google Scholar] [CrossRef]
- Karolewska, M.; Truszkiewicz, E.; Wściseł, M.; Mierzwa, B.; Kȩpiński, L.; Raróg-Pilecka, W. Ammonia synthesis over a Ba and Ce-promoted carbon-supported cobalt catalyst. Effect of the cerium addition and preparation procedure. J. Catal. 2013, 303, 130–134. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Kowalczyk, Z. Activated carbon as a template for creating catalyst precursors. Unsupported cobalt catalyst for ammonia synthesis. Catal. Commun. 2008, 9, 870–873. [Google Scholar] [CrossRef]
- Schwickardi, M.; Johann, T.; Schmidt, W.; Schüth, F. High-Surface-Area Oxides Obtained by an Activated Carbon Route. Chem. Mater. 2002, 14, 3913–3919. [Google Scholar] [CrossRef]
- Schüth, F. Endo- and Exotemplating to Create High-Surface-Area Inorganic Materials. Angew. Chemie Int. Ed. 2003, 42, 3604–3622. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Karolewska, M.; Truszkiewicz, E.E.; Iwanek, E.; Mierzwa, B. Cobalt Catalyst Doped with Cerium and Barium Obtained by Co-Precipitation Method for Ammonia Synthesis Process. Catal. Lett. 2011, 141, 678–684. [Google Scholar] [CrossRef]
- Kowalik, P.; Antoniak-Jurak, K.; Wiercioch, P.; Raróg-Pilecka, W.; Zybert, M.; Tarka, A. A Method for Obtaining Promoted Cobalt Catalysts for Ammonia Synthesis. EP3318326A1, 9 May 2018. [Google Scholar]
- Lin, B.; Liu, Y.; Heng, L.; Ni, J.; Lin, J.; Jiang, L. Effect of barium and potassium promoter on Co/CeO2 catalysts in ammonia synthesis. J. Rare Earths 2018, 36, 703–707. [Google Scholar] [CrossRef]
- Zybert, M.; Wyszyńska, M.; Tarka, A.; Patkowski, W.; Ronduda, H.; Mierzwa, B.; Kępiński, L.; Sarnecki, A.; Moszyński, D.; Raróg-Pilecka, W. Surface enrichment phenomenon in the Ba-doped cobalt catalyst for ammonia synthesis. Vacuum 2019, 168, 108831. [Google Scholar] [CrossRef]
