One Step Catalytic Conversion of Polysaccharides in Ulva prolifera to Lactic Acid and Value-Added Chemicals
Abstract
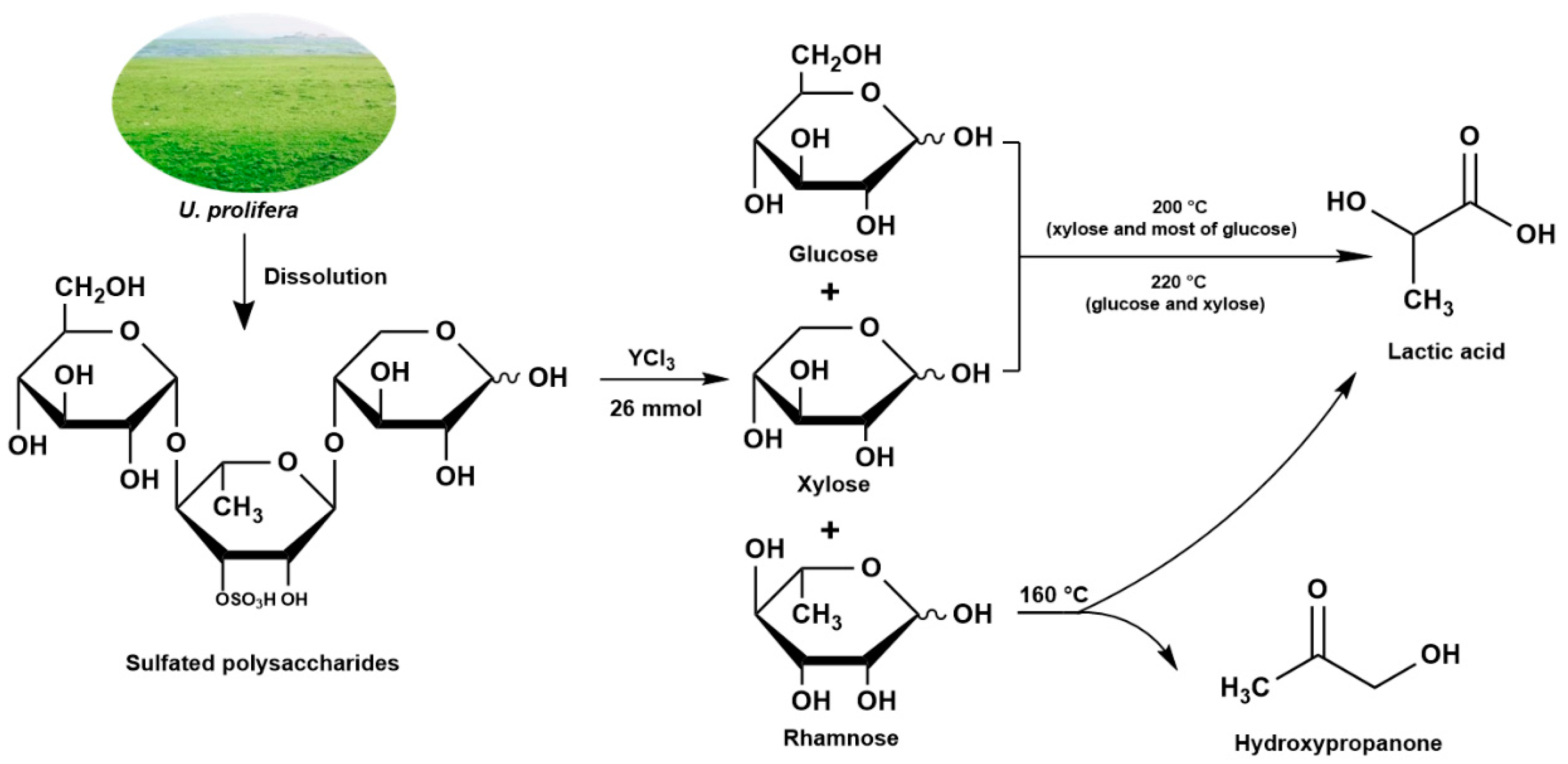
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Experiments of Ulva prolifera
2.3. Liquid Products Analysis
2.4. Characterization of Solid Samples
3. Results and Discussion
3.1. Composition of Ulva prolifera
3.2. Hydrothermal Conversion of Ulva prolifera
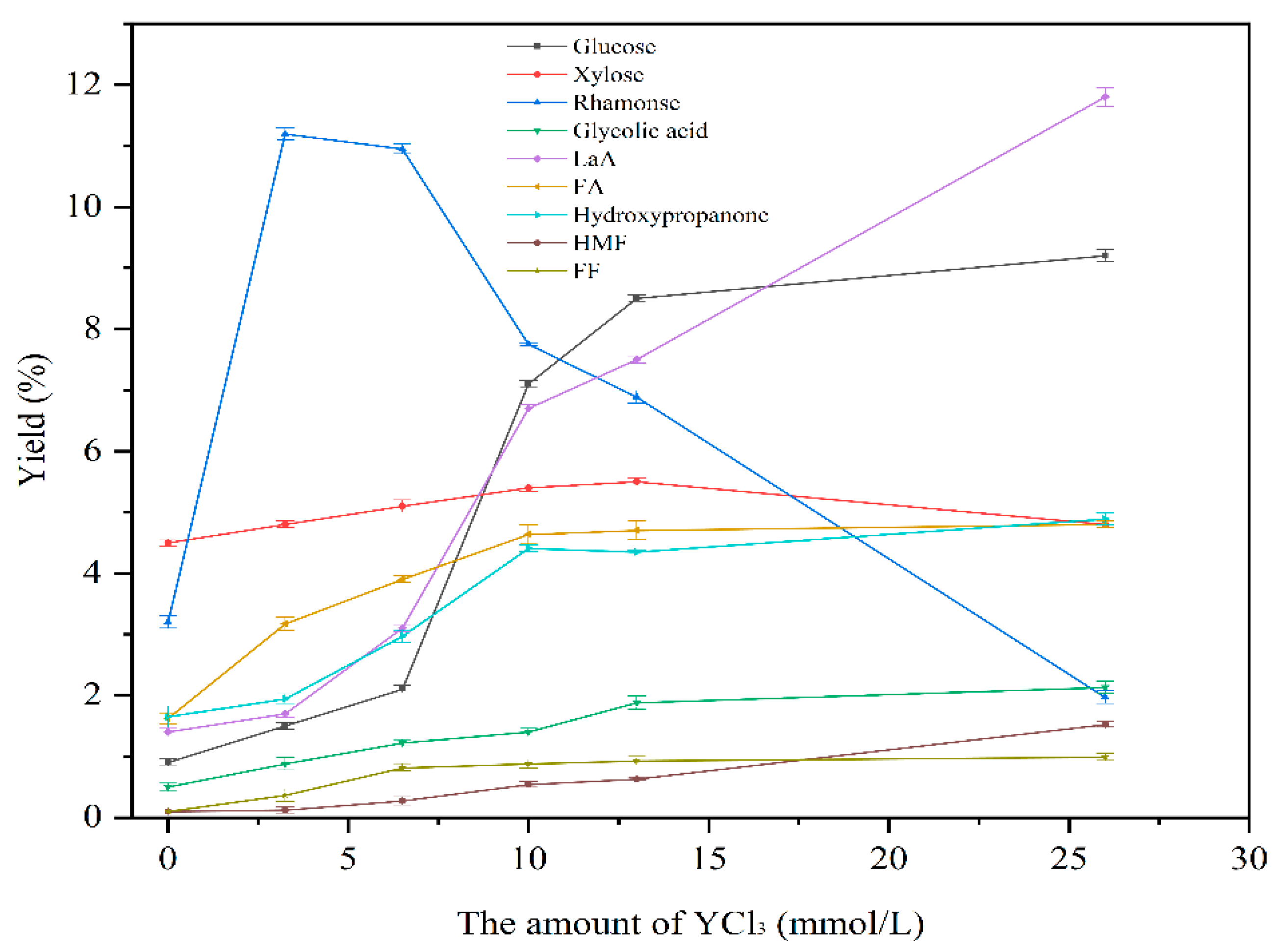
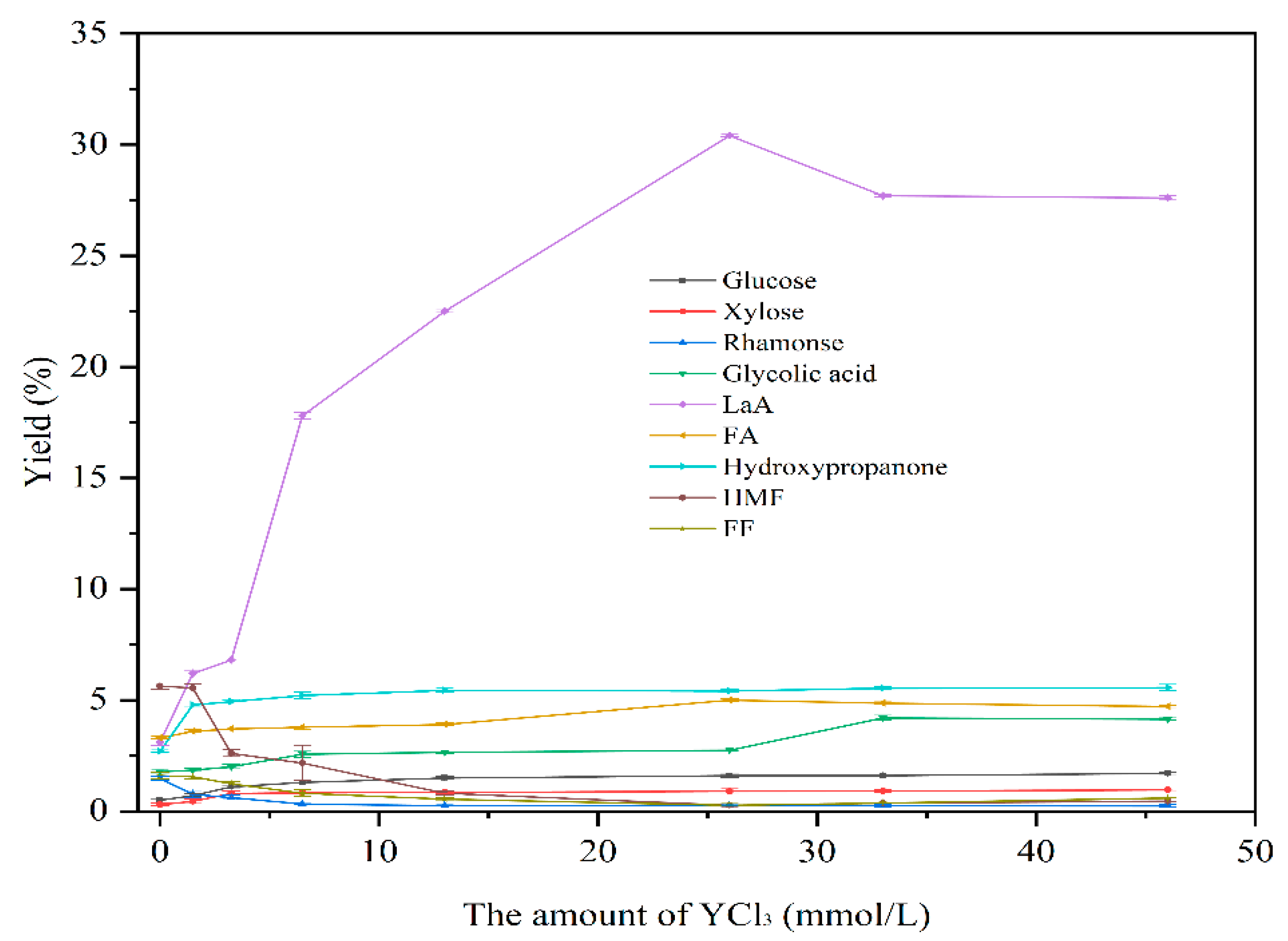
3.2.1. The Effect of Different Amounts of Y(III) Catalyst
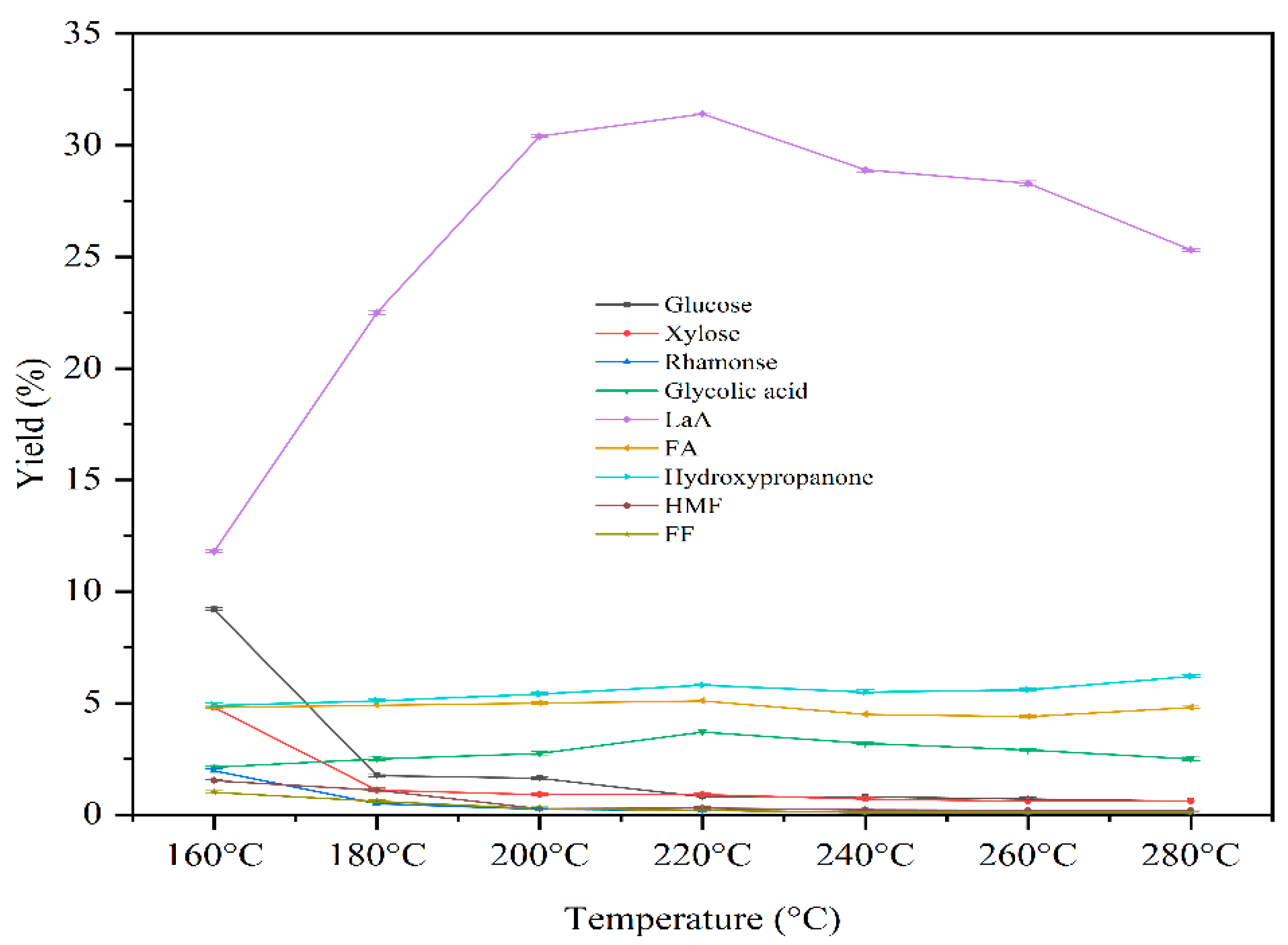
3.2.2. The Effect of Temperature on Catalytic Reaction
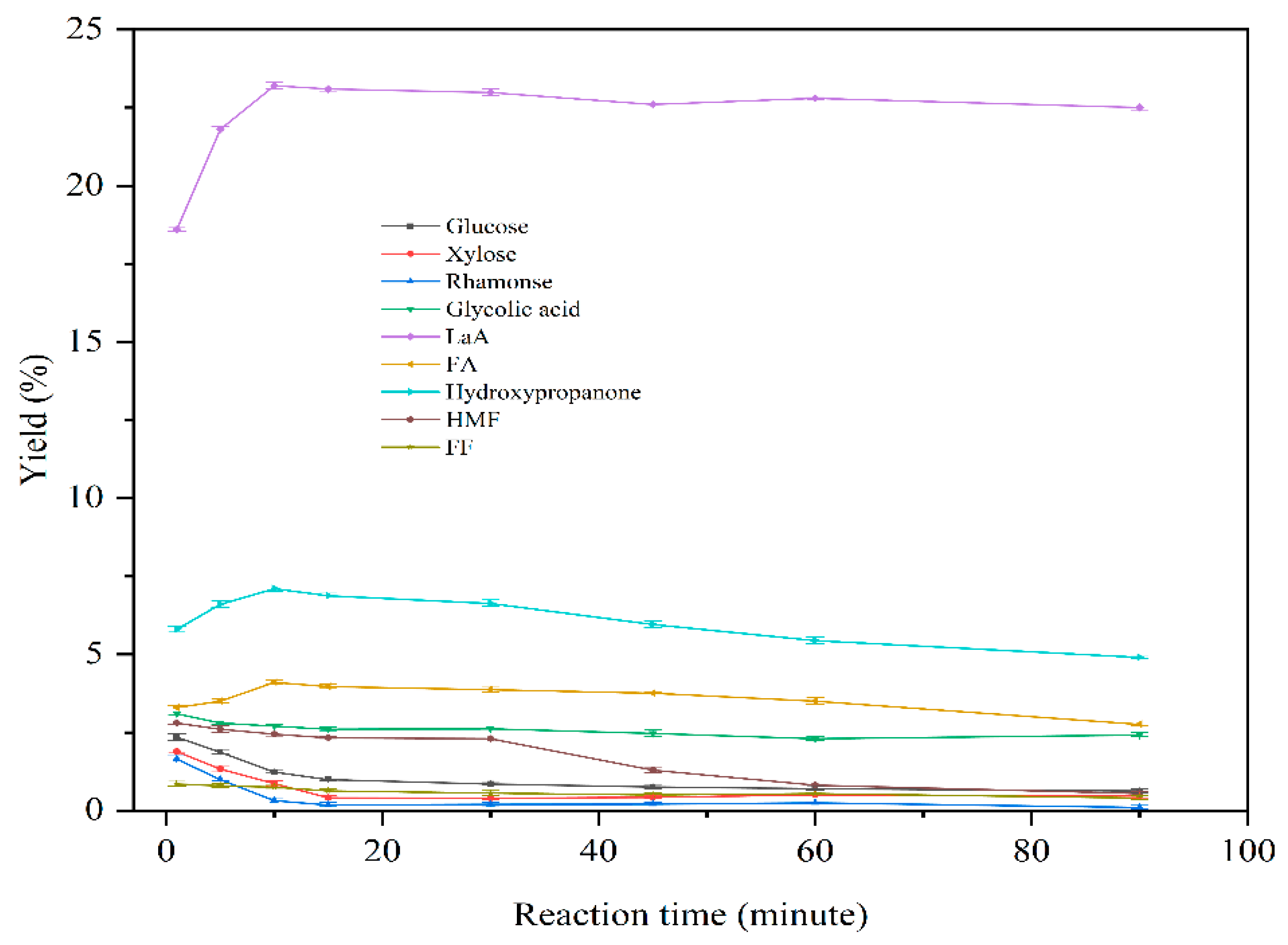
3.2.3. The Impact of Different Reaction Time
3.2.4. The GPC and ESI-MS Analysis of Liquid Products
3.3. The FT-IR Spectra of Solid Samples
3.4. Catalytic Reaction Pathways of Hydrothermal Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baco, S.; Klinksiek, M.; Ismail Bedawi Zakaria, R.; Antonia Garcia-Hernandez, E.; Mignot, M.; Legros, J.; Held, C.; Casson Moreno, V.; Leveneur, S. Solvent effect investigation on the acid-catalyzed esterification of levulinic acid by ethanol aided by a Linear Solvation Energy Relationship. Chem. Eng. Sci. 2022, 260, 117928. [Google Scholar] [CrossRef]
- Bhat, N.S.; Hegde, S.L.; Dutta, S.; Sudarsanam, P. Efficient Synthesis of 5-(Hydroxymethyl)furfural Esters from Polymeric Carbohydrates Using 5-(Chloromethyl)furfural as a Reactive Intermediate. ACS Sustain. Chem. Eng. 2022, 10, 5803–5809. [Google Scholar] [CrossRef]
