Abstract
Solving high electrical-energy input for pollutants degradation is one of the core requirements for the practical application of photoelectrocatalytic (PEC) technology. Herein, we developed a self-driven dual-photoelectrode PEC system (TiO2 NNs-Co3O4) composed of a TiO2 nanoneedle arrays (TiO2 NNs) photoanode and Co3O4 photocathode for the first time. Under light-emitting-diode (LED) illumination, the bias-free TiO2 NNs-Co3O4 PEC system exhibited excellent PEC performance, with an internal bias as high as 0.19 V, achieving near complete degradation (99.62%) of sulfamethazine (SMT) with a pseudo-first-order rate constant of 0.042 min−1. The influences of solution pH, typical inorganic anions, natural organic matter, and initial SMT concentration on the PEC performance were investigated. Moreover, the main reactive oxygen species (h+, •OH, •O2−) in the dual-photoelectrode PEC system for SMT decomposition were elaborated. The practical application feasibility for efficient water purification of this unbiased PEC system was evaluated. It was proved that the TiO2 NNs photoanode provided a negative bias while the Co3O4 photocathode provided a positive bias for the photoanode, which made this system operate without external bias. This work elucidated the cooperative mechanism of photoelectrodes, providing guidance to develop a sustainable, efficient, and energy-saving PEC system for wastewater treatment.
1. Introduction
As a typical sulfonamide antibiotic, sulfamethazine (SMT) has been used frequently in aquaculture and animal husbandry [1]. SMT has been detected in various aquatic environments, such as underground water, seawater, and river water, and its concentration in the aquatic environment ranges from ng L−1 to μg L−1 [2]. The residue of SMT in aquatic environments may increase bacterial resistance and can bring potential risk to the ecological system and human health through bioaccumulation in the food chain [3,4]. However, traditional bioprocesses cannot efficiently remove SMT, due to its biodegradation resistance [5,6]. Therefore, there is an urgent need to develop a cost-effective and environmentally friendly method to remove SMT.
Advanced oxidation processes (AOPs) have been widely used for degrading refractory organic pollutants [7]. Among various AOPs, photoelectrocatalysis (PEC) has attracted extensive attention for its efficient ability to remove organic pollutants without producing any harmful by-products [8,9]. Compared with photocatalytic technologies, PEC can accelerate the photogenerated carrier separation and transfer by applying the bias, which achieves a better photoelectric performance. Unfortunately, traditional PEC systems require an external bias to drive the PEC process, which means additional cost for the external power. To lower energy demand, a self-driven dual-photoelectrode PEC system connected with a photoanode and photocathode has been developed [10,11]. Specifically, the dual-photoelectrode PEC system includes two light absorbers: photoanode and photocathode, where the Fermi level difference between photoelectrodes will form an interior photovoltage [12]. The interior photovoltage can offset the electric energy input of the PEC system required to drive the reaction of pollutants removal [13].
In addition, the development of PEC technology has greatly promoted the application of energy-saving and green light-emitting diodes (LEDs). Compared with traditional light sources (such as xenon lamp, halogen lamp), the LED lamp has the advantages of low cost, long service life, low heat production, small size, and high energy conversion, and can be used as the excitation light source of semiconductor materials [14,15]. Therefore, the LED lamp with low energy consumption has great potential in PEC technology to solve environmental problems.
Herein, the novel dual-photoelectrode PEC system was developed, which could efficiently degrade SMT without external voltage. In this self-driven PEC system (named TiO2 NNs-Co3O4), two 30 W LED lamps were served as the light source, TiO2 nanoneedle arrays (TiO2 NNs, n-type semiconductor) were used as the photoanode, and Co3O4 (p-type semiconductor) was selected as the photocathode. Afterward, the catalytic performance and operating mechanism of the unbiased TiO2 NNs-Co3O4 PEC system under LED light irradiation was systematically studied. The effects of solution pH, typical anions, natural organic matter, initial SMT concentration, recycling, and actual water matrices on the catalytic performance of the TiO2 NNs-Co3O4 system were also deeply investigated to evaluate the practical feasibility.
2. Results and Discussion
2.1. Characterization of Photoelectrodes
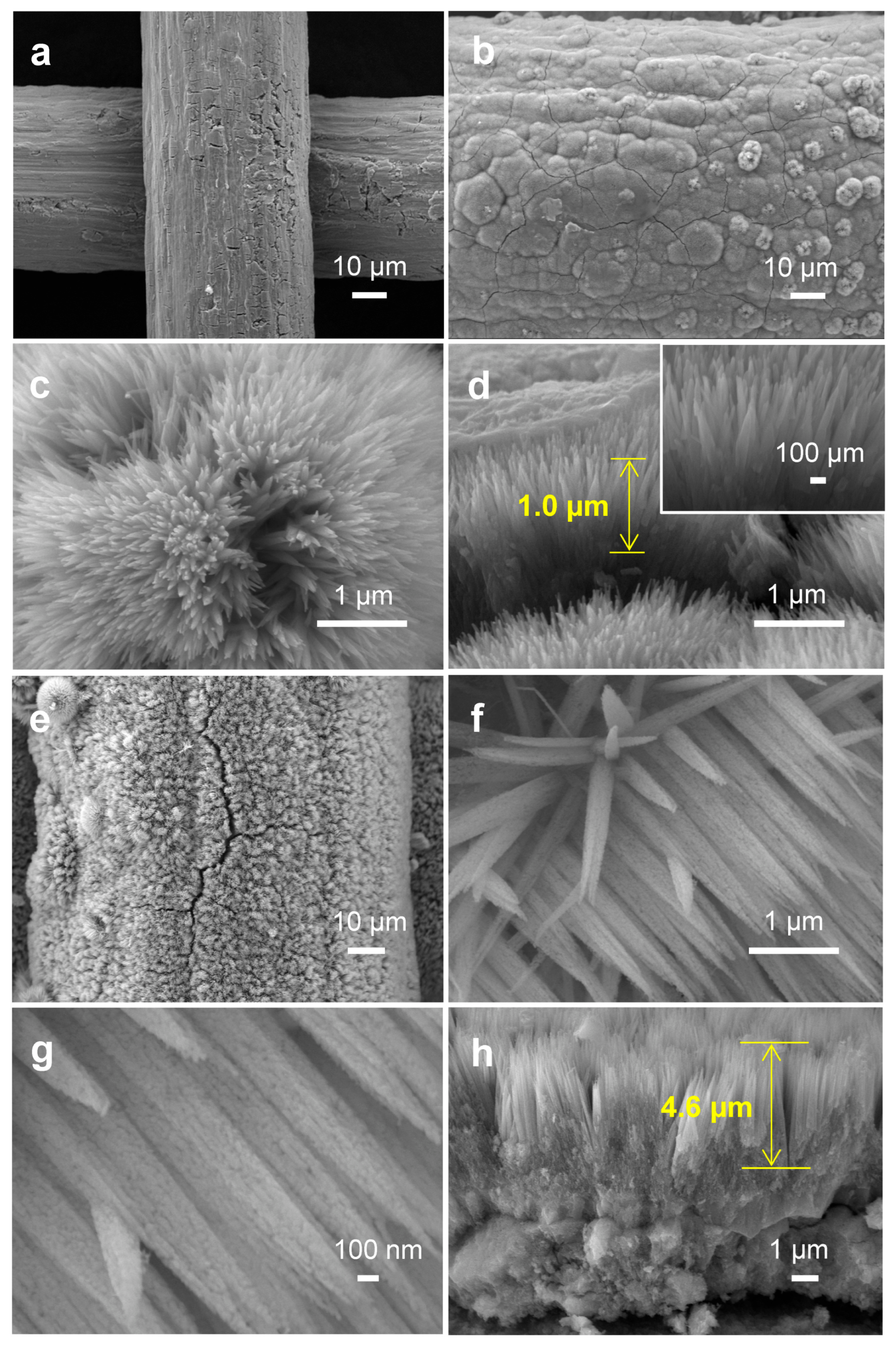
The FESEM images of the Ti mesh in Figure 1a manifested that the surface of the Ti mesh was rough and there were a large number of fine grids, which might be conducive to the growth of catalysts [16]. After hydrothermal and annealing treatment, TiO2 was densely and radially grown around Ti wires to form nanoneedle arrays, whose length was about 1.0 µm. The results were consistent with the literature (Figure 1b–d) [17]. In addition, the morphology of the Co3O4 photocathode was shown in Figure 1e–h. It could be seen from Figure 1e,f that Co3O4 nanoneedle arrays were evenly distributed onto the Ti mesh support, and the Co3O4 nanoneedle had a length of ~4.6 µm, which was composed of densely packed, small nanoparticles (Figure 1g,h) [18].
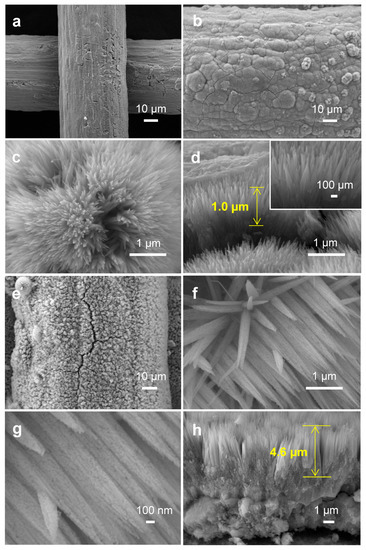
Figure 1.
(a) FESEM image of Ti mesh. (b,c) FESEM image and (d) cross-sectional FESEM image of TiO2 NNs. (e–g) FESEM image and (h) cross-sectional FESEM image of Co3O4.
