Photocatalytic Applications of SnO2 and Ag2O-Decorated SnO2 Coatings on Cement Paste
Abstract
:1. Introduction
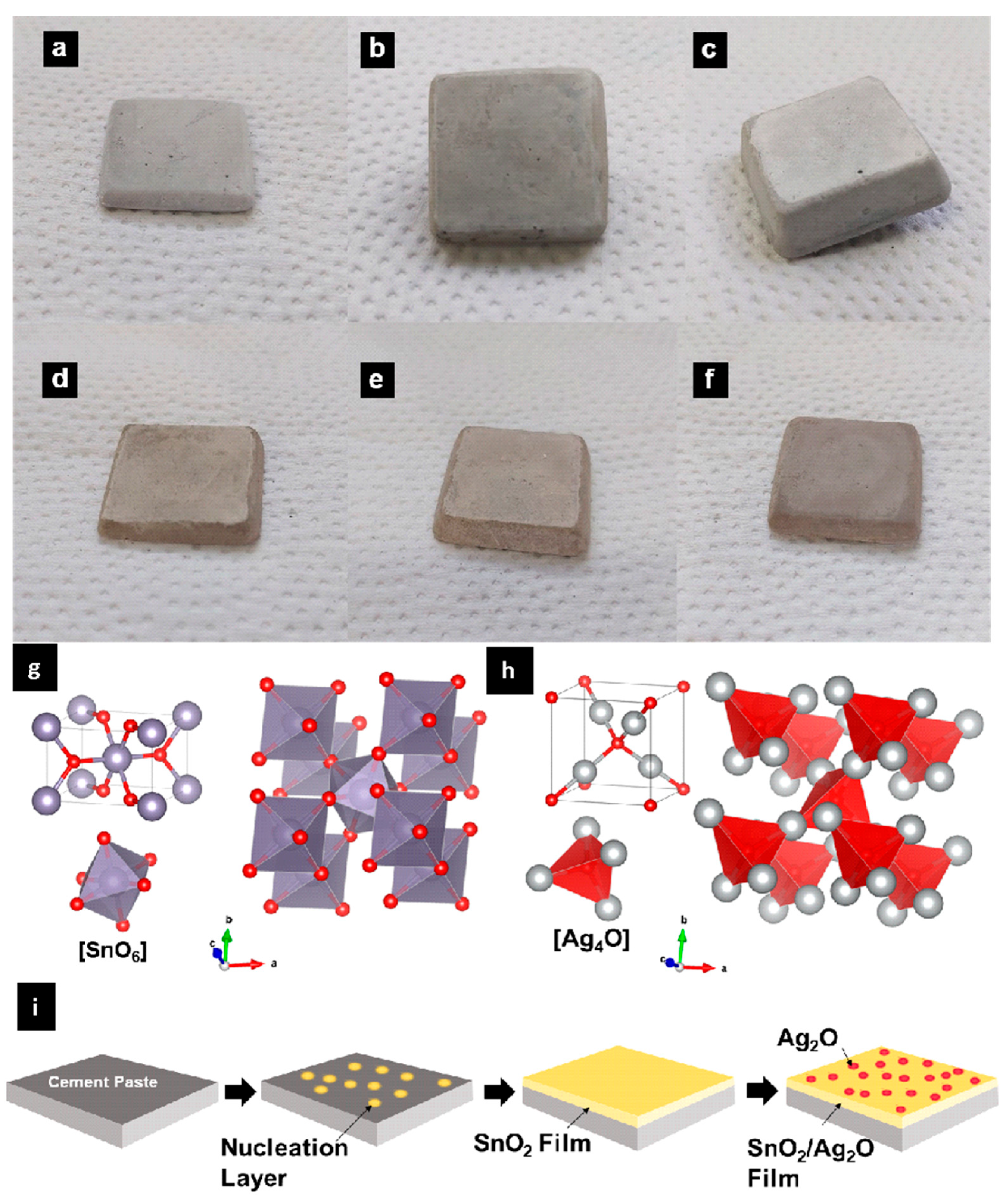
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Cemen Paste Mixture
3.3. SnO2 Coatings of Cement Paste Prepared by Hydrothermal Approaches
3.4. Ag2O-Decored SnO2/Cement Paste Composites
3.5. Photocatalytic Activity Evaluation
3.6. Photo-Fenton Activity Evaluation
3.7. Material Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Araújo, K.S.; Antonelli, R.; Gaydeczka, B.; Granato, A.C.; Malpass, G.R.P. Advanced oxidation processes: A review regarding the fundamentals and applications in wastewater treatment and industrial wastewater. Ambiente Agua-Interdiscip. J. Appl. Sci. 2016, 11, 387–401. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Qadri, H. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology; Hakeem, K., Bhat, R., Qadri, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Senthil Rathi, B.; Senthil Kumar, P.; Dai-Viet Vo, N. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, removal technologies and risk assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020, 6, e04691. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Benatto, V.G.; de Jesus, J.P.A.; de Castro, A.A.; Assis, L.; Ramalho, T.; La Porta, F. Prospects of ZnS and ZnO as smart semiconductor materials in light-activated antimicrobial coatings for mitigation of severe acute respiratory syndrome coronavirus-2 infection. Mater. Today Commun. 2023, 34, 105192. [Google Scholar] [CrossRef]
- Benatto, V.G.; Fabris, G.d.S.L.; Sambrano, J.R.; Taft, C.A.; Porta, F.d.A.L. Influence of structural disorder on the photocatalytic properties of ZnS nanocrystals prepared by the one-pot solvothermal approach. Eclética Química 2022, 47, 17–31. [Google Scholar] [CrossRef]
- Vaya, D.; Surolia, P.K. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Suzuki, V.Y.; Amorin, L.H.C.; Fabris, G.S.L.; Dey, S.; Sambrano, J.R.; Cohen, H.; Oron, D.; La Porta, F.A. Enhanced Photocatalytic and Photoluminescence Properties Resulting from Type-I Band Alignment in the Zn2GeO4/g-C3N4 Nanocomposites. Catalysts 2022, 12, 692. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-Based Adsorbents for Removal of Dyes from Wastewater: A Review. Front. Environ. Sci. 2021, 9, 764958. [Google Scholar] [CrossRef]
