Micron-Sized Hierarchical Beta Zeolites Templated by Mesoscale Cationic Polymers as Robust Catalysts for Acylation of Anisole with Acetic Anhydride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalysis Test
2.2.1. Acylation of AN with AA in a Batch Reactor
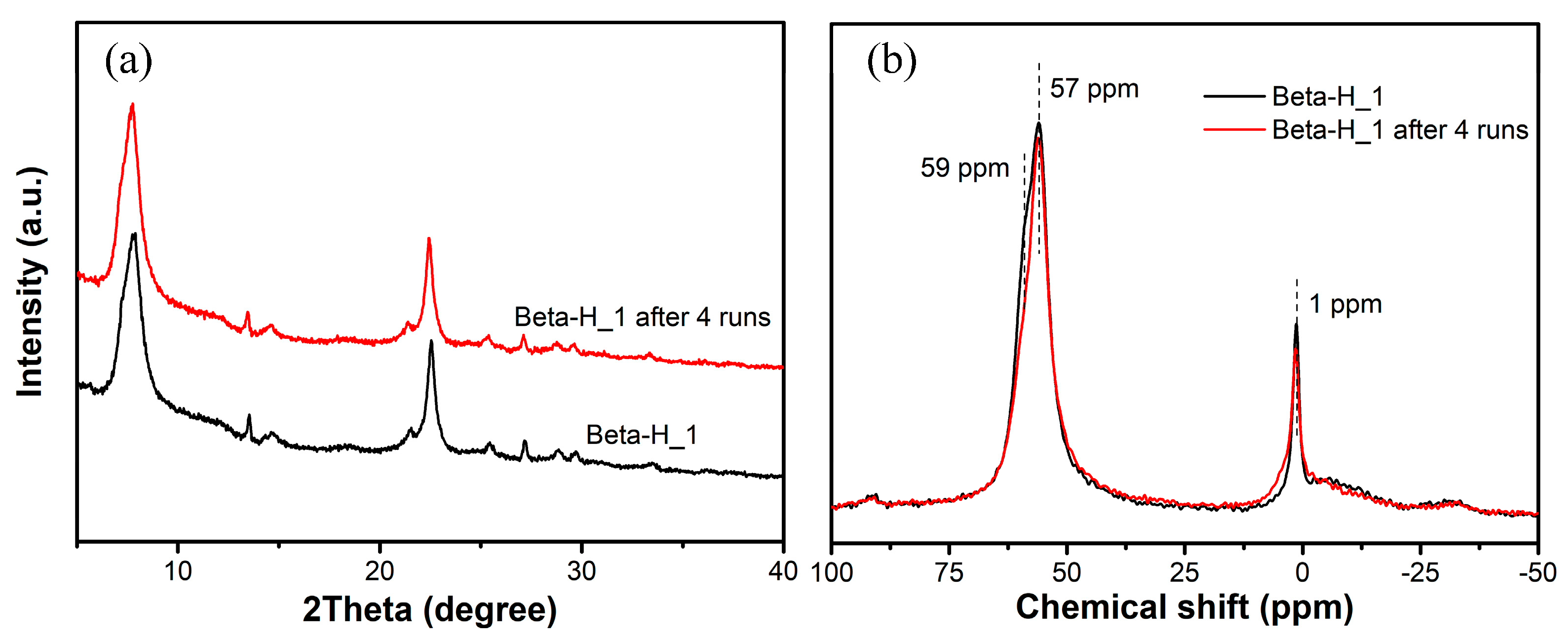
2.2.2. Acylation of AN with AA in a Fixed Bed Reactor
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Catalyst Preparation
3.3. Characterizations
3.4. Catalytic Tests
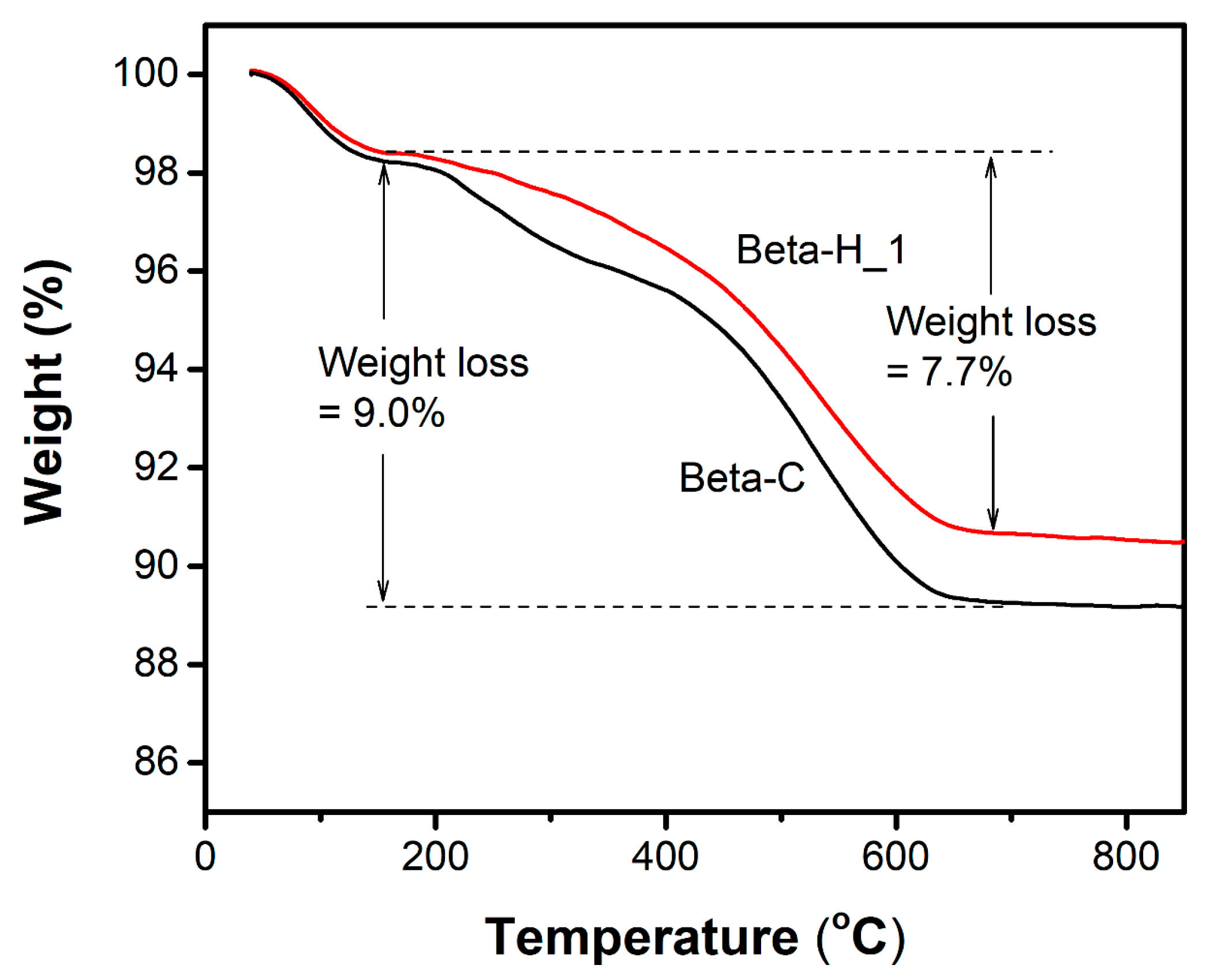
3.5. Analysis of the Spent Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jana, S.K. Advances in liquid-phase Friedel-Crafts acylation of aromatics catalyzed by heterogeneous solids. Catal. Surv. Asia 2006, 10, 98–109. [Google Scholar] [CrossRef]
- Sartori, G.; Maggi, R. Use of Solid Catalysts in Friedel−Crafts Acylation Reactions. Chem. Rev. 2006, 106, 1077–1104. [Google Scholar] [CrossRef]
- Bejblova, M.; Prochazkova, D.; Cejka, J. Acylation reactions over zeolites and mesoporous catalysts. ChemSusChem 2009, 2, 486–499. [Google Scholar] [CrossRef]
- Cardoso, L.A.M.; Alves, W., Jr.; Gonzaga, A.R.E.; Aguiar, L.M.G.; Andrade, H.M.C. Friedel–Crafts acylation of anisole with acetic anhydride over silica-supported heteropolyphosphotungstic acid (HPW/SiO2). J. Mol. Catal. A Chem. 2004, 209, 189–197. [Google Scholar] [CrossRef]
- Sarsani, V.; Lyon, C.; Hutchenson, K.; Harmer, M.; Subramaniam, B. Continuous acylation of anisole by acetic anhydride in mesoporous solid acid catalysts: Reaction media effects on catalyst deactivation. J. Catal. 2007, 245, 184–190. [Google Scholar] [CrossRef]
- Parida, K.M.; Mallick, S.; Pradhan, G.C. Acylation of anisole over 12-heteropolyacid of tungsten and molybdenum promoted zirconia. J. Mol. Catal. A Chem. 2009, 297, 93–100. [Google Scholar] [CrossRef]
- Arata, K.; Nakamura, H.; Shouji, M. Friedel–Crafts acylation of toluene catalyzed by solid superacids. Appl. Catal. A Gen. 2000, 197, 213–219. [Google Scholar] [CrossRef]