- Chang, C.; Wang, S.; Guo, P.; Xu, G.; Zheng, X.; Du, C.; Jiao, Y. Metal sulfate-catalyzed methanolysis of cellulose at high solid loadings: Heterogeneous degradation kinetics and levulinate synthesis. Chem. Eng. J. 2023, 453, 139873. [Google Scholar] [CrossRef]
- Deng, Q.; Hou, X.; Zhong, Y.; Zhu, J.; Wang, J.; Cai, J.; Zeng, Z.; Zou, J.J.; Deng, S.; Yoskamtorn, T.; et al. 2D MOF with Compact Catalytic Sites for the One-pot Synthesis of 2,5-Dimethylfuran from Saccharides via Tandem Catalysis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202205453. [Google Scholar] [CrossRef]
- Ching, T.W.; Haritos, V.; Tanksale, A. Microwave assisted conversion of microcrystalline cellulose into value added chemicals using dilute acid catalyst. Carbohydr. Polym. 2017, 157, 1794–1800. [Google Scholar] [CrossRef]
- Guo, W.; Kortenbach, T.; Qi, W.; Hensen, E.; Jan Heeres, H.; Yue, J. Selective tandem catalysis for the synthesis of 5-hydroxymethylfurfural from glucose over in-situ phosphated titania catalysts: Insights into structure, bi-functionality and performance in flow microreactors. Appl. Catal. B Environ. 2022, 301, 120800. [Google Scholar] [CrossRef]
- Hou, Q.; Bai, C.; Bai, X.; Qian, H.; Nie, Y.; Xia, T.; Lai, R.; Yu, G.; Rehman, M.L.U.; Ju, M. Roles of Ball Milling Pretreatment and Titanyl Sulfate in the Synthesis of 5-Hydroxymethylfurfural from Cellulose. ACS Sustain. Chem. Eng. 2022, 10, 1205–1213. [Google Scholar] [CrossRef]
- Kiwfo, K.; Yeerum, C.; Issarangkura Na Ayutthaya, P.; Kesonkan, K.; Suteerapataranon, S.; Panitsupakamol, P.; Chinwong, D.; Paengnakorn, P.; Chinwong, S.; Kotchabhakdi, N.; et al. Sustainable Education with Local-Wisdom Based Natural Reagent for Green Chemical Analysis with a Smart Device: Experiences in Thailand. Sustainability 2021, 13, 11147. [Google Scholar] [CrossRef]
