Recent Progress on Ruthenium-Based Electrocatalysts towards the Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Mechanisms of HER
3. Intermediate Adsorption on Ru-Based Catalyst Surfaces
3.1. Hydrogen Adsorption
3.2. Intermediate Adsorption Modification Strategies
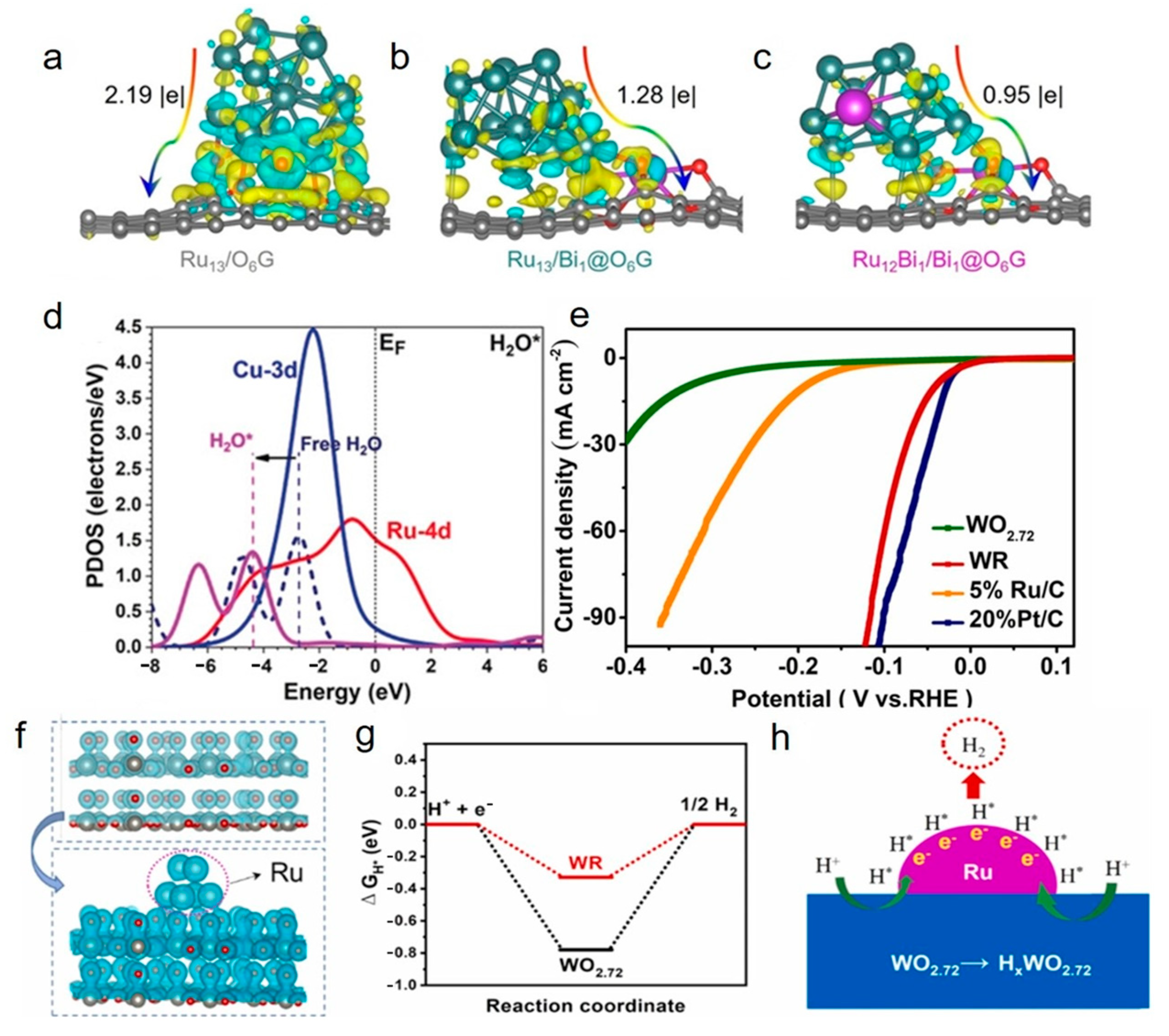
3.2.1. Alloying and Constructing Heterojunction
3.2.2. Doping and Combining with Supports
3.2.3. Other Modification Examples
4. Water Molecule Adsorption and Activation
4.1. Adsorption and Activation of H2O
4.2. Strengthening the Adsorption and Activation of Interfacial Water Molecules
5. Interfacial Water Behavior in HER
5.1. The Mechanism of Interfacial Water Behavior
5.2. Research on Interface Water Behavior
6. The Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Z.; Duan, Y.; Feng, X.; Yu, X.; Gao, M.; Yu, S. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, Y.; Li, J.; Chen, Q.; Du, Y.; Rao, P.; Li, R.; Jia, C.; Kang, Z.; Deng, P.; et al. Engineering Ruthenium-Based Electrocatalysts for Effective Hydrogen Evolution Reaction. Nano-Micro Lett. 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, 2108133. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xie, S.; Wang, X.; Pi, C.; Liu, Z.; Gao, B.; Hu, L.; Xiao, W.; Chu, P.K. Energy-Saving Hydrogen Production by the Methanol Oxidation Reaction Coupled with the Hydrogen Evolution Reaction Co-Catalyzed by a Phase Separation Induced Heterostructure. J. Mater. Chem. A 2022, 10, 20761–20769. [Google Scholar] [CrossRef]
- Özhava, D.; Özkar, S. Nanoceria Supported Rhodium(0) Nanoparticles as Catalyst for Hydrogen Generation from Methanolysis of Ammonia Borane. Appl. Catal. B Environ. 2018, 237, 1012–1020. [Google Scholar] [CrossRef]
- Meaburn, G.M.; Mellows, F.W.; Reiffsteck, A. Production of Hydrogen in the Radiolysis of Methanol Vapour. Nature 1964, 204, 1301–1302. [Google Scholar] [CrossRef]
- Lee, J.; Ga, S.; Lim, D.; Lee, S.; Cho, H.; Kim, J. Carbon-Free Green Hydrogen Production Process with Induction Heating-Based Ammonia Decomposition Reactor. Chem. Eng. J. 2023, 457, 141203. [Google Scholar] [CrossRef]
- Tabassum, H.; Mukherjee, S.; Chen, J.; Holiharimanana, D.; Karakalos, S.; Yang, X.; Hwang, S.; Zhang, T.; Lu, B.; Chen, M.; et al. Hydrogen Generation via Ammonia Decomposition on Highly Efficient and Stable Ru-Free Catalysts: Approaching Complete Conversion at 450 °C. Energy Environ. Sci. 2022, 15, 4190–4200. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-Temperature Ammonia Decomposition Catalysts for Hydrogen Generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Ramprakash, B.; Lindblad, P.; Eaton-Rye, J.J.; Incharoensakdi, A. Current Strategies and Future Perspectives in Biological Hydrogen Production: A Review. Renew. Sustain. Energy Rev. 2022, 168, 112773. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen Production from Biomass Using Dark Fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Wu, Y.; Ghalkhani, M.; Ashrafzadeh Afshar, E.; Karimi, F.; Xia, C.; Le, Q.V.; Vasseghian, Y. Recent progress in Biomass-derived nanoelectrocatalysts for the sustainable energy development. Fuel 2022, 323, 124349. [Google Scholar] [CrossRef]