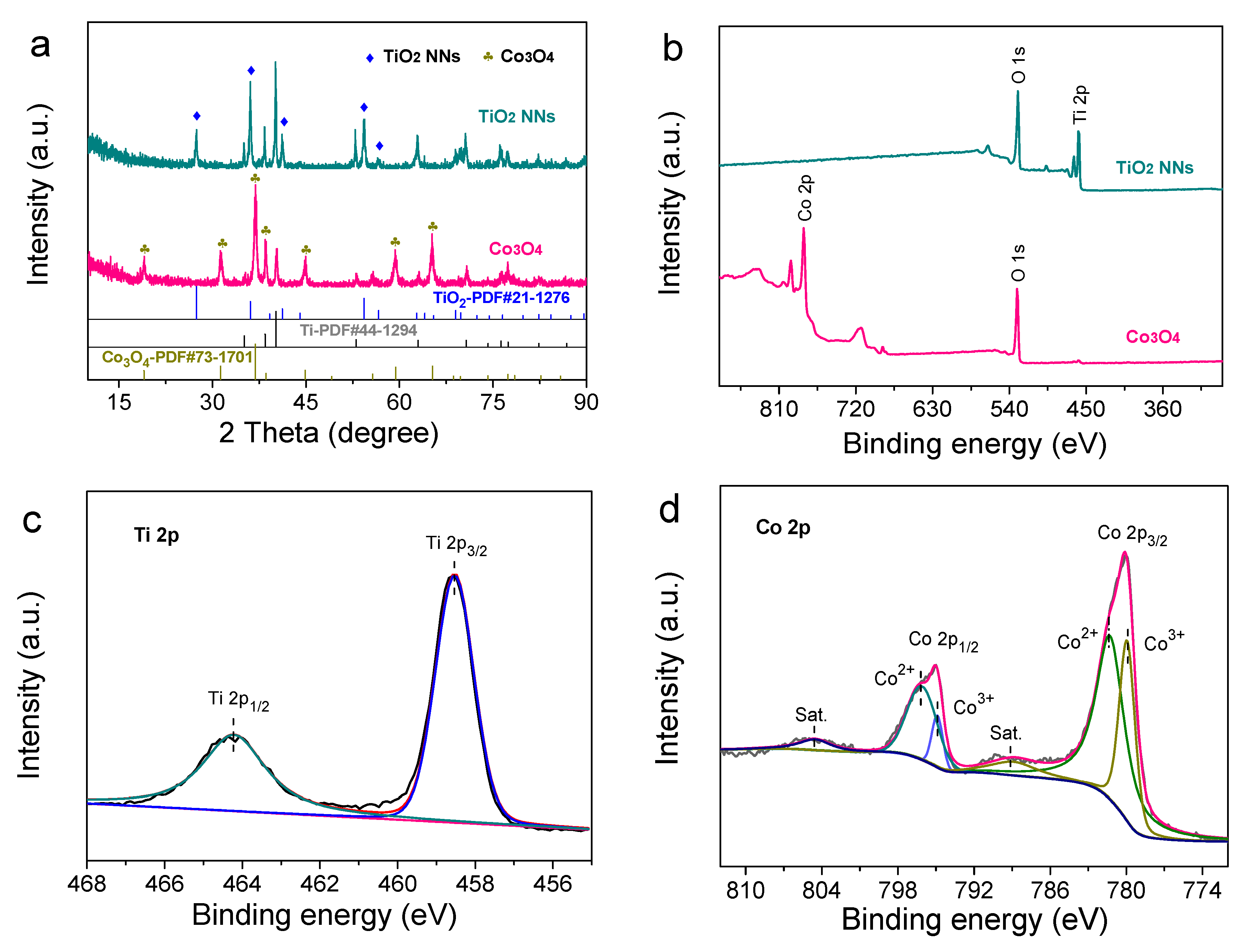
XRD patterns were recorded in Figure 2a. Notably, the dominant diffraction peaks of Co3O4 at 19.0°, 31.2°, 36.8°, 38.5°, 44.8°, 59.3°, and 65.2° might be assigned to the (111), (220), (311), (222), (400), (511), and (440) planes of cubic structure (JCPDS No. 73-1701). Similarly, through diffraction peaks of TiO2 NNs, it was postulated that the XRD pattern of the TiO2 NNs electrode was associated with tetragonal rutile TiO2 (JCPDS No. 21-1276), in agreement with a previous report [19]. The additional peaks on the XRD patterns of the two electrodes should be due to the Ti mesh, and there was no impurity diffraction peak [20]. The results confirmed that TiO2 NNs and Co3O4 photocatalysts had been successfully grown on the Ti mesh.
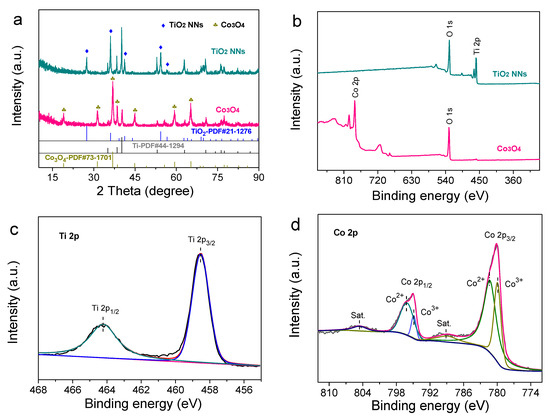
Figure 2.
(a) XRD patterns of TiO2 NNs, Co3O4. (b) The full XPS spectra recorded from the surface for TiO2 NNs and Co3O4. High-resolution XPS spectra of Ti 2p (c) and Co 2p (d).
In addition, XPS was employed to investigate the composition and chemical states of the element. It could be seen from Figure 2b that the Ti and O were included in the XPS full-survey spectra of the TiO2 NNs photoanode, and the Co and O were included in the XPS full-survey spectra of the Co3O4 photocathode. The peaks were located at 458.5 eV and 464.2 eV in the high-resolution Ti 2p XPS spectrum corresponding to the Ti4+ 2p3/2 and Ti4+ 2p1/2 in the TiO2 lattice, which was consistent with a previous report (Figure 2c) [21]. For the Co3O4 photocathode, the prominent peaks of Co 2p spectra were centered at 779.9 eV (Co3+), 781.2 eV (Co2+), 794.8 eV (Co3+), and 796.1 eV (Co2+), indicating that Co2+ and Co3+ coexisted (Figure 2d) [22]. Based on the above results, it could be perceived that the TiO2 NNs photoanode and Co3O4 photocathode were successfully prepared.
2.2. Photoelectric Properties
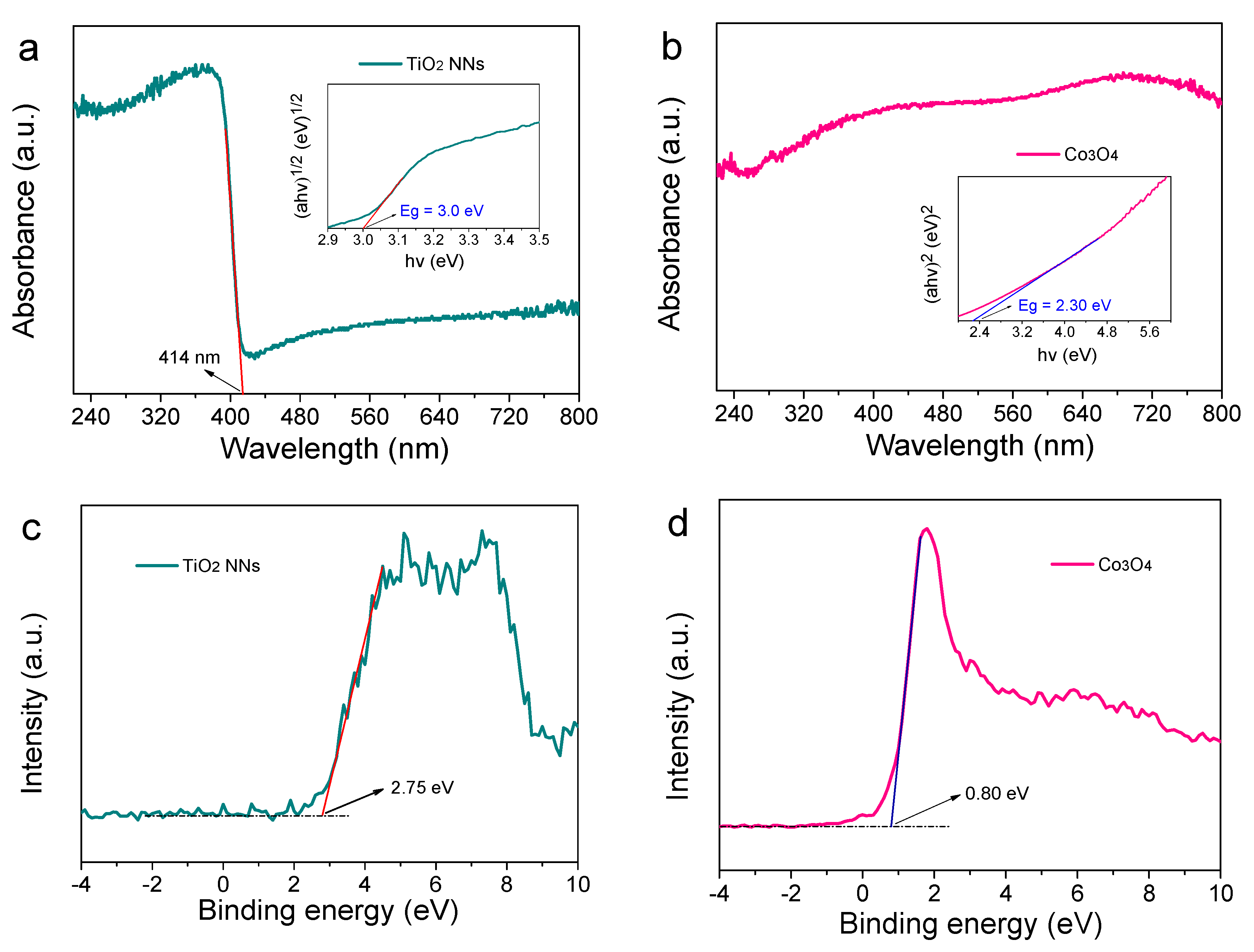
The light absorption ability and energy band position were studied. As demonstrated in Figure 3a,c, the absorption edge and valence band (EVB) of the TiO2 NNs photoanode were around 414 nm and 2.75 eV, respectively. The bandgap (Eg) and conduction band (ECB) were calculated by using Equations (1) and (2) [23].
