Steering Charge Directional Separation in MXenes/Titanium Dioxide for Efficient Photocatalytic Nitrogen Fixation
Abstract
:1. Introduction
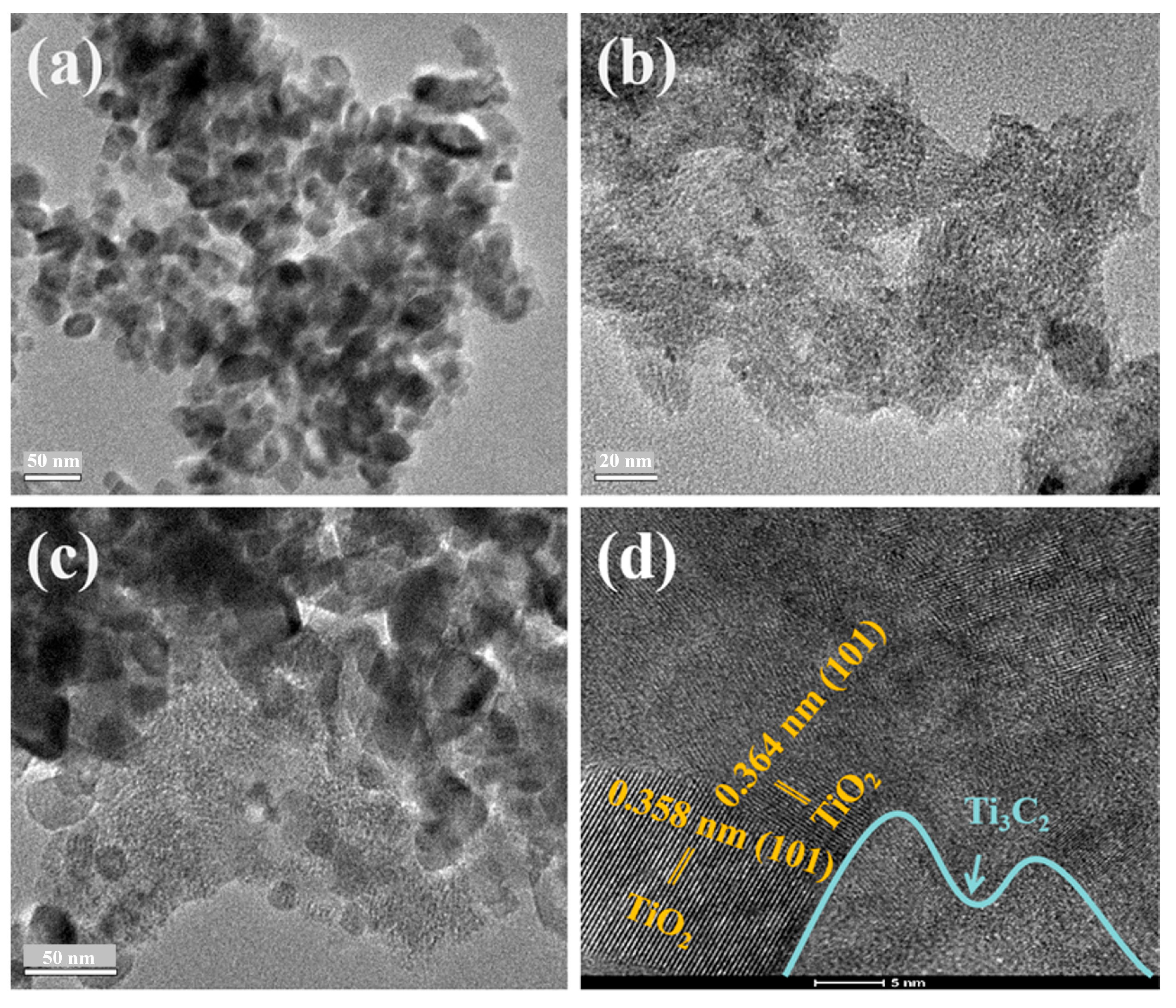
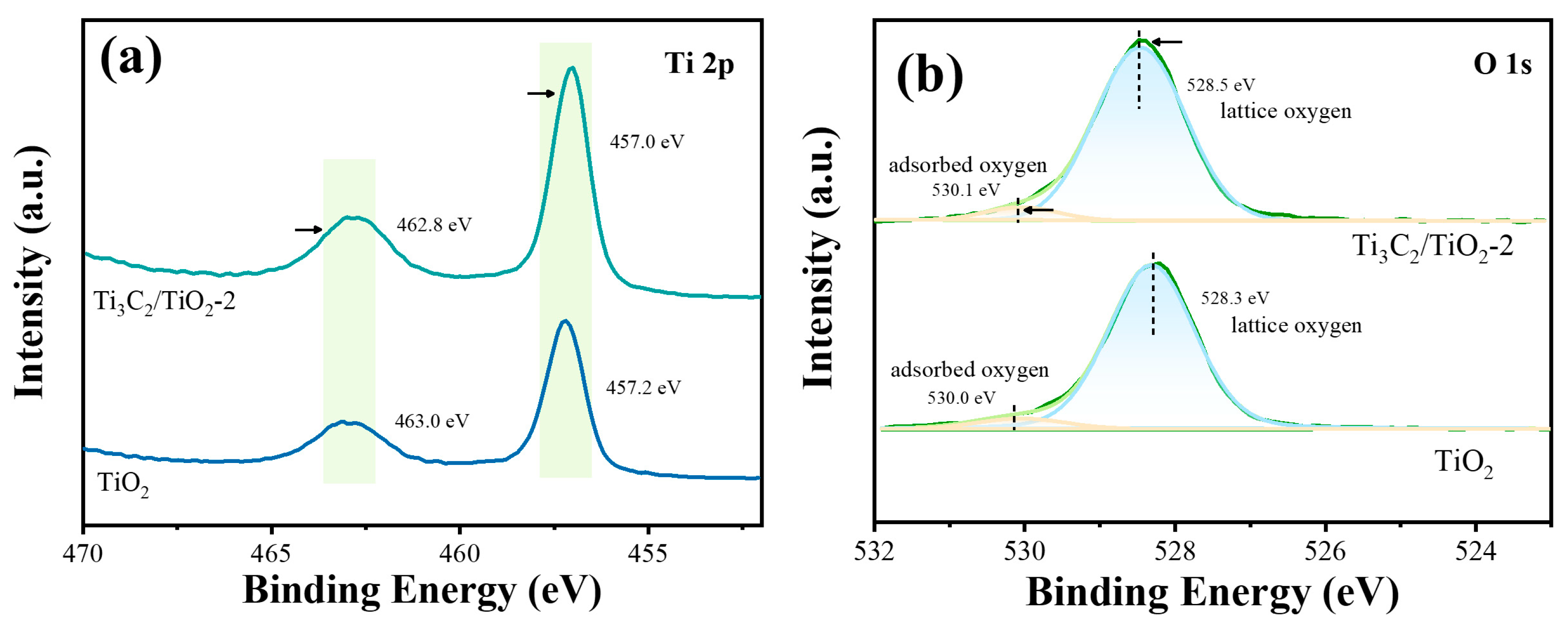
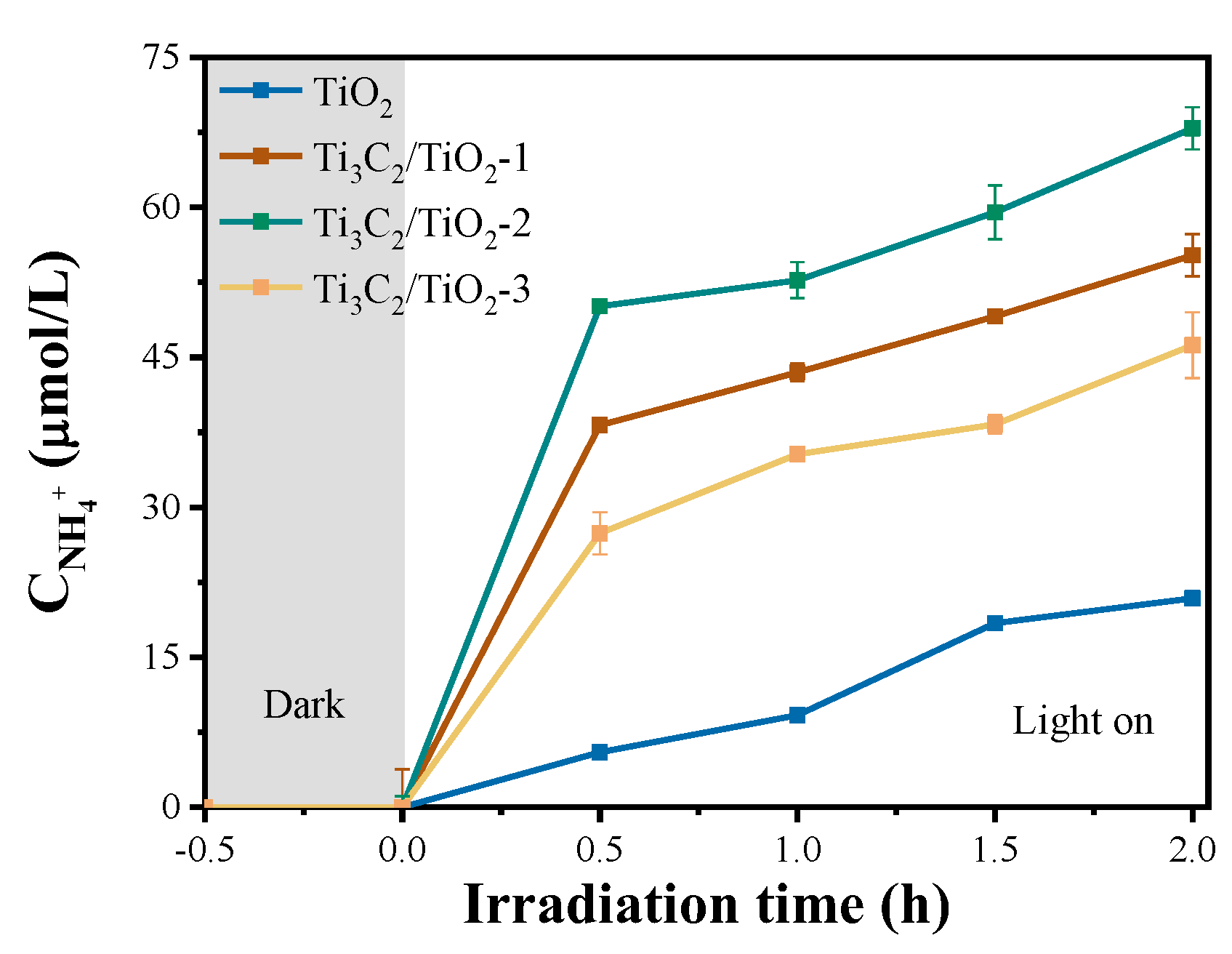
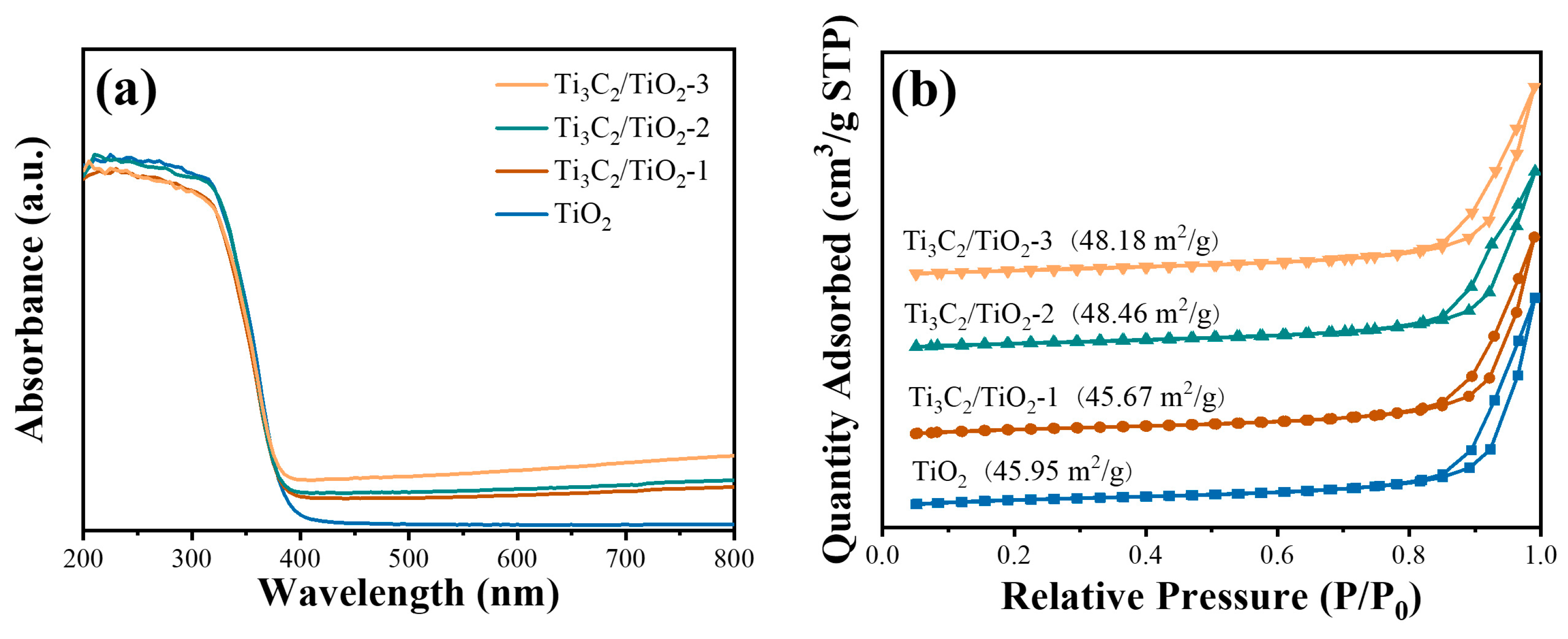
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Characterizations
3.2. Synthesis of the TiO2 Material
3.3. Synthesis of the Ti3C2 Ultrathin Nanosheets
3.4. Synthesis of the Ti3C2/TiO2 Composite Materials
3.5. Photocatalytic N2 Fixation Activity Experiments
3.6. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiao, F.; Xu, B. Electrochemical Ammonia Synthesis and Ammonia Fuel Cells. Adv. Mater. 2018, 31, 1805173. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Liang, C.; Niub, H.-Y.; Guo, H.; Niu, C.-G.; Yang, Y.-Y.; Liu, H.-Y.; Tang, W.-W.; Feng, H.-P. Efficient photocatalytic nitrogen fixation to ammonia over bismuth monoxide quantum dots-modified defective ultrathin graphitic carbon nitride. Chem. Eng. J. 2020, 406, 126868. [Google Scholar] [CrossRef]
- Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Zhang, S.; Zhang, T. Defect Engineering in Photocatalytic Nitrogen Fixation. ACS Catal. 2019, 9, 9739–9750. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Guth, T.D. Photolysis of Water and Photoreduction of Nitrogen on Titanium Dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, X.; Tang, Y.; Liu, M.; Liu, C. Efficient Photocatalytic Nitrogen Fixation: Enhanced Polarization, Activation, and Cleavage by Asymmetrical Electron Donation to N≡N Bond. Adv. Funct. Mater. 