- Salakkam, A.; Sittijunda, S.; Mamimin, C.; Phanduang, O.; Reungsang, A. Valorization of Microalgal Biomass for Biohydrogen Generation: A Review. Bioresour. Technol. 2021, 322, 124533. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H. Earth-Abundant Amorphous Catalysts for Electrolysis of Water. Chin. J. Catal. 2017, 38, 991–1005. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Zahid, R.; Khan, M.W.; Shaheen, M.; Aziz, U.; Aftab, S. Exploration of Catalytically Active Materials for Efficient Electrochemical Hydrogen and Oxygen Evolution Reactions. Int. J. Hydrog. Energy 2023, 48, 8045–8070. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, Z.; Yuan, Y.; Zheng, X.; Park, S.; Wei, S.; Li, L.; Ma, Y.; Liu, S.; Chen, J.; et al. Ultrafast Electrical Pulse Synthesis of Highly Active Electrocatalysts for Beyond-Industrial-Level Hydrogen Gas Batteries. Adv. Mater. 2023, 35, 2300502. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, X.; Wang, Z.; Cui, L.; Li, M.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Amorphization Activated RhPb Nanflowers for Energy-Saving Hydrogen Production by Hydrazine-Assisted Water Electrolysis. Chem. Eng. J. 2022, 440, 135848. [Google Scholar] [CrossRef]
- Cui, Z.; Jiao, W.; Huang, Z.; Chen, G.; Zhang, B.; Han, Y.; Huang, W. Design and Synthesis of Noble Metal-Based Alloy Electrocatalysts and Their Application in Hydrogen Evolution Reaction. Small 2023, 19, 2301465. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.; Lv, Y.; Zhang, G.; Huang, Y.; Liu, W.; Li, Y.; Chen, R.; Nuckolls, C.; Ni, H. An Effective Hybrid Electrocatalyst for the Alkaline HER: Highly Dispersed Pt Sites Immobilized by a Functionalized NiRu-Hydroxide. Appl. Catal. B Environ. 2020, 269, 118824. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, X.; Xu, R.; Dai, C.; Wu, B.; Yu, G.; Chen, B.; Wang, X.; Liu, N. H2 In Situ Inducing Strategy on Pt Surface Segregation Over Low Pt Doped PtNi5 Nanoalloy with Superhigh Alkaline HER Activity. Adv. Funct. Mater. 2021, 31, 2008298. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef]
- Kwon, T.; Yu, A.; Kim, S.; Kim, M.H.; Lee, C.; Lee, Y. Au-Ru Alloy Nanofibers as a Highly Stable and Active Bifunctional Electrocatalyst for Acidic Water Splitting. Appl. Surf. Sci. 2021, 563, 150293. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Yang, J.; Yan, Y.; Wang, D.; Hu, F.; Wang, X.; Zhang, R.; Feng, G. Ru/La2O3 Catalyst for Ammonia Decomposition to Hydrogen. Appl. Surf. Sci. 2019, 476, 928–936. [Google Scholar] [CrossRef]
- Li, J.; Tan, Y.; Zhang, M.; Gou, W.; Zhang, S.; Ma, Y.; Hu, J.; Qu, Y. Boosting Electrocatalytic Activity of Ru for Acidic Hydrogen Evolution through Hydrogen Spillover Strategy. ACS Energy Lett. 2022, 7, 1330–1337. [Google Scholar] [CrossRef]