- Bojanowska-Czajka, A. Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review. Appl. Sci. 2021, 11, 12032. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Wang, Y.; Sun, W. A review on degradation of perfluorinated compounds based on ultraviolet advanced oxidation. Environ. Pollut. 2021, 291, 118014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shaad, K.; Vollmer, D.; Ma, C. Treatment of Textile Wastewater Using Advanced Oxidation Processes—A Critical Review. Water 2021, 13, 3515. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.F.P.; Trovó, A.G.; Silva, M.R.A.d.; Villa, R.D.; Oliveira, M.C.d. Fundamentos e aplicações ambientais dos processos fenton e foto-fenton. Quím. Nova 2007, 30, 400–408. [Google Scholar] [CrossRef]
- Ramos, P.H.; La Porta, F.A.; Resende, E.C.; Giacoppo, J.O.S.; Guerreiro, M.C.; Ramalho, T.C. Fe-DPA as Catalyst for Oxidation of Organic Contaminants: Evidence of Homogeneous Fenton Process. Z. Für Anorg. Allg. Chem. 2015, 641, 780–785. [Google Scholar] [CrossRef]
- Suzuki, V.Y.; Amorin, L.H.C.; Lima, N.M.; Machado, E.G.; Carvalho, P.E.; Castro, S.B.R.; Alves, C.C.S.; Carli, A.P.; Li, M.S.; Longof, E.; et al. Characterization of the structural, optical, photocatalytic and in vitro and in vivo anti-inflammatory properties of Mn2+ doped Zn2GeO4 nanorods. J. Mater. Chem. C 2019, 7, 8216–8225. [Google Scholar] [CrossRef]
- De Souza, B.M. Avaliação de Processos Oxidativos Avançados Acoplados com Carvão Ativado Granulado com Biofilme para Reúso de Efluentes de Refinaria de Petróleo. Master’s Thesis, Federal University of Rio de Janeiro (COPPE-UFRJ), Rio de Janeiro, Brazil, 2010. Available online: http://portal.peq.coppe.ufrj.br/index.php/dissertacoes-de-mestrado/2010-1/130-avaliacao-de-processos-oxidativos-avancados-acoplados-com-carvao-ativado-granulado-com-biofilme-para-reuso-de-efluentes-de-refinaria-de-petroleo/file (accessed on 28 April 2022).
