Hydrothermal Co-Crystallization of Novel Copper Tungstate-Strontium Titanate Crystal Composite for Enhanced Photocatalytic Activity and Increased Electron–Hole Recombination Time
Abstract
:1. Introduction
2. Results and Discussion
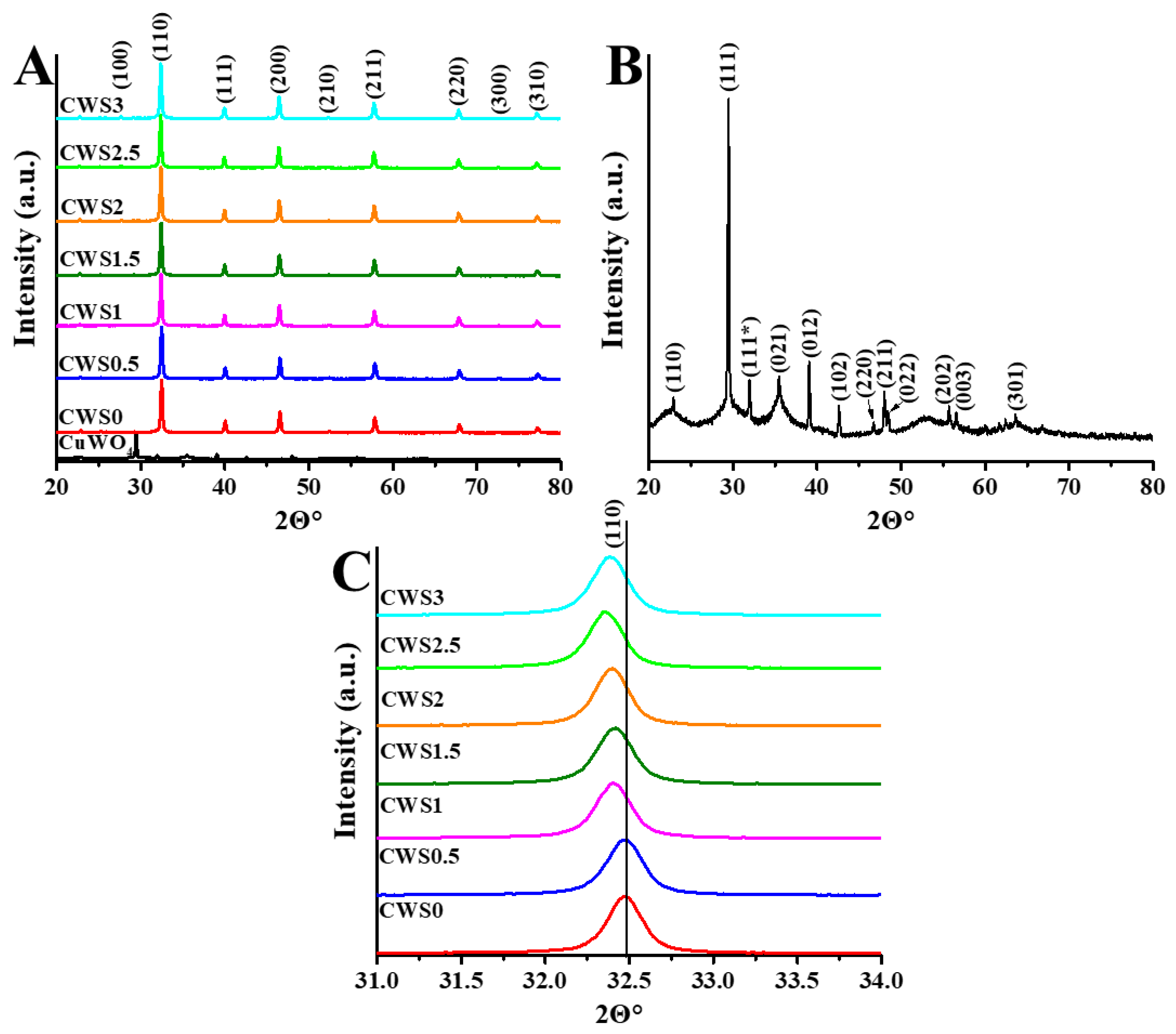
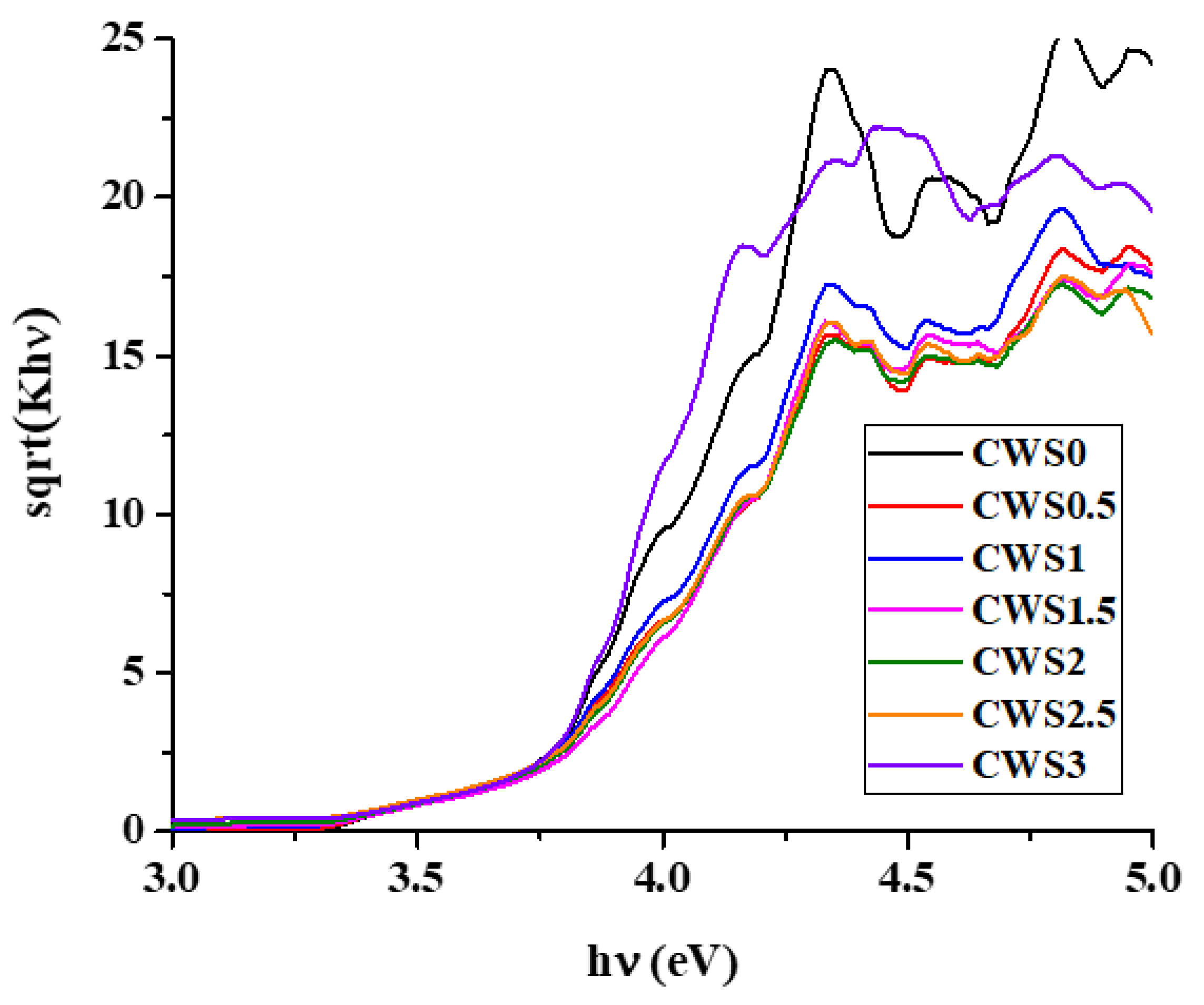
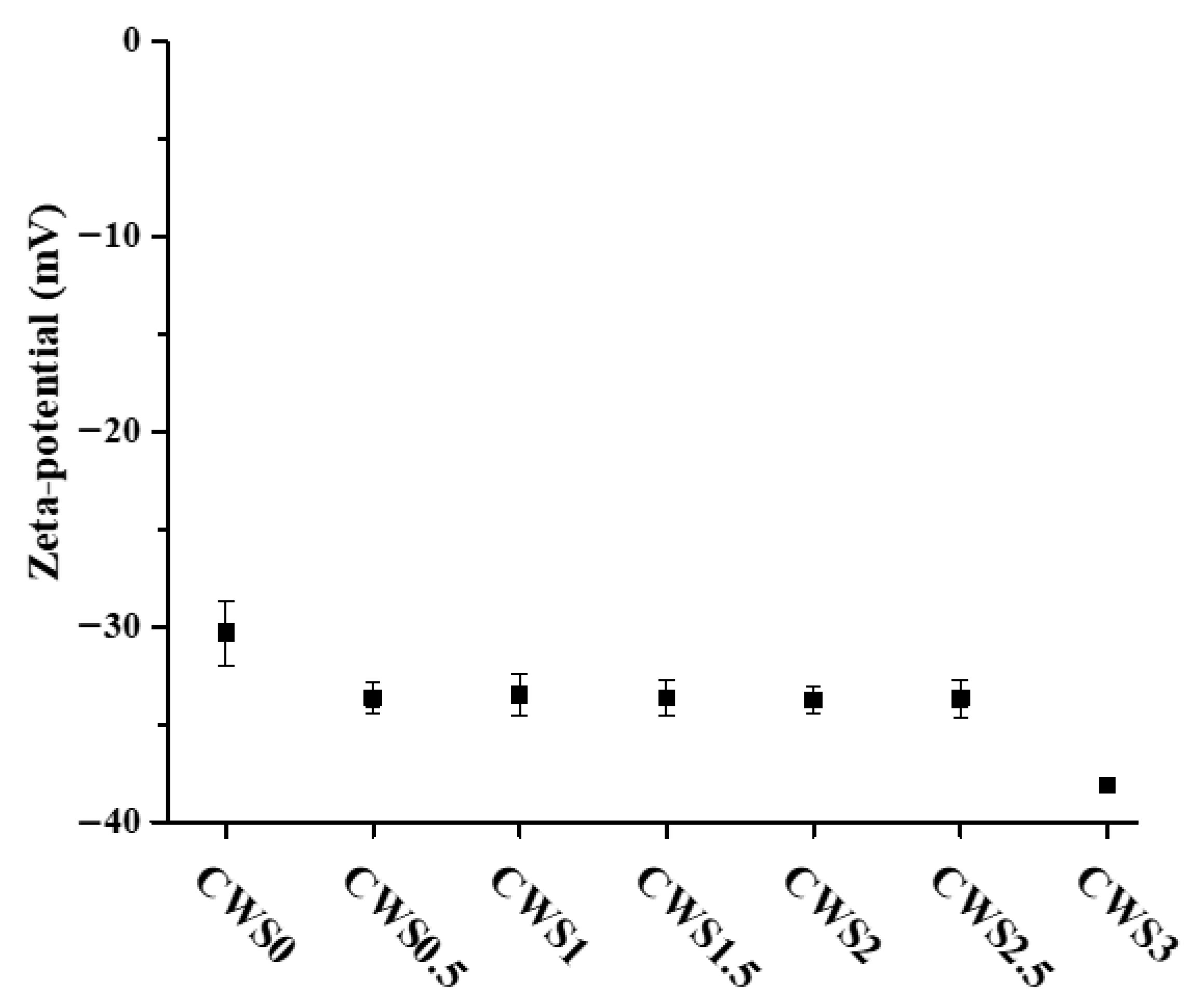
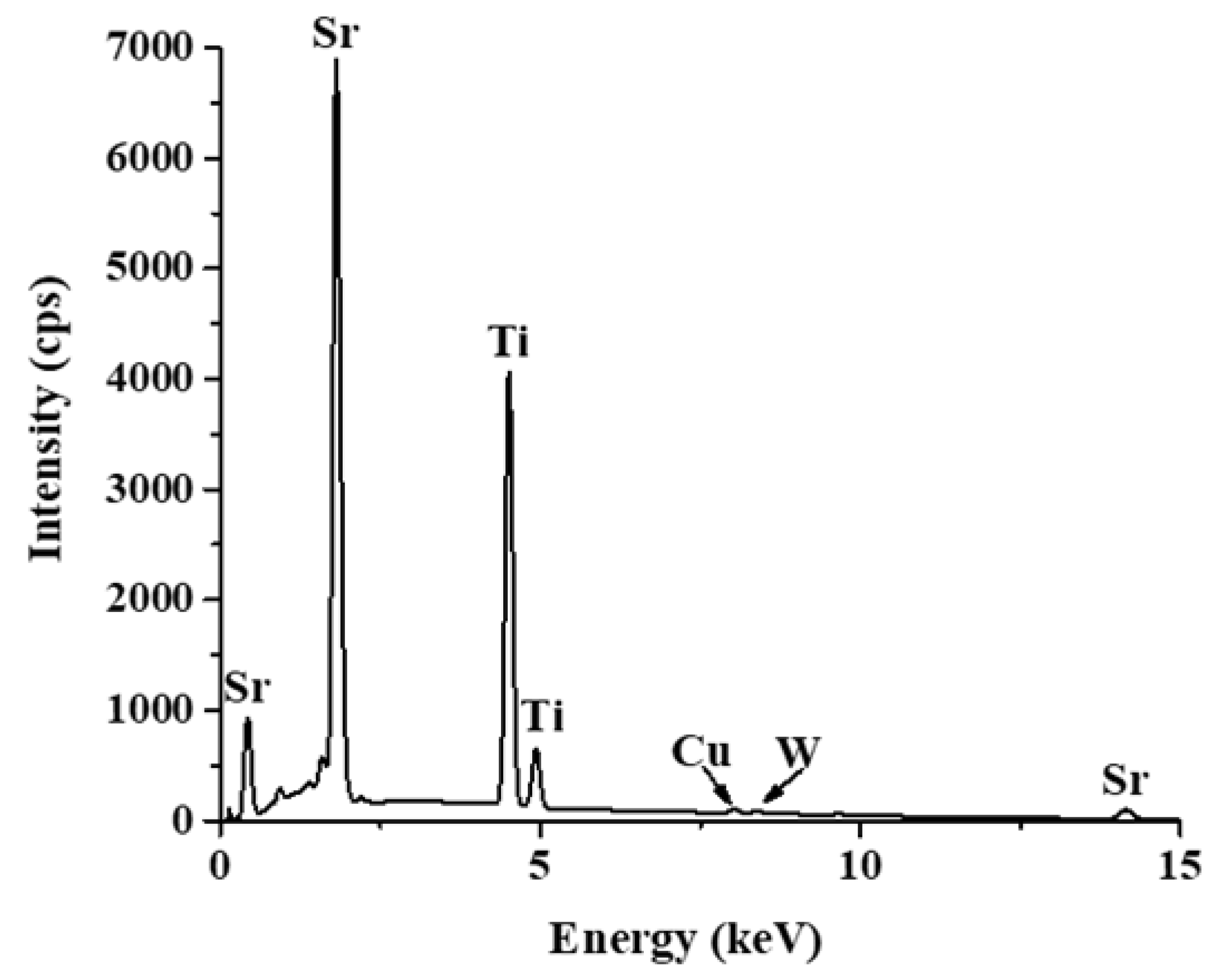
2.1. Characterization
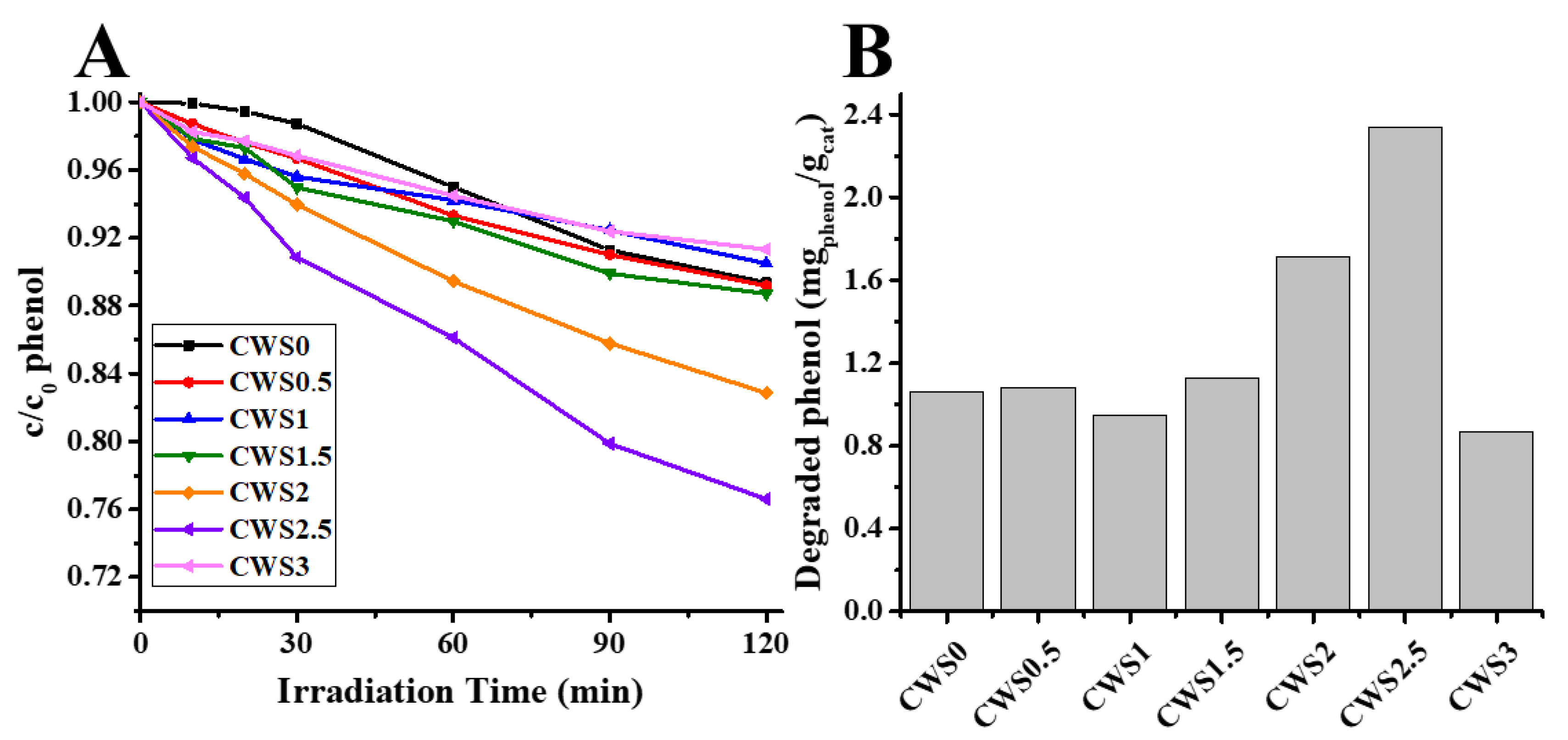
2.2. Photocatalytic Activity Measurements
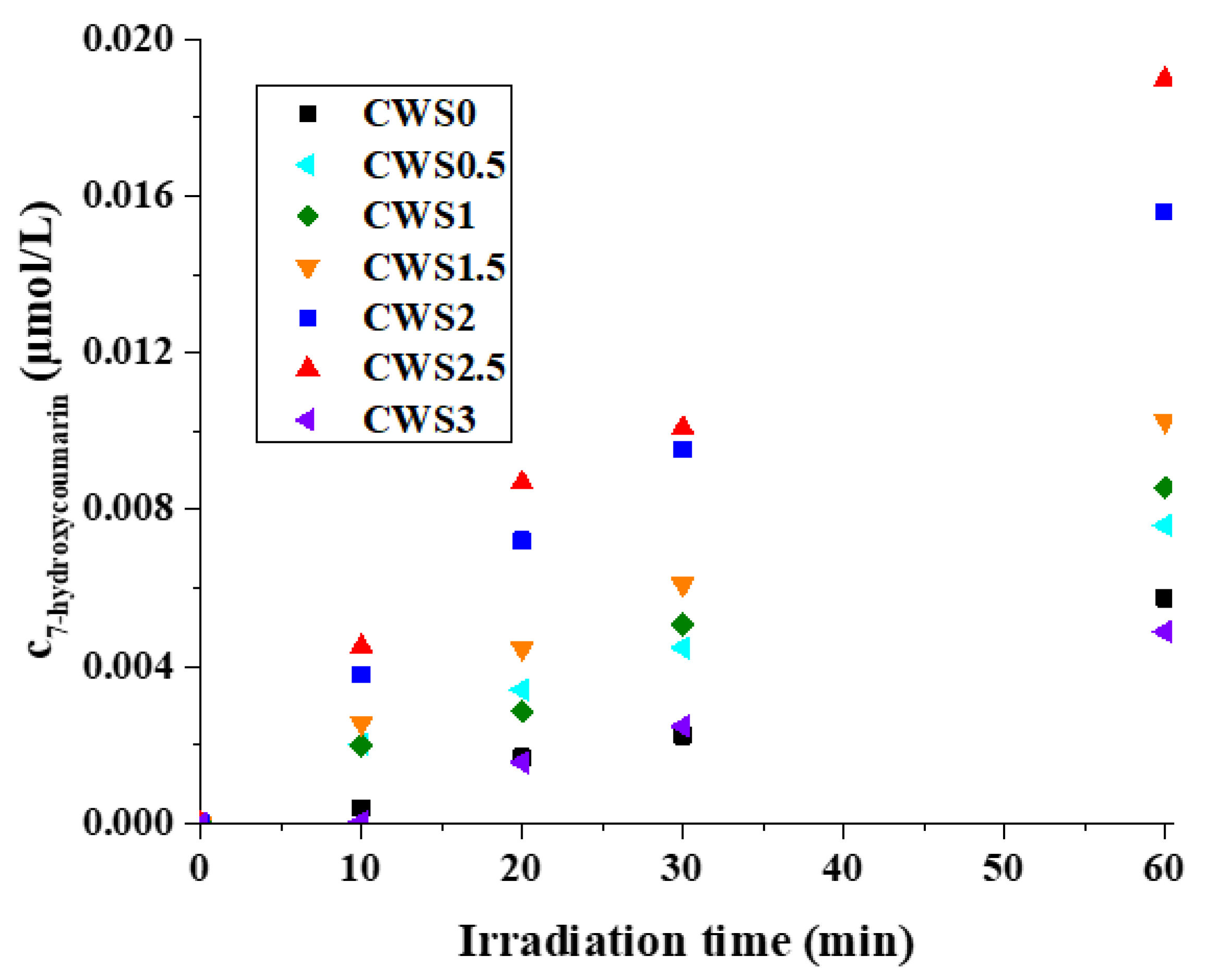
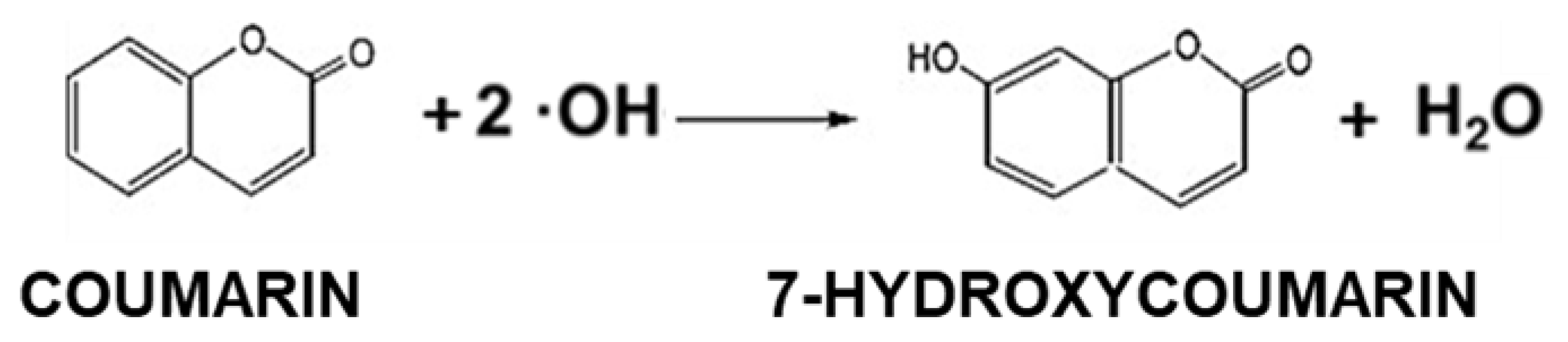
2.3. Detection of Hydroxyl Radical
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Photocatalytic Activity Measurements
3.4. Detection of Hydroxyl Radical
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodriguez, C.; Di Cara, A.; Renaud, F.N.R.; Freney, J.; Horvais, N.; Borel, R.; Puzenat, E.; Guillard, C. Antibacterial Effects of Photocatalytic Textiles for Footwear Application. Catal. Today 2014, 230, 41–46. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water Purification by Semiconductor Photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Ali, H.; Zaman, S.; Majeed, I.; Kanodarwala, F.K.; Nadeem, M.A.; Stride, J.A.; Nadeem, M.A. Porous