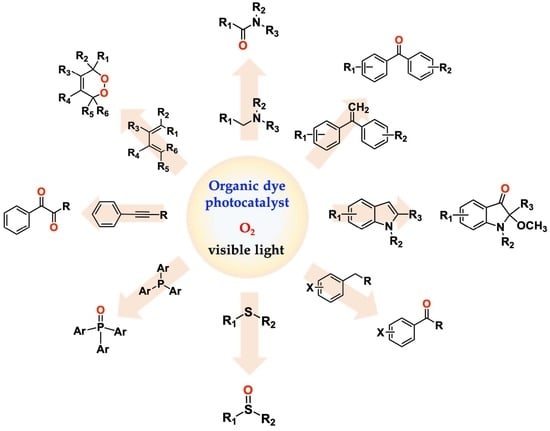
Photocatalyzed Oxygenation Reactions with Organic Dyes: State of the Art and Future Perspectives
Abstract
:1. Introduction
2. Organic-Dye-Promoted Photooxygenation Processes
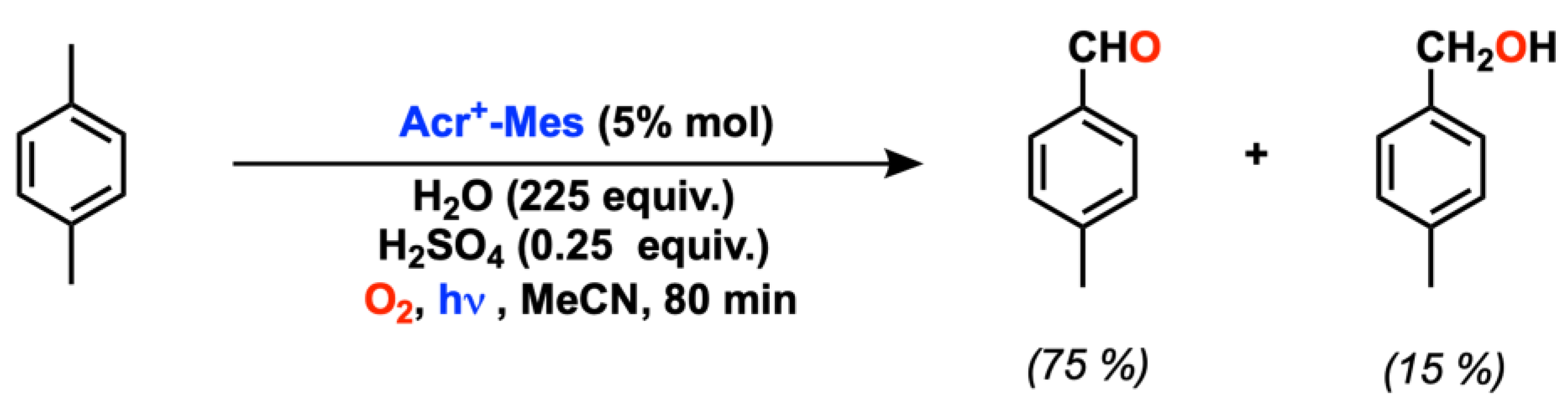
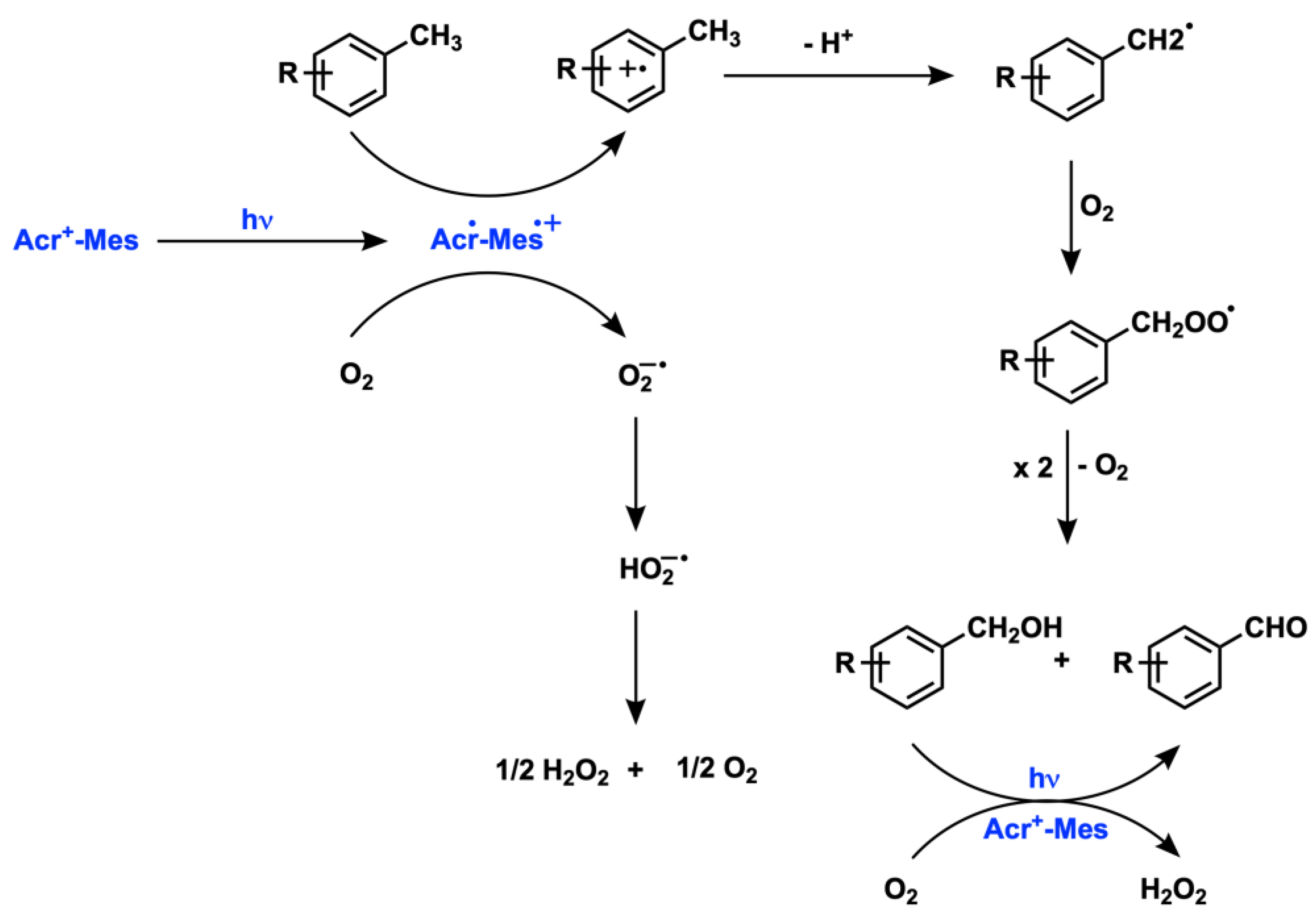
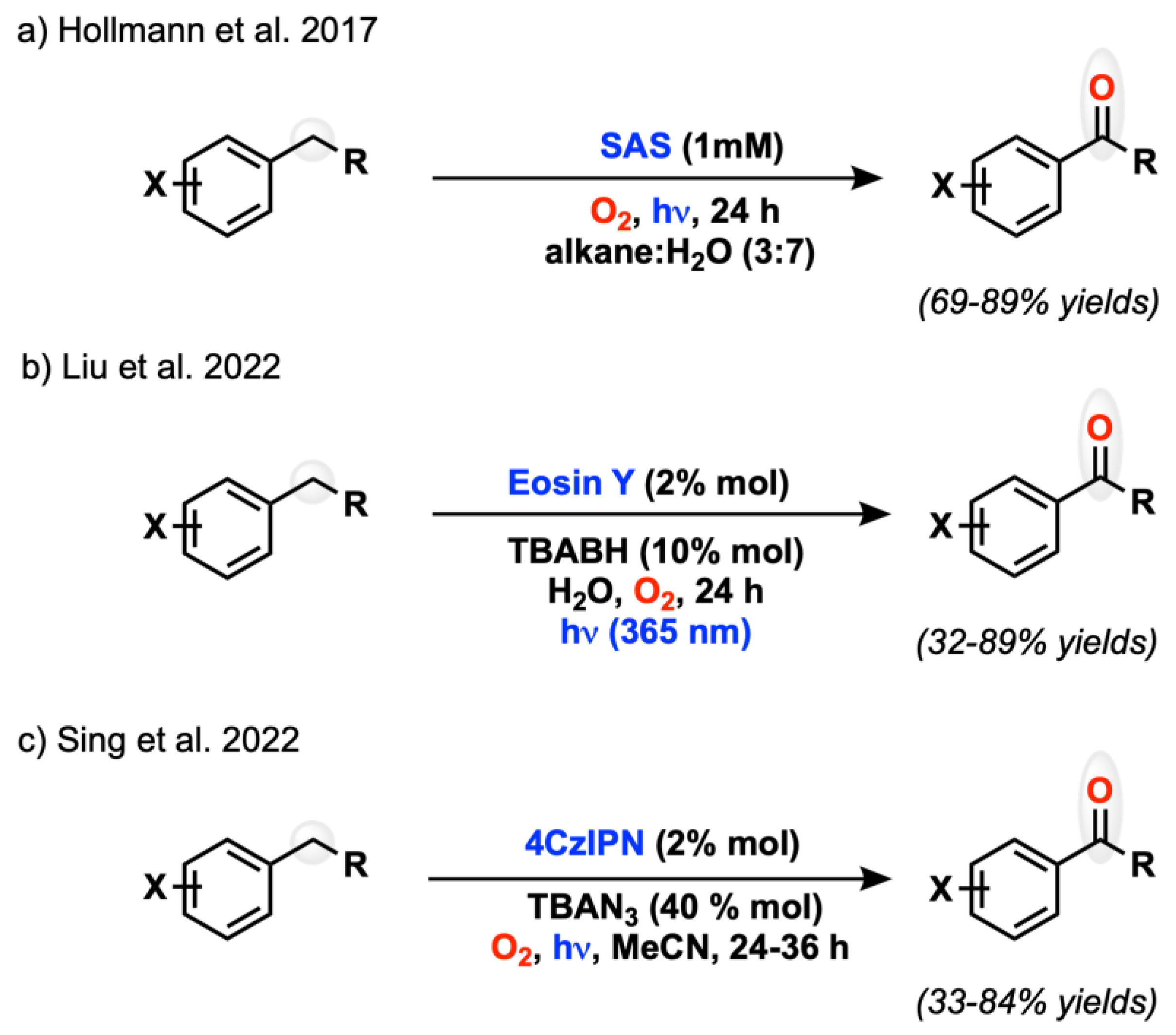
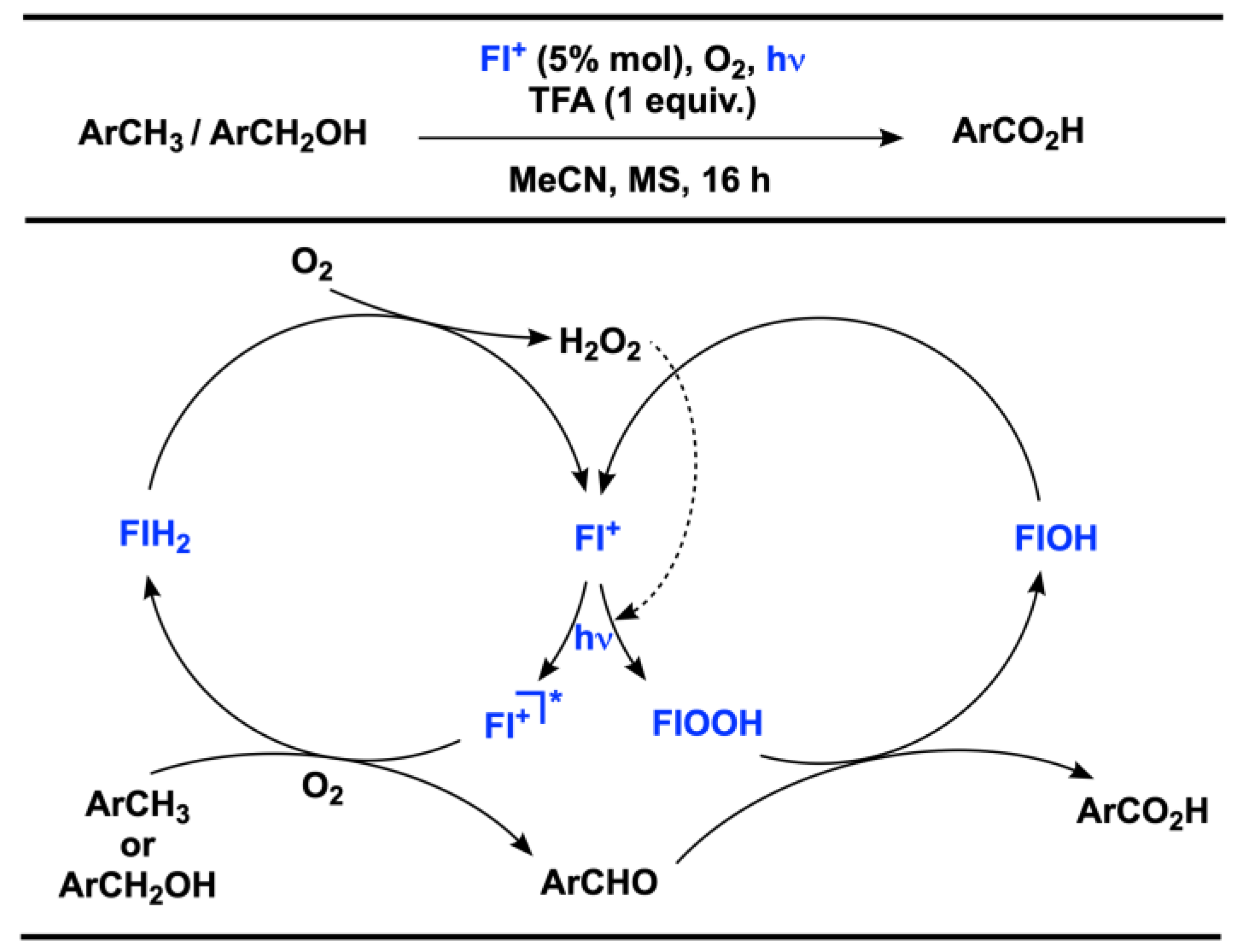
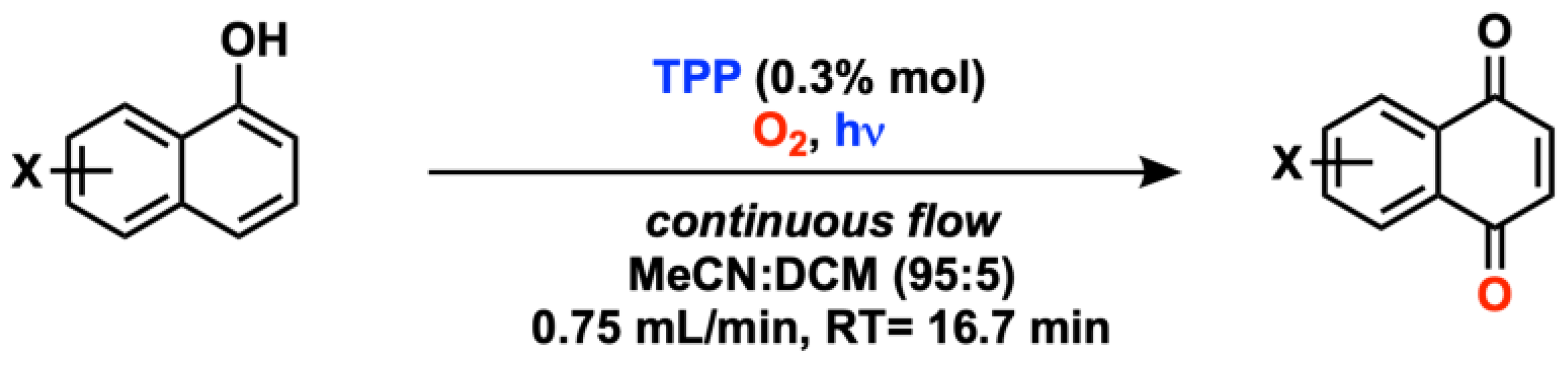
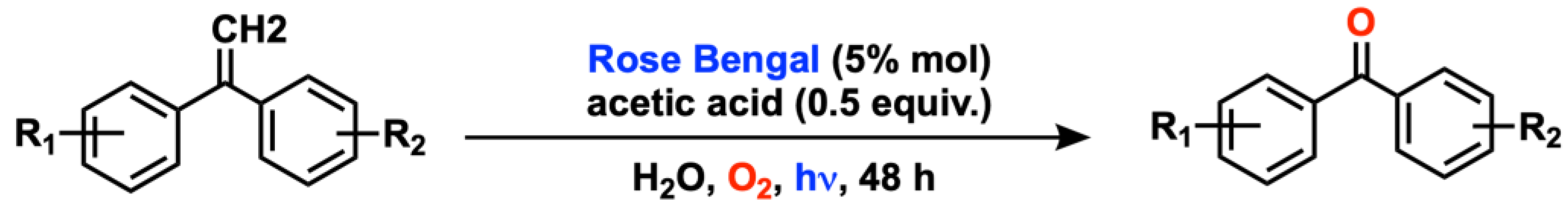
2.1. C(sp3) and Alkyl Arene (Benzyl) Oxygenation
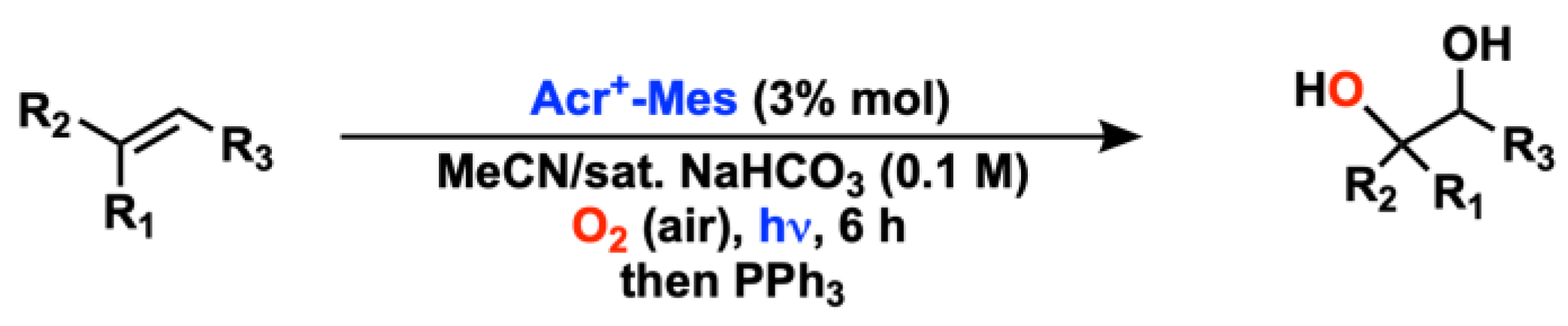
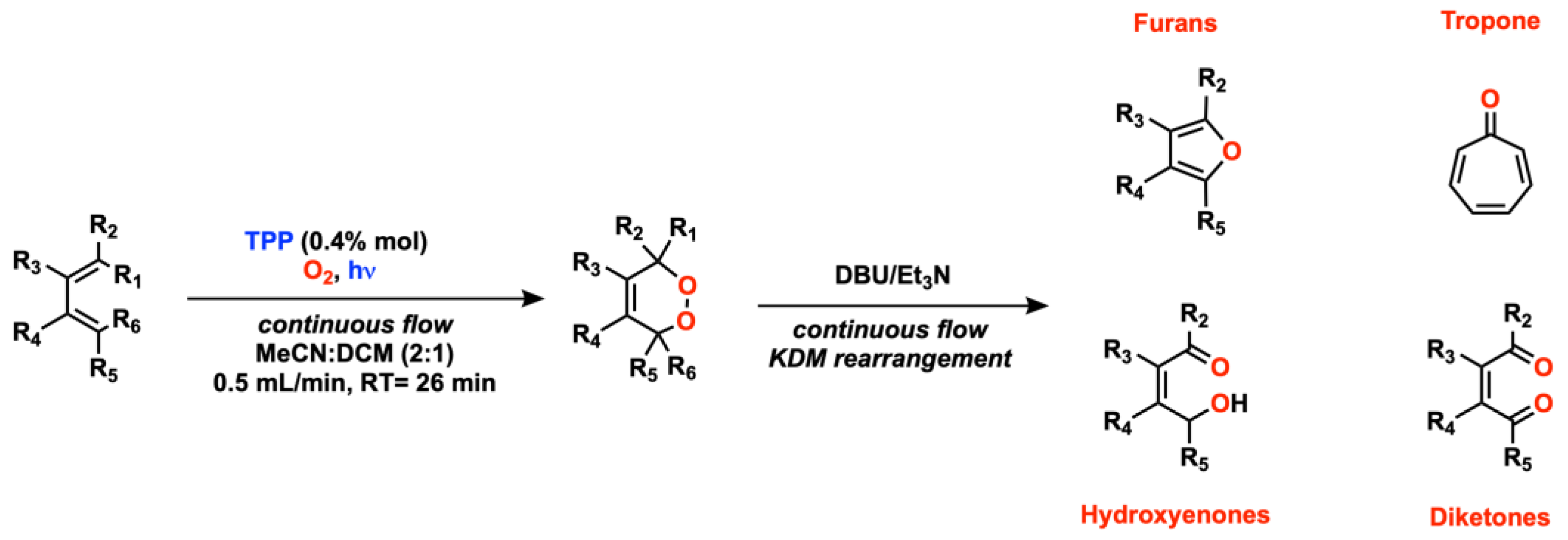