- Zhang, H.; Song, X.; Hu, D.; Zhang, W.; Jia, M. Iron-based nanoparticles embedded in a graphitic layer of carbon architectures as stable heterogeneous Friedel–Crafts acylation catalysts. Catal. Sci. Technol. 2019, 9, 3812–3819. [Google Scholar] [CrossRef]
- Zhang, H.; Song, X.; Zhao, C.; Hu, D.; Zhang, W.; Jia, M. N-Doped Porous Carbon–FexC Nanoparticle Composites as Catalysts for Friedel–Crafts Acylation. ACS Appl. Nano Mater. 2020, 3, 6664–6674. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Selvin, R.; Hsu, H.L.; Xiao, L.W. Efficient Acylation of Anisole over Hierarchical Porous ZSM-5 Structure. Chem. Eng. Technol. 2010, 33, 988–1002. [Google Scholar] [CrossRef]
- Inagaki, S.; Imai, H.; Tsujiuchi, S.; Yakushiji, H.; Yokoi, T.; Tatsumi, T. Enhancement of catalytic properties of interlayer-expanded zeolite Al-MWW via the control of interlayer silylation conditions. Microporous Mesoporous Mater. 2011, 142, 354–362. [Google Scholar] [CrossRef]
- Kore, R.; Srivastava, R.; Satpati, B. Synthesis of industrially important aromatic and heterocyclic ketones using hierarchical ZSM-5 and Beta zeolites. Appl. Catal. A Gen. 2015, 493, 129–141. [Google Scholar] [CrossRef]
- Wei, H.; Xie, S.; Gao, N.; Liu, K.; Liu, X.; Xin, W.; Li, X.; Liu, S.; Xu, L. Alkali treatment upon MCM-49 zeolite with various contents of HMI in the presence of CTAB and application in anisole acylation with acetic anhydride. Appl. Catal. A Gen. 2015, 495, 152–161. [Google Scholar] [CrossRef]
- Kim, J.-C.; Cho, K.; Lee, S.; Ryoo, R. Mesopore wall-catalyzed Friedel–Crafts acylation of bulky aromatic compounds in MFI zeolite nanosponge. Catal. Today 2015, 243, 103–108. [Google Scholar] [CrossRef]
- Xu, L.; Ji, X.; Li, S.; Zhou, Z.; Du, X.; Sun, J.; Deng, F.; Che, S.; Wu, P. Self-Assembly of Cetyltrimethylammonium Bromide and Lamellar Zeolite Precursor for the Preparation of Hierarchical MWW Zeolite. Chem. Mater. 2016, 28, 4512–4521. [Google Scholar] [CrossRef]
- Aleixo, R.; Elvas-Leitão, R.; Martins, F.; Carvalho, A.P.; Brigas, A.; Martins, A.; Nunes, N. Kinetic study of Friedel-Crafts acylation reactions over hierarchical MCM-22 zeolites. Mol. Catal. 2017, 434, 175–183. [Google Scholar] [CrossRef]