where , h, and are the coefficient, the frequency of light, and Plank’s constant, respectively; A is the proportionality constant; 0.5 indicates direct-bandgap semiconductors and 2 indicates indirect semiconductors. Thus, the Eg and ECB of the TiO2 NNs photoanode were 3.01 eV and –0.26 eV, respectively, consistent with the literature [21]. Similar analysis was also carried out for the Co3O4 photocathode and the results were shown in Figure 3b,d. The Co3O4 photocathode could absorb ultraviolet and light, whose Eg, EVB, and ECB were 2.30 eV, 0.80 eV, and –1.50 eV, respectively. As expected, the Fermi level of the n-type TiO2 NNs semiconductor was close to conduction band (–0.26 eV), and the Fermi level of the p-type Co3O4 semiconductor was close to the valence band (0.80 eV) [24]. The TiO2 NNs photoanode and Co3O4 photocathode were combined to construct a novel dual-photoelectrode PEC system [12]. As is known, the internal bias was generated by the Fermi level difference of the electrodes, which made it possible to establish a self-driven TiO2 NNs-Co3O4 PEC system [25].
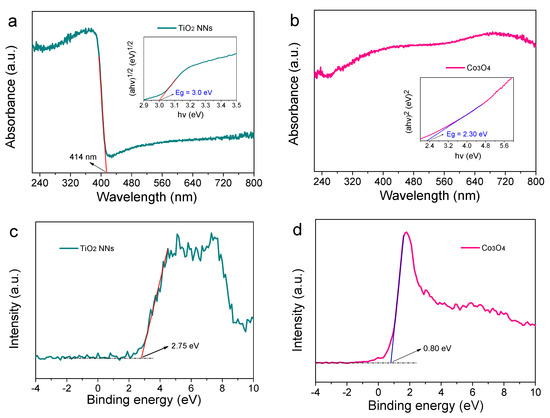
Figure 3.
UV-Vis diffuse reflectance spectra of TiO2 NNs photoanode with the inset of Taus’s plots of (αh)1/2 versus h (a), Co3O4 photocathode with the inset of Taus’s plots of (αh )2 versus h (b). VB XPS spectra of TiO2 NNs photoanode (c) and Co3O4 photocathode (d).
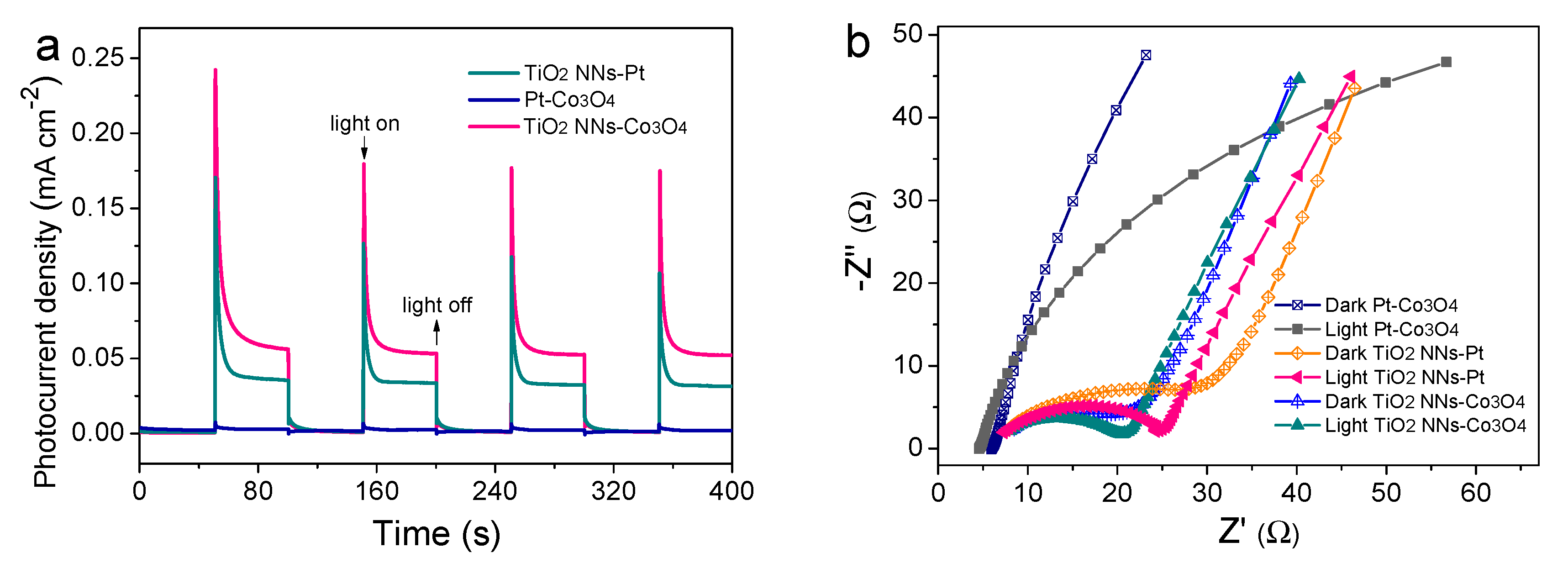
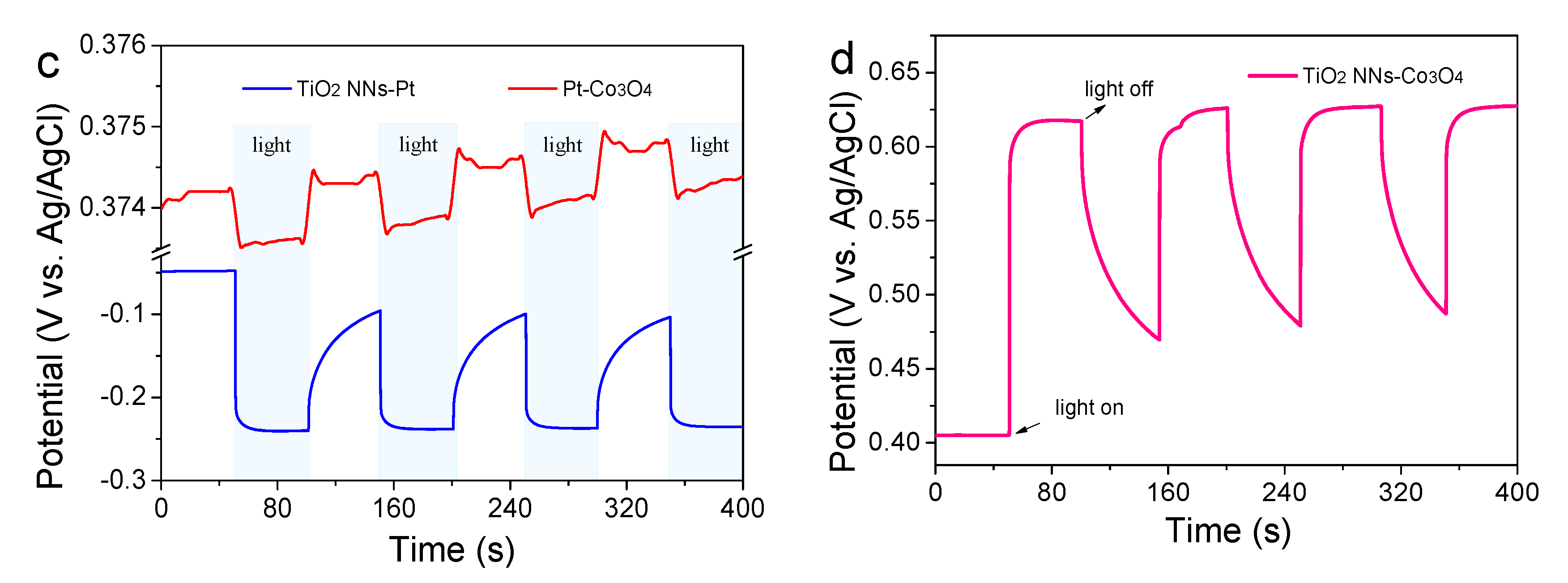
The transient photocurrent responses and EIS analysis were conducted in Na2SO4 solution with or without LED light illumination to examine the separation and transfer ability of photogenerated charge carriers. As shown in Figure 4a, the photocurrent of the dual-photoelectrode PEC system (TiO2 NNs-Co3O4) was 0.060 mA cm−2, which was about 1.67 times and 20 times higher than those of the TiO2 NNs-Pt (0.036 mA cm−2) and Pt-Co3O4 (0.004 mA cm−2) PEC system, respectively. In addition, it could be seen from Figure 4b and Table S2 that the radius of the TiO2 NNs-Co3O4 system was smaller than that of the single-photoelectrode (e.g., TiO2 NNs-Pt, Pt-Co3O4) PEC system both in the dark and under LED illumination, indicating that the dual-photoelectrode PEC system had lower charge transfer resistance [26]. Therefore, compared with TiO2 NNs-Pt and Pt-Co3O4, the dual-phot-electrode PEC system had excellent photogenerated charge carriers transfer performance, which was in agreement with recent research [25].