2.2. Alkene Oxygenation
2.3. Alkyne Oxygenation to 1,2-Diketones
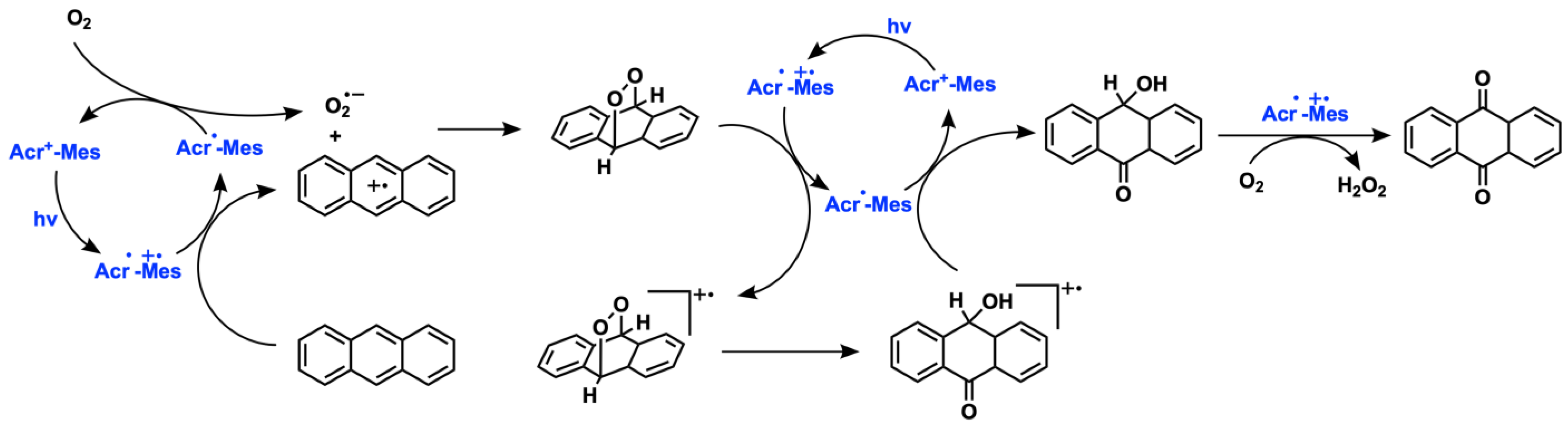
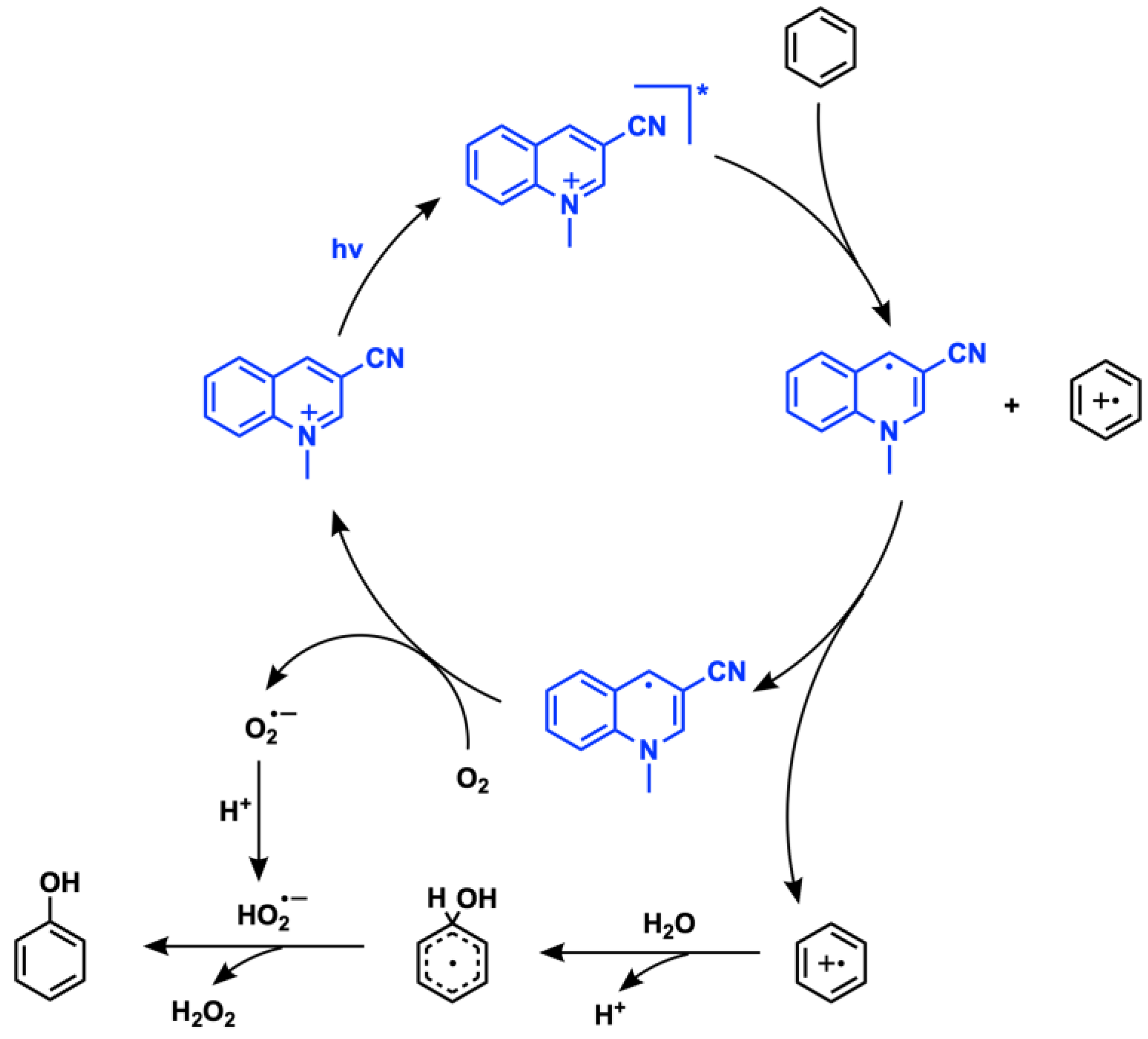
2.4. Aromatic Oxygenation
2.5. Oxidative C-C and C=C Cleavage
2.6. Amine Oxygenation
2.7. Indole Oxygenation
2.8. Triaryl Phosphine Oxygenation
2.9. Silane Oxygenation
2.10. Thioether Oxygenation to Sulfoxides
3. Conclusions
- -
- The heterogenization of organic photocatalysts on inert solid supports or their encapsulation into nano-porous systems. Heterogenization ensures efficient catalyst recovery, thus improving process efficiency and sustainability. In particular, catalyst recovery and reuse are aimed to enhance TONs, likely allowing for organic photocatalyst applications at the industrial level.
- -
- The development of additive-free photocatalytic systems. Photocatalytic reactions often require the use of electron/hole sacrificial additives in order to trigger or boost redox pathways. Considering the high versatility of organic photocatalysts, future challenges are intended to avoid the use of additives through appropriate organic photocatalyst structural modifications.