- Shao, Y.; Lu, W.; Meng, Y.; Zhou, D.; Zhou, Y.; Shen, D.; Long, Y. The formation of 5-hydroxymethylfurfural and hydrochar during the valorization of biomass using a microwave hydrothermal method. Sci. Total Environ. 2021, 755, 142499. [Google Scholar] [CrossRef]
- Vasudevan, S.V.; Kong, X.; Cao, M.; Wang, M.; Mao, H.; Bu, Q. Microwave-assisted liquefaction of carbohydrates for 5-hydroxymethylfurfural using tungstophosphoric acid encapsulated dendritic fibrous mesoporous silica as a catalyst. Sci. Total Environ. 2021, 760, 143379. [Google Scholar] [CrossRef]
- Padovan, D.; Endo, K.; Matsumoto, T.; Yokoi, T.; Fukuoka, A.; Kato, H.; Nakajima, K. Acid–Base Property of Tetragonal YNbO4 with Phosphate Groups and Its Catalysis for the Dehydration of Glucose to 5-Hydroxymethylfurfural. Small Struct. 2022, 2200224. [Google Scholar] [CrossRef]
- Peng, L.; Tao, C.; Yang, H.; Zhang, J.; Liu, H. Mechanistic insights into the effect of the feed concentration on product formation during acid-catalyzed conversion of glucose in ethanol. Green Chem. 2022, 24, 5219–5227. [Google Scholar] [CrossRef]
- Rezayan, A.; Wang, K.; Nie, R.; Lu, T.; Wang, J.; Zhang, Y.; Xu, C.C. Synthesis of bifunctional tin-based silica–carbon catalysts, Sn/KIT-1/C, with tunable acid sites for the catalytic transformation of glucose into 5-hydroxymethylfurfural. Chem. Eng. J. 2022, 429, 132261. [Google Scholar] [CrossRef]
- Shi, N.; Zhu, Y.; Qin, B.; Zhu, T.; Huang, H.; Liu, Y. Conversion of Cellulose into 5-Hydroxymethylfurfural in a Biphasic System Catalyzed by Aluminum Sulfate and Byproduct Characterization. ACS Sustain. Chem. Eng. 2022, 10, 10444–10456. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, Y.; Chen, W.-T.; Zhang, P.; Dong, Y. An investigation of reaction pathways of hydrothermal liquefaction using Chlorella pyrenoidosa and Spirulina platensis. Energy Convers. Manag. 2015, 96, 330–339. [Google Scholar] [CrossRef]
- Singh, A.; Olsen, S.I. A critical review of biochemical conversion, sustainability and life-cycle assessment of algal biofuels. Appl. Energy 2011, 88, 3548–3555. [Google Scholar] [CrossRef]
- Wu, X.-F.; Zhang, J.-J.; Li, M.-F.; Bian, J.; Peng, F. Catalytic hydrothermal liquefaction of eucalyptus to prepare bio-oils and product properties. Energy Convers. Manag. 2019, 199, 111955. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Beck, M.; Hornung, U.; Ronsse, F.; Kruse, A.; Prins, W. Suitability of hydrothermal liquefaction as a conversion route to produce biofuels from macroalgae. Algal Res. 2015, 11, 234–241. [Google Scholar] [CrossRef]
- Guo, J.; Zhuang, Y.; Chen, L.; Liu, J.; Li, D.; Ye, N. Process optimization for microwave-assisted direct liquefaction of Sargassum polycystum C.Agardh using response surface methodology. Bioresour. Technol. 2012, 120, 19–25. [Google Scholar] [CrossRef]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.; Zhou, Y.; Tong, D.; Hu, C. Selective Conversion of Hemicellulose in Macroalgae Enteromorpha prolifera to Rhamnose. ACS Omega 2019, 4, 7023–7028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zhou, Y.; Hu, C. Study on the pyrolysis behaviour of the macroalga Ulva prolifera. J. Appl. Phycol. 2020, 33, 91–99. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, S.; Fu, H.; Chen, J. Liquefaction of Macroalgae Enteromorpha prolifera in Sub-/Supercritical Alcohols: Direct Production of Ester Compounds. Energy Fuels 2012, 26, 2342–2351. [Google Scholar] [CrossRef]