- Wang, Y.; Torres, J.A.; Shviro, M.; Carmo, M.; He, T.; Ribeiro, C. Photocatalytic materials applications for sustainable agriculture. Prog. Mater. Sci. 2022, 130, 100965. [Google Scholar] [CrossRef]
- Fathima Beevi, A.; Sreekala, G.; Beena, B. Synthesis, characterization and photocatalytic activity of SnO2, ZnO nanoparticles against congo red: A comparative study. Mater. Today Proc. 2021, 45, 4045–4051. [Google Scholar] [CrossRef]
- La Porta, F.A.; Taft, C.A. Functional Properties of Advanced Engineering Materials and Biomolecules; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- La Porta, F.A.; Taft, C.A. Emerging Research in Science and Engineering Based on Advanced Experimental and Computational Strategies; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Longo, E.; La Porta, F.A. Recent Advances in Complex Functional Materials: From Design to Application; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- de Jesus, J.P.A.; Santos, A.C.L.; Pinto, F.M.; Taft, C.A.; La Porta, F.A. Review: Theoretical and experimental investigation of the intrinsic properties of Zn2GeO4 nanocrystals. J. Mater. Sci. 2021, 56, 4552–4568. [Google Scholar] [CrossRef]
- La Porta, F.A.; Andrés, J.; Vismara, M.V.G.; Graeff, C.F.O.; Sambrano, J.R.; Li, M.S.; Varela, J.A.; Longo, E. Correlation between structural and electronic order–disorder effects and optical properties in ZnO nanocrystals. J. Mater. Chem. C 2014, 47, 10164–10174. [Google Scholar] [CrossRef]
- Silva Junior, E.; La Porta, F.A.; Liu, M.S.; Andrés, J.; Varela, J.A.; Longo, E. A relationship between structural and electronic order–disorder effects and optical properties in crystalline TiO2 nanomaterials. Dalton Trans. 2015, 44, 3159–3175. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, W. Photochemical Preparation of Ag/SnO2 Composites and their Photocatalytic Properties. In Proceedings of the International Conference on Chemical, Material and Food Engineering, Kunming, China, 25–26 July 2015. [Google Scholar] [CrossRef]
- Ayeleru, O.O.; Dlova, S.; Ntuli, F.; Kupolati, W.K.; Olubambi, P.A. Synthesis and characterization of SnO2 nanofiller from recycled expanded polystyrene. Procedia Manuf. 2019, 30, 635–641. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.A.; Hidalgo, M.C. Enhanced UV and visible light photocatalytic properties of synthesized AgBr/SnO2 composites. Sep. Purif. Technol. 2021, 257, 117948. [Google Scholar] [CrossRef]
- do Nascimento, J.L.A.; Chantelle, L.; dos Santos, I.M.G.; de Oliveira, A.L.M.; Alves, M.C.F. The Influence of Synthesis Methods and Experimental Conditions on the Photocatalytic Properties of SnO2: A Review. Catalysts 2022, 12, 428. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. J. Chem. Eng. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Akhir, M.A.M.; Rezan, S.; Mohamed, K.; Arafat, M.; Haseeb, A.; Lee, H. Synthesis of SnO2 Nanoparticles via Hydrothermal Method and Their Gas Sensing Applications for Ethylene Detection. Mater. Today Proc. 2019, 17, 810–819. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, K.; Ke, J.; Guo, Z.; Deng, X.; Bai, C.; Sun, Y.; Wang, Q.; Yang, B.; Dong, H.; et al. Synthesis of ternary SnO2–MoO3–C composite with nanosheet structure as high-capacity, high-rate and long-lifetime anode for lithium-ion batteries. Ceram. Int. 2021, 47, 9303–9309. [Google Scholar] [CrossRef]