- -
- The design of tandem oxidative-reductive processes. Following the oxidation of an organic substrate, the reduced photocatalyst participates in a subsequent redox process to restore the native photocatalyst.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shaikh, I.R. Organocatalysis: Key Trends in Green Synthetic Chemistry, Challenges, Scope towards Heterogenization, and Importance from Research and Industrial Point of View. J. Catal. 2014, 2014, 402860. [Google Scholar] [CrossRef] [Green Version]
- Ciamician, G. The Photochemistry of the Future. Science 1912, 36, 385–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 30. [Google Scholar]
- Albini, A.; Fagnoni, M. Green Chemistry and Photochemistry Were Born at the Same Time. Green Chem. 2004, 6, 1. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Xiao, W.J. Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6838. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.-F.; Jiao, N. Oxygenation via C–H/C–C Bond Activation with Molecular Oxygen. Acc. Chem. Res. 2017, 50, 1640–1653. [Google Scholar] [CrossRef]
- Sideri, I.K.; Voutyritsa, E.; Kokotos, C.G. PhotoOrganocatalysis, Small Organic Molecules and Light in the Service of Organic Synthesis: The Awakening of a Sleeping Giant. Org. Biomol. Chem. 2018, 16, 4596–4614. [Google Scholar] [CrossRef]
- Zhang, Y.; Schilling, W.; Das, S. Metal-Free Photocatalysts for C−H Bond Oxygenation Reactions with Oxygen as the Oxidant. ChemSusChem 2019, 12, 2898–2910. [Google Scholar] [CrossRef]
- Amos, S.G.E.; Garreau, M.; Buzzetti, L.; Waser, J. Photocatalysis with Organic Dyes: Facile Access to Reactive Intermediates for Synthesis. Beilstein J. Org. Chem. 2020, 16, 1163–1187. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; Nguyen, T.M. Recent Applications of Organic Dyes as Photoredox Catalysts in Organic Synthesis. ACS Catal. 2014, 4, 355–360. [Google Scholar] [CrossRef]
- Ravelli, D.; Fagnoni, M. Dyes as Visible Light Photoredox Organocatalysts. ChemCatChem 2012, 4, 169–171. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. Organic Synthetic Transformations Using Organic Dyes as Photoredox Catalysts. Org. Biomol. Chem. 2014, 12, 6059–6071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tlili, A.; Lakhdar, S. Acridinium Salts and Cyanoarenes as Powerful Photocatalysts: Opportunities in Organic Synthesis. Angew. Chem. Int. Ed. 2021, 60, 19526–19549. [Google Scholar] [CrossRef] [PubMed]
- Bosveli, A.; Montagnon, T.; Kalaitzakis, D.; Vassilikogiannakis, G. Eosin: A Versatile Organic Dye Whose Synthetic Uses Keep Expanding. Org. Biomol. Chem. 2021, 19, 3303–3317. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A. Recent advances in photocatalytic manipulations of Rose Bengal in organic synthesis. Org. Biomol. Chem. 2019, 17, 4394–4405. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Srivastava, A.; Singh, P.P. Synthetic Applications of Flavin Photocatalysis: A Review. RSC Adv. 2021, 11, 14251–14259. [Google Scholar] [CrossRef]
- Cervantes-González, J.; Vosburg, D.A.; Mora-Rodriguez, S.E.; Vázquez, M.A.; Zepeda, L.G.; Villegas Gómez, C.; Lagunas-Rivera, S. Anthraquinones: Versatile Organic Photocatalysts. ChemCatChem 2020, 12, 3811–3827. [Google Scholar] [CrossRef]
- Nikitas, N.F.; Gkizis, P.L.; Kokotos, C.G. Thioxanthone: A Powerful Photocatalyst for Organic Reactions. Org. Biomol. Chem. 2021, 19, 5237–5253. [Google Scholar] [CrossRef]
- Iqbal, S.; Amjad, A.; Javed, M.; Alfakeer, M.M.; Rabea, S.; Elkaeed, E.B.; Pashameah, R.A.; Alzahrani, E.; Farouk, A.E. Boosted spatial charge carrier separation of binary ZnFe2O4/S-g-C3N4 heterojunction for visible-light-driven photocatalytic activity and antimicrobial performance. Front. Chem. 2022, 10, 975355. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Comp. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S.; Jin, W. Recent advances in non-metal modification of graphitic carbon nitride for photocatalysis: A historic review. Catal. Sci. Technol. 2016, 6, 7002–7023. [Google Scholar] [CrossRef]
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic Photocatalysts for the Oxidation of Pollutants and Model Compounds. Chem. Rev. 2012, 112, 1710–1750. [Google Scholar] [CrossRef]
- Li, Q.; Li, F. Recent Advances in Molecular Oxygen Activation via Photocatalysis and Its Application in Oxidation Reactions. Chem. Eng. J. 2021, 421, 129915. [Google Scholar] [CrossRef]
- Bäckvall, J.E. Modern Oxidation Methods, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 1–465. [Google Scholar] [CrossRef]
- Poulos, T.L. Heme Enzyme Structure and Function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Qiu, X.; Cheng, Z.; Jiao, N. Molecular Oxygen-Mediated Oxygenation Reactions Involving Radicals. Chem. Soc. Rev. 2021, 50, 8067–8101. [Google Scholar] [CrossRef]
- Zhang, X.; Rakesh, K.P.; Ravindar, L.; Qin, H.L. Visible-Light Initiated Aerobic Oxidations: A Critical Review. Green Chem. 2018, 20, 4790–4833. [Google Scholar] [CrossRef]
- Metz, M.; Solomon, E.I. Dioxygen Binding to Deoxyhemocyanin: Electronic Structure and Mechanism of the Spin-Forbidden Two-Electron Reduction of O2. J. Am. Chem. Soc. 2001, 123, 4938–4950. [Google Scholar] [CrossRef]
- Vo, N.T.; Mekmouche, Y.; Tron, T.; Guillot, R.; Banse, F.; Halime, Z.; Sircoglou, M.; Leibl, W.; Aukauloo, A. A Reversible Electron Relay to Exclude Sacrificial Electron Donors in the Photocatalytic Oxygen Atom Transfer Reaction with O2 in Water. Angew. Chem. Int. Ed. 2019, 58, 16023–16027. [Google Scholar] [CrossRef]
- Romero, E.; Gómez Castellanos, J.R.; Gadda, G.; Fraaije, M.W.; Mattevi, A. Same Substrate, Many Reactions: Oxygen Activation in Flavoenzymes. Chem. Rev. 2018, 118, 1742–1769. [Google Scholar] [CrossRef] [Green Version]
- Qian, K.; Du, L.; Zhu, X.; Liang, S.; Chen, S.; Kobayashi, H.; Yan, X.; Xu, M.; Dai, Y.; Li, R. Directional Oxygen Activation by Oxygen-Vacancy-Rich WO2 Nanorods for Superb Hydrogen Evolution via Formaldehyde Reforming. J. Mater. Chem. A 2019, 7, 14592–14601. [Google Scholar] [CrossRef]