- Dave, N.; Varadavenkatesan, T.; Singh, R.S.; Giri, B.S.; Selvaraj, R.; Vinayagam, R. Evaluation of seasonal variation and the optimization of reducing sugar extraction from Ulva prolifera biomass using thermochemical method. Environ. Sci. Pollut. Res. 2021, 28, 58857–58871. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, J.; Zhang, G.; Liu, Z.; Guan, H.; Hwang, H.; Aker, W.G.; Wang, P. Optimization study on the hydrogen peroxide pretreatment and production of bioethanol from seaweed Ulva prolifera biomass. Bioresour. Technol. 2016, 214, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Varadavenkatesan, T.; Selvaraj, R.; Vinayagam, R. Modelling of fermentative bioethanol production from indigenous Ulva prolifera biomass by Saccharomyces cerevisiae NFCCI1248 using an integrated ANN-GA approach. Sci. Total Environ. 2021, 791, 148429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, M.; Chen, Y.; Hu, C. Conversion of polysaccharides in Ulva prolifera to valuable chemicals in the presence of formic acid. J. Appl. Phycol. 2020, 33, 101–110. [Google Scholar] [CrossRef]
- Garrido, R.; Cabeza, L.F.; Falguera, V.; Pérez Navarro, O. Potential Use of Cow Manure for Poly(Lactic Acid) Production. Sustainability 2022, 14, 16753. [Google Scholar] [CrossRef]
- Malacara-Becerra, A.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Riquelme-Jiménez, L.M.; Mansouri, S.S.; Iqbal, H.M.N.; Parra-Saldívar, R. Bioconversion of Corn Crop Residues: Lactic Acid Production through Simultaneous Saccharification and Fermentation. Sustainability 2022, 14, 11799. [Google Scholar] [CrossRef]