2020, 30, 1906983. [Google Scholar] [CrossRef]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Zhang, S.; Yin, L.; Yang, Y. Defective titanium dioxide nanobamboo arrays architecture for photocatalytic nitrogen fixation up to 780 nm. Chem. Eng. J. 2020, 401, 126033. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Chisholm, M.F.; Zhong, J.; Chen, C.; Cao, X.; Dong, F.; Chi, Z.; Chen, H.; Weng, Y.X.; et al. Defect-Tailoring Mediated Electron-Hole Separation in Single-Unit-Cell Bi3O4Br Nanosheets for Boosting Photocatalytic Hydrogen Evolution and Nitrogen Fixation. Adv. Mater. 2019, 31, 1807576. [Google Scholar] [CrossRef]
- Shen, Z.-K.; Cheng, M.; Yuan, Y.-J.; Shen, Z.-K.; Cheng, M.; Yuan, Y.-J.; Pei, L.; Zhong, J.; Guan, J.; Li, X.; et al. Identifying the role of interface chemical bonds in activating charge transfer for enhanced photocatalytic nitrogen fixation of Ni2P-black phosphorus photocatalysts. Appl. Catal. B Environ. 2021, 295, 120274. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillaia, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, Z.; Liu, X.; Qiu, P.; Cui, H.; Tian, J. Noble metal-like behavior of plasmonic Bi particles deposited on reduced TiO2 microspheres for efficient full solar spectrum photocatalytic oxygen evolution. Chin. J. Catal. 2020, 41, 333–340. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hua, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Bakhteeva, I.A.; Medvedeva, I.V.; Zhakov, S.V.; Byzov, I.V.; Filinkova, M.S.; Uimin, M.A.; Murzakaev, A.M. Magnetic separation of water suspensions containing TiO2. Sep. Purif. Technol. 2021, 269, 118716. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Bao, X.; Li, H.; Wang, Z.; Tong, F.; Liu, M.; Zheng, Z.; Wang, P.; Cheng, H.; Liu, Y.; Dai, Y.; et al. TiO2/Ti3C2 as an efficient photocatalyst for selective oxidation of benzyl alcohol to benzaldehyde. Appl. Catal. B Environ. 2021, 286, 119885. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, D.; Zhang, Y.; Jiao, S.; Tang, W.; Wang, Z.; Wu, N.; Wang, Y.; Zhong, W.; Zhang, A.; et al. Integrated unit-cell-thin MXene and Schottky electric field into piezo-photocatalyst for enhanced photocarrier separation and hydrogen evolution. Chem. Eng. J. 2022, 439, 135640. [Google Scholar] [CrossRef]
- Chang, H.; Li, X.; Shi, L.; Zhu, Y.-R.; Yi, T.-F. Towards high-performance electrocatalysts and photocatalysts: Design and construction of MXenes-based nanocomposites for water splitting. Chem. Eng. J. 2021, 421, 129944. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Tang, R.; Xiong, S.; Gong, D.; Deng, Y.; Wang, Y.; Su, L.; Ding, C.; Yang, L.; Liao, C. Ti3C2 2D MXene: Recent Progress and Perspectives in Photocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 56663–56680. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Sasaki, T.; Yunoki, S. Electronic properties and applications of MXenes: A theoretical review. J. Mater. Chem. C 2017, 5, 2488–2503. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, R.; Qin, J.; Hu, H.; Fan, X.; Cao, X.; Wang, D. MIL-100(Fe)/Ti3C2 MXene as a Schottky Catalyst with Enhanced Photocatalytic Oxidation for Nitrogen Fixation Activities. ACS Appl. Mater. Interfaces 2019, 11, 44249–44262. [Google Scholar] [CrossRef]
- Li, Z.; Huang, W.; Liu, J.; Lv, K.; Li, Q. Embedding CdS@Au into Ultrathin Ti3−xC2Ty to Build Dual Schottky Barriers for Photocatalytic H2 Production. ACS Catal. 2021, 11, 8510–8520. [Google Scholar] [CrossRef]