- Gervillié, C.; Boisard, A.; Labbé, J.; Guérin, K.; Berthon-Fabry, S. Relationship between tin environment of SnO2 nanoparticles and their electrochemical behaviour in a lithium ion battery. Mater. Chem. Phys. 2021, 257, 123461. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, R.; Ge, W.; Guo, R.; Shirsath, S.E.; Zhu, J. Facile one-step hydrothermal synthesis of SnO2 microspheres with oxygen vacancies for superior ethanol sensor. J. Alloys Compd. 2020, 814, 152266. [Google Scholar] [CrossRef]
- Prakash, K.; Senthil Kumar, P.; Pandiaraj, S.; Saravanakumar, K.; Karuthapandian, S. Controllable synthesis of SnO2 photocatalyst with superior photocatalytic activity for the degradation of methylene blue dye solution. J. Exp. Nanosci. 2016, 11, 1138–1155. [Google Scholar] [CrossRef]
- Tuan, P.V.; Hieu, L.T.; Tan, V.T.; Phuong, T.T.; Qu, H.T.T.; Khiem, T.N. The dependence of morphology, structure, and photocatalytic activity of SnO2/rGO nanocomposites on hydrothermal temperature. Mater. Res. Express 2019, 6, 106204. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, R.J.; Yu, Z.; Han, X.-n.; Zhao, W.-X.; Ma, F. Synthesis of BiPO4/SnO2 heterojunction for the photocatalytic degradation of RhB under visible light emitting diode irradiation. J. Chin. Chem. Soc. 2021, 68, 1663–1672. [Google Scholar] [CrossRef]
- Pham, V.V.; Mai, D.Q.; Bui, D.P.; Van Man, T.; Zhu, B.; Zhang, L.; Sangkaworn, J.; Tantirungrotechai, J.; Reutrakul, V.; Cao, T.M. Emerging 2D/0D g-C3N4/SnO2 S-scheme photocatalyst: New generation architectural structure of heterojunctions toward visible-light-driven NO degradation. Environ. Pollut. 2021, 286, 117510. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Ni, Y.; Zhou, Q.; Kou, J.; Lu, C.; Xu, Z. New g-C3N4 based photocatalytic cement with enhanced visible-light photocatalytic activity by constructing muscovite sheet/SnO2 structures. Constr. Build. Mater. 2018, 179, 315–325. [Google Scholar] [CrossRef]
- Rajput, R.B.; Jamble, N.S.; Kale, B.R. A review on TiO2/SnO2 heterostructures as a photocatalyst for the degradation of dyes and organic pollutants. J. Environ. Manag. 2022, 307, 114533. [Google Scholar] [CrossRef]
- Kumar, M.R.; Murugadoss, G.; Venkatesh, N.; Sakthivel, P. Synthesis of Ag2O-SnO2 and SnO2-Ag2O Nanocomposites and Investigation on Photocatalytic Performance under Direct Sun Light. ChemistrySelect 2020, 5, 6946–6953. [Google Scholar] [CrossRef]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714. [Google Scholar] [CrossRef]
- Viet, P.V.; Thi, C.M.; Hieu, L.V. The High Photocatalytic Activity of SnO2 Nanoparticles Synthesized by Hydrothermal Method. J. Nanomater. 2016, 2016, 4231046. [Google Scholar] [CrossRef]
- Qi, Y.; Xiu-Juan, G.; Ru, F.; Li, M.-J.; Zhang, J.-F.; Zhang, Q.-D.; Han, Y.-Z. MoO3-SnO2 catalyst prepared by hydrothermal synthesis method for dimethyl ether catalytic oxidation. J. Fuel Chem. Technol. 2019, 47, 934–941. [Google Scholar]
- Sudrajat, H.; Hartuti, S.; Babel, S.; Nguyen, T.K.; Tong, H.D. SnO2/ZnO heterostructured nanorods: Structural properties and mechanistic insights into the enhanced photocatalytic activity. J. Phys. Chem. Solids 2021, 149, 109762. [Google Scholar] [CrossRef]
