Lipase Production by Yarrowia lipolytica in Solid-State Fermentation Using Amazon Fruit By-Products and Soybean Meal as Substrate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Agro-Industrial By-Product Characterization
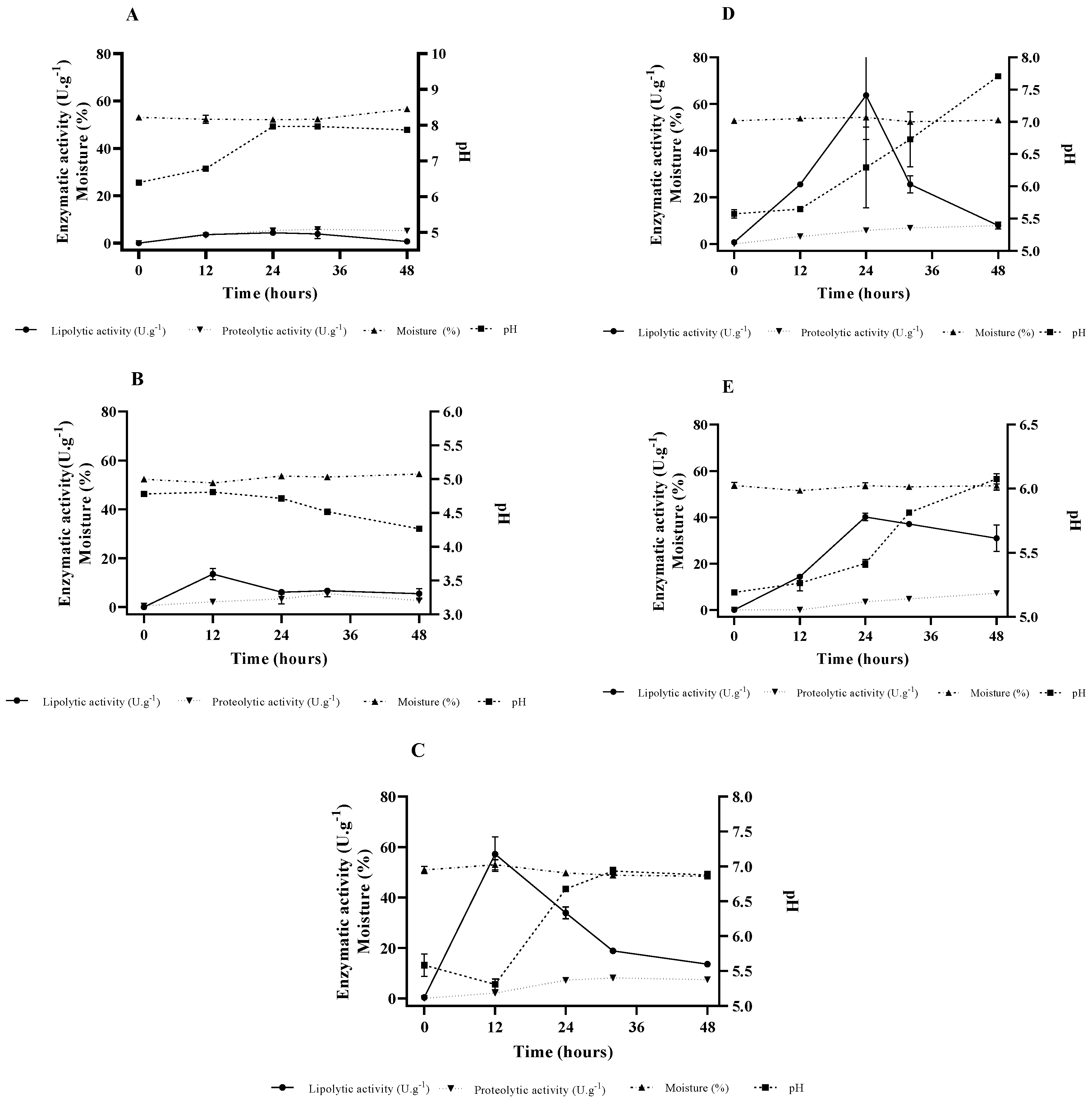
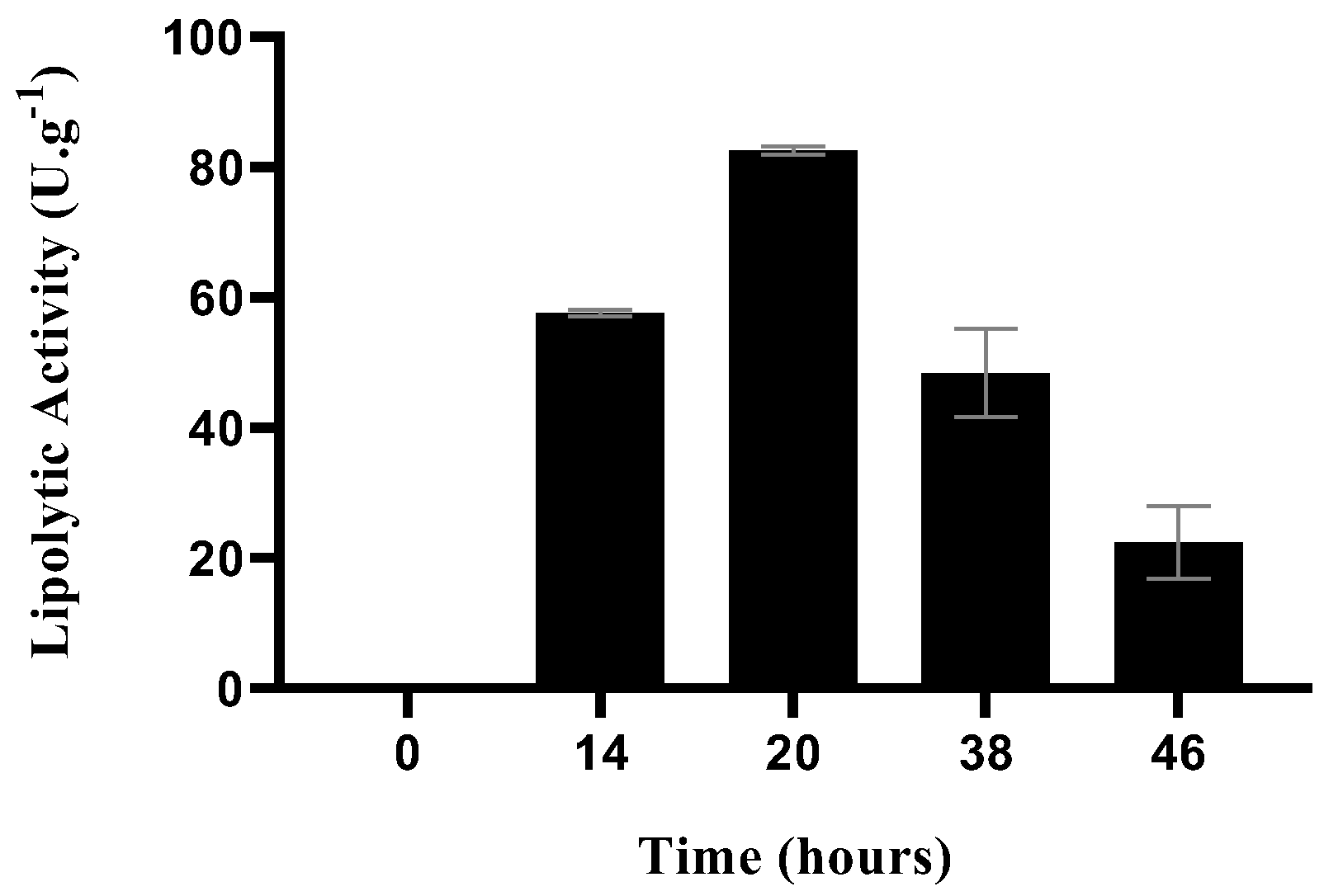
2.2. Lipase Production by SSF
2.3. Fermentation Matrix Supplementation
2.4. SDS-PAGE
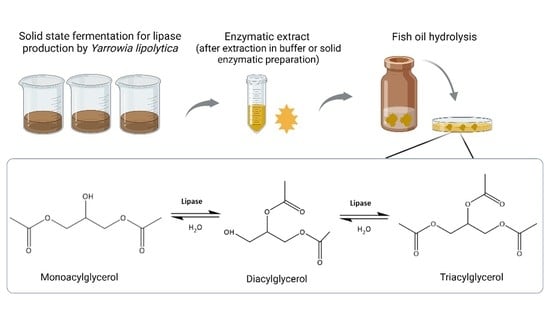
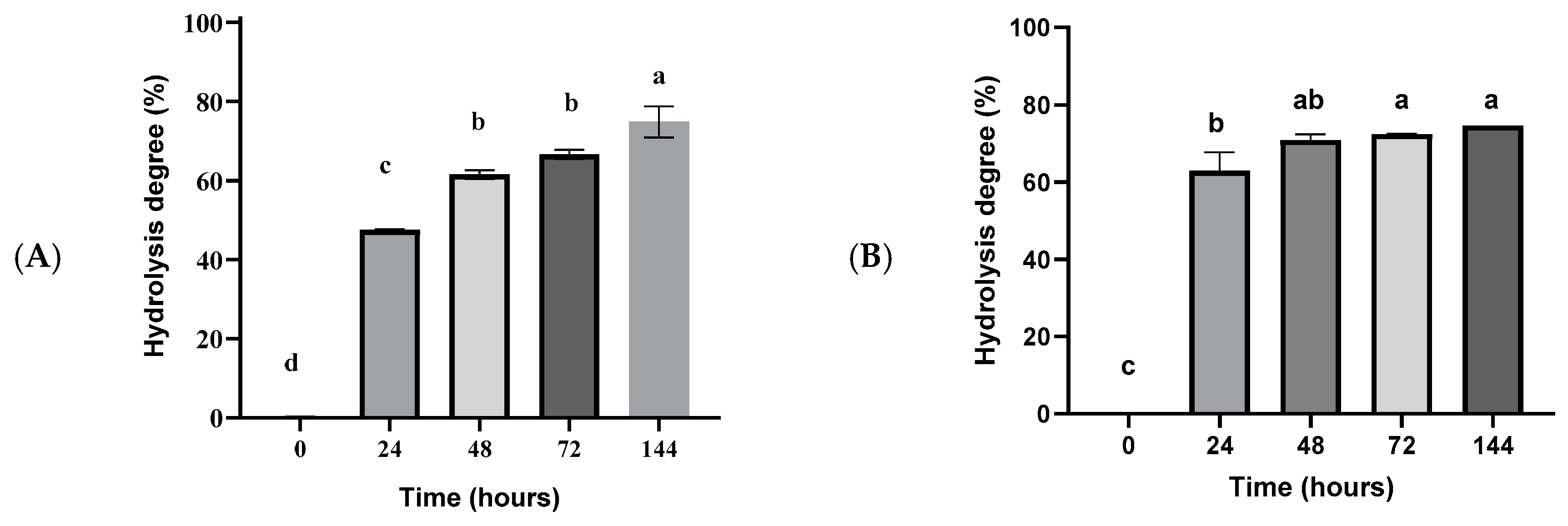
2.5. Fish Oil Hydrolysis
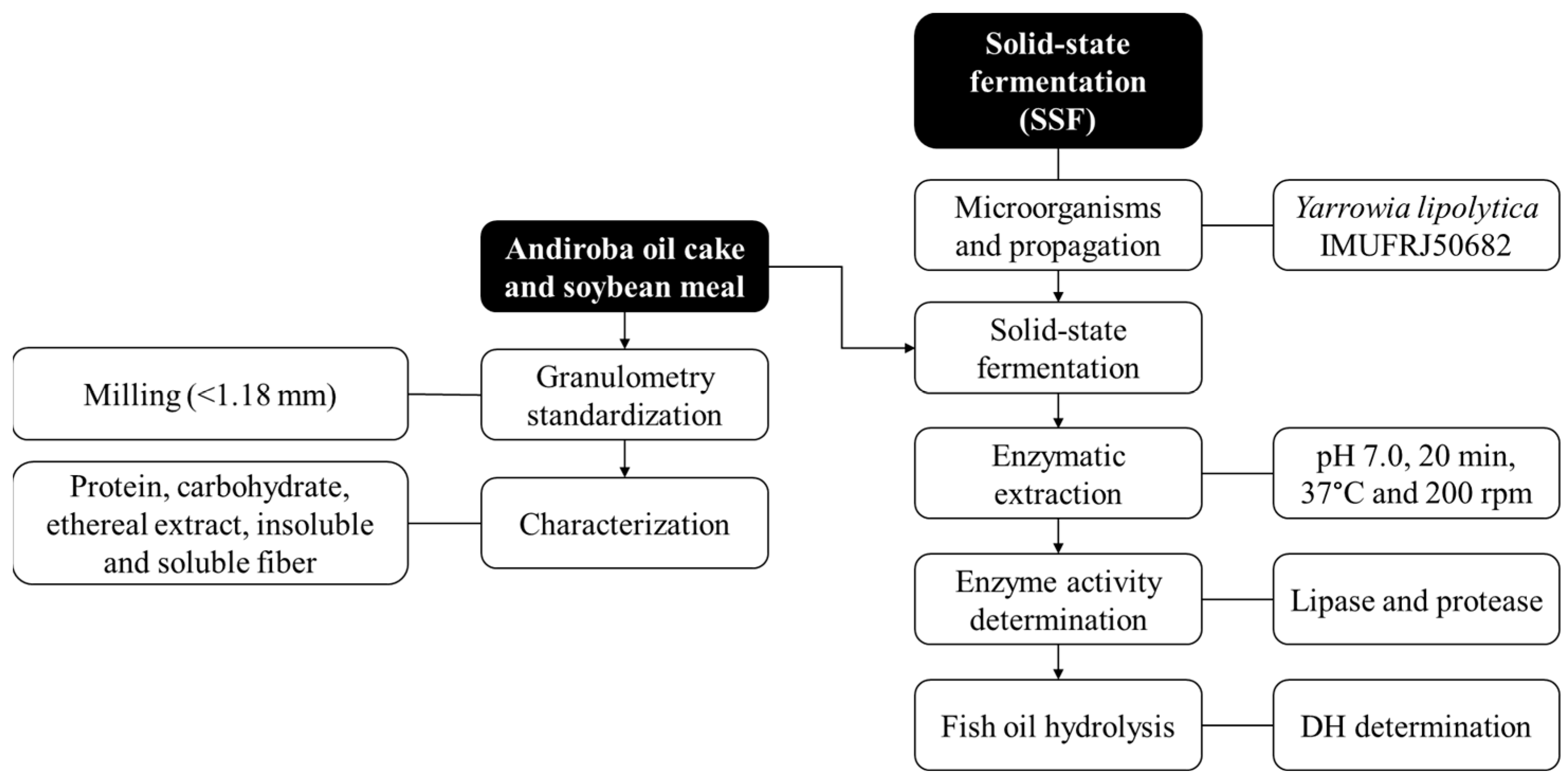
3. Materials and Methods
3.1. Material