- Zang, Y.; Gong, L.; Mei, L.; Gu, Z.; Wang, Q. Bi2WO6 Semiconductor Nanoplates for Tumor Radiosensitization through High-Z Effects and Radiocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 18942–18952. [Google Scholar] [CrossRef] [PubMed]
- Brugmans, J.P.; Thienpont, D.C.; van Wijngaarden, I.; Vanparijs, O.F.; Schuermans, V.L.; Lauwers, H.L. Mebendazole in Enterobiasis Radiochemical and Pilot Clinical Study in 1,278 Subjects. JAMA 1971, 217, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, A. Analgesic Value of Fenbufen in Postoperative Patients. A Comparative Oral Analgesic Study of Fenbufen, Aspirin, and Placebo. J. Clin. Pharmacol. 1975, 15, 591–597. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Pyriofenone. EFS2 2013, 11, 4302. [Google Scholar] [CrossRef]
- Rusch, F.; Schober, J.C.; Brasholz, M. Visible-Light Photocatalytic Aerobic Benzylic C(sp3)−H Oxygenations with the 3DDQ*/tert -Butyl Nitrite Co-Catalytic System. ChemCatChem 2016, 8, 2881–2884. [Google Scholar] [CrossRef]
- Ohkubo, K.; Mizushima, K.; Iwata, R.; Souma, K.; Suzuki, N.; Fukuzumi, S. Simultaneous Production of p-Tolualdehyde and Hydrogen Peroxide in Photocatalytic Oxygenation of p-Xylene and Reduction of Oxygen with 9-Mesityl-10-Methylacridinium Ion Derivatives. Chem. Commun. 2010, 46, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhang, Q.; Yang, L.; Deng, Y.; Yang, B.; Liu, Y.; Zhang, C.; Fu, Z. Regulating Effects of Anthraquinone Substituents and Additives in Photo-Catalytic Oxygenation of p-Xylene by Molecular Oxygen under Visible Light Irradiation. Renew. Energy 2021, 174, 928–938. [Google Scholar] [CrossRef]
- Morrison, G.; Bannon, R.; Wharry, S.; Moody, T.S.; Mase, N.; Hattori, M.; Manyar, H.; Smyth, M. Continuous Flow Photooxidation of Alkyl Benzenes Using Fine Bubbles for Mass Transfer Enhancement. Tetrahedron Lett. 2022, 90, 153613. [Google Scholar] [CrossRef]
- Zhang, W.; Gacs, J.; Arends, I.W.C.E.; Hollmann, F. Selective Photooxidation Reactions Using Water-Soluble Anthraquinone Photocatalysts. ChemCatChem 2017, 9, 3821–3826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, C.; Bu, Q.; Liu, J.; Dai, B.; Liu, N. Photocatalytic Benzylic Oxidation Promoted by Eosin Y in Water. ACS Sust. Chem. Eng. 2022, 10, 1822–1828. [Google Scholar] [CrossRef]
- Shee, M.; Singh, N.D.P. Photogenerated Azido Radical Mediated Oxidation: Access to Carbonyl Functionality from Alcohols, Alkylarenes, and Olefins via Organophotoredox. Adv. Synth. Catal. 2022, 364, 2032–2039. [Google Scholar] [CrossRef]
- Zelenka, J.; Svobodová, E.; Tarábek, J.; Hoskovcová, I.; Boguschová, V.; Bailly, S.; Sikorski, M.; Roithová, J.; Cibulka, R. Combining Flavin Photocatalysis and Organocatalysis: Metal-Free Aerobic Oxidation of Unactivated Benzylic Substrates. Org. Lett. 2019, 21, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Pokluda, A.; Anwar, Z.; Boguschová, V.; Anusiewicz, I.; Skurski, P.; Sikorski, M.; Cibulka, R. Robust Photocatalytic Method Using Ethylene-Bridged Flavinium Salts for the Aerobic Oxidation of Unactivated Benzylic Substrates. Adv. Synth. Catal. 2021, 363, 4371–4379. [Google Scholar] [CrossRef]
- Hermans, I.; Spier, E.S.; Neuenschwander, U.; Turrà, N.; Baiker, A. Selective Oxidation Catalysis: Opportunities and Challenges. Top. Catal. 2009, 52, 1162–1174. [Google Scholar] [CrossRef]
- Musser, M.T. Cyclohexanol and Cyclohexanone. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2010; Volume 11, pp. 49–60. [Google Scholar] [CrossRef]
- Ohkubo, K.; Fujimoto, A.; Fukuzumi, S. Metal-Free Oxygenation of Cyclohexane with Oxygen Catalyzed by 9-Mesityl-10-Methylacridinium and Hydrogen Chloride under Visible Light Irradiation. Chem. Commun. 2011, 47, 8515. [Google Scholar] [CrossRef]
- Ohkubo, K.; Hirose, K.; Fukuzumi, S. Photooxygenation of Alkanes by Dioxygen with p-Benzoquinone Derivatives with High Quantum Yields. Photochem. Photobiol. Sci. 2016, 15, 731–734. [Google Scholar] [CrossRef]
- Tate, E.W.; Dixon, D.J.; Ley, S.V. A Highly Enantioselective Total Synthesis of (+)-Goniodiol. Org. Biomol. Chem. 2006, 4, 1698–1706. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Zeng, A.P. Biosynthesizing Structurally Diverse Diols via a General Route Combining Oxidative and Reductive Formations of OH-Groups. Nat. Commun. 2022, 13, 1595. [Google Scholar] [CrossRef]
- Yang, B.; Lu, Z. Visible Light-Promoted Dihydroxylation of Styrenes with Water and Dioxygen. Chem. Commun. 2017, 53, 12634–12637. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Tian, J.; Yuan, M.; Peng, W.Q.; Wang, Y.Z.; Wang, P.; Liu, L.; Gou, Q.; Huang, H.; Chen, T. Visible Light-Induced Aerobic Dioxygenation of α,β-Unsaturated Amides/Alkenes toward Selective Synthesis of β-Oxy Alcohols Using Rose Bengal as a Photosensitizer. Org. Chem. Front. 2021, 8, 2215–2223. [Google Scholar] [CrossRef]
- De Souza, J.M.; Brocksom, T.J.; McQuade, D.T.; De Oliveira, K.T. Continuous Endoperoxidation of Conjugated Dienes and Subsequent Rearrangements Leading to C–H Oxidized Synthons. J. Org. Chem. 2018, 83, 7574–7585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Tang, H.; Liang, D.; Wu, J.; Huang, D. Pyrenediones as Versatile Photocatalysts for Oxygenation Reactions with in Situ Generation of Hydrogen Peroxide under Visible Light. Green Chem. 2020, 22, 22–27. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Zheng, X.; Chen, S.; Lai, Q.; Zheng, C.; Huang, M.; Cai, K.; Cai, Z.; Cai, S. Visible-Light-Enabled Allylic C–H Oxidation: Metal-Free Photocatalytic Generation of Enones. ACS Catal. 2022, 12, 1375–1381. [Google Scholar] [CrossRef]
- Zhao, Z.; Wisnoski, D.D.; Wolkenberg, S.E.; Leister, W.H.; Wang, Y.; Lindsley, C.W. General Microwave-Assisted Protocols for the Expedient Synthesis of Quinoxalines and Heterocyclic Pyrazines. Tetrahedron Lett. 2004, 45, 4873–4876. [Google Scholar] [CrossRef]