- Meng, G.; Tian, H.; Peng, L.; Ma, Z.; Chen, Y.; Chen, C.; Chang, Z.; Cui, X.; Shi, J. Ru to W Electron Donation for Boosted HER from Acidic to Alkaline on Ru/WNO Sponges. Nano Energy 2021, 80, 105531. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Kong, X. Ru-O-Cu Center Constructed by Catalytic Growth of Ru for Efficient Hydrogen Evolution. Nano Energy 2023, 111, 108403. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Zhu, B.; Wan, X.; Hua, S.; Tang, H. A Bottom-up Method to Construct Ru-Doped FeP Nanosheets on Foam Iron with Ultra-High Activity for Hydrogen Evolution Reaction. Int. J. Hydrog. Energy 2023, 48, 4686–4693. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Li, J.; Wang, Y.; Xu, H.; Wang, C.; Guo, J.; Du, Y. Ultralow Ru Doping Induced Interface Engineering in MOF Derived Ruthenium-Cobalt Oxide Hollow Nanobox for Efficient Water Oxidation Electrocatalysis. Chem. Eng. J. 2021, 420, 129805. [Google Scholar] [CrossRef]
- Sun, X.; Gao, X.; Chen, J.; Wang, X.; Chang, H.; Li, B.; Song, D.; Li, J.; Li, H.; Wang, N. Ultrasmall Ru Nanoparticles Highly Dispersed on Sulfur-Doped Graphene for HER with High Electrocatalytic Performance. ACS Appl. Mater. Interfaces 2020, 12, 48591–48597. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Suo, H.; Zhang, T.; Liao, C.; Wang, Y.; Zhao, Q.; Lai, W. Recent Advances in Seawater Electrolysis. Catalysts 2022, 12, 123. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Shen, J.; Wu, H.; Chen, H.; Yuan, C.; Wu, N.; Liu, G.; Guo, D.; Liu, X. Self-Supported Electrocatalysts for the Hydrogen Evolution Reaction. Mater. Chem. Front. 2023, 7, 567–606. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, R.; Zou, J.; Yang, H. Surface Design Strategy of Catalysts for Water Electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, G.; Zheng, X.; Jiang, P.; Yi, J.; Zhou, H.; Gao, X.; Yu, Z.-Q.; Wu, Y. A Double Atomic-Tuned RuBi SAA/Bi@OG Nanostructure with Optimum Charge Redistribution for Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2023, 62, e202300879. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Zhang, N.; Sun, M.; Shao, Q.; Huang, X. Channel-Rich RuCu Nanosheets for pH-Universal Overall Water Splitting Electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 13983–13988. [Google Scholar] [CrossRef]
- Peng, L.; Su, L.; Yu, X.; Wang, R.; Cui, X.; Tian, H.; Cao, S.; Xia, B.Y.; Shi, J. Electron Redistribution of Ruthenium-Tungsten Oxides Mott-Schottky Heterojunction for Enhanced Hydrogen Evolution. Appl. Catal. B Environ. 2022, 308, 121229. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Wang, Y.; Zhou, S.; Zhang, Y.; Abdukayum, A.; Jin, Z.; Zhang, H.; Hu, G. Effect of In-Plane Mott-Schottky on the Hydroxyl Deprotonation in MoS2@Co3S4/NC Heterostructure for Efficient Overall Water Splitting. J. Colloid Interface Sci. 2023, 649, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jang, H.; Liu, S.; Li, Z.; Kim, M.G.; Qin, Q.; Liu, X.; Cho, J. The Synergistic Effect of Hf-O-Ru Bonds and Oxygen Vacancies in Ru/HfO2 for Enhanced Hydrogen Evolution. Nat. Commun. 2022, 13, 1270. [Google Scholar] [CrossRef]