3.2. Microorganism and Inoculum Cultivation Conditions
3.3. Agro-Industrial By-Product Characterization
3.4. Lipase Production by SSF
3.5. Enzyme Extraction and Production of Solid Biocatalyst
3.6. Analytical Determinations
3.6.1. Lipase Activity
3.6.2. Protease Activity
3.6.3. Moisture Content and pH
3.7. SDS-PAGE
3.8. Fish Oil Hydrolysis: A Potential Application
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serra, J.L.; Rodrigues, A.M.d.C.; Freitas, R.A.; Meirelles, A.J.d.A.; Darnet, S.H.; Silva, L.H.M.d. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Penido, C.; Conte, F.P.; Chagas, M.S.; Rodrigues, C.A.; Pereira, J.F.; Henriques, M.G. Antiinflammatory effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-induced arthritis in mice. Inflamm. Res. 2006, 55, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Penido, C.; Costa, K.A.; Pennaforte, R.J.; Costa, M.F.; Pereira, J.F.; Siani, A.C.; Henriques, M.G. Anti-allergic effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on allergen-induced vascular permeability and hyperalgesia. Inflamm. Res. 2005, 54, 295–303. [Google Scholar] [CrossRef]
- Santos, K.I.P.; Benjamim, J.K.F.; Costa, K.A.D.; Reis, A.S.; Souza Pinheiro, W.B.; Santos, A.S. Metabolomics techniques applied in the investigation of phenolic acids from the agro-industrial by-product of Carapa guianensis Aubl. Arab. J. Chem. 2021, 14, 103421. [Google Scholar] [CrossRef]
- Lourenço, J.N.P.; Ferreira, L.M.M.; Martins, G.C.; Nascimento, D.G. Produção, Biometria de Frutos e Sementes e Extração do Óleo de Andiroba (Carapa Guianensis Aublet; Sob Manejo Comunitário em Parintins, AM: Brasília, Brazil, 2017; p. 36. [Google Scholar]
- Organic, S. Óleo de Andiroba. Available online: https://simpleorganic.com.br/products/oleo-andiroba#! (accessed on 19 December 2022).