Carbon/RGO Composite: An Ideal Support Material of Highly Efficient Palladium Electrocatalysts for the Formic Acid Oxidation Reaction. ChemElectroChem 2017, 4, 3126–3133. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Huang, L.; Zaman, S.; Lei, K.; Yue, T.; Li, Z.A.; You, B.; Xia, B.Y. Engineering 2D Photocatalysts toward Carbon Dioxide Reduction. Adv. Energy Mater. 2021, 11, 2003159. [Google Scholar] [CrossRef]
- Noureen, L.; Xie, Z.; Gao, Y.; Li, M.; Hussain, M.; Wang, K.; Zhang, L.; Zhu, J. Multifunctional Ag3PO4-RGO-Coated Textiles for Clean Water Production by Solar-Driven Evaporation, Photocatalysis, and Disinfection. ACS Appl. Mater. Interfaces 2020, 12, 6343–6350. [Google Scholar] [CrossRef]
- Sasikala, R.; Kandasamy, M.; Suresh, S.; Ragavendran, V.; Sasirekha, V.; Pearce, J.M.; Murugesan, S.; Mayandi, J. Enhanced Dye-Sensitized Solar Cell Performance Using Strontium Titanate Perovskite Integrated Photoanodes Modified with Plasmonic Silver Nanoparticles. J. Alloys Compd. 2021, 889, 161693. [Google Scholar] [CrossRef]