- Huang, G.; Ji, P.; Xu, H.; Jiang, J.-G.; Chen, L.; Wu, P. Fast synthesis of hierarchical Beta zeolites with uniform nanocrystals from layered silicate precursor. Microporous Mesoporous Mater. 2017, 248, 30–39. [Google Scholar] [CrossRef]
- Makihara, M.; Aoki, H.; Komura, K. Reaction Profiles of High Silica MOR Zeolite Catalyzed Friedel–Crafts Acylation of Anisole Using Acetic Anhydride in Acetic Acid. Catal. Lett. 2018, 148, 2974–2979. [Google Scholar] [CrossRef]
- Silva, D.S.A.; Castelblanco, W.N.; Piva, D.H.; de Macedo, V.; Carvalho, K.T.G.; Urquieta-González, E.A. Tuning the Brønsted and Lewis acid nature in HZSM-5 zeolites by the generation of intracrystalline mesoporosity—Catalytic behavior for the acylation of anisole. Mol. Catal. 2020, 492, 111026. [Google Scholar] [CrossRef]
- Hu, W.-H.; Liu, M.-N.; Luo, Q.-X.; Zhang, J.; Chen, H.; Xu, L.; Sun, M.; Ma, X.; Hao, Q.-Q. Friedel-Crafts acylation of anisole with acetic anhydride over single- to multiple-layer MWW zeolites: Catalytic behavior and kinetic mechanism. Chem. Eng. J. 2023, 466, 143098. [Google Scholar] [CrossRef]
- Kantam, M.L.; Ranganath, K.V.S.; Sateesh, M.; Kumar, K.B.S.; Choudary, B.M. Friedel–Crafts acylation of aromatics and heteroaromatics by beta zeolite. J. Mol. Catal. A Chem. 2005, 225, 15–20. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the Art and Perspectives of Hierarchical Zeolites: Practical Overview of Synthesis Methods and Use in Catalysis. Adv. Mater. 2020, 32, e2004690. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Neves, V.; Moutinho, J.; Nunes, N.; Carvalho, A.P. Friedel-Crafts acylation reaction over hierarchical Y zeolite modified through surfactant mediated technology. Microporous Mesoporous Mater. 2021, 323, 111167. [Google Scholar] [CrossRef]
- Escola, J.M.; Serrano, D.P.; Sanz, R.; Garcia, R.A.; Peral, A.; Moreno, I.; Linares, M. Synthesis of hierarchical Beta zeolite with uniform mesopores: Effect on its catalytic activity for veratrole acylation. Catal. Today 2018, 304, 89–96. [Google Scholar] [CrossRef]