- Li, L.; Qiu, H.; Zhu, Y.; Chen, G.; She, S.; Guo, X.; Li, H.; Liu, T.; Lin, Z.; Zhou, H.; et al. Atomic Ruthenium Modification of Nickel-Cobalt Alloy for Enhanced Alkaline Hydrogen Evolution. Appl. Catal. B Environ. 2023, 331, 122710. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Harzandi, A.M.; Ha, M.; Sultan, S.; Myung, C.W.; Park, H.J.; Kim, D.Y.; Thangavel, P.; Singh, A.N.; Sharma, P.; et al. Hydrogen Evolution: High-Performance Hydrogen Evolution by Ru Single Atoms and Nitrided-Ru Nanoparticles Implanted on N-Doped Graphitic Sheet. Adv. Energy Mater. 2019, 9, 1970101. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, K.-W.; He, J.-W.; Li, F.-M.; Huang, H.; Chen, P.; Chen, Y. Nitrogen-Doped Graphene Aerogel-Supported Ruthenium Nanocrystals for pH-Universal Hydrogen Evolution Reaction. Chin. J. Catal. 2022, 43, 1535–1543. [Google Scholar] [CrossRef]
- Wang, W.; Tang, H.; Liu, H.; Li, S.; Liu, G.; Zhang, W.; Wang, Y.; Wang, Q.; Liu, Q. Modified Graphene Supported Ruthenium as an Efficient Electrocatalyst for Hydrogen Evolution Reaction in Alkaline Media. Catal. Lett. 2023. [Google Scholar] [CrossRef]
- Yu, R.; Cao, X.; Chen, Q.; Li, W.; Huang, A.; Wei, X.; Mao, J. D-Band Center Optimization of Edge-Rich Ultrathin RuZn Nanosheets with Moiré Superlattices for pH-Universal Hydrogen Evolution Reaction. Small 2023, 19, e2303440. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Liu, Q.; Fan, C.; Sun, D.; Tang, Y.; Sun, H.; Feng, X. Competitive Adsorption: Reducing the Poisoning Effect of Adsorbed Hydroxyl on Ru Single-Atom Site with SnO2 for Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 134, e202209486. [Google Scholar] [CrossRef]
- Huang, X.; Lu, R.; Cen, Y.; Wang, D.; Jin, S.; Chen, W.; Geoffrey, I.; Waterhouse, N.; Wang, Z.; Tian, S.; et al. Micropore-Confined Ru Nanoclusters Catalyst for Efficient pH-Universal Hydrogen Evolution Reaction. Nano Res. 2023, 16, 9073–9080. [Google Scholar] [CrossRef]
- Qin, X.; Ola, O.; Zhao, J.; Yang, Z.; Tiwari, S.K.; Wang, N.; Zhu, Y. Recent Progress in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 1806. [Google Scholar] [CrossRef]
- Wu, C.; Ding, S.; Liu, D.; Li, D.; Chen, S.; Wang, H.; Qi, Z.; Ge, B.; Song, L. A Unique Ru-N4-P Coordinated Structure Synergistically Waking Up the Nonmetal P Active Site for Hydrogen Production. Research 2020, 2020, 5860712. [Google Scholar] [CrossRef]
- Cao, L.; Luo, Q.; Chen, J.; Wang, L.; Lin, Y.; Wang, H.; Liu, X.; Shen, X.; Zhang, W.; Liu, W.; et al. Dynamic Oxygen Adsorption on Single-Atomic Ruthenium Catalyst with High Performance for Acidic Oxygen Evolution Reaction. Nat. Commun. 2019, 10, 4849. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Gao, L.; Wang, Y.; Wang, X.; Zhu, J.; Jin, Z.; Liu, C.; Chen, H.; Li, G.; Ge, J.; et al. Engineering Energy Level of Metal Center: Ru Single-Atom Site for Efficient and Durable Oxygen Reduction Catalysis. J. Am. Chem. Soc. 2019, 141, 19800–19806. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhou, Y.; Shou, H.; Wang, X.; Zhang, P.; Xu, W.; Qiao, S.; Wu, C.; Liu, H.; Liu, D.; et al. Synergic Reaction Kinetics over Adjacent Ruthenium Sites for Superb Hydrogen Generation in Alkaline Media. Adv. Mater. 