- Mort, R.; Peters, E.; Curtzwiler, G.; Jiang, S.; Vorst, K. Biofillers Improved Compression Modulus of Extruded PLA Foams. Sustainability 2022, 14, 5521. [Google Scholar] [CrossRef]
- Pal, P.; Sikder, J.; Roy, S.; Giorno, L. Process intensification in lactic acid production: A review of membrane based processes. Chem. Eng. Process. Process Intensif. 2009, 48, 1549–1559. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, X.; Li, Y.; Li, Y.-Y.; Wang, Q. Lactic Acid Production by Fermentation of Biomass: Recent Achievements and Perspectives. Sustainability 2022, 14, 14434. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem. Rev. 2014, 114, 1909–1971. [Google Scholar] [CrossRef]
- Yang, L.; Su, J.; Carl, S.; Lynam, J.G.; Yang, X.; Lin, H. Catalytic conversion of hemicellulosic biomass to lactic acid in pH neutral aqueous phase media. Appl. Catal. B Environ. 2015, 162, 149–157. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Li, J.; Wu, Y.; Xiao, Y.; Hu, C. D-Excess-LaA Production Directly from Biomass by Trivalent Yttrium Species. iScience 2019, 12, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Deng, W.; Wang, B.; Zhang, Q.; Wan, X.; Tang, Z.; Wang, Y.; Zhu, C.; Cao, Z.; Wang, G.; et al. Chemical synthesis of lactic acid from cellulose catalysed by lead(II) ions in water. Nat. Commun. 2013, 4, 2141. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Jiang, Z.; Wu, P.; Yi, J.; Li, J.; Hu, C. Fractionation for further conversion: From raw corn stover to lactic acid. Sci. Rep. 2016, 6, 38623. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Y.; Li, J.; He, T.; Xiao, Y.; Zhou, C.; Hu, C. Directing the Simultaneous Conversion of Hemicellulose and Cellulose in Raw Biomass to Lactic Acid. ACS Sustain. Chem. Eng. 2020, 8, 4244–4255. [Google Scholar] [CrossRef]