- Manrique, C.; Guzmán, A.; Pérez-Pariente, J.; Márquez-Álvarez, C.; Echavarría, A. Vacuum gas-oil hydrocracking performance of Beta zeolite obtained by hydrothermal synthesis using carbon nanotubes as mesoporous template. Fuel 2016, 182, 236–247. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Wang, G.; Chen, F.; Zhu, J.; Wang, C.; Bian, C.; Pan, S.; Xiao, F.-S. Hierarchical Sn-Beta Zeolite Catalyst for the Conversion of Sugars to Alkyl Lactates. ACS Sustain. Chem. Eng. 2017, 5, 3123–3131. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Chu, W.; Wang, X.; Zhu, X.; Li, X.; Xie, S.; Wang, H.; Liu, S.; Xu, L. Direct synthesis of hollow single-crystalline zeolite beta using a small organic lactam as a recyclable hollow-directing agent. J. Mater. Chem. A 2019, 7, 10795–10804. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, B.; Huang, J.; Zhang, K.; Li, F.; Xi, H. Cationic surfactant-directed synthesis of hollow Beta zeolite with hierarchical structure. Inorg. Chem. Commun. 2019, 107, 107468. [Google Scholar] [CrossRef]
- Möller, K.; Yilmaz, B.; Müller, U.; Bein, T. Hierarchical Zeolite Beta via Nanoparticle Assembly with a Cationic Polymer. Chem. Mater. 2011, 23, 4301–4310. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Wang, M.; Yan, X.; Li, C.; Xi, H. Synthesis and catalytic performance of hierarchically structured beta zeolites by a dual-functional templating approach. New J. Chem. 2017, 41, 3950–3956. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.; Sukenaga, S.; Ando, M.; Shibata, H.; Okubo, T.; Wakihara, T. Ultrafast synthesis of *BEA zeolite without the aid of aging pretreatment. Microporous Mesoporous Mater. 2018, 268, 1–8. [Google Scholar] [CrossRef]
- Al-Eid, M.; Ding, L.; Saleem, Q.; Badairy, H.; Sitepu, H.; Al-Malki, A. A facile method to synthesize hierarchical nano-sized zeolite beta. Microporous Mesoporous Mater. 2019, 279, 99–106. [Google Scholar] [CrossRef]
- Bok, T.O.; Andriako, E.P.; Knyazeva, E.E.; Ivanova, I.I. Engineering of zeolite BEA crystal size and morphology via seed-directed steam assisted conversion. RSC Adv. 2020, 10, 38505–38514. [Google Scholar] [CrossRef]