- Conab. Boletim da Sociobiodiversidade; Conab: Brasília, Brazil, 2017; Volume 1, p. 67.
- Souza, C.R.; Lima, R.M.B.; Azevedo, C.P.; Rossi, L.M.B. Andiroba (Carapa guianensis Aubl.); Embrapa Amazônia Ocidental: Brasilia, Brazil, 2006; 21p. [Google Scholar]
- Oliveira, F.; Souza, C.E.; Peclat, V.R.O.L.; Salgado, J.M.; Ribeiro, B.D.; Coelho, M.A.Z.; Venâncio, A.; Belo, I. Optimization of lipase production by Aspergillus ibericus from oil cakes and its application in esterification reactions. Food Bioprod. Process. 2017, 102, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Enhancing the bioproduction of value-added aroma compounds via solid-state fermentation of sugarcane bagasse and sugar beet molasses: Operational strategies and scaling-up of the process. Bioresour. Technol. 2018, 263, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ano, T.; Jin, G.Y.; Mizumoto, S.; Rahman, M.S.; Okuno, K.; Shoda, M. Solid state fermentation of lipopeptide antibiotic iturin A by using a novel solid state fermentation reactor system. J. Environ. Sci. 2009, 21 (Suppl. S1), S162–S165. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Karp, S.G.; Oliveira, P.Z.; Carvalho, J.C.; Rodrigues, C.; Soccol, C.R. Chapter 18—Solid-State Fermentation for the Production of Organic Acids. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 415–434. [Google Scholar]
- Sala, A.; Vittone, S.; Barrena, R.; Sánchez, A.; Artola, A. Scanning agro-industrial wastes as substrates for fungal biopesticide production: Use of Beauveria bassiana and Trichoderma harzianum in solid-state fermentation. J. Environ. Manag. 2021, 295, 113113. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.S.; Sant’Ana, G.C.F.; Amaral, P.F.F. Mango agro-industrial wastes for lipase production from Yarrowia lipolytica and the potential of the fermented solid as a biocatalyst. Food Bioprod. Process. 2019, 115, 68–77. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Amaral, P.F.F.; Coelho, M.A.Z.; Gonçalves, L.R.B. Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2014, 101, 148–158. [Google Scholar] [CrossRef]
- Treichel, H.; Oliveira, D.; Mazutti, M.A.; Di Luccio, M.; Oliveira, J.V. A Review on Microbial Lipases Production. Food Bioprocess Technol. 2010, 3, 182–196. [Google Scholar] [CrossRef]
- Mehta, A.; Guleria, S.; Sharma, R.; Gupta, R. 6—The lipases and their applications with emphasis on food industry. In Microbial Biotechnology in Food and Health; Ray, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 143–164. [Google Scholar]
- Aarthy, M.; Saravanan, P.; Ayyadurai, N.; Gowthaman, M.K.; Kamini, N.R. A two step process for production of omega 3-polyunsaturated fatty acid concentrates from sardine oil using Cryptococcus sp. MTCC 5455 lipase. J. Mol. Catal. B Enzym. 2016, 125, 25–33. [Google Scholar] [CrossRef]
- Nascimento, F.V.; Castro, A.M.; Secchi, A.R.; Coelho, M.A.Z. Insights into media supplementation in solid-state fermentation of soybean hulls by Yarrowia lipolytica: Impact on lipase production in tray and insulated packed-bed bioreactors. Biochem. Eng. J. 2021, 166, 107866. [Google Scholar] [CrossRef]
- Sales, J.C.S.; Castro, A.M.; Ribeiro, B.D.; Coelho, M.A.Z. Supplementation of watermelon peels as an enhancer of lipase and esterase production by Yarrowia lipolytica in solid-state fermentation and their potential use as biocatalysts in poly(ethylene terephthalate) (PET) depolymerization reactions. Biocatal. Biotransform. 2020, 38, 457–468. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; de Barros, D.S.N.; Fernandez-Lafuente, R.; Freire, D.M.G. Production of lipases in cottonseed meal and application of the fermented solid as biocatalyst in esterification and transesterification reactions. Renew. Energy 2019, 130, 574–581. [Google Scholar] [CrossRef]