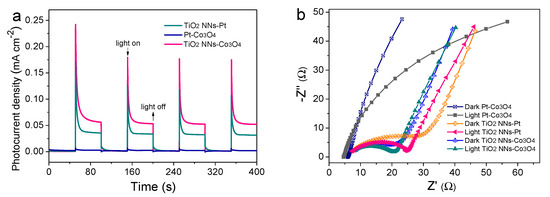
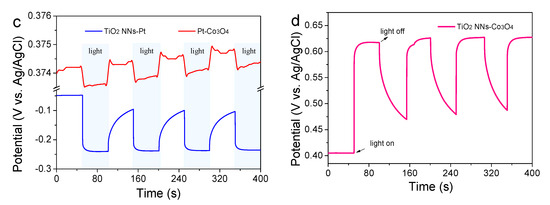
Figure 4.
(a) Transient photocurrent responses of TiO2 NNs-Pt, Pt-Co3O4, and TiO2 NNs-Co3O4. (b) EIS Nyquist plots with or without light irradiation. The Voc of (c) TiO2 NNs-Pt and Pt-Co3O4 PEC system in the dark and under LED light illumination, and (d) TiO2 NNs-Co3O4 PEC system.
To further clarify the peculiarity of the novel TiO2 NNs-Co3O4 system, we measured the open-circuit voltage (Voc) of different PEC systems (Figure 4c,d). Notably, the VOC values in the dark of the TiO2 NNs-Pt and Pt-Co3O4 PEC system were measured to be about 0.048 and 0.374 V vs. Ag/AgCl, respectively. Under illumination, the TiO2 NNs photoanode absorbed LED light to make Voc change to 0.238 V vs. Ag/AgCl, while the Voc of the Co3O4 photocathode showed a slight change. As reported in [12], the theoretical Voc of the TiO2 NNs-Co3O4 system was calculated to be about 0.422 V vs. Ag/AgCl in the dark and 0.612 V vs. Ag/AgCl with LED-light irradiation, which was almost consistent with the Voc curve of the dual-photoelectrode PEC system in Figure 4d. Consequently, the internal bias of the TiO2 NNs-Co3O4 system was around 0.19 V, where the TiO2 NNs photoanode provided a negative bias for the photocathode and the Co3O4 photocathode provided a positive bias for the photoanode, in agreement with LSV results (Figure S1) [27]. This phenomenon confirmed that the TiO2 NNs-Co3O4 PEC system would produce larger internal bias than the single-photoelectrode system (TiO2 NNs-Pt, Pt-Co3O4) under light irradiation, which had the potential to enable this dual-photoelectrode PEC system to self-drive and efficiently degrade organic pollutants without external bias [12,24].
2.3. Photoelectrochemical Degradation of SMT in TiO2 NNs-Co3O4 System
2.3.1. Photoelectrochemical Performance
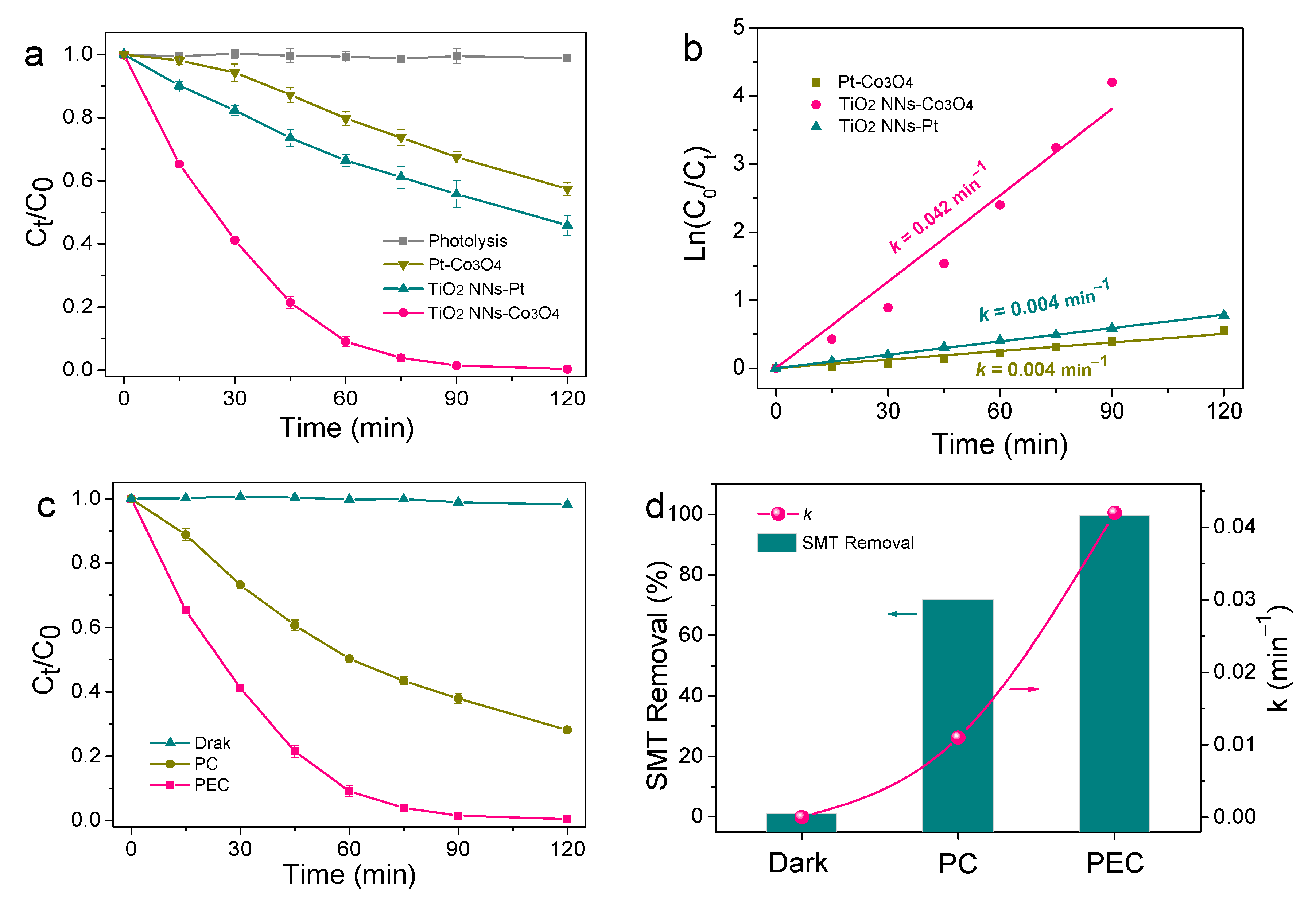
Photoelectrocatalytic activities of the TiO2 NNs-Pt, Pt-Co3O4, and TiO2 NNs-Co3O4 PEC system for SMT degradation were tested under LED lamp irradiation. As seen from Figure 5a, no appreciable photolysis of SMT was observed. In comparison, the degradation efficiency of SMT in the TiO2 NNs-Pt and Pt-Co3O4 PEC system increased to 54.04% and 42.53%, respectively. These enhanced catalytic activities might be due to the production of photogenerated electrons and holes with redox activity by TiO2 NNs or Co3O4 under LED light illumination. Obviously, about 99.62% SMT could be removed within 120 min of the self-driven TiO2 NNs-Co3O4 PEC system, possibly associating with the increased light absorbance ability and elevated separation rate of photogenerated electrons and holes [12]. Moreover, the k of the self-driven TiO2 NNs-Co3O4 PEC system for SMT removal was 0.042 min−1, which was about 6 and 10.5 times as high as that of TiO2 NNs-Pt (0.007 min−1) and Pt-Co3O4 (0.004 min−1), respectively (Figure 5b). Therefore, the dual-photoelectrode PEC system of TiO2 NNs-Co3O4 possessed increased photoelectrocatalytic activity and water purification performance.

Figure 5.
(a) Degradation of SMT by direct photolysis, in Pt-Co3O4, TiO2 NNs-Pt, and TiO2 NNs-Co3O4; (b) the kinetics curves of the above systems. (c,d) Removal of SMT by dark, photocatalysis, and photoelectrochemical process using TiO2 NNs-Co3O4 PEC system.
The degradation efficiency of SMT in the TiO2 NNs-Co3O4 PEC system was also evaluated in the dark (without light), PC (without Cu wire), and self-driven PEC (with light and Cu wire) processes. As shown in Figure 5c,d, the removals of SMT in the TiO2 NNs-Co3O4 system were 1.12, 71.88, and 99.62% after 120 min of reaction, and the corresponding k were about 0, 0.011, and 0.042 min−1 in the processes of dark, PC, and self-driven PEC, respectively. The unbiased PEC process showed greatly improved performance in organic pollutant degradation compared to the dark and PC processes. This phenomenon could be attributed to the effectively suppressed recombination of photogenerated charge carriers in the PEC process under the external circuit [28]. Specifically, both the TiO2 NNs photoanode and Co3O4 photocathode could generate electrons and holes under the illumination of an LED lamp. Because the Fermi level of the TiO2 NNs photoanode was more negative than that of the Co3O4 photocathode, the TiO2 NNs-Co3O4 system produced a stable internal photovoltage (0.19 V) [12]. This photovoltage was key to establish a bias-free photoelectrocatalytic degradation system by the TiO2 NNs photoanode and Co3O4 photocathode [29]. In this case, the photovoltage drove the directional transfer of photoinduced charge carriers through the external circuit, thus enhancing the separation rate of photogenerated electron–hole pairs and finally exhibiting higher pollutant degradation activity. The performance comparison of the unbiased dual-photoelectrode PEC system in the literature for pollutant degradation is summarized in Table 1. It was clear that the self-driven TiO2 NNs-Co3O4 system had excellent performance and the energy consumption of the light source (LED lamp) used in this system was significantly lower than that of other unbiased PEC systems (Xe lamp).