- Sun, M.-H.; Chen, L.-H.; Yu, S.; Li, Y.; Zhou, X.-G.; Hu, Z.-Y.; Sun, Y.-H.; Xu, Y.; Su, B.-L. Micron-Sized Zeolite Beta Single Crystals Featuring Intracrystal Interconnected Ordered Macro-Meso-Microporosity Displaying Superior Catalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 19582–19591. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; She, P.; Chang, X.; Zhao, C.; Sun, Y.; Lei, Z.; Sun, S.; Zhang, W.; Jia, M. Synthesis of beta nanozeolite aggregates with hierarchical pores via steam-assisted conversion of dry gel and their catalytic properties for Friedel-Crafts acylation. Microporous Mesoporous Mater. 2022, 334, 111777. [Google Scholar] [CrossRef]
- Soltanali, S.; Darian, J.T. Synthesis of mesoporous beta catalysts in the presence of carbon nanostructures as hard templates in MTO process. Microporous Mesoporous Mater. 2019, 286, 169–175. [Google Scholar] [CrossRef]
- Egeblad, K.; Kustova, M.; Klitgaard, S.K.; Zhu, K.; Christensen, C.H. Mesoporous zeolite and zeotype single crystals synthesized in fluoride media. Microporous Mesoporous Mater. 2007, 101, 214–223. [Google Scholar] [CrossRef]
- Truong, T.T.T.; Vu, T.N.; Dinh, T.D.; Pham, T.T.; Nguyen, T.A.H.; Nguyen, M.H.; Nguyen, T.D.; Yusa, S.-I.; Pham, T.D. Adsorptive removal of cefixime using a novel adsorbent based on synthesized polycation coated nanosilica rice husk. Prog. Org. Coat. 2021, 158, 106361. [Google Scholar] [CrossRef]
- Nguyen, Q.K.; Hoang, T.H.; Bui, X.T.; Nguyen, T.A.H.; Pham, T.D.; Pham, T.N.M. Synthesis and application of polycation-stabilized gold nanoparticles as a highly sensitive sensor for molecular cysteine determination. Microchem. J. 2021, 168, 106481. [Google Scholar] [CrossRef]
- Xiao, F.S.; Wang, L.; Yin, C.; Lin, K.; Di, Y.; Li, J.; Xu, R.; Su, D.S.; Schlogl, R.; Yokoi, T.; et al. Catalytic properties of hierarchical mesoporous zeolites templated with a mixture of small organic ammonium salts and mesoscale cationic polymers. Angew. Chem. Int. Ed. Engl. 2006, 45, 3090–3093. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhu, L.; Rigutto, M.; van der Made, A.; Yang, C.; Pan, S.; Wang, L.; Zhu, L.; Jin, Y.; et al. Highly mesoporous single-crystalline zeolite beta synthesized using a nonsurfactant cationic polymer as a dual-function template. J. Am. Chem. Soc. 2014, 136, 2503–2510. [Google Scholar] [CrossRef]