- Ágoston, Á.; Balog, Á.; Narbutas, Š.; Dékány, I.; Janovák, L. Optimization of Plasmonic Copper Content at Copper-Modified Strontium Titanate (Cu-SrTiO3): Synthesis, Characterization, Photocatalytic Activity. Catalysts 2022, 12, 1041. [Google Scholar] [CrossRef]
- Farré, M.J.; Franch, M.I.; Malato, S.; Ayllón, J.A.; Peral, J.; Doménech, X. Degradation of Some Biorecalcitrant Pesticides by Homogeneous and Heterogeneous Photocatalytic Ozonation. Chemosphere 2005, 58, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Reydellet, L.-H.; Roche, P.; Glattli, D.C.; Etienne, B.; Jin, Y. Quantum Partition Noise of Photon-Created Electron-Hole Pairs. Phys. Rev. Lett. 2003, 90, 176803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, Y.; Leichtweis, J.; Foletto, E.L.; Silvestri, S. Reactive Oxygen Species-Induced Heterogeneous Photocatalytic Degradation of Organic Pollutant Rhodamine B by Copper and Zinc Aluminate Spinels. J. Chem. Technol. Biotechnol. 2020, 95, 791–797. [Google Scholar] [CrossRef]
- He, R.A.; Cao, S.W.; Yu, J.G. Recent Advances in Morphology Control and Surface Modification of Bi-Based Photocatalysts. Wuli Huaxue Xuebao/Acta Phys.-Chim. Sin. 2016, 32, 2841–2870. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Sulfur-Doped TiO2: Structure and Surface Properties. Catalysts 2017, 7, 214. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of Photocatalytic Activity by Metal Deposition: Characterisation and Photonic Efficiency of Pt, Au and Pd Deposited on TiO2 Catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Hadibarata, T.; Zon, N.F. Salmiati Characterization of Titanium Dioxide Doped with Nitrogen and Sulfur and Its Photocatalytic Appraisal for Degradation of Phenol and Methylene Blue. J. Chin. Chem. Soc. 2017, 64, 1333–1339. [Google Scholar] [CrossRef]
- Rosy, A.; Kalpana, G. Reduced Graphene Oxide/Strontium Titanate Heterostructured Nanocomposite as Sunlight Driven Photocatalyst for Degradation of Organic Dye Pollutants. Curr. Appl. Phys. 2018, 18, 1026–1033. [Google Scholar] [CrossRef]
- Xu, J.; Wei, Y.; Huang, Y.; Wang, J.; Zheng, X.; Sun, Z.; Fan, L.; Wu, J. Solvothermal Synthesis Nitrogen Doped SrTiO3 with High Visible Light Photocatalytic Activity. Ceram. Int. 2014, 40, 10583–10591. [Google Scholar] [CrossRef]
- Choi, Y.; Jung, M.; Lee, Y.K. Effect of Heating Rate on the Activation Energy for Crystallization of Amorphous Ge2Sb2Te5 Thin Film. Electrochem. Solid-State Lett. 2009, 12, F17. [Google Scholar] [CrossRef]