- Souza, C.E.C.; Farias, M.A.; Ribeiro, B.D.; Coelho, M.A.Z. Adding Value to Agro-industrial Co-products from Canola and Soybean Oil Extraction Through Lipase Production Using Yarrowia lipolytica in Solid-State Fermentation. Waste Biomass Valorization 2017, 8, 1163–1176. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Zhang, L.; Ding, Z.; Shi, G. Understanding and application of Bacillus nitrogen regulation: A synthetic biology perspective. J. Adv. Res. 2022; in press. [Google Scholar] [CrossRef]
- Almeida, A.F.; Taulk-Tornisielo, S.M.; Carmona, E.C. Influence of carbon and nitrogen sources on lipase production by a newly isolated Candida viswanathii strain. Ann. Microbiol. 2013, 63, 1225–1234. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z.; AbdulKarim, M.I.; Salleh, H.M. Lipase production: An insight in the utilization of renewable agricultural residues. Resour. Conserv. Recycl. 2012, 58, 36–44. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; He, A.; Xia, J.; He, J.; Deng, Y.; Xu, N.; Qiu, Z.; Wang, X.; Zhao, P. One-pot fermentation for erythritol production from distillers grains by the co-cultivation of Yarrowia lipolytica and Trichoderma reesei. Bioresour. Technol. 2022, 351, 127053. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, H.; Alahuhta, M.; Chen, X.; Hyman, D.; Johnson, D.K.; Zhang, M.; Himmel, M.E. Heterologous Expression of Xylanase Enzymes in Lipogenic Yeast Yarrowia lipolytica. PLoS ONE 2014, 9, e111443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, F.V.d.; Lemes, A.C.; Castro, A.M.d.; Secchi, A.R.; Zarur Coelho, M.A. A Temporal Evolution Perspective of Lipase Production by Yarrowia lipolytica in Solid-State Fermentation. Processes 2022, 10, 381. [Google Scholar] [CrossRef]
- Mandari, V.; Nema, A.; Devarai, S.K. Sequential optimization and large scale production of lipase using tri-substrate mixture from Aspergillus niger MTCC 872 by solid state fermentation. Process Biochem. 2020, 89, 46–54. [Google Scholar] [CrossRef]
- Mala, J.G.; Edwinoliver, N.G.; Kamini, N.R.; Puvanakrishnan, R. Mixed substrate solid state fermentation for production and extraction of lipase from Aspergillus niger MTCC 2594. J. Gen. Appl. Microbiol. 2007, 53, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Barth, G.; Gaillardin, C. Yarrowia lipolytica. In Nonconventional Yeasts in Biotechnology; Wolf, K., Ed.; Springer: New York, NY, USA, 1996; pp. 313–388. [Google Scholar]
- Moftah, O.A.; Grbavčić, S.; Zuža, M.; Luković, N.; Bezbradica, D.; Knežević-Jugović, Z. Adding value to the oil cake as a waste from oil processing industry: Production of lipase and protease by Candida utilis in solid state fermentation. Appl. Biochem. Biotechnol. 2012, 166, 348–364. [Google Scholar] [CrossRef]
- Contesini, F.J.; da Silva, V.C.; Maciel, R.F.; de Lima, R.J.; Barros, F.F.; de Oliveira Carvalho, P. Response surface analysis for the production of an enantioselective lipase from Aspergillus niger by solid-state fermentation. J. Microbiol. 2009, 47, 563–571. [Google Scholar] [CrossRef]
- Rigo, E.; Ninow, J.L.; Di Luccio, M.; Oliveira, J.V.; Polloni, A.E.; Remonatto, D.; Arbter, F.; Vardanega, R.; Oliveira, D.; Treichel, H. Lipase production by solid fermentation of soybean meal with different supplements. LWT Food Sci. Technol. 2010, 43, 1132–1137. [Google Scholar] [CrossRef]