- Atta, S.; Pennington, A.M.; Celik, F.E.; Fabris, L. TiO2 on Gold Nanostars Enhances Photocatalytic Water Reduction in the Near Infrared Regime. Chem 2018, 4, 2140–2153. [Google Scholar] [CrossRef]
- Tran, N.M.; Ta, Q.T.; Noh, J.S. Unusual synthesis of safflower-shaped TiO2/Ti3C2 heterostructures initiated from two-dimensional Ti3C2 MXene. Appl. Surf. Sci. 2021, 538, 148023. [Google Scholar] [CrossRef]
- Lu, X.; Li, S.; Guo, W.; Zhang, F.; Qu, F. covalent organic polymer-TiO2/Ti3C2 heterostructure as nonenzymatic biosensor for voltammetric detection of dopamine and uric acid. Microchim. Acta. 2021, 188, 95. [Google Scholar] [CrossRef]
- Xue, Q.; Pei, Z.; Huang, Y.; Zhu, M.; Tang, Z.; Li, H.; Huang, Y.; Li, N.; Zhang, H.; Zhi, C. Mn3O4 Nanoparticles on Layer-Structured Ti3C2 MXene towards Oxygen Reduction Reaction and Zinc-air Battery. J Mater Chem A 2017, 5, 20818–20823. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Wang, H.-D.; Tang, R.; Cheng, Q.; Yuan, Y.-J. Rutile TiO2 Nanoparticles with Oxygen Vacancy for Photocatalytic Nitrogen Fixation. ACS Appl. Nano Mater. 2021, 4, 8674–8679. [Google Scholar] [CrossRef]
- Ke, T.; Shen, S.; Rajavel, K.; Yang, K.; Lin, D. In situ growth of TiO2 nanoparticles on nitrogen-doped Ti3C2 with isopropyl amine toward enhanced photocatalytic activity. J. Hazard. Mater. 2021, 402, 124066. [Google Scholar] [CrossRef]
- Ji, M.; Shao, Y.; Nkudede, E.; Liu, Z.; Sun, X.; Zhao, J.; Chen, Z.; Yin, S.; Li, H.; Xia, J. Oxygen vacancy triggering the broad-spectrum photocatalysis of bismuth oxyhalide solid solution for ciprofloxacin removal. J. Colloid Interface Sci. 2022, 626, 221–230. [Google Scholar] [CrossRef]