- Da Silva, L.F.; Avansi, W.; Moreira, M.L.; Mesquita, A.; Maia, L.J.Q.; Andrés, J.; Longo, E.; Mastelaro, V.R. Relationship between Crystal Shape, Photoluminescence, and Local Structure in SrTiO3 Synthesized by Microwave-Assisted Hydrothermal Method. J. Nanomater. 2012, 2012, 890397. [Google Scholar] [CrossRef] [Green Version]
- WEITZEL, H. Kristallstrukturverfeinerung von Wolframiten Und Columbiten. Zeitschrift für Krist.-Cryst. Mater. 1976, 144, 238–258. [Google Scholar] [CrossRef]
- Gillespie, J.B.; Lindberg, J.D.; Laude, L.S. Kubelka-Munk Optical Coefficients for a Barium Sulfate White Reflectance Standard. Appl. Opt. 1975, 14, 807–809. [Google Scholar] [CrossRef]
- Lévy, F.; Mercier, A.; Voitchovsky, J.P. Band-Edge Photoluminescence of PbI2. Solid State Commun. 1974, 15, 819–822. [Google Scholar] [CrossRef]
- Siebenhofer, M.; Viernstein, A.; Morgenbesser, M.; Fleig, J.; Kubicek, M. Photoinduced Electronic and Ionic Effects in Strontium Titanate. Mater. Adv. 2021, 2, 7583–7619. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, Z.; Cheung, G.; Doughty, R.M.; Ballestas-Barrientos, A.R.; Hirmez, B.; Han, R.; Maschmeyer, T.; Osterloh, F.E. Role of Surface States in Photocatalytic Oxygen Evolution with CuWO 4 Particles. J. Electrochem. Soc. 2019, 166, H3014–H3019. [Google Scholar] [CrossRef]