- Du, S.; Sun, Q.; Wang, N.; Chen, X.; Jia, M.; Yu, J. Synthesis of hierarchical TS-1 zeolites with abundant and uniform intracrystalline mesopores and their highly efficient catalytic performance for oxidation desulfurization. J. Mater. Chem. A 2017, 5, 7992–7998. [Google Scholar] [CrossRef]
- Du, S.; Chen, X.; Sun, Q.; Wang, N.; Jia, M.; Valtchev, V.; Yu, J. A non-chemically selective top-down approach towards the preparation of hierarchical TS-1 zeolites with improved oxidative desulfurization catalytic performance. Chem. Commun. 2016, 52, 3580–3583. [Google Scholar] [CrossRef]
- Song, X.; Yang, X.; Zhang, T.; Zhang, H.; Zhang, Q.; Hu, D.; Chang, X.; Li, Y.; Chen, Z.; Jia, M.; et al. Controlling the Morphology and Titanium Coordination States of TS-1 Zeolites by Crystal Growth Modifier. Inorg. Chem. 2020, 59, 13201–13210. [Google Scholar] [CrossRef]
- Miao, S.; Liu, Y.; Zhang, H.; Chang, X.; Sun, H.; Zhao, C.; Zhang, W.; Jia, M. Effect of Triton X-100 additive on the synthesis of Beta zeolites and their catalytic application in acylation of anisole with acetic anhydride. Mater. Chem. Phys. 2021, 278, 125618. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Newsam, J.M. Two new three-dimensional twelve-ring zeolite frameworks of which zeolite beta is a disordered intergrowth. Nature 1988, 322, 249–251. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Yin, C.; Shan, Z.; Xiao, F.-S. Hierarchical mesoporous zeolites with controllable mesoporosity templated from cationic polymers. Microporous Mesoporous Mater. 2010, 131, 58–67. [Google Scholar] [CrossRef]
- Bisio, C.; Martra, G.; Coluccia, S.; Massiani, P. FT-IR Evidence of Two Distinct Protonic Sites in BEA Zeolite Consequences on Cationic Exchange and on Acido-Basic Properties in the Presence of Cesium. J. Phys. Chem. C 2008, 112, 10520–10530. [Google Scholar] [CrossRef]