- Nagaraju, Y.S.; Ganesha, H.; Veeresha, S.; Vandana, M.; Ashokkumar, S.P.; Vijeth, H.; Devendrappa, H. Single crystalline hierarchical SnO2 microsphere and fluoride-mediated hollow structures for photocatalytic activity. Mater. Today Proc. 2021, 45, 3833–3836. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, J.; Song, P.; Yang, Z.; Wang, Q. Synthesis of reduced graphene oxide/SnO2 nanosheets/Au nanoparticles ternary composites with enhanced formaldehyde sensing performance. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 118, 113953. [Google Scholar] [CrossRef]
- Dobrovolski, M.E.G.; Munhoz, G.S.; Pereira, E.; Medeiros-Junior, R.A. Effect of crystalline admixture and polypropylene microfiber on the internal sulfate attack in Portland cement composites due to pyrite oxidation. Constr. Build Mater. 2021, 308, 125018. [Google Scholar] [CrossRef]
- Wang, L.; Ma, H.; Li, Z.; Ma, G.; Guan, J. Cementitious composites blending with high belite sulfoaluminate and medium-heat Portland cements for largescale 3D printing. Addit. Manuf. 2021, 46, 102189. [Google Scholar] [CrossRef]
- Luo, D.; Wei, J. Hydration kinetics and phase evolution of Portland cement composites containing sodium-montmorillonite functionalized with a Non-Ionic surfactant. Constr. Build Mater. 2022, 333, 127386. [Google Scholar] [CrossRef]
- Claverie, J.; Wang, Q.; Kamali-Bernard, S.; Bernard, F. Assessment of the reactivity and hydration of Portland cement clinker phases from atomistic simulation: A critical review. Cem. Concr. Res. 2022, 154, 106711. [Google Scholar] [CrossRef]
- Natalli, J.F.; Thomaz, E.C.S.; Mendes, J.C.; Peixoto, R.A.F. A review on the evolution of Portland cement and chemical admixtures in Brazil. Rev. Ibracon Estrut. Mater. 2021, 14, 14603. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Liu, Z.; He, F.; Zheng, K. Effect of transitional aluminas on Portland cement hydration, phase assemblage and the correlation to ASR preventing effectiveness. Cem. Concr. Res. 2022, 151, 106622. [Google Scholar] [CrossRef]
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M. Applications of using nano material in concrete: A review. Constr. Build. Mater. 2017, 133, 91–97. [Google Scholar] [CrossRef]
- Chen, X.-F.; Kou, S.-C.; Sun Poon, C. Rheological behaviour, mechanical performance, and NOx removal of photocatalytic mortar with combined clay brick sands-based and recycled glass-based nano-TiO2 composite photocatalysts. Constr. Build. Mater. 2020, 240, 117698. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Stephan, D.; Huang, S.; Zhang, L.; Yang, P.; Cheng, X. SiO2/TiO2 composite powders deposited on cement-based materials: Rhodamine B removal and the bonding mechanism. Constr. Build. Mater. 2020, 241, 118124. [Google Scholar] [CrossRef]
- Flores, Y.C.; Cordeiro, G.C.; Toledo Filho, R.D.; Tavares, L.M. Performance of Portland cement pastes containing nano-silica and different types of silica. Constr. Build. Mater. 2017, 146, 524–530. [Google Scholar] [CrossRef]
- Nogueira, G.S.F.; Schwantes-Cezario, N.; Souza, I.C.; Cavaleiro, C.D.; Porto, M.F.; Toralles, B.M. Incorporação de nanossílica em compósitos cimentícios. Matér. Rio Jan. 2018, 23, e12182. [Google Scholar] [CrossRef]