- Kong, L.; Li, G.; Wang, H.; He, W.; Ling, F. Hydrothermal catalytic conversion of biomass for lactic acid production. J. Chem. Technol. Biotechnol. 2008, 83, 383–388. [Google Scholar] [CrossRef]
- Younas, R.; Zhang, S.; Zhang, L.; Luo, G.; Chen, K.; Cao, L.; Liu, Y.; Hao, S. Lactic acid production from rice straw in alkaline hydrothermal conditions in presence of NiO nanoplates. Catal. Today 2016, 274, 40–48. [Google Scholar] [CrossRef]
- Gao, X.; Qu, H.; Shan, S.; Song, C.; Baranenko, D.; Li, Y.; Lu, W. A novel polysaccharide isolated from Ulva Pertusa: Structure and physicochemical property. Carbohydr. Polym. 2020, 233, 115849. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Xing, R.; Li, K.; Li, R.; Qin, Y.; Wang, X.; Wei, Z.; Li, P. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013, 92, 1991–1996. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Cai, B.; Li, J.; Tong, D.; Hu, C. The degradation of the lignin in Phyllostachys heterocycla cv. pubescens in an ethanol solvothermal system. Green Chem. 2014, 16, 3107–3116. [Google Scholar] [CrossRef]
- Fu, X.; Hu, Y.; Zhang, Y.; Zhang, Y.; Tang, D.; Zhu, L.; Hu, C. Solvent Effects on Degradative Condensation Side Reactions of Fructose in Its Initial Conversion to 5-Hydroxymethylfurfural. ChemSusChem 2020, 13, 501–512. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
| Algae Characterization | Content | Test Methods |
|---|---|---|
| Proximate (wt%) | ||
| Moisture | 4.3 | Drying at 80 °C according to [27] |
| Combustible | 68.9 | Burning at 800 °C in air |