- Maier, S.M.; Jentys, A.; Lercher, J.A. Steaming of Zeolite BEA and Its Effect on Acidity: A Comparative NMR and IR Spectroscopic Study. J. Phys. Chem. C 2011, 115, 8005–8013. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Chen, Y.; Wen, X.; Li, H.; Yuan, D.; Guo, Q.; Ren, S.; Pang, X.; Shen, B. Mild-acid-assisted thermal or hydrothermal dealumination of zeolite beta, its regulation to Al distribution and catalytic cracking performance to hydrocarbons. J. Catal. 2018, 362, 94–105. [Google Scholar] [CrossRef]
- Wei, H.; Liu, K.; Xie, S.; Xin, W.; Li, X.; Liu, S.; Xu, L. Determination of different acid sites in Beta zeolite for anisole acylation with acetic anhydride. J. Catal. 2013, 307, 103–110. [Google Scholar] [CrossRef]
- Rohan, D.; Canaff, C.; Fromentin, E.; Guisnet, M. Acetylation of anisole by acetic anhydride over a HBEA zeolite—Origin of deactivation of the catalyst. J. Catal. 1998, 177, 296–305. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.; Zhang, J. Effects of Modified Beta Zeolites with Acid on Anisole Acetylation in a Fixed Bed Reactor. Catal. Lett. 2008, 126, 188–192. [Google Scholar] [CrossRef]
- Alalq, I.; Nguyen-Phu, H.; Crossley, S. Shifts in catalyst deactivation mechanisms as a function of surface coverage during Friedel-Crafts acylation in zeolites. J. Catal. 2023, 426, 222–233. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, X.; Zhu, Y.; Emwas, A.-H.; Zhang, D.; Tian, Q.; Han, Y. Investigating the Influence of Mesoporosity in Zeolite Beta on Its Catalytic Performance for the Conversion of Methanol to Hydrocarbons. ACS Catal. 2015, 5, 5837–5845. [Google Scholar] [CrossRef]
- Wang, Y.; Otomo, R.; Tatsumi, T.; Yokoi, T. Dealumination of organic structure-directing agent (OSDA) free beta zeolite for enhancing its catalytic performance in n-hexane cracking. Microporous Mesoporous Mater. 2016, 220, 275–281. [Google Scholar] [CrossRef]
- Kim, J.; Choi, M.; Ryoo, R. Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process. J. Catal. 2010, 269, 219–228. [Google Scholar] [CrossRef]
- Derouane, E.G.; Dillon, C.J.; Bethell, D.; Hamid, S.B.D.-A. Zeolite Catalysts as Solid Solvents in Fine Chemicals Synthesis_1. Catalyst Deactivation in the Friedel–Crafts Acetylation of Anisole. J. Catal. 1999, 187, 209–218. [Google Scholar] [CrossRef]