- Balassa, L.; Ágoston, Á.; Kása, Z.; Hornok, V.; Janovák, L. Surface Sulfate Modified TiO2 Visible Light Active Photocatalyst for Complex Wastewater Purification: Preparation, Characterization and Photocatalytic Activity. J. Mol. Struct. 2022, 1260, 132860. [Google Scholar] [CrossRef]
- Shukla, S.; Pandey, H.; Singh, P.; Tiwari, A.K.; Baranwal, V.; Pandey, A.C. Synergistic Impact of Photocatalyst and Dopants on Pharmaceutical-Polluted Waste Water Treatment: A Review. Environ. Pollut. Bioavailab. 2021, 33, 347–364. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, H.; Lyu, M.; Wang, Q.; Yun, J.-H.; Wang, L. Composition-Dependent Photoluminescence Intensity and Prolonged Recombination Lifetime of Perovskite CH3NH3PbBr3−xClx Films. Chem. Commun 2014, 50, 11727. [Google Scholar] [CrossRef] [Green Version]
- Jayathilaka, P.B.; Pathiraja, G.C.; Bandara, A.; Subasinghe, N.D.; Nanayakkara, N. Theoretical Study of Phenol and Hydroxyl Radical Reaction Mechanism in Aqueous Medium by the DFT/B3LYP/6-31+G(d,p)/CPCM Model. Can. J. Chem. 2014, 92, 809–813. [Google Scholar] [CrossRef]