- Sun, S.Y.; Xu, Y. Solid-state fermentation for ‘whole-cell synthetic lipase’ production from Rhizopus chinensis and identification of the functional enzyme. Process Biochem. 2008, 43, 219–224. [Google Scholar] [CrossRef]
- Farias, M.A.; Valoni, E.A.; Castro, A.M.; Coelho, M.A.Z. Lipase Production by Yarrowia Lipolytica in Solid State Fermentation Using Different Agro Industrial Residues. Chem. Eng. Trans. 2014, 38, 301–306. [Google Scholar]
- Sidhu, P.; Sharma, R.; Soni, S.K.; Gupta, J.K. Production of extracellular alkaline lipase by a new thermophilicBacillus sp. Folia Microbiol. 1998, 43, 51–54. [Google Scholar] [CrossRef]
- Corzo, G.; Revah, S. Production and characteristics of the lipase from Yarrowia lipolytica 681. Bioresour. Technol. 1999, 70, 173–180. [Google Scholar] [CrossRef]
- Souza, C.E.C.; Ribeiro, B.D.; Coelho, M.A.Z. Characterization and Application of Yarrowia lipolytica Lipase Obtained by Solid-State Fermentation in the Synthesis of Different Esters Used in the Food Industry. Appl. Biochem. Biotechnol. 2019, 189, 933–959. [Google Scholar] [CrossRef]
- Fickers, P.; Fudalej, F.; Dall, M.T.L.; Casaregola, S.; Gaillardin, C.; Thonart, P.; Nicaud, J.M. Identification and characterisation of LIP7 and LIP8 genes encoding two extracellular triacylglycerol lipases in the yeast Yarrowia lipolytica. Fungal Genet. Biol. 2005, 42, 264–274. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Hsiao, F.S.-H.; Wen, C.-M.; Wu, C.-Y.; Dybus, A.; Yu, Y.-H. Mixed fermentation of soybean meal by protease and probiotics and its effects on the growth performance and immune response in broilers. J. Appl. Anim. Res. 2019, 47, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, K.; Paul Raj, R. Selective enrichment of n−3 polyunsaturated fatty acids with C18–C20 acyl chain length from sardine oil using Pseudomonas fluorescens MTCC 2421 lipase. Food Chem. 2009, 114, 142–150. [Google Scholar] [CrossRef]
- Zarai, Z.; Eddehech, A.; Rigano, F.; Oteri, M.; Micalizzi, G.; Dugo, P.; Mondello, L.; Cacciola, F. Characterization of monoacylglycerols and diacylglycerols rich in polyunsaturated fatty acids produced by hydrolysis of Musteleus mustelus liver oil catalyzed by an immobilized bacterial lipase. J. Chromatogr. A 2020, 1613, 460692. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Wang, L.; Cui, Q. Comprehensive analysis of metabolic alterations in Schizochytrium sp. strains with different DHA content. J. Chromatogr. B 2020, 1160, 122193. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, W.; Cheng, X.; Dong, Z.; Chang, M.; Wang, X. Enzymatic enrichment of n-3 polyunsaturated fatty acid glycerides by selective hydrolysis. Food Chem. 2021, 346, 128743. [Google Scholar] [CrossRef]
- Okada, T.; Morrissey, M.T. Production of n-3 polyunsaturated fatty acid concentrate from sardine oil by immobilized Candida rugosa lipase. J. Food Sci. 2008, 73, C146–C150. [Google Scholar] [CrossRef]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chu, W.; Sun, J.; Mao, X. Identification of an alkaline lipase capable of better enrichment of EPA than DHA due to fatty acids selectivity and regioselectivity. Food Chem. 2020, 330, 127225. [Google Scholar] [CrossRef]