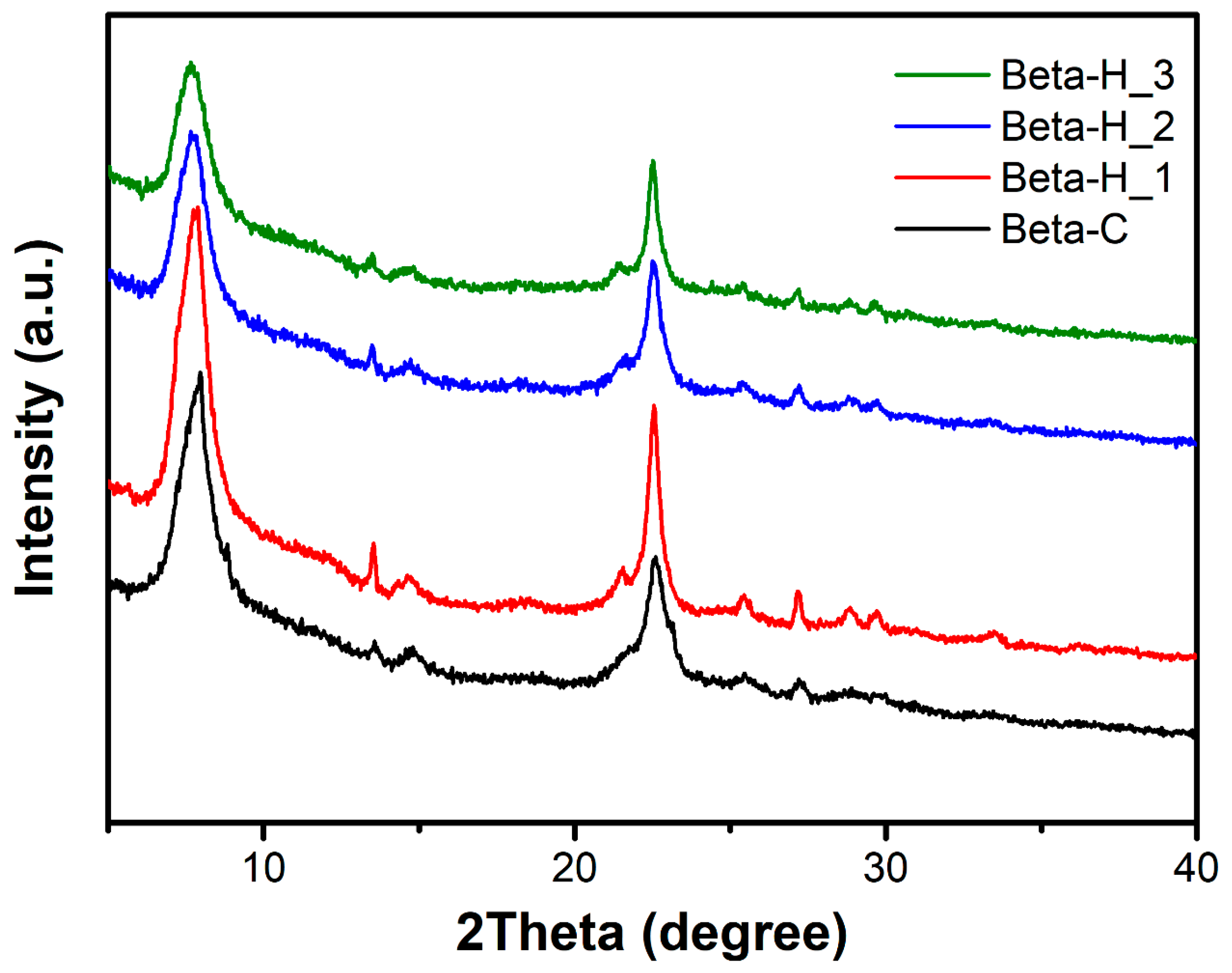

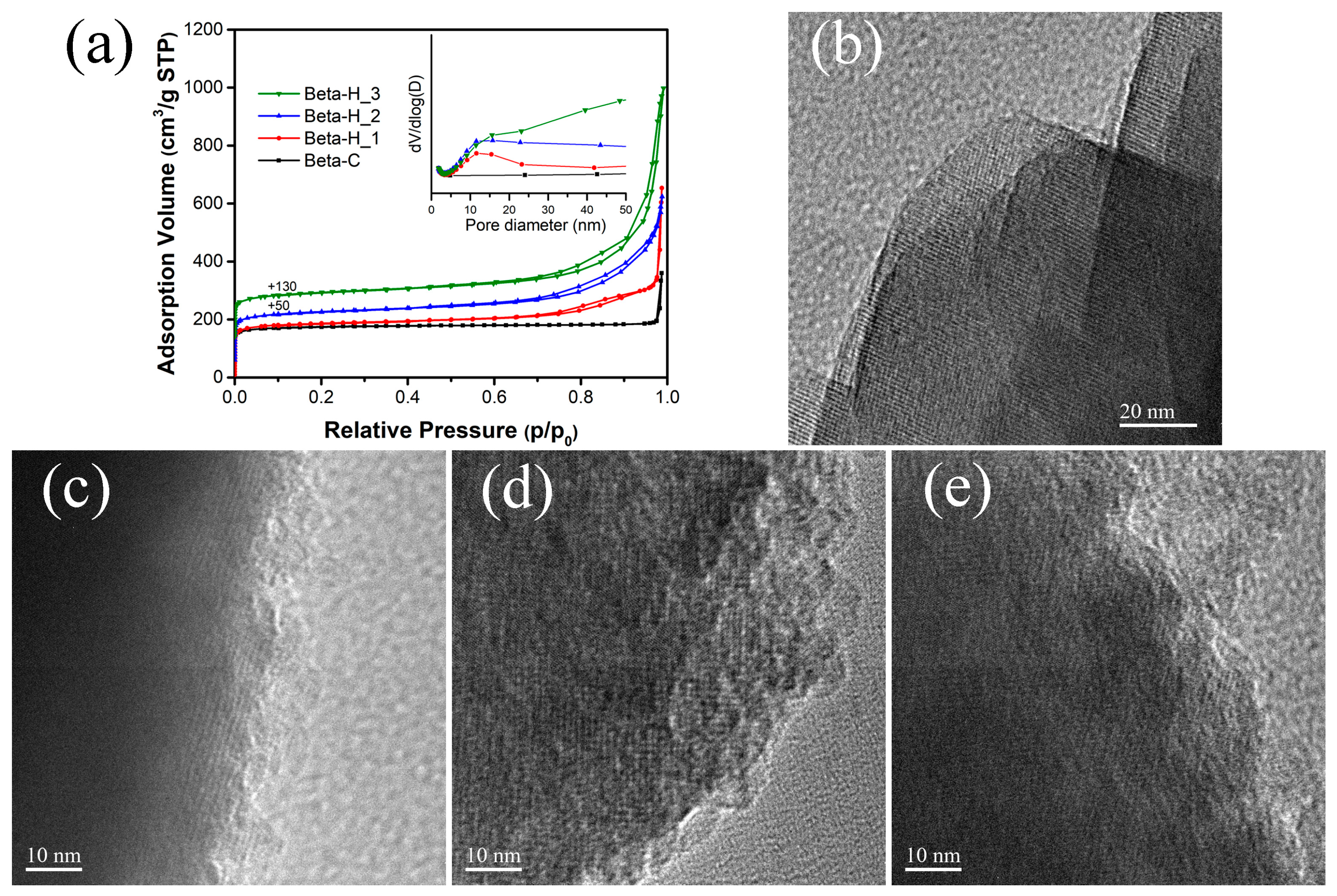
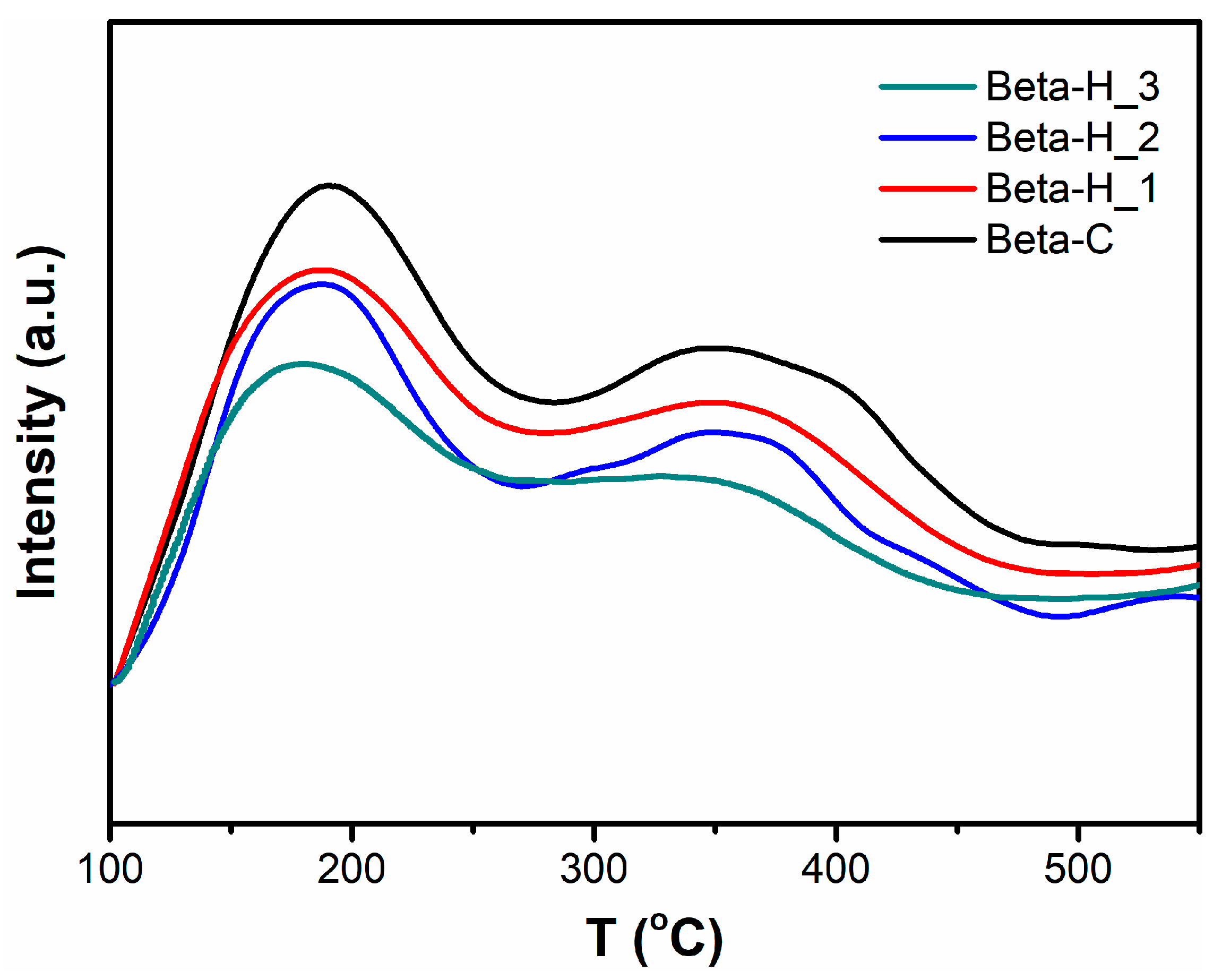


| Samples | Si/Al a | SBET (m2/g) | Smicro b (m2/g) | Vmicro b (cm3/g) | Vmeso c (cm3/g) | Yield d (%) | RC e (%) |
|---|---|---|---|---|---|---|---|
| Beta-C | 21.3 | 532 | 453 | 0.23 | 0.32 | 73 | 77 |
| Beta-H_1 | 18.5 | 574 | 447 | 0.23 | 0.78 | 74 | 100 |
| Beta-H_2 | 19.3 | 555 | 375 | 0.19 | 0.69 | 74 | 73 |
| Beta-H_3 | 20.4 | 517 | 311 | 0.16 | 1.18 | 76 | 69 |
| Samples | SBET (m2/g) | Smicro a (m2/g) | Vmicro a (cm3/g) | Vmeso b (cm3/g) |
|---|---|---|---|---|
| Beta-C | 532 | 453 | 0.23 | 0.32 |
| spent Beta-C | 237 | 186 (41%) c | 0.10 (43%) c | 0.11 |
| Beta-H_1 | 574 | 447 | 0.23 | 0.78 |
| spent Beta-H_1 | 354 | 250 (56%) c | 0.13 (56%) c | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, S.; Sun, S.; Lei, Z.; Sun, Y.; Zhao, C.; Zhan, J.; Zhang, W.; Jia, M. Micron-Sized Hierarchical Beta Zeolites Templated by Mesoscale Cationic Polymers as Robust Catalysts for Acylation of Anisole with Acetic Anhydride. Catalysts 2023, 13, 1517. https://doi.org/10.3390/catal13121517
Miao S, Sun S, Lei Z, Sun Y, Zhao C, Zhan J, Zhang W, Jia M. Micron-Sized Hierarchical Beta Zeolites Templated by Mesoscale Cationic Polymers as Robust Catalysts for Acylation of Anisole with Acetic Anhydride. Catalysts. 2023; 13(12):1517. https://doi.org/10.3390/catal13121517
Chicago/Turabian StyleMiao, Songsong, Shuaishuai Sun, Zhenyu Lei, Yuting Sun, Chen Zhao, Junling Zhan, Wenxiang Zhang, and Mingjun Jia. 2023. "Micron-Sized Hierarchical Beta Zeolites Templated by Mesoscale Cationic Polymers as Robust Catalysts for Acylation of Anisole with Acetic Anhydride" Catalysts 13, no. 12: 1517. https://doi.org/10.3390/catal13121517
APA StyleMiao, S., Sun, S., Lei, Z., Sun, Y., Zhao, C., Zhan, J., Zhang, W., & Jia, M. (2023). Micron-Sized Hierarchical Beta Zeolites Templated by Mesoscale Cationic Polymers as Robust Catalysts for Acylation of Anisole with Acetic Anhydride. Catalysts, 13(12), 1517. https://doi.org/10.3390/catal13121517