- Menon, P.G.; Rao, T.S.R.P.; Prasada Rao, T.S.R.R. Surface Enrichment in Catalysts. Catal. Rev. 1979, 20, 97–120. [Google Scholar] [CrossRef]
- Karolewska, M.; Truszkiewicz, E.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Ammonia synthesis over cobalt catalysts doped with cerium and barium. Effect of the ceria loading. Appl. Catal. A Gen. 2012, 445–446, 280–286. [Google Scholar] [CrossRef]
- Tarka, A.; Zybert, M.; Kindler, Z.; Szmurło, J.; Mierzwa, B.; Raróg-Pilecka, W. Effect of precipitating agent on the properties of cobalt catalysts promoted with cerium and barium for NH3 synthesis obtained by co-precipitation. Appl. Catal. A Gen. 2017, 532, 19–25. [Google Scholar] [CrossRef]
- Tarka, A.; Patkowski, W.; Zybert, M.; Ronduda, H.; Wieciński, P.; Adamski, P.; Sarnecki, A.; Moszyński, D.; Raróg-Pilecka, W. Synergistic interaction of cerium and barium-new insight into the promotion effect in cobalt systems for ammonia synthesis. Catalysts 2020, 10, 658. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Li, J. Highly Effective Ru/BaCeO3 Catalysts on Supports with Strong Basic Sites for Ammonia Synthesis. Chem. Asian J. 2019, 14, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, B.; Lin, J. Highly effective perovskite-type BaZrO3 supported Ru catalyst for ammonia synthesis. Appl. Catal. A Gen. 2013, 458, 130–136. [Google Scholar] [CrossRef]
- Ahmadi, S.; Kaghazchi, P. On the origin of high activity of hcp metals for ammonia synthesis. Phys. Chem. Chem. Phys. 2016, 18, 5291–5298. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Chen, P.-P.; Liu, J.-X.; Su, H.-Y.; Li, W.-X. Influence of Cobalt Crystal Structures on Activation of Nitrogen Molecule: A First-Principles Study. J. Phys. Chem. C 2019, 123, 10956–10966. [Google Scholar] [CrossRef]