- Nath, R.K.; Zain, M.F.M.; Alam, R.; Kadhum, A.A.H.; Kaish, A.B.M.A. Hybrid photocatalyst for corrosion reducing and sustainable concrete construction. Int. J. Sustain. Constr. Eng. Technol. 2012, 3, 38–44. [Google Scholar]
- Fernandes, C.N.; Ferreira, R.L.S.; Bernardo, R.D.S.; Avelino, F.; Bertini, A.A. Using TiO2 nanoparticles as a SO2 catalyst in cement mortars. Constr. Build. Mater. 2020, 257, 119542. [Google Scholar] [CrossRef]
- Barbudo, A.; Lozano-Lunar, A.; López-Uceda, A.; Galvín, A.P.; Ayuso, J. Photocatalytic Recycled Mortars: Circular Economy as a Solution for Decontamination. Appl. Sci. 2020, 10, 7305. [Google Scholar] [CrossRef]
- Tobón, J.I.; Cohen, J.D.; Dorkis, L.I. Photocatalytic activity under visible light irradiation of cement based materials containing TiO2-xNy nanoparticles. Rev. Fac. Ing. Univ. Antioq. 2020, 94, 87–96. [Google Scholar] [CrossRef]
- Sakthivel, R.; Kitcha, T.A.; Dhanabal, M.; Aravindan, V.; Aravindh, S. Experimental Study of Photocatalytic Concrete using Titanium Dioxide. Int. J. Innov. Res. Sci. Technol. 2018, 4, 117–123. [Google Scholar]
- Luna, M.; Delgado, J.J.; Romero, I.; Montini, T.; Almoraima Gil, M.L.; Martínez-López, J.; Fornasiero, P.; Mosquera, M.J. Photocatalytic TiO2 nanosheets-SiO2 coatings on concrete and limestone: An enhancement of de-polluting and self-cleaning properties by nanoparticle design. Constr. Build. Mater. 2022, 338, 127349. [Google Scholar] [CrossRef]
- Torabi, S.; Mansoorkhani, M.J.K.; Majedi, A.; Motevalli, S. Review: Synthesis, Medical and Photocatalyst Applications of Nano-Ag2O. J. Coord. Chem. 2020, 73, 1861–1880. [Google Scholar] [CrossRef]
- Zancan, P.H. Influência dos Parâmetros de Deposição na Molhabilidade de Filmes de a-C:H. Master’s Thesis, The University of the State of Santa Catarina (UDESC), Joinville, Brazil, 2017. Available online: https://www.udesc.br/arquivos/cct/id_cpmenu/877/Disserta__o_Paulo_Zancan_15180956415105_877.pdf (accessed on 28 April 2022).
- Talinungsang; Paul, N.; Purkayastha, D.D.; Krishna, M.G. TiO2/SnO2 and SnO2/TiO2 heterostructures as photocatalysts for degradation of stearic acid and methylene blue under UV irradiation. Superlattices Microstruct. 2019, 129, 105–114. [Google Scholar] [CrossRef]
- Katz, L. X-ray diffraction in crystals, imperfect crystals, and amorphous bodies (Guinier, A.). J. Chem. Educ. 1964, 41, 292. [Google Scholar] [CrossRef]
- GNU Octave (Version 8.1.0): A High-Level Interactive Language for Numerical Computations. Available online: https://www.gnu.org/software/octave/doc/v8.1.0/ (accessed on 10 August 2023).
- Wu, J.; Jin, X.; Mi, S.; Tang, S. An effective method to compute the box-counting dimension based on the mathematical definition and intervals. Results Eng. 2020, 6, 100–106. [Google Scholar] [CrossRef]
- Suzuki, V.Y.; Amorin, L.H.C.; de Paula, N.H.; Albuquerque, A.R.; Li, M.S.; Sambrano, J.R.; Longo, E.; La Porta, F.A. New insights into the nature of the bandgap of CuGeO3 nanofibers: Synthesis, electronic structure, and optical and photocatalytic properties. Mater. Today Commun. 2021, 26, 101701. [Google Scholar] [CrossRef]