- Maier, A.C.; Iglebaek, E.H.; Jonsson, M. Confirming the Formation of Hydroxyl Radicals in the Catalytic Decomposition of H2O2 on Metal Oxides Using Coumarin as a Probe. ChemCatChem 2019, 11, 5435–5438. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Rahimi-Nasrabadi, M.; Khalilian-Shalamzari, M.; Ghaeni, H.R.; Hajimirsadeghi, S.S. Facile Chemical Synthesis and Characterization of Copper Tungstate Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2014, 24, 333–339. [Google Scholar] [CrossRef]
- Gallardo, V.; Morales, M.E.; Ruiz, M.A.; Delgado, A.V. An Experimental Investigation of the Stability of Ethylcellulose Latex: Correlation between Zeta Potential and Sedimentation. Eur. J. Pharm. Sci. 2005, 26, 170–175. [Google Scholar] [CrossRef]

| Catalyst Name | Nominal Cu Content (wt%) | Measured CuWO4 Content (wt%) * | Specific Surface Area (m2/g) |
|---|---|---|---|
| CWS0 | 0 | 0 | 40.30 ± 1.60 |
| CWS0.5 | 0.50 | 0.21 | 38.38 ± 0.40 |
| CWS1 | 1.00 | 1.34 | 41.04 ± 0.35 |
| CWS1.5 | 1.50 | 2.16 | 39.30 ± 0.55 |
| CWS2 | 2.00 | 2.86 | 40.81 ± 1.95 |
| CWS2.5 | 2.50 | 4.28 | 40.92 ± 5.43 |