- Tarka, A.; Zybert, M.; Ronduda, H.; Patkowski, W.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. On Optimal Barium Promoter Content in a Cobalt Catalyst for Ammonia Synthesis. Catalysts 2022, 12, 199. [Google Scholar] [CrossRef]
- Patkowski, W.; Kowalik, P.; Antoniak-Jurak, K.; Zybert, M.; Ronduda, H.; Mierzwa, B.; Próchniak, W.; Raróg-Pilecka, W. On the Effect of Flash Calcination Method on the Characteristics of Cobalt Catalysts for Ammonia Synthesis Process. Eur. J. Inorg. Chem. 2021, 2021, 1518–1529. [Google Scholar] [CrossRef]
- Lin, B.; Qi, Y.; Wei, K.; Lin, J. Effect of pretreatment on ceria-supported cobalt catalyst for ammonia synthesis. RSC Adv. 2014, 4, 38093. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Y.; Ding, L.; Xu, L.; Wu, Z.; Yuan, Q.; Huang, W. Reactivity of hydroxyls and water on a CeO2(111) thin film surface: The role of oxygen vacancy. J. Phys. Chem. C 2013, 117, 5800–5810. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Heng, L.; Ni, J.; Lin, J.; Jiang, L. Effect of ceria morphology on the catalytic activity of Co/CeO2 catalyst for ammonia synthesis. Catal. Commun. 2017, 101, 15–19. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhang, T.; Lin, B.; Ni, J.; Au, C.-T.; Jiang, L. Strong metal-support interactions of Co-based catalysts facilitated by dopamine for highly efficient ammonia synthesis: In situ XPS and XAFS spectroscopy coupled with TPD studies. Chem. Commun. 2019, 55, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Liu, Y.; Heng, L.; Lin, J.; Ni, J.; Jiang, L. Cobalt-Based Ammonia Synthesis Catalyst with Cerium Oxide as Carrier and Preparation Method of Cobalt-Based Ammonia Synthesis Catalyst. CN107008335A, 4 August 2017. [Google Scholar]