- Samanta, S.; Roy, D.; Khamarui, S.; Maiti, D.K. Ni(II)–Salt Catalyzed Activation of Primary Amine-sp3Cα–H and Cyclization with 1,2-Diketone to Tetrasubstituted Imidazoles. Chem. Commun. 2014, 50, 2477–2480. [Google Scholar] [CrossRef]
- Irannejad, H.; Nadri, H.; Naderi, N.; Rezaeian, S.N.; Zafari, N.; Foroumadi, A.; Amini, M.; Khoobi, M. Anticonvulsant Activity of 1,2,4-Triazine Derivatives with Pyridyl Side Chain: Synthesis, Biological, and Computational Study. Med. Chem. Res. 2015, 24, 2505–2513. [Google Scholar] [CrossRef]
- Liu, X.; Cong, T.; Liu, P.; Sun, P. Synthesis of 1,2-Diketones via a Metal-Free, Visible-Light-Induced Aerobic Photooxidation of Alkynes. J. Org. Chem. 2016, 81, 7256–7261. [Google Scholar] [CrossRef]
- Qin, H.T.; Xu, X.; Liu, F. Aerobic Oxidation of Alkynes to 1,2-Diketones by Organic Photoredox Catalysis. ChemCatChem 2017, 9, 1409–1412. [Google Scholar] [CrossRef]
- Kotani, H.; Ohkubo, K.; Fukuzumi, S. Photocatalytic Oxygenation of Anthracenes and Olefins with Dioxygen via Selective Radical Coupling Using 9-Mesityl-10-Methylacridinium Ion as an Effective Electron-Transfer Photocatalyst. J. Am. Chem. Soc. 2004, 126, 15999–16006. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, K.; Kobayashi, T.; Fukuzumi, S. Direct Oxygenation of Benzene to Phenol Using Quinolinium Ions as Homogeneous Photocatalysts. Angew. Chem. Int. Ed. 2011, 50, 8652–8655. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, K.T.; Miller, L.Z.; McQuade, D.T. Exploiting Photooxygenations Mediated by Porphyrinoid Photocatalysts under Continuous Flow Conditions. RSC Adv. 2016, 6, 12717–12725. [Google Scholar] [CrossRef]
- Morcillo, S.P. Radical-Promoted C−C Bond Cleavage: A Deconstructive Approach for Selective Functionalization. Angew. Chem. Int. Ed. 2019, 58, 14044–14054. [Google Scholar] [CrossRef]
- Bolleddula, J.; Chowdhury, S.K. Carbon–Carbon Bond Cleavage and Formation Reactions in Drug Metabolism and the Role of Metabolic Enzymes. Drug Metabol. Rev. 2015, 47, 534–557. [Google Scholar] [CrossRef]
- Leonard, D.K.; Li, W.; Junge, K.; Beller, M. Improved Bimetallic Cobalt–Manganese Catalysts for Selective Oxidative Cleavage of Morpholine Derivatives. ACS Catal. 2019, 9, 11125–11129. [Google Scholar] [CrossRef]
- Dong, C.; Huang, L.; Guan, Z.; Huang, C.; He, Y. Visible-Light-Mediated Aerobic Oxidative C(sp3)−C(sp3) Bond Cleavage of Morpholine Derivatives Using 4CzIPN as a Photocatalyst. Adv Synth Catal 2021, 363, 3803–3811. [Google Scholar] [CrossRef]
- Singh, A.K.; Chawla, R.; Yadav, L.D.S. Eosin Y Catalyzed Visible Light Mediated Aerobic Photo-Oxidative Cleavage of the C–C Double Bond of Styrenes. Tetrahedron Lett. 2015, 56, 653–656. [Google Scholar] [CrossRef]
- Deng, Y.; Wei, X.J.; Wang, H.; Sun, Y.; Noël, T.; Wang, X. Disulfide-Catalyzed Visible-Light-Mediated Oxidative Cleavage of C=C Bonds and Evidence of an Olefin–Disulfide Charge-Transfer Complex. Angew. Chem. Int. Ed. 2017, 56, 832–836. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.P.; Gao, Y.; Wei, L. Recent Advances in Transition-Metal-Free Oxygenation of Alkene C=C Double Bonds for Carbonyl Generation. Chem. Asian J. 2016, 11, 2092–2102. [Google Scholar] [CrossRef]
- Wise, D.E.; Gogarnoiu, E.S.; Duke, A.D.; Paolillo, J.M.; Vacala, T.L.; Hussain, W.A.; Parasram, M. Photoinduced Oxygen Transfer Using Nitroarenes for the Anaerobic Cleavage of Alkenes. J. Am. Chem. Soc. 2022, 144, 15437–15442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; He, J.T.; Wu, M.C.; Liu, Z.L.; Tang, K.; Xia, P.J.; Chen, K.; Xiang, H.Y.; Chen, X.Q.; Yang, H. Photochemical Organocatalytic Aerobic Cleavage of C=C Bonds Enabled by Charge-Transfer Complex Formation. Org. Lett. 2022, 24, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yue, X.; Liang, C.; Zhao, J.; Yu, W.; Zhang, P. Photo-Induced Oxidative Cleavage of C-C Double Bonds of Olefins in Water. Tetrahedron Lett. 2021, 80, 153321. [Google Scholar] [CrossRef]
- Balskus, E.P.; Jacobsen, E.N. α,β-Unsaturated β-Silyl Imide Substrates for Catalytic, Enantioselective Conjugate Additions: A Total Synthesis of (+)-Lactacystin and the Discovery of a New Proteasome Inhibitor. J. Am. Chem. Soc. 2006, 128, 6810–6812. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhu, X.; Kang, E.T.; Neoh, K.G. Modification of Poly(Ether Imide) Membranes via Surface-Initiated Atom Transfer Radical Polymerization. Macromolecules 2006, 39, 1660–1663. [Google Scholar] [CrossRef]
- Sood, A.; Panchagnula, R. Peroral Route: An Opportunity for Protein and Peptide Drug Delivery. Chem. Rev. 2001, 101, 3275–3304. [Google Scholar] [CrossRef]
- Castral, T.C.; Matos, A.P.; Monteiro, J.L.; Araujo, F.M.; Bondancia, T.M.; Batista-Pereira, L.G.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.G.F.; Corrêa, A.G. Synthesis of a Combinatorial Library of Amides and Its Evaluation against the Fall Armyworm, Spodoptera Frugiperda. J. Agric. Food Chem. 2011, 59, 4822–4827. [Google Scholar] [CrossRef]
- Forbis, R.M.; Rinehart, K.L. Nybomycin. VII. Preparative Routes to Nybomycin and Deoxynybomycin. J. Am. Chem. Soc. 1973, 95, 5003–5013. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, P.K.; Singh, P.P. Eosin Y Catalysed Visible-Light Mediated Aerobic Oxidation of Tertiary Amines. Tetrahedron Lett. 2019, 60, 151041. [Google Scholar] [CrossRef]
- Zhang, Y.; Riemer, D.; Schilling, W.; Kollmann, J.; Das, S. Visible-Light-Mediated Efficient Metal-Free Catalyst for α-Oxygenation of Tertiary Amines to Amides. ACS Catal. 2018, 8, 6659–6664. [Google Scholar] [CrossRef]
- Zhang, Y.; Schilling, W.; Riemer, D.; Das, S. Metal-Free Photocatalysts for the Oxidation of Non-Activated Alcohols and the Oxygenation of Tertiary Amines Performed in Air or Oxygen. Nat. Protoc. 2020, 15, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Neerathilingam, N.; Anandhan, R. Metal-Free Photoredox-Catalyzed Direct α-Oxygenation of N,N-Dibenzylanilines to Imides under Visible Light. RSC Adv. 2022, 12, 8368–8373. [Google Scholar] [CrossRef] [PubMed]