- Tang, L.; Lv, Z.-Q.; Xue, Y.-C.; Xu, L.; Qiu, W.-H.; Zheng, C.-M.; Chen, W.-Q.; Wu, M.-H. MIL-53(Fe) incorporated in the lamellar BiOBr: Promoting the visible-light T catalytic capability on the degradation of rhodamine B and carbamazepine. Chem. Eng. J. 2019, 374, 975–982. [Google Scholar] [CrossRef]
- Ji, M.; Di, J.; Zhao, J.; Chen, C.; Zhang, Y.; Liu, Z.; Li, H.; Xia, J.; He, M.; Li, H. Orientated dominating charge separation via crystal facet homojunction inserted into BiOBr for solar-driven CO2 conversion. J. CO2 Util. 2022, 59, 101957. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Huang, F.; Yu Quan, Y.; Liu, G.; Zhang, X.; Xiong, F.; Huang, C.; Ji, M.; Li, H.; et al. Porous edge confinement: High carrier potential and low activation energy barrier synergistically boosting the efficiency of selective photocatalytic CO2 conversion. Appl. Catal. B Environ. 2023, 325, 122304. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Liu, G.; Chen, H.; Weng, Y.-W.; Li, H.; Chu, P.K.; Xia, J. Excited electron-rich Bi(3−x)+ sites: A quantum well-like structure for highly-promoted selective photocatalytic CO2 reduction performance. Adv. Funct. Mater. 2022, 32, 2202885. [Google Scholar] [CrossRef]
- Yue, X.; Cheng, L.; Li, F.; Xiang, Q. Highly strained Bi-MOF on bismuth oxyhalide support with tailored intermediate adsorption/desorption capability for robust CO2 photoreduction. Angew. Chem. Int. Ed. 2022, 61, e202208414. [Google Scholar] [CrossRef]
- Dong, J.; Chen, F.; Xu, L.; Yan, P.; Qian, J.; Chen, Y.; Yang, M.; Li, H. Fabrication of sensitive photoelectrochemical aptasensor using Ag nanoparticles sensitized bismuth oxyiodide for determination of chloramphenicol. Microchem. J. 2022, 178, 107317. [Google Scholar] [CrossRef]