- Ni, J.; Zhou, L.; Lin, B.; Lin, J.; Jiang, L. Cerium Oxide-Loaded High-Surface-Defect Cobalt Oxide Ammonia Synthesis Catalyst. CN113181926A, 30 July 2021. [Google Scholar]
- Zybert, M.; Karasińska, M.; Truszkiewicz, E.; Mierzwa, B.; Raróg-Pilecka, W. Properties and activity of the cobalt catalysts for NH3 synthesis obtained by co-precipitation—The effect of lanthanum addition. Pol. J. Chem. Technol. 2015, 17, 138–143. [Google Scholar] [CrossRef]
- Zybert, M.; Tarka, A.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Promotion effect of lanthanum on the Co/La/Ba ammonia synthesis catalysts—The influence of lanthanum content. Appl. Catal. A Gen. 2016, 515, 16–24. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Ostrowski, A.; Jodłowski, P.; Szymański, D.; Kępiński, L.; Raróg-Pilecka, W. Development of cobalt catalyst supported on MgO-Ln2O3 (Ln = La, Nd, Eu) mixed oxide systems for ammonia synthesis. Int. J. Hydrogen Energy 2022, 47, 6666–6678. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Ostrowski, A.; Jodłowski, P.; Szymański, D.; Raróg-Pilecka, W. Co supported on Mg-La mixed oxides as an efficient catalyst for ammonia synthesis. Int. J. Hydrogen Energy 2022, 47, 35689–35700. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Dziewulska, A.; Patkowski, W.; Sobczak, K.; Ostrowski, A.; Raróg-Pilecka, W. Ammonia synthesis using Co catalysts supported on MgO-Nd2O3 mixed oxide systems: Effect of support composition. Surf. Interfaces 2023, 36, 102530. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Tarka, A.; Jodłowski, P.; Kępiński, L.; Sarnecki, A.; Moszyński, D.; Raróg-Pilecka, W. Tuning the catalytic performance of Co/Mg-La system for ammonia synthesis via the active phase precursor introduction method. Appl. Catal. A Gen. 2020, 598, 117553. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Sobczak, K.; Moszyński, D.; Albrecht, A.; Sarnecki, A.; Raróg-Pilecka, W. On the effect of metal loading on the performance of Co catalysts supported on mixed MgO-La2O3 oxides for ammonia synthesis. RSC Adv. 2022, 12, 33876–33888. [Google Scholar] [CrossRef] [PubMed]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Moszyński, D.; Albrecht, A.; Sobczak, K.; Małolepszy, A.; Raróg-Pilecka, W. Co nanoparticles supported on mixed magnesium–lanthanum oxides: Effect of calcium and barium addition on ammonia synthesis catalyst performance. RSC Adv. 2023, 13, 4787–4802. [Google Scholar] [CrossRef] [PubMed]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Tarka, A.; Ostrowski, A.; Raróg-Pilecka, W. Kinetic studies of ammonia synthesis over a barium-promoted cobalt catalyst supported on magnesium–lanthanum mixed oxide. J. Taiwan Inst. Chem. Eng. 2020, 114, 241–248. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Ostrowski, A.; Jodłowski, P.; Szymański, D.; Kępiński, L.; Raróg-Pilecka, W. Boosting the Catalytic Performance of Co/Mg/La Catalyst for Ammonia Synthesis by Selecting a Pre-Treatment Method. Catalysts 2021, 11, 941. [Google Scholar] [CrossRef]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Ostrowski, A.; Jodłowski, P.; Szymański, D.; Kępiński, L.; Raróg-Pilecka, W. A high performance barium-promoted cobalt catalyst supported on magnesium-lanthanum mixed oxide for ammonia synthesis. RSC Adv. 2021, 11, 14218–14228. [Google Scholar] [CrossRef]