| Ash | 26.8 | Burning at 800 °C in air |
| Elemental (wt%) | ||
| C | 41.1 | FLASH 1112SERIES Element Analyzer |
| H | 7.6 | FLASH 1112SERIES Element Analyzer |
| N | 1.6 | FLASH 1112SERIES Element Analyzer |
| S | 3.4 | FLASH 1112SERIES Element Analyzer |
| O | 46.3 | Calculated by difference (O=100-C-H-N-S) |
| Biochemical (wt%) | ||
| Carbohydrates | 44.5 | DNS colorimetric analysis |
| Proteins | 8.1 | N (wt%) multiplied by conversion factor of 6.25 |
| Lipids | 1.5 | Bligh and Dyer method |
| Carbohydrates (wt%) | ||
| Glucose | 13.5 | Hydrolyzing of polysaccharides in feedstock |
| Xylose | 8.4 | Hydrolyzing of polysaccharides in feedstock |
| Rhamnose | 18.1 | Hydrolyzing of polysaccharides in feedstock |
| Others | 4.5 | Calculated by difference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhou, Y.; Hu, C. One Step Catalytic Conversion of Polysaccharides in Ulva prolifera to Lactic Acid and Value-Added Chemicals. Catalysts 2023, 13, 262. https://doi.org/10.3390/catal13020262
Li M, Zhou Y, Hu C. One Step Catalytic Conversion of Polysaccharides in Ulva prolifera to Lactic Acid and Value-Added Chemicals. Catalysts. 2023; 13(2):262. https://doi.org/10.3390/catal13020262
Chicago/Turabian StyleLi, Mingyu, Yingdong Zhou, and Changwei Hu. 2023. "One Step Catalytic Conversion of Polysaccharides in Ulva prolifera to Lactic Acid and Value-Added Chemicals" Catalysts 13, no. 2: 262. https://doi.org/10.3390/catal13020262
APA StyleLi, M., Zhou, Y., & Hu, C. (2023). One Step Catalytic Conversion of Polysaccharides in Ulva prolifera to Lactic Acid and Value-Added Chemicals. Catalysts, 13(2), 262. https://doi.org/10.3390/catal13020262