- Martins, P.A.; Trobo-Maseda, L.; Lima, F.A.; de Morais Júnior, W.G.; De Marco, J.L.; Salum, T.F.C.; Guisán, J.M. Omega-3 production by fish oil hydrolysis using a lipase from Burkholderia gladioli BRM58833 immobilized and stabilized by post-immobilization techniques. Biochem. Biophys. Rep. 2022, 29, 101193. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C.; Egea, M.B.; Oliveira Filho, J.G.d.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds from Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C.; Sala, L.; Ores, J.D.C.; Braga, A.R.C.; Egea, M.B.; Fernandes, K.F. A Review of the Latest Advances in Encrypted Bioactive Peptides from Protein-Rich Waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [Green Version]
- Lemes, A.C.; Coelho, M.A.Z.; Gautério, G.V.; Paula, L.C.; Filho, J.G.d.O.; Egea, M.B. Chapter 11—Industrial Wastes and By-products: A Source of Functional Foods, Nutraceuticals, and Biopolymers. In Biopolymers in Nutraceuticals and Functional Foods; The Royal Society of Chemistry: London, UK, 2023; pp. 329–360. [Google Scholar]
- Pintado, M.E.; Teixeira, J.A. Valorização de subprodutos da indústria alimentar: Obtenção de ingredientes de valor acrescentado. Bol. Biotecnol. 2015, 1, 10–12. [Google Scholar]
- Hagler, A.N.; Mendonça-Hagler, L.C. Yeasts from marine and estuarine waters with different levels of pollution in the state of rio de janeiro, Brazil. Appl. Environ. Microbiol. 1981, 41, 173–178. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Castro, A.M.d.; Castilho, L.d.R.; Freire, D.M.G. Characterization of babassu, canola, castor seed and sunflower residual cakes for use as raw materials for fermentation processes. Ind. Crops Prod. 2016, 83, 140–148. [Google Scholar] [CrossRef]
- Freire, D.M.; Teles, E.M.; Bon, E.P.; Sant’ Anna, G.L., Jr. Lipase production by Penicillium restrictum in a bench-scale fermenter: Effect of carbon and nitrogen nutrition, agitation, and aeration. Appl. Biochem. Biotechnol. 1997, 63–65, 409–421. [Google Scholar] [CrossRef]
- Charney, J.; Tomarelli, R.M. A colorimetric method for the determination of the proteolytic activity of duodenal juice. J. Biol. Chem. 1947, 171, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]


| Component | Andiroba Oil Cake (%) | Soybean Meal (%) |
|---|---|---|
| Proteins | 13.7 ± 1.2 | 48.3 ± 0.64 |
| Ethereal extract | 26.9 ± 0.66 | 2.4 ± 0.04 |
| Carbohydrates | 0.4 ± 0.92 | 14.0 ± 3.68 |
| Ashes | 4.5 ± 0.00 | 6.3 ± 0.05 |
| Insoluble fibers | 45.6 ± 0.95 | 18.0 ± 1.58 |
| Cellulose | 14.4 ± 0.85 | 6.9 ± 0.40 |
| Lignin | 22.7± 1.02 | 0.2 ± 0.10 |
| Hemicellulose | 8.5 ± 0.85 | 10.9 ± 0.58 |
| Porosity (m3air·m−3bed) | 0.425 | 0.412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.S.S.; Sales, J.C.S.; Nascimento, F.V.d.; Ribeiro, B.D.; Souza, C.E.C.d.; Lemes, A.C.; Coelho, M.A.Z. Lipase Production by Yarrowia lipolytica in Solid-State Fermentation Using Amazon Fruit By-Products and Soybean Meal as Substrate. Catalysts 2023, 13, 289. https://doi.org/10.3390/catal13020289
Carvalho ASS, Sales JCS, Nascimento FVd, Ribeiro BD, Souza CECd, Lemes AC, Coelho MAZ. Lipase Production by Yarrowia lipolytica in Solid-State Fermentation Using Amazon Fruit By-Products and Soybean Meal as Substrate. Catalysts. 2023; 13(2):289. https://doi.org/10.3390/catal13020289
Chicago/Turabian StyleCarvalho, Aparecida Selsiane Sousa, Júlio Cesar Soares Sales, Felipe Valle do Nascimento, Bernardo Dias Ribeiro, Carlos Eduardo Conceição de Souza, Ailton Cesar Lemes, and Maria Alice Zarur Coelho. 2023. "Lipase Production by Yarrowia lipolytica in Solid-State Fermentation Using Amazon Fruit By-Products and Soybean Meal as Substrate" Catalysts 13, no. 2: 289. https://doi.org/10.3390/catal13020289