| CWS3 | 3.00 | 5.22 | 40.13 ± 1.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ágoston, Á.; Janovák, L. Hydrothermal Co-Crystallization of Novel Copper Tungstate-Strontium Titanate Crystal Composite for Enhanced Photocatalytic Activity and Increased Electron–Hole Recombination Time. Catalysts 2023, 13, 287. https://doi.org/10.3390/catal13020287
Ágoston Á, Janovák L. Hydrothermal Co-Crystallization of Novel Copper Tungstate-Strontium Titanate Crystal Composite for Enhanced Photocatalytic Activity and Increased Electron–Hole Recombination Time. Catalysts. 2023; 13(2):287. https://doi.org/10.3390/catal13020287
Chicago/Turabian StyleÁgoston, Áron, and László Janovák. 2023. "Hydrothermal Co-Crystallization of Novel Copper Tungstate-Strontium Titanate Crystal Composite for Enhanced Photocatalytic Activity and Increased Electron–Hole Recombination Time" Catalysts 13, no. 2: 287. https://doi.org/10.3390/catal13020287
APA StyleÁgoston, Á., & Janovák, L. (2023). Hydrothermal Co-Crystallization of Novel Copper Tungstate-Strontium Titanate Crystal Composite for Enhanced Photocatalytic Activity and Increased Electron–Hole Recombination Time. Catalysts, 13(2), 287. https://doi.org/10.3390/catal13020287