Table 1.
The performance comparison of recently self-driven PEC system for wastewater treatment.
2.3.2. Effect of Solution pH
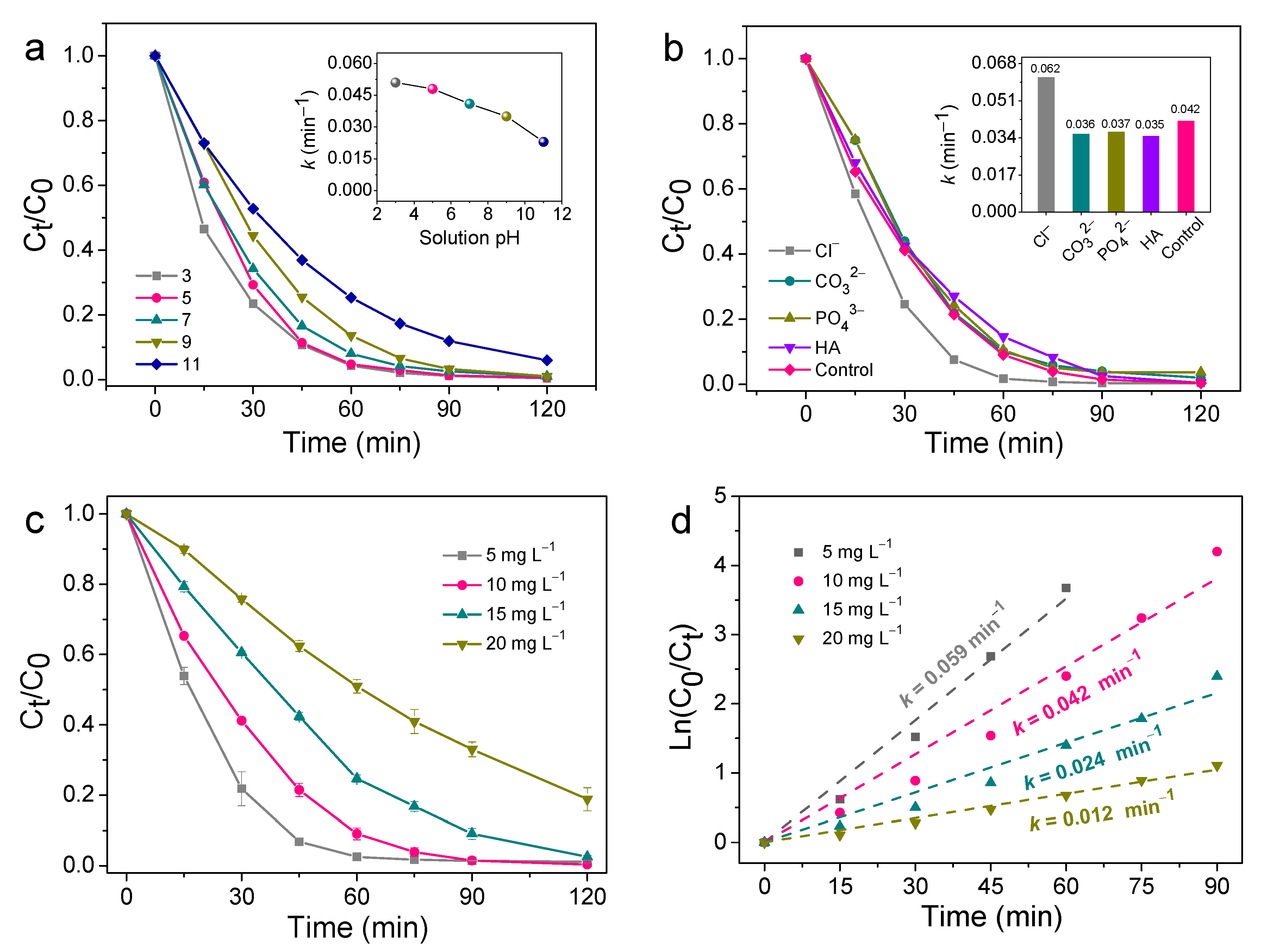
Figure 6a manifested how the initial solution pH affected SMT degradation in the unbiased TiO2 NNs-Co3O4 PEC system. When the pH was 3.0, 5.0, 7.0, 9.0, and 11.0, the degradation efficiency of SMT was 98.92% (0.051 min–1), 98.73% (0.048 min–1), 97.44% (0.041 min–1), 96.77% (0.035 min–1), and 80.89% (0.023 min–1) after 90 min of reaction. It was clear that the decomposition rate of SMT decreased with the increase in initial pH value. This might be due to the fact that pH was closely related to the formation of ROS and the interaction between electrolyte and electrode. Specifically, the •OH and h+ worked as the dominating role of SMT degradation, which were obtained by quenching experiments (part 2.4.1). There was a large amount of OH− in the alkaline environment, which could consume h+ to generate •OH, and the oxidation ability of •OH was lower than that of h+ [15]. In addition, according to the Nernst equation, the oxidation potential of •OH in basic medium was also lower than that in acidic conditions [36]. On the other hand, acidic circumstances had been reported to facilitate charge transfer between the photoelectrode and electrolyte in PEC degradation of organic pollutants [37]. Hence, the novel TiO2 NNs-Co3O4 PEC system had superior operative performance in acidic and slightly alkaline environments.
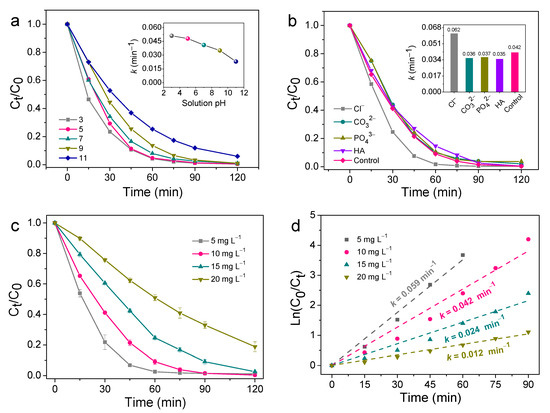
Figure 6.
Effects of (a) solution pH and (b) background species, and (c,d) initial SMT concentration on unbiased TiO2 NNs-Co3O4 PEC system for removal of SMT.
2.3.3. Effect of Background Species
Inorganic anions and natural organic matter (NOM) were ubiquitous in actual water matrix. Therefore, the effects of 10 mM typical anions (Cl−, CO32−, PO43−) and 10 mg L−1 of NOM (representing by HA) on the degradation of SMT by the self-driven TiO2 NNs-Co3O4 PEC system were investigated to evaluate the practical application prospects of this system. In the presence of CO32–, PO43–, and HA, the degradation rates of SMT were 97.95, 96.28, and 99.52%, and the corresponding k values were 0.36, 0.37, and 0.35 min–1, respectively. This slight inhibition effect might be attributed to the consumption of •OH or h+ by CO32–, PO43–, or HA, while •OH and h+ participated in the degradation of SMT (Figure 6b) [38,39,40]. Impressively, the presence of Cl− significantly accelerated SMT removal (99.60%, 0.062 min–1), which could be attributed to the formation of active chlorine species (•Cl, •ClO, HClO) in the bias-free TiO2 NNs-Co3O4 PEC system [41,42,43]. Therefore, bias-free TiO2 NNs-Co3O4 PEC had the potential for saline wastewater treatment.
2.3.4. Effect of Initial SMT Concentration
The degradation ability of the bias-free TiO2 NNs-Co3O4 PEC system at different SMT initial concentrations is shown in Figure 6c,d. The k for degradation of 5 mg/L of SMT was 0.059 min−1, which was about 1.40, 2.46, and 4.92 times higher than those of 10, 15, and 20 mg/L of SMT, respectively (Figure 6d). Additionally, in the case of 5 mg/L of SMT, about 98% SMT was decomposed within 60 min of irradiation, and the degradation rate still reached more than 97% after 90 (for concentration of 10 mg/L) and 120 min (for concentration of 15 mg/L) of LED light irradiation. When the initial concentration was increased to 20 mg/L, the removal rate was only 81.16% after 120 min of reaction, which could be attributed to the fact that the reactive oxygen species (ROS) generated in the PEC system were exhausted by excessive SMT (Figure 6c) [44]. Although k of SMT removal in this self-driven PEC system decreased with the increase in initial concentration, the degradation efficiency of SMT was high when the illumination time was prolonged.