- Zybert, M.; Tarka, A.; Patkowski, W.; Ronduda, H.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Structure Sensitivity of Ammonia Synthesis on Cobalt: Effect of the Cobalt Particle Size on the Activity of Promoted Cobalt Catalysts Supported on Carbon. Catalysts 2022, 12, 1285. [Google Scholar] [CrossRef]
- Patkowski, W.; Zybert, M.; Ronduda, H.; Gawrońska, G.; Albrecht, A.; Moszyński, D.; Fidler, A.; Dłużewski, P.; Raróg-Pilecka, W. The Influence of Active Phase Content on Properties and Activity of Nd2O3-Supported Cobalt Catalysts for Ammonia Synthesis. Catalysts 2023, 13, 405. [Google Scholar] [CrossRef]
- Liu, J.-X.; Li, W.-X. Theoretical study of crystal phase effect in heterogeneous catalysis. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 571–583. [Google Scholar] [CrossRef]
- Weststrate, C.J.; Mahmoodinia, M.; Farstad, M.H.; Svenum, I.-H.; Strømsheim, M.D.; Niemantsverdriet, J.W.; Venvik, H.J. Interaction of hydrogen with flat (0001) and corrugated (11–20) and (10–12) cobalt surfaces: Insights from experiment and theory. Catal. Today 2020, 342, 124–130. [Google Scholar] [CrossRef]
- Weststrate, C.J.; Rodriguez, D.G.; Sharma, D.; (Hans) Niemantsverdriet, J.W. Structure-dependent adsorption and desorption of hydrogen on FCC and HCP cobalt surfaces. J. Catal. 2022, 405, 303–312. [Google Scholar] [CrossRef]
- Ogawa, T.; Masaki, Y.; Keiichi, I. Techno-economic analysis on recent heterogeneous catalysts for ammonia synthesis. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Mayer, P.; Ramirez, A.; Pezzella, G.; Winter, B.; Sarathy, S.M.; Gascon, J.; Bardow, A. Blue and green ammonia production: A techno-economic and life cycle assessment perspective. iScience 2023, 26, 107389. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shin, Y.; Jeong, E.; Han, M. Techno-economic analysis of green and blue hybrid processes for ammonia production. Korean J. Chem. Eng. 2023, 40, 2657–2670. [Google Scholar] [CrossRef]
- Alvero, R.; Odriozola, J.A.; Trillo, J.M.; Bernal, S. Lanthanide oxides: Preparation and ageing. J. Chem. Soc. Dalt. Trans. 1984, 2, 87. [Google Scholar] [CrossRef]
- Alvero, R.; Bernal, A.; Carrizosa, I.; Odriozola, J.A.; Trillo, J.M. The kinetics of Ln2O3 Hydration under mild conditions. J. Therm. Anal. 1987, 32, 637–643. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; Calvino, J.J.; Omil, J.A.P.; Pintado, J.M. Some major aspects of the chemical behavior of rare earth oxides: An overview. J. Alloys Compd. 2006, 408–412, 496–502. [Google Scholar] [CrossRef]
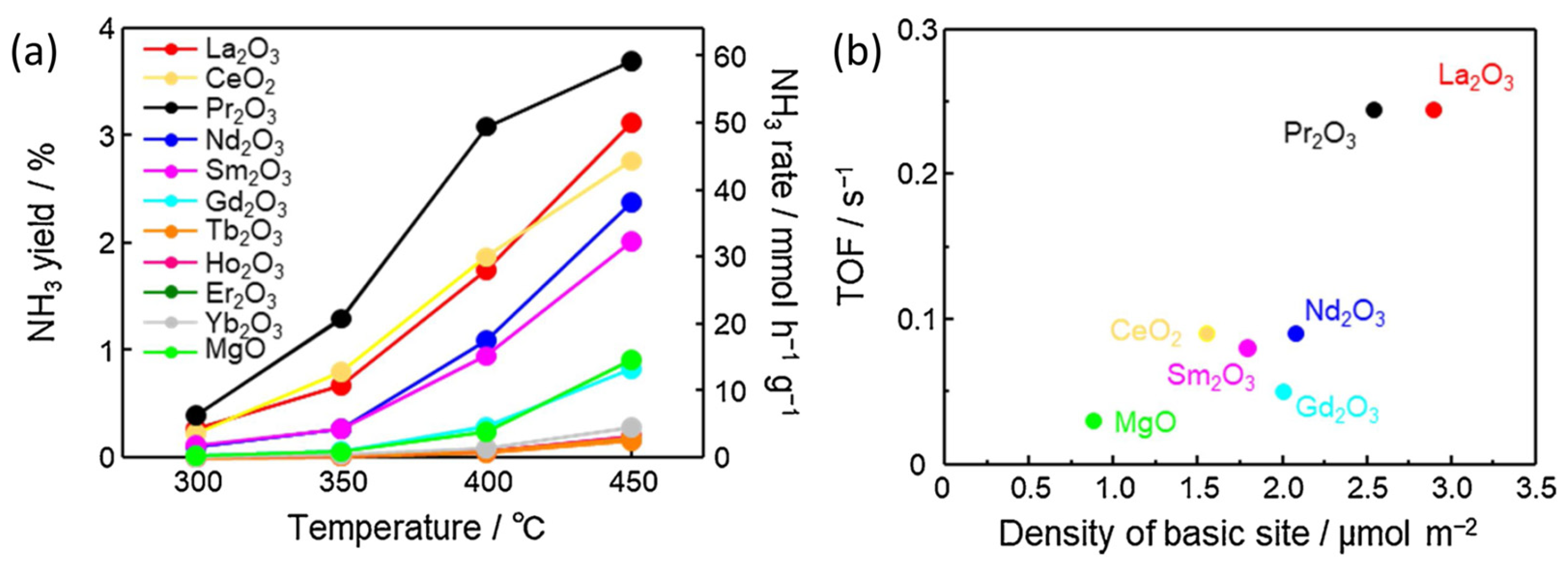
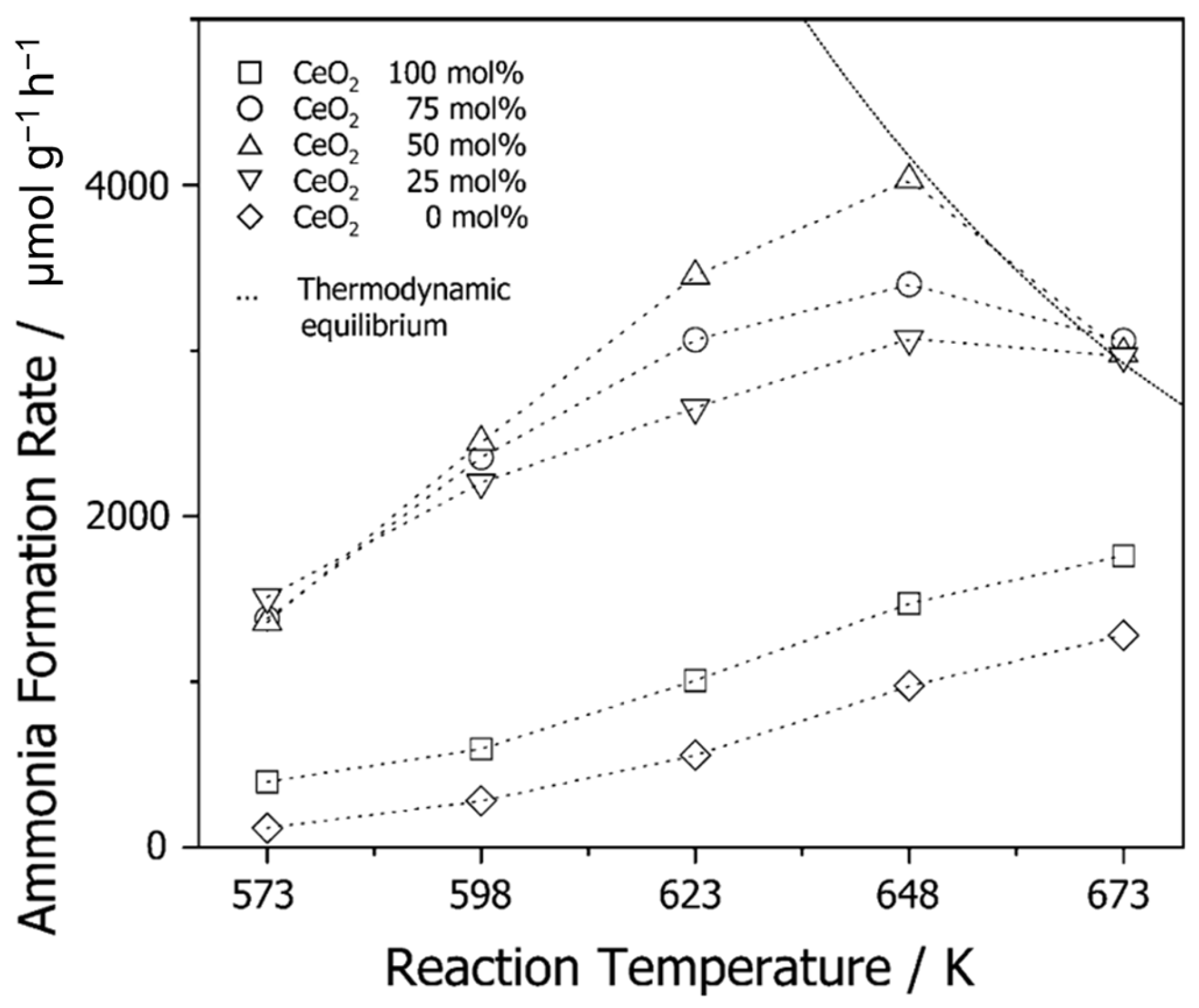
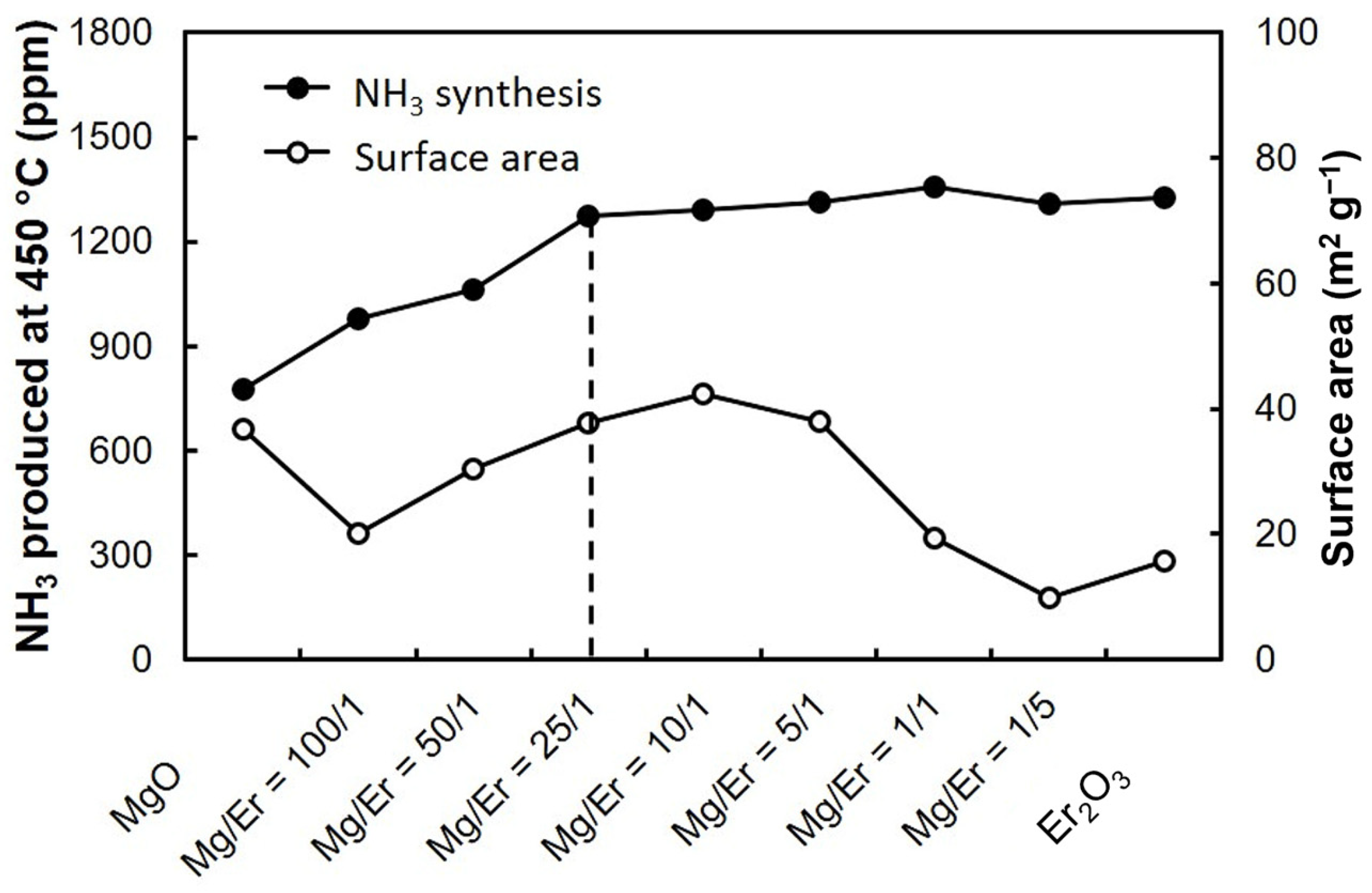
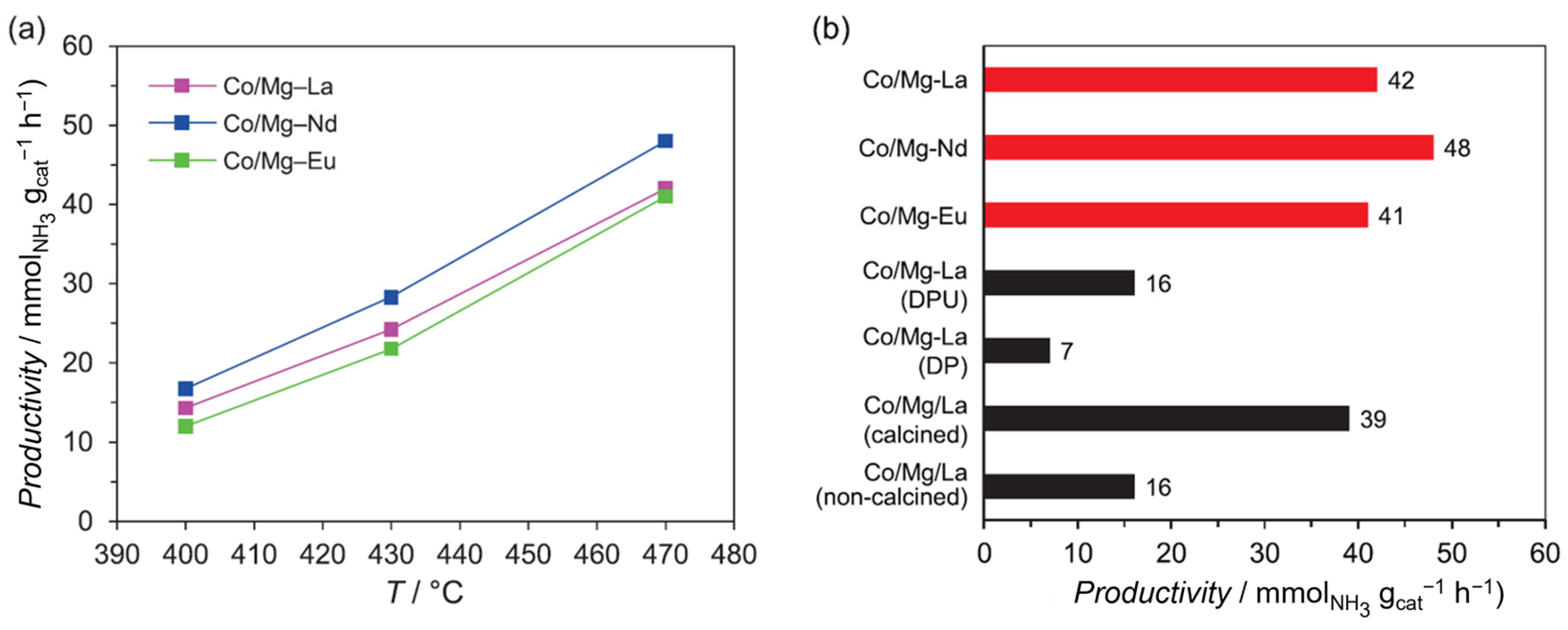

| Element | Electronic Configuration | A Sum of the First Three Ionisation Potentials [kJ mol−1] | Fourth Ionisation Potential [kJ mol−1] | Ln3+ Ionic Radius [pm] [27,28] |
|---|---|---|---|---|
| La | 5d1 6s2 | 3455 | 4819 | 117 |
| Ce | 4f1 5d1 6s2 | 3523 | 3547 | 115 |
| Pr | 4f3 6s2 | 3627 | 3761 | 113 |
| Nd | 4f4 6s2 | 3697 | 3899 | 112 |
| Pm | 4f5 6s2 | 3740 | 3966 | 111 |
| Sm | 4f6 6s2 | 3869 | 3994 | 110 |
| Eu | 4f7 6s2 | 4036 | 4110 | 109 |
| Gd | 4f7 5d1 6s2 | 3749 | 4245 | 108 |
| Tb | 4f9 6s2 | 3791 | 3839 | 106 |
| Dy | 4f10 6s2 | 3911 | 4001 | 105 |
| Ho | 4f11 6s2 | 3924 | 4101 | 104 |
| Er | 4f12 6s2 | 3934 | 4115 | 103 |
| Tm | 4f13 6s2 | 4045 | 4119 | 102 |
| Yb | 4f14 6s2 | 4194 | 4220 | 101 |