2022, 34, 2110604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Du, X.; Li, S.; Guan, J.; Fang, Y.; Li, X.; Dai, Y.; Zhang, M. Application of Heteroatom Doping Strategy in Electrolyzed Water Catalytic Materials. J. Electroanal. Chem. 2022, 921, 116679. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, J.; Sun, M.; Lin, F.; Huang, B.; Lv, F.; Zeng, L.; Zhang, Q.; Gu, L.; Luo, M.; et al. Cu-Doped Heterointerfaced Ru/RuSe2 Nanosheets with Optimized H and H2O Adsorption Boost Hydrogen Evolution Catalysis. Adv. Mater. 2023, 35, 2300980. [Google Scholar] [CrossRef]
- Li, L.; Bu, L.; Huang, B.; Wang, P.; Shen, C.; Bai, S.; Chan, T.-S.; Shao, Q.; Hu, Z.; Huang, X. Compensating Electronic Effect Enables Fast Site-to-Site Electron Transfer over Ultrathin RuMn Nanosheet Branches toward Highly Electroactive and Stable Water Splitting. Adv. Mater. 2021, 33, 2105308. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, C.; Wang, P.; Chen, D.; Xiao, M.; Chen, L.; Liu, S.; Yu, J.; Mu, S. Nearly Hollow Ru–Cu–MoO2 Octahedrons Consisting of Clusters and Nanocrystals for High Efficiency Hydrogen Evolution Reaction. J. Mater. Chem. A 2022, 10, 12341–12349. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Zhang, D.; Qin, Y.; Wang, M.; Han, Y.; Zhan, T.; Yang, B.; Li, S.; Lai, J.; et al. Solvent-Free Microwave Synthesis of Ultra-Small Ru-Mo2C@CNT with Strong Metal-Support Interaction for Industrial Hydrogen Evolution. Nat. Commun. 2021, 12, 4018. [Google Scholar] [CrossRef]
- Liu, J.; Ding, G.; Yu, J.; Liu, X.; Zhang, X.; Guo, J.; Zhang, J.; Ren, W.; Che, R. Visualizing Spatial Potential and Charge Distribution in Ru/N-Doped Carbon Electrocatalysts for Superior Hydrogen Evolution Reaction. J. Mater. Chem. A 2019, 7, 18072–18080. [Google Scholar] [CrossRef]
- Yu, J.; Wang, A.; Yu, W.; Liu, X.; Li, X.; Liu, H.; Hu, Y.; Wu, Y.; Zhou, W. Tailoring the Ruthenium Reactive Sites on N Doped Molybdenum Carbide Nanosheets via the Anti-Ostwald Ripening as Efficient Electrocatalyst for Hydrogen Evolution Reaction in Alkaline Media. Appl. Catal. B Environ. 2020, 277, 119236. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Qin, M.; Li, B.; Lin, B.; Mao, Q.; Yang, H.; Liu, B.; Wang, Y. Reversible Hydrogen Spillover in Ru-WO3−x Enhances Hydrogen Evolution Activity in Neutral pH Water Splitting. Nat. Commun. 2022, 13, 5382. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, M.; Huang, Z.; Zhu, W.; Zheng, J.; Jiang, Q.; Wang, Z.; Liang, H. Surface Reconstruction of Water Splitting Electrocatalysts. Adv. Energy Mater. 2022, 12, 2201713. [Google Scholar] [CrossRef]
- Lao, M.; Li, P.; Jiang, Y.; Pan, H.; Dou, S.X.; Sun, W. From fundamentals and theories to heterostructured electrocatalyst design: An in-depth understanding of alkaline hydrogen evolution reaction. Nano Energy 2022, 98, 107231. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.-T.; Le, J.-B.; Li, S.-M.; Wang, X.; Zhang, Y.-J.; Radjenovic, P.; Zhao, Y.; Wang, Y.-H.; Lin, X.-M.; et al. Revealing the Role of Interfacial Water and Key Intermediates at Ruthenium Surfaces in the Alkaline Hydrogen Evolution Reaction. Nat. Commun. 