2.4. Mechanism in TiO2 NNs-Co3O4 System
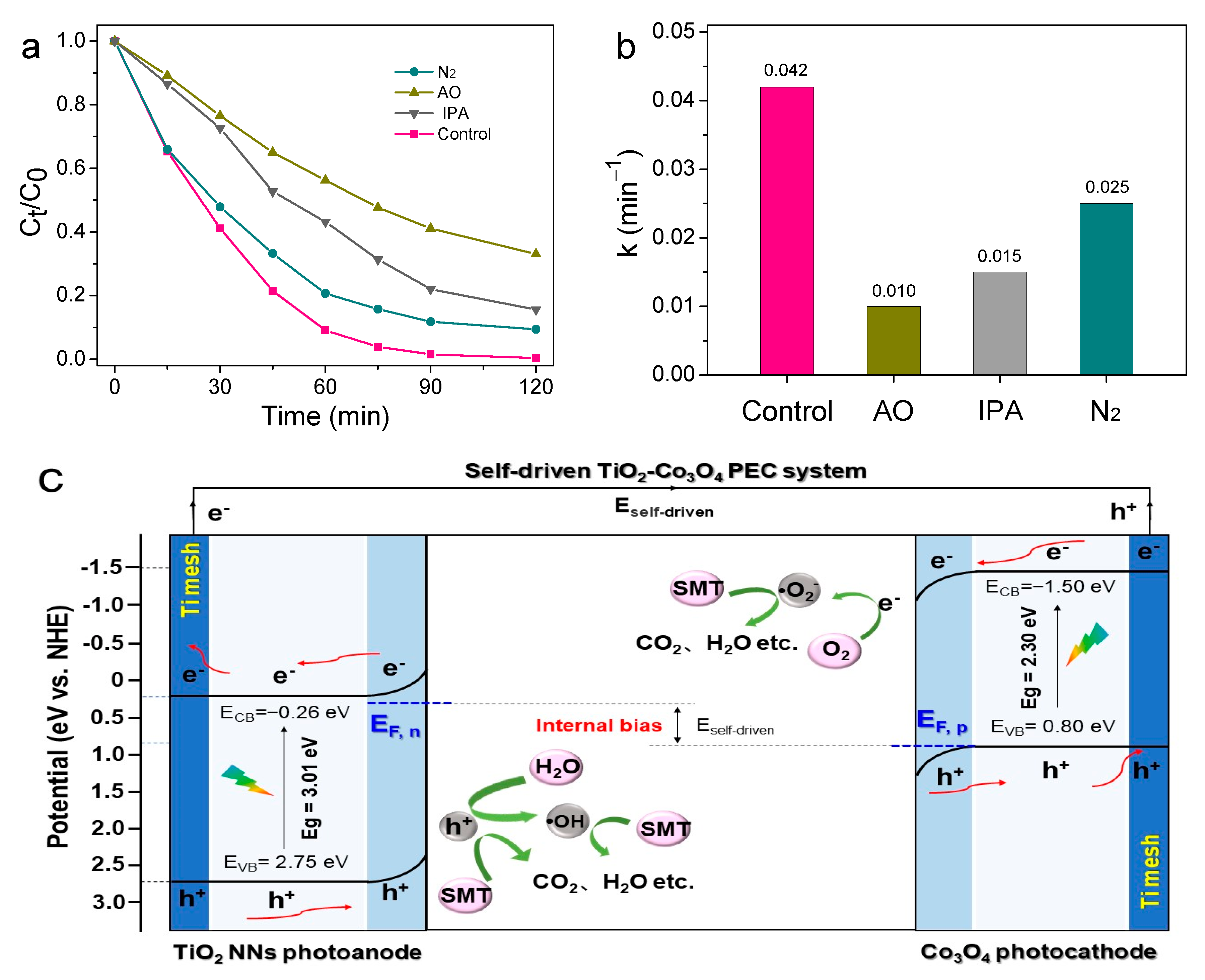
The quenching experiments were studied to clarify the contribution of the main ROS during SMT degradation in the unbiased TiO2 NNs-Co3O4 PEC system under visible LED light irradiation. Isopropanol (IPA, 100 mM) and ammonium oxalate (AO, 50 mM) were applied as h+ and •OH quenchers, respectively, and the results were displayed in Figure 7a. After adding AO to the reaction system, the degradation efficiency of SMT decreased to the lowest (66.96%) within 120 min, indicating that h+ was the dominant ROS. When IPA was added, the efficiency was reduced to 84.37%, implying that •OH participated in the degradation process of SMT. Moreover, we further confirmed the role of •O2− in the system by purging N2. The decomposition efficiency was slightly suppressed to 90.56%. To sum up, the contribution of different ROS for SMT removal in the unbiased TiO2 NNs-Co3O4 PEC system was in the order: h+ (0.010 min−1) > •OH (0.015 min−1) > •O2− (0.025 min−1) (Figure 7b).
Figure 7.
Effects of different quenching scavengers on SMT degradation efficiency (a) and reaction rate constant (b). Possible mechanism of SMT degradation of unbiased TiO2 NNs-Co3O4 PEC system under visible LED light irradiation (c).
Given these results, the operating mechanism of the unbiased TiO2 NNs-Co3O4 PEC system for SMT degradation was inferred in-depth. As observed in Figure 7c, under the LED light irradiation, the dual-photoelectrode PEC system produced internal bias (0.19 V) due to the difference in the Fermi level of the TiO2 NNs photoanode and Co3O4 photocathode [30]. Then, the photoinduced electrons of the TiO2 NNs photoanode were swiftly transferred to the Co3O4 photocathode driven by internal bias, which improved the separation rate of electron–hole pairs [45]. As a result, there would be more photoinduced holes in the photoanode and photogenerated electrons in the photocathode to participate in the oxidation–reduction reaction to generate ROS (•OH, •O2−), leading to the efficient degradation of SMT.
2.5. Practicability of TiO2 NNs-Co3O4 PEC System
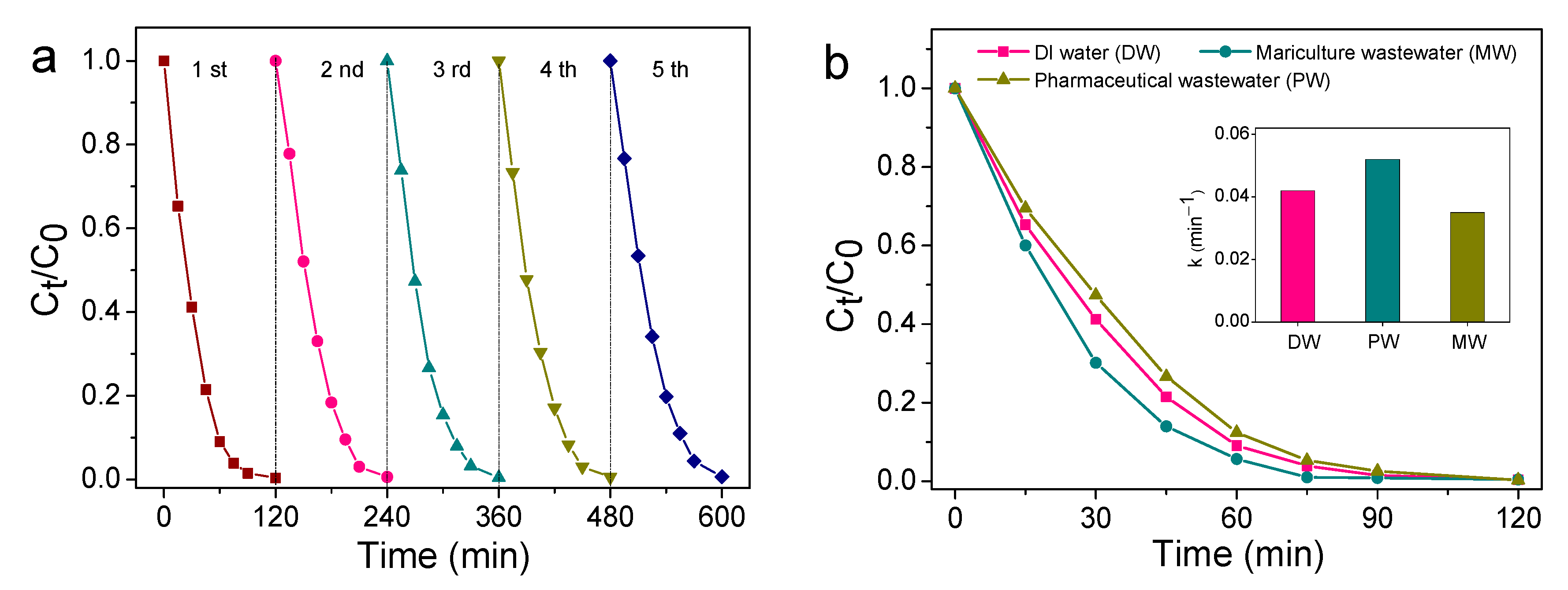
The practicability of the unbiased TiO2 NNs-Co3O4 PEC system was evaluated by the cycling experiment. As presented in Figure 8a, the degradation efficiency of SMT in the first, second, third, fourth, and fifth cycle experiments was 99.62%, 99.38%, 99.52%, 99.33%, and 99.40%, respectively. It was clear that the degradation capacity of SMT was almost unchanged after five degradation cycles, indicating that the bias-free TiO2 NNs-Co3O4 PEC system had superior stability. Furthermore, the catalytic performance of this unbiased dual-photoelectrode PEC system was detected in pharmaceutical wastewater and mariculture wastewater. The quality parameters of actual pharmaceutical wastewater (PW) and mariculture wastewater (MW) are listed in Table S1. As can be seen from Figure 8b, the k value of SMT degradation in pharmaceutical wastewater and mariculture wastewater was 0.035 min−1 and 0.052 min−1, respectively. The inhibitory action of pharmaceutical wastewater might be due to its complex composition, and the promoting effect of mariculture wastewater could be attributed to the presence of high concentrations of Cl−. This phenomenon was consistent with the results of the influence of background species. The remarkable catalytic performance for SMT degradation in pharmaceutical wastewater (99.70%, 120 min) and mariculture wastewater (98.98%, 75 min) indicated that self-driven TiO2 NNs-Co3O4 was a promising PEC system for water purification.