| Lu | 4f14 5d1 6s2 | 3887 | 4360 | 100 |
| Lanthanide Oxide | TB [°C] | E1 (T < TB) [eV] | E2 (T > TB) [eV] | σ 400 °C [×109 Ω−1 cm−1] | σ 650 °C [×109 Ω−1 cm−1] |
|---|---|---|---|---|---|
| La2O3 | 270 | 0.7 | 1.05 | 230 | 1700 |
| Pr2O3 | 320 | 0.4 | 0.95 | 300 | 3450 |
| Nd2O3 | - | - | 1.15 | 25 | 1450 |
| Sm2O3 | 560 | 0.6 | 1.28 | 20 | 880 |
| Eu2O3 | 570 | 0.6 | 1.35 | 5 | 150 |
| Gd2O3 | 560 | 0.5 | 1.57 | 5 | 130 |
| Tb2O3 | 280 | 0.4 | 0.95 | 3 | 200 |
| Ho2O3 | 575 | 0.7 | 1.61 | 5 | 160 |
| Yb2O3 | 605 | 0.5 | 1.61 | 3 | 50 |
| Promoter | Rate [µmolNH3 g−1 h−1] |
|---|---|
| - | 60 |
| Na+ | 361 |
| K+ | 536 |
| Rb+ | 581 |
| Cs+ | 690 |
| Ca2+ | 135 |
| Sr2+ | 113 |
| Ba2+ | 213 |
| La3+ | 115 |
| Ce3+ | 100 |
| Pr3+ | 129 |
| Nd3+ | 140 |
| Sm3+ | 86 |
| Gd3+ | 93 |
| Dy3+ | 118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patkowski, W.; Zybert, M.; Ronduda, H.; Raróg-Pilecka, W. Lanthanide Oxides in Ammonia Synthesis Catalysts: A Comprehensive Review. Catalysts 2023, 13, 1464. https://doi.org/10.3390/catal13121464
Patkowski W, Zybert M, Ronduda H, Raróg-Pilecka W. Lanthanide Oxides in Ammonia Synthesis Catalysts: A Comprehensive Review. Catalysts. 2023; 13(12):1464. https://doi.org/10.3390/catal13121464
Chicago/Turabian StylePatkowski, Wojciech, Magdalena Zybert, Hubert Ronduda, and Wioletta Raróg-Pilecka. 2023. "Lanthanide Oxides in Ammonia Synthesis Catalysts: A Comprehensive Review" Catalysts 13, no. 12: 1464. https://doi.org/10.3390/catal13121464
APA StylePatkowski, W., Zybert, M., Ronduda, H., & Raróg-Pilecka, W. (2023). Lanthanide Oxides in Ammonia Synthesis Catalysts: A Comprehensive Review. Catalysts, 13(12), 1464. https://doi.org/10.3390/catal13121464