- Liao, Y.; Qian, J.; Xie, G.; Han, Q.; Dang, W.; Wang, Y.; Lv, L.; Zhao, S.; Luo, L.; Zhang, W.; et al. 2D-layered Ti3C2 MXenes for promoted synthesis of NH3 on P25 photocatalysts. Appl. Catal. B Environ. 2020, 273, 119054. [Google Scholar] [CrossRef]
- Xia, J.; Yang, S.-A.; Wang, B.; Wu, P.; Popovs, I.; Li, H.; Irle, S.; Dai, S.; Zhu, H. Boosting electrosynthesis of ammonia on surface-engineered MXene Ti3C2. Nano Energy 2020, 72, 104681. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Tang, R.; Li, K.; Wang, B.; Zhao, J.; Xu, Q.; Ji, M.; Xia, J. Steering Charge Directional Separation in MXenes/Titanium Dioxide for Efficient Photocatalytic Nitrogen Fixation. Catalysts 2023, 13, 1487. https://doi.org/10.3390/catal13121487
Liu N, Tang R, Li K, Wang B, Zhao J, Xu Q, Ji M, Xia J. Steering Charge Directional Separation in MXenes/Titanium Dioxide for Efficient Photocatalytic Nitrogen Fixation. Catalysts. 2023; 13(12):1487. https://doi.org/10.3390/catal13121487
Chicago/Turabian StyleLiu, Nianhua, Rong Tang, Kai Li, Bin Wang, Junze Zhao, Qing Xu, Mengxia Ji, and Jiexiang Xia. 2023. "Steering Charge Directional Separation in MXenes/Titanium Dioxide for Efficient Photocatalytic Nitrogen Fixation" Catalysts 13, no. 12: 1487. https://doi.org/10.3390/catal13121487
APA StyleLiu, N., Tang, R., Li, K., Wang, B., Zhao, J., Xu, Q., Ji, M., & Xia, J. (2023). Steering Charge Directional Separation in MXenes/Titanium Dioxide for Efficient Photocatalytic Nitrogen Fixation. Catalysts, 13(12), 1487. https://doi.org/10.3390/catal13121487