- Testoni, G.O.; Amoresi, R.A.C.; Lustosa, G.M.M.M.; Costa, J.P.C.; Nogueira, M.V.; Ruiz, M.; Zaghete, M.A.; Perazolli, L. Increased photocatalytic activity induced by TiO2/Pt/SnO2 heterostructured films. Solid State Sci. 2018, 76, 65–73. [Google Scholar] [CrossRef]
- Ben Ameur, S.; Bel Hadjltaief, H.; Barhoumi, A.; Duponchel, B.; Leroy, G.; Amlouk, M.; Guermazi, H. Physical investigations and photocatalytic activities on ZnO and SnO2 thin films deposited on flexible polymer substrate. Vacuum 2018, 155, 546–552. [Google Scholar] [CrossRef]
- Badr, Y.; Abd El-Wahed, M.G.; Mahmoud, M.A. Photocatalytic degradation of methyl red dye by silica nanoparticles. J. Hazard. Mater. 2008, 154, 245–253. [Google Scholar] [CrossRef]
- Comparelli, R.; Cozzoli, P.D.; Curri, M.L.; Agostiano, A.; Mascolo, G.; Lovecchio, G. Photocatalytic degradation of methyl-red by immobilised nanoparticles of TiO2 and ZnO. Water Sci. Technol. 2004, 49, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, R.; Debnath, N.C. Computation of X-ray powder diffractograms of cement components and its application to phase analysis and hydration performance of OPC cement. Bull. Mater. Sci. 2011, 34, 1137–1150. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Senthilnathan, R.; Megarajan, S.; Anbazhagan, V. Sunlight mediated synthesis of silver nanoparticles using redox phytoprotein and their application in catalysis and colorimetric mercury sensing. J. Photochem. Photobiol. B 2015, 151, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Lv, Y.; Zhang, W.; Yang, B.; Chi, F.; Ran, S.; Liu, X. One-step Synthesis of Ag3PO4/Ag Photocatalyst with Visible-light Photocatalytic Activity. Mater. Res. 2015, 18, 939–945. [Google Scholar] [CrossRef]
- Venkatesh, D.; Pavalamalar, S.; Anbalagan, K. Selective Photodegradation on Dual Dye System by Recoverable Nano SnO2 Photocatalyst. J. Inorg. Organomet. Polym. Mater. 2019, 29, 939–953. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, F.; Li, X.; Wang, D.; Zhong, Y.; Zeng, G. Self-assembly Z-scheme heterostructured photocatalyst of Ag2O@Ag-modified bismuth vanadate for efficient photocatalytic degradation of single and dual organic pollutants under visible light irradiation. RSC Adv. 2016, 6, 60291–60307. [Google Scholar] [CrossRef]
- Lebedev, A.; Anariba, F.; Li, X.; Seng Hwee Leng, D.; Wu, P. Ag/Ag2O/BiNbO4 structure for simultaneous photocatalytic degradation of mixed cationic and anionic dyes. Sol. Energy 2019, 178, 257–267. [Google Scholar] [CrossRef]
- Reshma, T.S.; Pan, S.; Das, A. Uncapped SnO2 quantum dots for selective adsorption, separation and photocatalytic degradation of a mixture of dyes. New J. Chem. 2023, 47, 16136–16147. [Google Scholar] [CrossRef]
- Groeneveld, I.; Kanelli, M.; Ariese, F.; van Bommel, M.R. Parameters that affect the photodegradation of dyes and pigments in solution and on substrate—An overview. Dyes Pigm. 2023, 210, 110999. [Google Scholar] [CrossRef]
- Gorup, L.F.; Amorin, L.H.; Camargo, E.R.; Sequinel, T.; Cincotto, F.H.; Ramesar, G.B.N.; La Porta, F.A. Methods for design and fabrication of nanosensors: The case of ZnO-based nanosensor. In Micro and Nano Technologies, Nanosensors for Smart Cities; Han, B., Tomer, V.K., Nguyen, T.A., Farmani, A., Singh, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 9–30. [Google Scholar] [CrossRef]