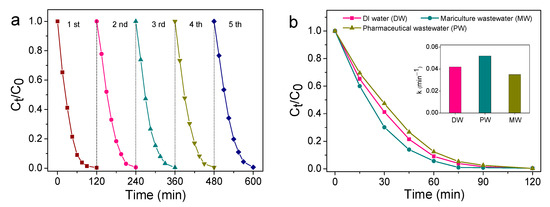
Figure 8.
Five consecutive cycling tests using TiO2 NNs-Co3O4 PEC system (a). PEC degradation of SMT in actual pharmaceutical wastewater and mariculture wastewater of TiO2 NNs-Co3O4 system (b).
3. Experimental Section
3.1. Photoelectrodes Preparation
The TiO2 NNs were grown on Ti mesh through a hydrothermal method reported previously [17]. Ti mesh (2.8 cm × 4 cm × 0.1 mm) was pretreated with acetone, ethanol, and deionized water before use. Briefly, 0.3 mL of titanium isopropoxide was dissolved in acetylacetone to form a yellow solution. Then, the above solution was slowly added to Na2EDTA solution and stirred for 30 min. The solution was then transferred into a Teflon-lined stainless-steel autoclave, which was heated at 200 °C for 12 h. The sample was annealed in a Muffle furnace at 500 °C (ramping rate: 2 °C min−1) for 1 h to obtain the TiO2 NNs electrode.
The Co3O4 electrode was synthesized by using a NH4F-assisted hydrothermal method recently reported [46]. Typically, 2.9 mmol Co(NO3)2•6H2O, 5.7 mmol NH4F, and 14.3 mmol CO(NH2)2 were dissolved in deionized water to form a homogeneous solution. Then, this solution along with pretreated Ti mesh was transferred into a 50 mL Teflon-lined autoclave, which was heated at 120 °C for 5 h. Subsequently, the obtained sample was calcined in air atmosphere at 350 °C for 2 h.
3.2. Characterizations
SEM images and structures of the prepared electrodes were investigated by field-emission scanning electron microscopy (FESEM, LEO-1530VP), and X-ray diffraction (XRD, Philips-12045 B/3 diffractometer with Cu-Kα irradiation). The X-ray photoelectron spectra (XPS) were determined on a Thermo Scientifc ESCALAB 250Xi with a monochromatic Al-Kα source. The UV-vis diffuse reflectance spectra (UV-vis DRS) were collected on a Shimadzu 2600 UV spectrophotometer by using BaSO4 as a reference. The cobalt leaching concentration after photoelectrocatalytic reaction was analyzed by ICP-MS (PerkinElmer ELAN DRC-e, Waltham, MA, USA). The ROS were detected by electron paramagnetic resonance (EPR, Bruker EMX Nano, Heidelberg, Germany) by employing 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin-trapping agent.
3.3. Photoelectrochemical Measurement
The photoelectrochemical measurement was analyzed using the standard three-electrode system on a CHI760E electrochemical station. All tests were carried out in 0.1 M Na2SO4 electrolyte by using Ag/AgCl as a reference electrode, and Pt foil and the as-prepared photoelectrode as the counter electrode and working electrode, respectively. The light source was two 30 W LED lamps. Electrochemical impedance spectroscopy (EIS) was measured at an open-circuit voltage from 100 kHz to 0.01 Hz. The transient photocurrent responses were collected without external bias under LED light illumination and in the dark. Linear sweep voltammetry (LSV) curves were recorded with the scan rate of 10 mV s−1.
3.4. Construction and Evaluation of Established PEC Systems
For simplicity, we named the TiO2 NNs photoanode-coupled Pt cathode, Pt anode-coupled Co3O4 photocathode, and TiO2 NNs photoanode-coupled Co3O4 photocathode as TiO2 NNs-Pt, Pt-Co3O4, and TiO2 NNs-Co3O4, respectively. Then, taking SMT as the model pollutant, the degradation experiments were carried out in a single-chamber electrolysis cell with a two-electrode system to evaluate the performance of the above-mentioned PEC systems. The 70 mL reaction solution contained 0.1 M Na2SO4 and 10 mg/L of SMT. The established PEC systems were connected via Cu wire without applied voltage, and the LED light was turned on to start the photoelectrocatalytic reaction.
3.5. Analytical Methods
The concentration of SMT was determined by high-performance liquid chromatography (HPLC, Ultimate 3000 ThermoFisher, Acclaim™ 120 C18 column at 30 °C, a 35:65 v/v acetonitrile/water solution as the mobile phase, flow rate of 0.5 mL/min, and detection wavelength of 270 nm). The degradation kinetics of SMT was fitted by the first-order reaction using Equation (3).
where Ct (mg/L) is the SMT concentration at a certain time t (min), C0 (mg/L) is the initial SMT concentrations, and k is the pseudo-first-order rate constant of SMT (min−1).
4. Conclusions
In short, an unbiased dual-photoelectrode (TiO2 NNs-Co3O4) PEC system encompassing a TiO2 NNs photoanode and Co3O4 photocathode was established for the first time, which could efficiently degrade SMT (99.62%, 0.042 min−1) under LED light irradiation. The photoelectrocatalytic activity of the self-driven TiO2 NNs-Co3O4 PEC system for SMT degradation was around 6 and 10.5 times as high as those of the TiO2 NNs-Pt (0.007 min−1) and Pt-Co3O4 (0.004 min−1) system, respectively. The improved catalytic performance could be attributed to the internal photovoltage (0.19 V) generated by the potential difference of Femi level between the photoelectrode. In addition, the slight influence of solution pH, typical inorganic anions, and NOM on SMT degradation efficiency (≥94.03%) suggested that the bias-free TiO2 NNs-Co3O4 PEC system had excellent catalytic performance under environment-related conditions. In addition, quenching experiments indicated that h+, •OH, and •O2− were predominant ROS during the decomposition process of SMT in this dual-photoelectrode PEC system. Moreover, the outstanding performance in reusability (99.40% of the fifth cycles), pharmaceutical wastewater (99.56%), and mariculture wastewater (99.70%) further revealed its potential for practical environmental remediation. This work provides novel inspirations for designing an efficient, sustainable, and strong-stable PEC system to purify wastewater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13020327/s1, Figure S1: Linear sweep voltammetry (LSV) curves of the as-prepared TiO2 NNs photoanode (a) and Co3O4 photocathode (b); Table S1: The quality parameters of the actual environmental matrices; Table S2: Equivalent circuits fitting of EIS data for TiO2 NNs-Co3O4, TiO2 NNs-Pt, and Pt-Co3O4 system with or without LED lamp illumination.
Author Contributions
Z.H.: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing—original draft, Writing—review & editing. R.L.: Conceptualization, Methodology, Formal analysis, Investigation, Data curation. X.S. and H.W.: Investigation, Resources, Data curation. J.S. and J.L.: Data curation. M.Z.: Conceptualization, Supervision, Funding acquisition, Resources, Writing—review & editing. O.A.A.: Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key R&D Program International Cooperation Project (2021YFE0106500); Key Project of Natural Science Foundation of Tianjin (No. 21JCZDJC00320); Fundamental Research Funds for the Central Universities, Nankai University, and National Research Foundation IRG—China/South Africa Research Cooperation Program (Grant Number 132793).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest or other interest that might be perceived to influence the results and/or discussion reported in this paper.