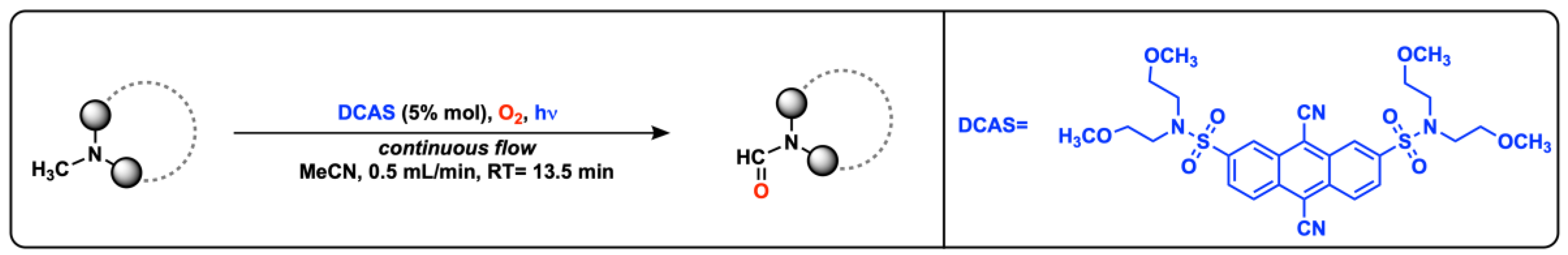
- Mandigma, M.J.P.; Žurauskas, J.; MacGregor, C.I.; Edwards, L.J.; Shahin, A.; d’Heureuse, L.; Yip, P.; Birch, D.J.S.; Gruber, T.; Heilmann, J.; et al. An Organophotocatalytic Late-Stage N–CH3 Oxidation of Trialkylamines to N-Formamides with O2 in Continuous Flow. Chem. Sci. 2022, 13, 1912–1924. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Idei, H.; Kanyiva, K.S.; Hiyama, T. Hydrocarbamoylation of Unsaturated Bonds by Nickel/Lewis-Acid Catalysis. J. Am. Chem. Soc. 2009, 131, 5070–5071. [Google Scholar] [CrossRef]
- Ding, S.; Jiao, N. N,N-Dimethylformamide: A Multipurpose Building Block. Angew. Chem. Int. Ed. 2012, 51, 9226–9237. [Google Scholar] [CrossRef]
- Kohnen-Johannsen, K.; Kayser, O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef] [Green Version]
- Nakai, S.; Yatabe, T.; Suzuki, K.; Sasano, Y.; Iwabuchi, Y.; Hasegawa, J.; Mizuno, N.; Yamaguchi, K. Methyl-Selective α-Oxygenation of Tertiary Amines to Formamides by Employing Copper/Moderately Hindered Nitroxyl Radical (DMN-AZADO or 1-Me-AZADO). Angew. Chem. Int. Ed. 2019, 58, 16651–16659. [Google Scholar] [CrossRef]
- Su, J.; Ma, X.; Ou, Z.; Song, Q. Deconstructive Functionalizations of Unstrained Carbon–Nitrogen Cleavage Enabled by Difluorocarbene. ACS Cent. Sci. 2020, 6, 1819–1826. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, B.; Hu, X.; Xu, Q.; Lu, Z. Visible-Light-Promoted Metal-Free Aerobic Oxidation of Primary Amines to Acids and Lactones. Chem. Eur. J. 2016, 22, 17566–17570. [Google Scholar] [CrossRef]
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structure, Reactions Synthesis and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 1–556. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Bureš, F.; Lee, R.; Ye, X.; Jiang, Z. Visible Light Photocatalytic Aerobic Oxygenation of Indoles and pH as a Chemoselective Switch. ACS Catal. 2016, 6, 6853–6860. [Google Scholar] [CrossRef]
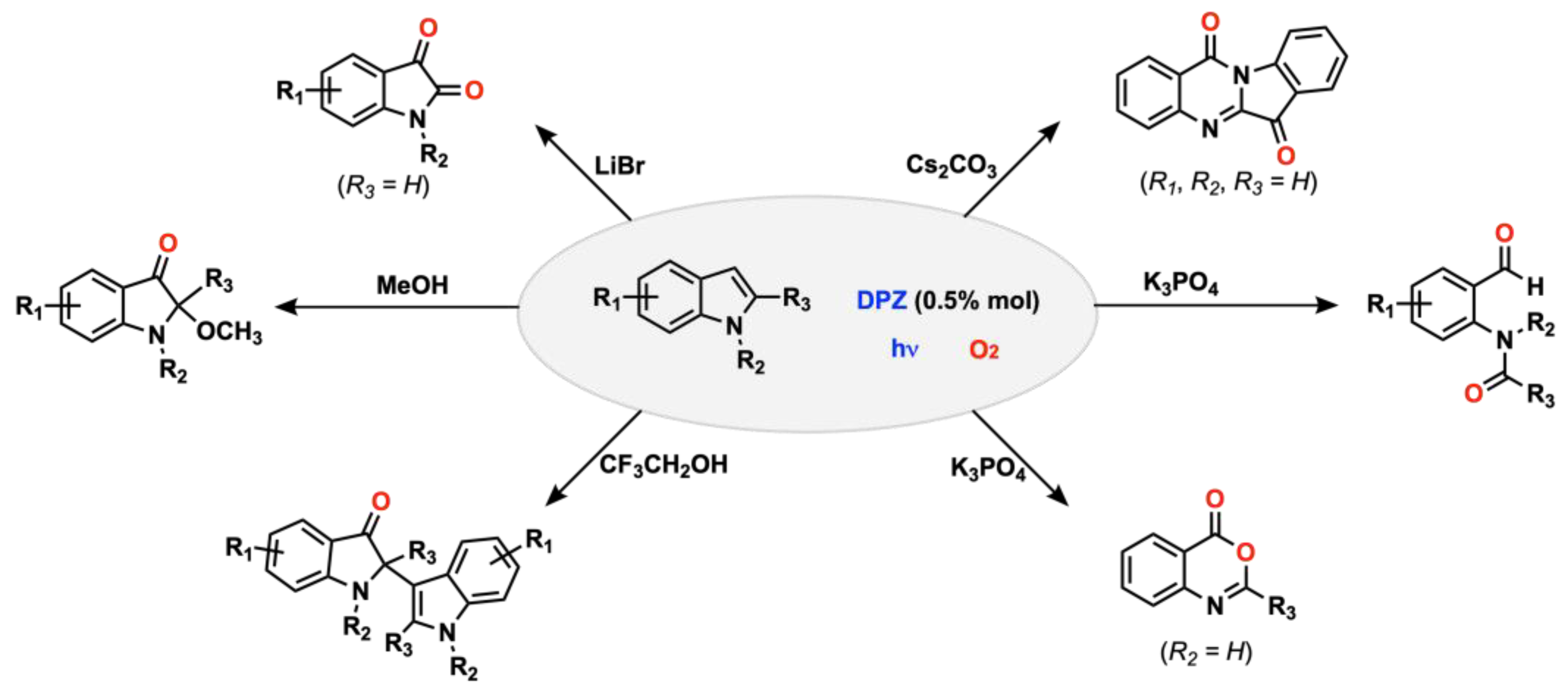
- Schilling, W.; Zhang, Y.; Riemer, D.; Das, S. Visible-Light-Mediated Dearomatisation of Indoles and Pyrroles to Pharmaceuticals and Pesticides. Chem. Eur. J. 2020, 26, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushwaha, A.K.; Maury, S.K.; Kumari, S.; Kamal, A.; Singh, H.K.; Kumar, D.; Singh, S. Visible-Light-Initiated Oxidative Coupling of Indole and Active Methylene Compounds Using Eosin Y as a Photocatalyst. Synthesis 2022, 54, 5099–5109. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, R. Recent Developments in Metal Catalyzed Asymmetric Addition of Phosphorus Nucleophiles. Chem. Soc. Rev. 2012, 41, 2095–2108. [Google Scholar] [CrossRef] [PubMed]
- Clarion, L.; Jacquard, C.; Sainte-Catherine, O.; Loiseau, S.; Filippini, D.; Hirlemann, M.-H.; Volle, J.N.; Virieux, D.; Lecouvey, M.; Pirat, J.L.; et al. Oxaphosphinanes: New Therapeutic Perspectives for Glioblastoma. J. Med. Chem. 2012, 55, 2196–2211. [Google Scholar] [CrossRef] [PubMed]
- Hibner-Kulicka, P.; Joule, J.A.; Skalik, J.; Bałczewski, P. Recent Studies of the Synthesis, Functionalization, Optoelectronic Properties and Applications of Dibenzophospholes. RSC Adv. 2017, 7, 9194–9236. [Google Scholar] [CrossRef] [Green Version]
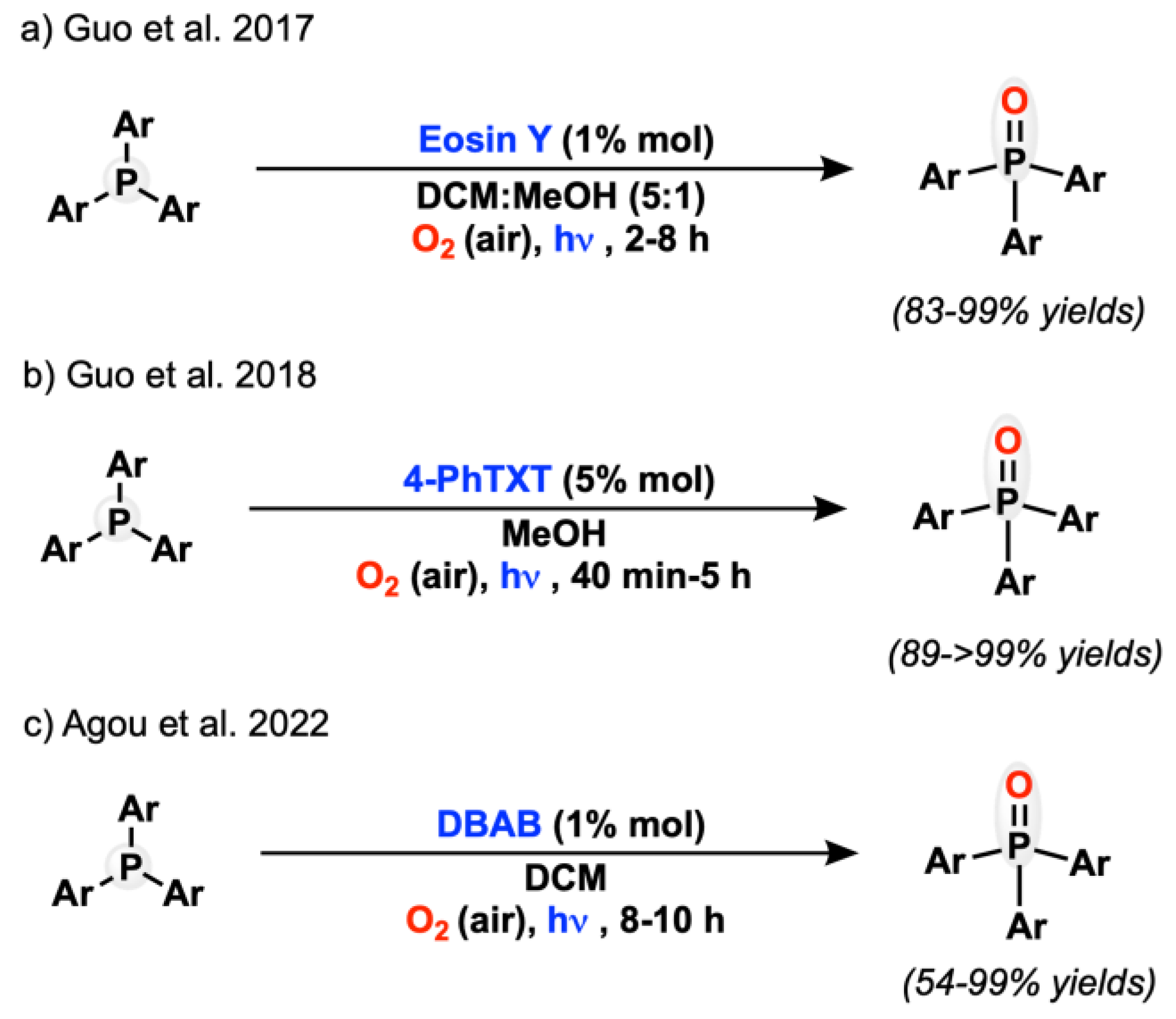
- Zhang, Y.; Ye, C.; Li, S.; Ding, A.; Gu, G.; Guo, H. Eosin Y-Catalyzed Photooxidation of Triarylphosphines under Visible Light Irradiation and Aerobic Conditions. RSC Adv. 2017, 7, 13240–13243. [Google Scholar] [CrossRef] [Green Version]
- Ding, A.; Li, S.; Chen, Y.; Jin, R.; Ye, C.; Hu, J.; Guo, H. Visible Light-Induced 4-Phenylthioxanthone-Catalyzed Aerobic Oxidation of Triarylphosphines. Tetrahedron Lett. 2018, 59, 3880–3883. [Google Scholar] [CrossRef]
- Kondo, M.; Agou, T. Catalytic Aerobic Photooxidation of Triarylphosphines Using Dibenzo-Fused 1,4-Azaborines. Chem. Commun. 2022, 58, 5001–5004. [Google Scholar] [CrossRef]
- Denmark, S.E. The Interplay of Invention, Discovery, Development, and Application in Organic Synthetic Methodology: A Case Study. J. Org. Chem. 2009, 74, 2915–2927. [Google Scholar] [CrossRef] [Green Version]
- Mewald, M.; Schiffner, J.A.; Oestreich, M. A New Direction in C-H Alkenylation: Silanol as a Helping Hand. Angew. Chem. Int. Ed. 2012, 51, 1763–1765. [Google Scholar] [CrossRef]
- Tran, N.T.; Wilson, S.O.; Franz, A.K. Cooperative Hydrogen-Bonding Effects in Silanediol Catalysis. Org. Lett. 2012, 14, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Sieburth, S. McN. Synthesis and Properties of a Sterically Unencumbered δ-Silanediol Amino Acid. J. Org. Chem. 2012, 77, 2901–2906. [Google Scholar] [CrossRef]
- Franz, A.K.; Wilson, S.O. Organosilicon Molecules with Medicinal Applications. J. Med. Chem. 2013, 56, 388–405. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Supported gold nanoparticlecatalyst for the selective oxidation of silanes to silanols in water. Chem. Commun. 2009, 35, 5302–5304. [Google Scholar] [CrossRef]
- Jeon, M.; Han, J.; Park, J. Catalytic Synthesis of Silanols from Hydrosilanes and Applications. ACS Catal. 2012, 2, 1539–1549. [Google Scholar] [CrossRef]
- Adam, W.; Mello, R.; Curci, R. O-Atom Insertion into Si-H Bonds by Dioxiranes: A Stereospecific and Direct Conversion of Silanes into Silanols. Angew. Chem. Int. Ed. Engl. 1990, 29, 890–891. [Google Scholar] [CrossRef]
- Li, J.; Xu, D.; Shi, G.; Liu, X.; Zhang, J.; Fan, B. Oxidation of Silanes to Silanols with Oxygen via Photoredox Catalysis. ChemistrySelect 2021, 6, 8345–8348. [Google Scholar] [CrossRef]
- Arzumanyan, A.V.; Goncharova, I.K.; Novikov, R.A.; Milenin, S.A.; Boldyrev, K.L.; Solyev, P.N.; Tkachev, Y.V.; Volodin, A.D.; Smol’yakov, A.F.; Korlyukov, A.A.; et al. Aerobic Co or Cu/NHPI-Catalyzed Oxidation of Hydride Siloxanes: Synthesis of Siloxanols. Green Chem. 2018, 20, 1467–1471. [Google Scholar] [CrossRef]
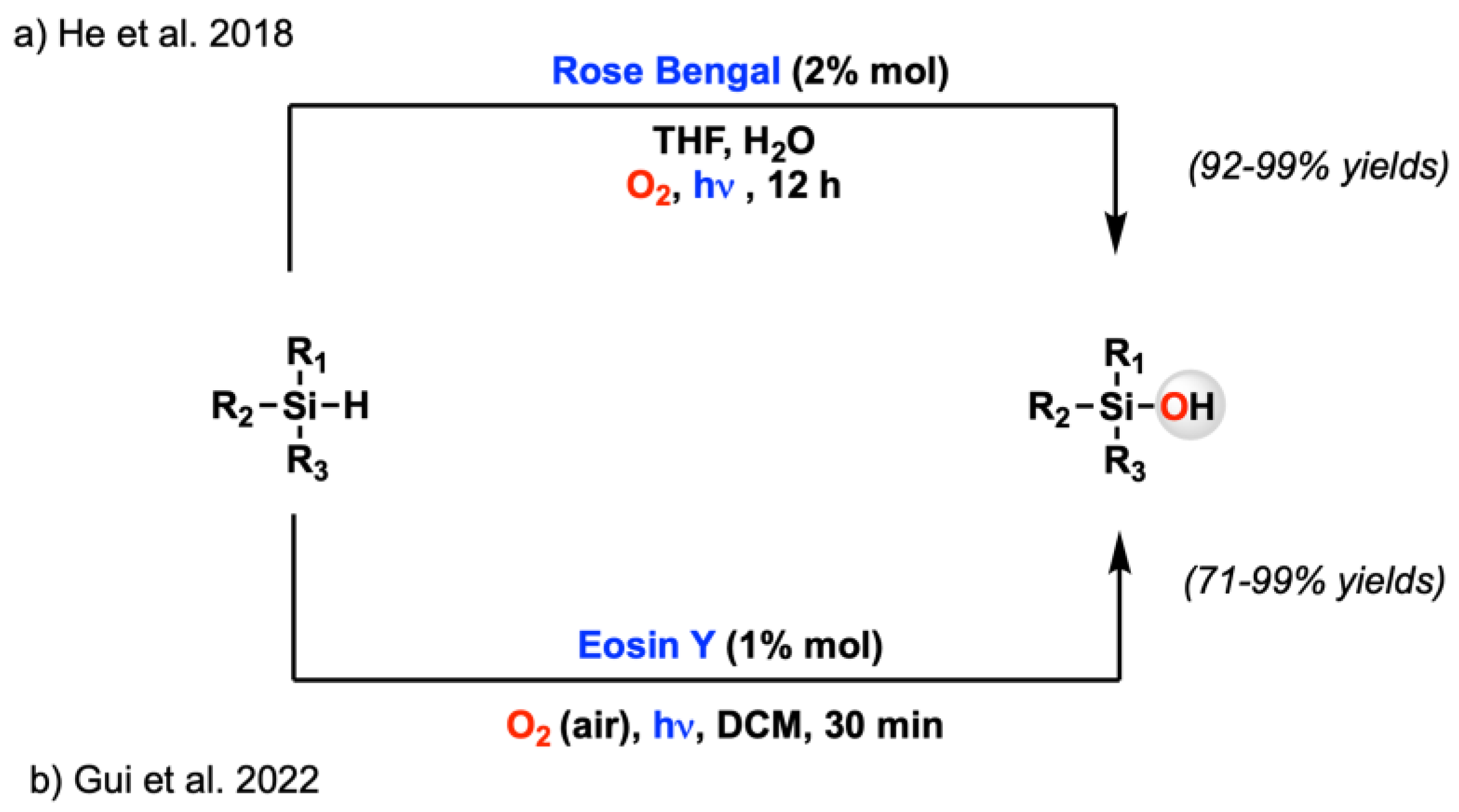