| Sample | Contact Angle (θ) |
|---|---|
| Cement paste | 126° |
| Nucleation layer | 30° |
| Film | 11° |
| Ag1 | 23° |
| Ag2 | 23° |
| Ag3 | 23° |
| Samples | Crystallite Size (tc) |
|---|---|
| SnO2—Powder | 6 nm |
| SnO2—Film | 33 nm |
| SnO2—Ag1 | 26 nm |
| SnO2—Ag2 | 32 nm |
| SnO2—Ag3 | 29 nm |
| Ag2O—Ag1 | 37 nm |
| Ag2O—Ag2 | 35 nm |
| Ag2O—Ag3 | 33 nm |
| Scavenger | Kinetic Constant (k) (min−1) | Degradation (%) | Adsorption (%) | R2 |
|---|---|---|---|---|
| WS | 0.0059 | 64 | 27 | 0.9865 |
| Ag | 0.0023 | 31 | 21 | 0.9271 |
| AO | 0.0035 | 46 | 22 | 0.9925 |
| ISO | 0.0033 | 42 | 27 | 0.9947 |
| p-BQ | 0.0026 | 37 | 18 | 0.9941 |
| Sample | k (min−1) | Degradation (%) | Adsorption (%) | R2 |
|---|---|---|---|---|
| Ref | 0.0090 | 82 | 28 | 0.9808 |
| Ag1 (0.01 mMol) | 0.0079 | 76 | 28 | 0.9954 |
| Ag2 (0.02 mMol) | 0.0142 | 91 | 34 | 0.9873 |
| Ag3 (0.04 mMol) | 0.0154 | 93 | 22 | 0.9929 |
| Sample | k (min−1) | Degradation (%) | Adsorption (%) | R2 |
|---|---|---|---|---|
| Ref | 0.0060 | 63 | 31 | 0.9491 |
| Ag1 (0.01 mMol) | 0.0019 | 29 | 28 | 0.9463 |
| Ag2 (0.02 mMol) | 0.0014 | 27 | 18 | 0.8895 |
| Ag3 (0.04 mMol) | 0.0007 | 12 | 22 | 0.9020 |
| Samples | k (min−1) | Degradation (%) | Adsorption (%) | R2 |
|---|---|---|---|---|
| Photo-Fenton—Wavelength 665 nm (MB) | ||||
| Ref | 0.0092 | 79 | 25 | 0.9301 |
| Ag1 (0.01 mMol) | 0.0058 | 63 | 19 | 0.9730 |
| Ag2 (0.02 mMol) | 0.0075 | 73 | 14 | 0.9993 |
| Ag3 (0.04 mMol) | 0.0084 | 79 | 30 | 0.9712 |
| Photo-Fenton—Wavelength 425 nm (MR) | ||||
| Ref | 0.0021 | 34 | 29 | 0.9298 |
| Ag1 (0.01 mMol) | 0.0012 | 19 | 31 | 0.9220 |
| Ag2 (0.02 mMol) | 0.0021 | 31 | 22 | 0.9735 |
| Ag3 (0.04 mMol) | 0.0032 | 45 | 23 | 0.9282 |
| pH | k (min−1) | Degradation (%) | Adsorption (%) | R2 |
|---|---|---|---|---|
| Wavelength 665 nm (MB) | ||||
| 2 | 0.0322 | 100 | 22 | 0.9509 |
| 4 | 0.0205 | 100 | 19 | 0.9292 |
| 6 | 0.0084 | 79 | 30 | 0.9712 |
| Wavelength 425 nm (pH 6) and 533 nm (pH 2 and 4) (MR) | ||||
| 2 | 0.0440 | 100 | 16 | 0.9427 |
| 4 | 0.1106 | 100 | 18 | 0.9008 |
| 6 | 0.0032 | 45 | 23 | 0.9282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendramini, D.d.S.; Benatto, V.G.; Ashtiani, A.M.; La Porta, F.d.A. Photocatalytic Applications of SnO2 and Ag2O-Decorated SnO2 Coatings on Cement Paste. Catalysts 2023, 13, 1479. https://doi.org/10.3390/catal13121479
Vendramini DdS, Benatto VG, Ashtiani AM, La Porta FdA. Photocatalytic Applications of SnO2 and Ag2O-Decorated SnO2 Coatings on Cement Paste. Catalysts. 2023; 13(12):1479. https://doi.org/10.3390/catal13121479
Chicago/Turabian StyleVendramini, Danilo da Silva, Victoria Gabriela Benatto, Alireza Mohebi Ashtiani, and Felipe de Almeida La Porta. 2023. "Photocatalytic Applications of SnO2 and Ag2O-Decorated SnO2 Coatings on Cement Paste" Catalysts 13, no. 12: 1479. https://doi.org/10.3390/catal13121479
APA StyleVendramini, D. d. S., Benatto, V. G., Ashtiani, A. M., & La Porta, F. d. A. (2023). Photocatalytic Applications of SnO2 and Ag2O-Decorated SnO2 Coatings on Cement Paste. Catalysts, 13(12), 1479. https://doi.org/10.3390/catal13121479