2023, 14, 5289. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wu, X.; Zhuang, Z.; Liu, L.; Fang, J.; Zeng, L.; Ma, J.; Liu, S.; Li, J.; Dai, R.; et al. Interfacial Water Engineering Boosts Neutral Water Reduction. Nat. Commun. 2022, 13, 6260. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, L.; Han, G.; Sheng, J.; Yu, Y.; Yang, W. Design of Rich Defects Carbon Coated MnFe2O4/LaMnO3/LaFeO3 Heterostructure Nanocomposites for Broadband Electromagnetic Wave Absorption. Chem. Eng. J. 2023, 476, 146199. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, S.; Liu, X.; Qiu, L.; Sheng, J.; Yang, W.; Yu, Y. Regulating the Peroxymonosulfate Activation on N Doped δ-MnO2 Nanosheets for Tetracycline Degradation: N Species as the Degradation Pathways Switcher to Convert Radical to Nonradical. Chem. Eng. J. 2023, 477, 147050. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Bao, S.; Tian, F.; Sheng, J.; Yang, W.; Yu, Y. Visible-Light Enhanced Peroxymonosulfate Activation on Co3O4/MnO2 for the Degradation of Tetracycline: Cooperation of Radical and Non-Radical Mechanisms. Sep. Purif. Technol. 2023, 316, 123779. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Sun, X. Recent Progress on Earth Abundant Electrocatalysts for Hydrogen Evolution Reaction (HER) in Alkaline Medium to Achieve Efficient Water Splitting—A Review. J. Energy Chem. 2019, 34, 111–160. [Google Scholar] [CrossRef]
- Lin, F.; Li, M.; Zeng, L.; Luo, M.; Guo, S. Intermetallic Nanocrystals for Fuel-Cells-Based Electrocatalysis. Chem. Rev. 2023, 123, 12507–12593. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, K.; Li, M.; Xu, D.; Ma, Z. Engineering the Spin Configuration of Electrocatalysts for Electrochemical Renewable Conversions. Mater. Chem. Front. 2024. [Google Scholar] [CrossRef]






| Condition | Acidic | Neutral/Alkaline |
|---|---|---|
| Total reaction | 2H + + 2e− → H2 | 2H2O + 2e− → H2 + 2OH− |
| Volmer | H + + e− → H* | H2O + e− → H* + OH− |
| Tafel | 2H* → H2 | 2H* → H2 |
| Heyrovsky | H + + e− + H* → H2 | H2O + e− + H* → H2 + OH− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Tian, F.; Qiu, L.; Wu, F.; Yang, W.; Yu, Y. Recent Progress on Ruthenium-Based Electrocatalysts towards the Hydrogen Evolution Reaction. Catalysts 2023, 13, 1497. https://doi.org/10.3390/catal13121497
Li L, Tian F, Qiu L, Wu F, Yang W, Yu Y. Recent Progress on Ruthenium-Based Electrocatalysts towards the Hydrogen Evolution Reaction. Catalysts. 2023; 13(12):1497. https://doi.org/10.3390/catal13121497
Chicago/Turabian StyleLi, Lulu, Fenyang Tian, Longyu Qiu, Fengyu Wu, Weiwei Yang, and Yongsheng Yu. 2023. "Recent Progress on Ruthenium-Based Electrocatalysts towards the Hydrogen Evolution Reaction" Catalysts 13, no. 12: 1497. https://doi.org/10.3390/catal13121497
APA StyleLi, L., Tian, F., Qiu, L., Wu, F., Yang, W., & Yu, Y. (2023). Recent Progress on Ruthenium-Based Electrocatalysts towards the Hydrogen Evolution Reaction. Catalysts, 13(12), 1497. https://doi.org/10.3390/catal13121497