- Li, H.; Chen, L.; Duan, P.; Zhang, W. Highly Active and Selective Photocatalytic Oxidation of Organosilanes to Silanols. ACS Sust. Chem. Eng. 2022, 10, 4642–4649. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Liu, L.C.; Jiang, C.; He, T.; He, W. Metal-Free Visible-Light-Mediated Aerobic Oxidation of Silanes to Silanols. Sci. China Chem. 2018, 61, 1594–1599. [Google Scholar] [CrossRef]
- He, P.; Xhang, F.; Si, X.; Jiang, W.; Shen, Q.; Li, Z.; Zhu, Z.; Tang, S.; Gui, Q.W. Visible-light-induced aerobic oxidation of tertiary silanes to silanols using molecular oxygen as an oxidant. Synthesis 2022. [Google Scholar] [CrossRef]
- Zeng, Q.; Gao, S.; Chelashaw, A. Advances in Titanium-Catalyzed Synthesis of Chiral Sulfoxide Drugs. Mini-Reviews in Org. Chem. 2013, 10, 198–206. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, J.; Wu, Y.Q.; Zhang, Y.; Xia, J.Y.; Zhao, Q.; Lin, G.Q.; Yu, H.L.; Xu, J.H. Enzymatic Preparation of the Chiral (S)-Sulfoxide Drug Esomeprazole at Pilot-Scale Levels. Org. Process Res. Dev. 2020, 24, 1124–1130. [Google Scholar] [CrossRef]
- Peng, T.; Cheng, X.; Chen, Y.; Yang, J. Sulfoxide Reductases and Applications in Biocatalytic Preparation of Chiral Sulfoxides: A Mini-Review. Front. Chem. 2021, 9, 714899. [Google Scholar] [CrossRef]
- Yoshida, Y.; Otsuka, S.; Nogi, K.; Yorimitsu, H. Palladium-catalyzed amination of aryl sulfoxides. Org. Lett. 2018, 20, 1134–1137. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Xu, W.; Wang, R.; Zhang, M.; Xie, C.; Zeng, X.; Wang, M. Potassium Tert-Butoxide-Mediated Condensation Cascade Reaction: Transition Metal-Free Synthesis of Multisubstituted Aryl Indoles and Benzofurans. Org. Lett. 2019, 21, 3658–3662. [Google Scholar] [CrossRef]
- Venier, C.G.; Squires, T.G.; Chen, Y.Y.; Smith, B.F. Peroxytrifluoroacetic acid oxidation of sulfides to sulfoxides and sulfones. J. Org. Chem. 1982, 47, 3773–3774. [Google Scholar] [CrossRef]
- Colonna, S.; Gaggero, N. Enantioselective oxidation of sulphides by dioxiranes in the presence of bovine serum albumin. Tetrahedron Lett. 1989, 30, 6233–6236. [Google Scholar] [CrossRef]
- Kropp, P.J.; Breton, G.W.; Fields, J.D.; Tung, J.C.; Loomis, B.R. Surface-Mediated Reactions. 8. Oxidation of Sulfides and Sulfoxides with tert-Butyl Hydroperoxide and Oxone. J. Am. Chem. Soc. 2000, 122, 4280–4285. [Google Scholar] [CrossRef]
- Li, Q.; Lan, X.; An, G.; Ricardez-Sandoval, L.; Wang, Z.; Bai, G. Visible-Light-Responsive Anthraquinone Functionalized Covalent Organic Frameworks for Metal-Free Selective Oxidation of Sulfides: Effects of Morphology and Structure. ACS Catal. 2020, 10, 6664–6675. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, J.; Ding, A. Synthesis of an anthraquinone-containing polymeric photosensitizer and its application in aerobic photooxidation of thioethers. RSC Adv. 2020, 10, 10661–10665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, M.; Deng, Y.; Hu, W.; Su, A.; Yang, B.; Mao, F.; Zhang, C.; Liu, Y.; Fu, Z. 9,10-Dihydroanthracene auto-photooxidation efficiently triggered photo-catalytic oxidation of organic compounds by molecular oxygen under visible light. Mol. Catal. 2020, 494, 111127. [Google Scholar] [CrossRef]
- Dang, C.; Zhu, L.; Guo, H.; Xia, H.; Zhao, J.; Dick, B. Flavin Dibromide as an Efficient Sensitizer for Photooxidation of Sulfides. ACS Sustainable Chem. Eng. 2018, 6, 15254–15263. [Google Scholar] [CrossRef]
- Guo, H.; Xia, H.; Ma, X.; Chen, K.; Dang, C.; Zhao, J.; Dick, B. Efficient Photooxidation of Sulfides with Amidated Alloxazines as Heavy-Atom-Free Photosensitizers. ACS Omega 2020, 5, 10586–10595. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xie, Z.; Jing, X. BODIPY photocatalyzed oxidation of thioanisole under visible light. Catal. Commun. 2011, 16, 94–97. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Xiao, H.; Qi, R.; Huang, Y.; Xie, Z.; Jing, X.; Zhang, H. Iodo-BODIPY: A visible-light-driven, highly efficient and photostable metal-free organic photocatalyst. RSC Adv. 2013, 3, 13417–13421. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, H.; Zhang, Y.; Tang, C.; Fan, W. Visible-light photocatalytic aerobic oxidation of sulfides to sulfoxides with a perylene diimide photocatalyst. Org. Biomol. Chem. 2019, 17, 7144–7149. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, Y.; Ding, A.; Hu, Y.; Guo, H. Visible Light Sensitizer-Catalyzed Highly Selective Photo Oxidation from Thioethers into Sulfoxides under Aerobic Condition. Sci. Rep. 2018, 8, 2205. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Hammond, G.B.; Xu, B. Modulation of Photochemical Oxidation of Thioethers to Sulfoxides or Sulfones Using an Aromatic Ketone as the Photocatalyst. Tetrahedron Lett. 2021, 82, 153376. [Google Scholar] [CrossRef]
- Forchetta, M.; Sabuzi, F.; Stella, L.; Conte, V.; Galloni, P. KuQuinone as a Highly Stable and Reusable Organic Photocatalyst in Selective Oxidation of Thioethers to Sulfoxides. J. Org. Chem. 2022, 87, 14016–14025. [Google Scholar] [CrossRef]




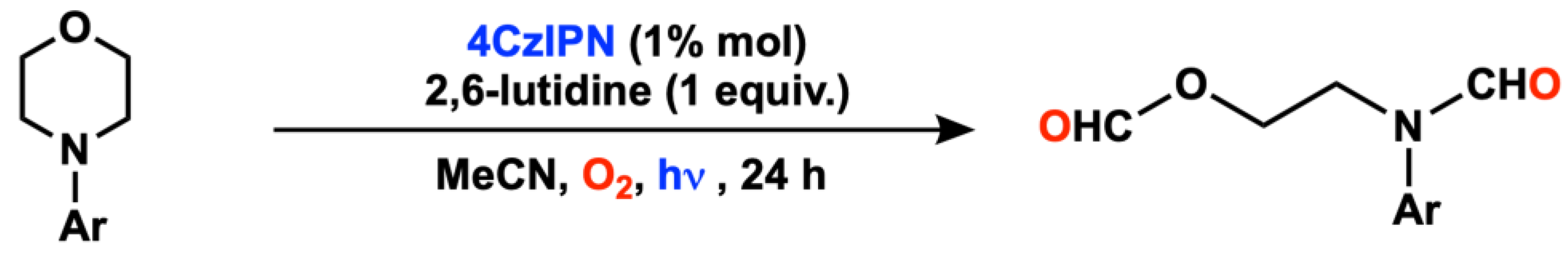





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forchetta, M.; Valentini, F.; Conte, V.; Galloni, P.; Sabuzi, F. Photocatalyzed Oxygenation Reactions with Organic Dyes: State of the Art and Future Perspectives. Catalysts 2023, 13, 220. https://doi.org/10.3390/catal13020220
Forchetta M, Valentini F, Conte V, Galloni P, Sabuzi F. Photocatalyzed Oxygenation Reactions with Organic Dyes: State of the Art and Future Perspectives. Catalysts. 2023; 13(2):220. https://doi.org/10.3390/catal13020220
Chicago/Turabian StyleForchetta, Mattia, Francesca Valentini, Valeria Conte, Pierluca Galloni, and Federica Sabuzi. 2023. "Photocatalyzed Oxygenation Reactions with Organic Dyes: State of the Art and Future Perspectives" Catalysts 13, no. 2: 220. https://doi.org/10.3390/catal13020220
APA StyleForchetta, M., Valentini, F., Conte, V., Galloni, P., & Sabuzi, F. (2023). Photocatalyzed Oxygenation Reactions with Organic Dyes: State of the Art and Future Perspectives. Catalysts, 13(2), 220. https://doi.org/10.3390/catal13020220