References
- Xu, G.; Zhang, B.; Wang, X.; Li, N.; Liu, L.; Lin, J.-M.; Zhao, R.-S. Nitrogen-doped flower-like porous carbon nanostructures for fast removal of sulfamethazine from water. Environ. Pollut. 2019, 255, 113229. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Y.; Zhou, M.; Cai, J.; Tian, Y. Enhanced removal of emerging contaminants using persulfate activated by UV and pre-magnetized Fe0. Chem. Eng. J. 2019, 361, 908–918. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can Microalgae Remove Pharmaceutical Contaminants from Water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, D.; Baccar, R.; Jaabiri, I.; Bouzid, J.; Kallel, M.; Ayadi, H.; Zhou, J.L. Fate of selected estrogenic hormones in an urban sewage treatment plant in Tunisia (North Africa). Sci. Total Environ. 2015, 505, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, Y.; Li, X.; Yu, H.; Wang, N.; Li, H.; Du, P.; Jiang, Y.; Fan, X.; Zhou, Z. Degradation of sulfamethazine by persulfate activated with nanosized zero-valent copper in combination with ultrasonic irradiation. Sep. Purif. Technol. 2020, 239, 116537. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef]
- Pan, Y.; Bu, Z.; Sang, C.; Guo, H.; Zhou, M.; Zhang, Y.; Tian, Y.; Cai, J.; Wang, W. EDTA enhanced pre-magnetized Fe0/H2O2 process for removing sulfamethazine at neutral pH. Sep. Purif. Technol. 2020, 250, 117281. [Google Scholar] [CrossRef]
- Zhang, X.; Nengzi, L.-c.; Li, B.; Liu, L.; Cheng, X. Design and construction of a highly efficient photoelectrocatalytic system based on dual-Pd/TNAs photoelectrodes for elimination of triclosan. Sep. Purif. Technol. 2020, 235, 116232. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.H. A comprehensive review on catalysts for electrocatalytic and photoelectrocatalytic degradation of antibiotics. Chem. Eng. J. 2021, 409, 127739. [Google Scholar] [CrossRef]
- Lu, Y.; Chu, Y.; Zheng, W.; Huo, M.; Huo, H.; Qu, J.; Yu, H.; Zhao, Y. Significant tetracycline hydrochloride degradation and electricity generation in a visible-light-driven dual photoelectrode photocatalytic fuel cell using BiVO4/TiO2 NT photoanode and Cu2O/TiO2 NT photocathode. Electrochim. Acta 2019, 320, 134617. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, J.W.; Jo, Y.H.; Abdi, F.F.; Lee, Y.H.; van de Krol, R.; Lee, J.S. Hetero-type dual photoanodes for unbiased solar water splitting with extended light harvesting. Nat. Commun. 2016, 7, 13380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, J.; Li, X.; Huang, K.; Zhou, B.; Cai, W.; Shangguan, W. Visible-light responsive photocatalytic fuel cell based on WO3/W photoanode and Cu2O/Cu photocathode for simultaneous wastewater treatment and electricity generation. Environ. Sci. Technol. 2012, 46, 11451–11458. [Google Scholar] [CrossRef]
- Luo, T.; Bai, J.; Li, J.; Zeng, Q.; Ji, Y.; Qiao, L.; Li, X.; Zhou, B. Self-Driven photoelectrochemical splitting of H2S for S and H2 recovery and simultaneous electricity generation. Environ. Sci. Technol. 2017, 51, 12965–12971. [Google Scholar] [CrossRef] [PubMed]
- Zuarez-Chamba, M.; Tuba-Guamán, D.; Quishpe, M.; Vizuete, K.; Debut, A.; Herrera-Robledo, M. Photocatalytic degradation of bisphenol A on BiOI nanostructured films under visible LED light irradiation. J. Photochem. Photobiol. A Chem. 2022, 431, 114021. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, X.; Li, S.; Song, M.; Liang, G.; Zhao, J.; Wang, Z. Rational construct CQDs/BiOCOOH/uCN photocatalyst with excellent photocatalytic performance for degradation of sulfathiazole. Chem. Eng. J. 2021, 404, 126541. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. A three-dimensional branched cobalt-doped alpha-Fe2O3 nanorod/MgFe2O4 heterojunction array as a flexible photoanode for efficient photoelectrochemical water oxidation. Angew. Chem. Int. Ed. 2013, 52, 1248–1252. [Google Scholar] [CrossRef]
- Song, R.; Chi, H.; Ma, Q.; Li, D.; Wang, X.; Gao, W.; Wang, H.; Wang, X.; Li, Z.; Li, C. Highly efficient degradation of persistent pollutants with 3D nanocone TiO2-Based photoelectrocatalysis. J. Am. Chem. Soc. 2021, 143, 13664–13674. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.-B.; Yang, Z.; Zheng, G. Reduced mesoporous Co3O4 nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Zhong, D.; Cai, B.; Wang, X.; Yang, Z.; Xing, Y.; Miao, S.; Zhang, W.-H.; Li, C. Synthesis of oriented TiO2 nanocones with fast charge transfer for perovskite solar cells. Nano Energy 2015, 11, 409–418. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Ge, R.; Ren, X.; Ren, J.; Yang, D.; Zhang, L.; Sun, X. Phosphorus-Doped Co3O4 Nanowire Array: A highly efficient bifunctional electrocatalyst for overall water splitting. ACS Catal. 2018, 8, 2236–2241. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Li, L.; Wang, J.; Zhang, Y.; Li, J.; Bai, J.; Xia, L.; Xu, Q.; Rahim, M.; et al. Carbon quantum dots modified anatase/rutile TiO2 photoanode with dramatically enhanced photoelectrochemical performance. Appl. Catal. B-Environ. 2020, 269, 118776. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Zhang, X.; Meng, Y.; Xiao, D. Three-Dimensional Co3O4 Nanowires@Amorphous Ni(OH)2 Ultrathin Nanosheets Hierarchical Structure for Electrochemical Energy Storage. Electrochim. Acta 2016, 191, 758–766. [Google Scholar] [CrossRef]
- Zheng, Z.; Lo, I.M.C. Fabrication of MoS2@BL-BiVO4 photoanode with promoted charge separation for photoelectrochemical sewage treatment to simultaneously degrade PPCPs, disinfect E. coli, and produce H2: Performance, mechanisms, and influence factors. Appl. Catal. B-Environ. 2021, 299, 120636. [Google Scholar] [CrossRef]
- Pan, H.; Liao, W.; Sun, N.; Murugananthan, M.; Zhang, Y. Highly efficient and visible light responsive heterojunction composites as dual photoelectrodes for photocatalytic fuel cell. Catalysts 2018, 8, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Lan, H.; Qu, J.; Liu, H. Synergetic hydroxyl radical oxidation with atomic hydrogen reduction lowers the organochlorine conversion barrier and potentiates effective contaminant mineralization. Environ. Sci. Technol. 2021, 55, 3296–3304. [Google Scholar] [CrossRef]
- Chatzitakis, A.; Papaderakis, A.; Karanasios, N.; Georgieva, J.; Pavlidou, E.; Litsardakis, G.; Poulios, I.; Sotiropoulos, S. Comparison of the photoelectrochemical performance of particulate and nanotube TiO2 photoanodes. Catal. Today 2017, 280, 14–20. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, H.; Zou, Y.; Wang, R.; Lyu, Y.; Jiang, S.P.; Wang, S. Efficiency and stability of narrow-gap semiconductor-based photoelectrodes. Energy Environ. Sci. 2019, 12, 2345–2374. [Google Scholar] [CrossRef]
- Pan, D.; Xiao, S.; Chen, X.; Li, R.; Cao, Y.; Zhang, D.; Pu, S.; Li, Z.; Li, G.; Li, H. Efficient photocatalytic fuel cell via simultaneous visible-photoelectrocatalytic degradation and electricity generation on a porous coral-like WO3/W photoelectrode. Environ. Sci. Technol. 2019, 53, 3697–3706. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Tong, F.; Liu, X.; Wang, C.; Wang, M.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; et al. Bias-free solar water splitting by Tetragonal Zircon BiVO4 nanocrystal photocathode and Monoclinic Scheelite BiVO4 nanoporous photoanode. Adv. Funct. Mater. 2020, 31, 2008656. [Google Scholar] [CrossRef]
- Xia, L.; Bai, J.; Li, J.; Zeng, Q.; Li, X.; Zhou, B. A highly efficient BiVO4/WO3/W heterojunction photoanode for visible-light responsive dual photoelectrode photocatalytic fuel cell. Appl. Catal. B-Environ. 2016, 183, 224–230. [Google Scholar] [CrossRef]
- Bai, J.; Wang, R.; Li, Y.; Tang, Y.; Zeng, Q.; Xia, L.; Li, X.; Li, J.; Li, C.; Zhou, B. A solar light driven dual photoelectrode photocatalytic fuel cell (PFC) for simultaneous wastewater treatment and electricity generation. J. Hazard. Mater. 2016, 311, 51–62. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Chen, Q.; Bai, J.; Zhou, B. Converting hazardous organics into clean energy using a solar responsive dual photoelectrode photocatalytic fuel cell. J. Hazard. Mater. 2013, 262, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Zhong, D.; Yao, H.; Zeng, Y.; Zhong, N.; Luo, H. BiVO4@PDA/TiO2/Ti photoanode with polydopamine as electron transfer mediator for efficient visible-light driven photocatalytic fuel cell. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125941. [Google Scholar] [CrossRef]
- Yu, H.; Xue, Y.; Lu, Y.; Wang, X.; Zhu, S.; Qin, W.; Huo, M. Novel application of a Z-scheme photocatalyst of Ag3PO4@g-C3N4 for photocatalytic fuel cells. J. Environ. Manag. 2020, 254, 109738. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, C.; Wen, R.; Tian, J.; Huang, W.; Wei, H.; Lu, J. Membraneless Photocatalytic Fuel Cell with Double Photoelectrodes for Simultaneous Electricity Generation and Pollutant Degradation. J. Electrochem. Soc. 2022, 169, 026502. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent developments and challenges in practical application of visible-light-driven TiO2-based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Mazierski, P.; Borzyszkowska, A.F.; Wilczewska, P.; Bialk-Bielinska, A.; Zaleska-Medynska, A.; Siedlecka, E.M.; Pieczynska, A. Removal of 5-fluorouracil by solar-driven photoelectrocatalytic oxidation using Ti/TiO2(NT) photoelectrodes. Water Res. 2019, 157, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Yang, Z.; Xu, H.; Song, P.; Xiong, W.; Cao, J.; Zhang, Y.; Xiang, Y.; Hu, J.; Zhou, C.; et al. Integrating N and F co-doped TiO2 nanotubes with ZIF-8 as photoelectrode for enhanced photo-electrocatalytic degradation of sulfamethazine. Chem. Eng. J. 2020, 388, 124388. [Google Scholar] [CrossRef]
- Wu, H.; Hu, Z.; Liang, R.; Nkwachukwu, O.V.; Arotiba, O.A.; Zhou, M. Novel Bi2Sn2O7 quantum dots/TiO2 nanotube arrays S-scheme heterojunction for enhanced photoelectrocatalytic degradation of sulfamethazine. Appl. Catal. B-Environ. 2023, 321, 122053. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Wu, J.; Tao, Y.; Zhang, C.; Zhu, Q.; Zhang, D.; Li, G. Activation of chloride by oxygen vacancies-enriched TiO(2) photoanode for efficient photoelectrochemical treatment of persistent organic pollutants and simultaneous H(2) generation. J. Hazard. Mater. 2023, 443, 130363. [Google Scholar] [CrossRef]
- Koo, M.S.; Chen, X.; Cho, K.; An, T.; Choi, W. In situ photoelectrochemical chloride activation using a WO3 electrode for oxidative treatment with simultaneous H2 evolution under visible light. Environ. Sci. Technol. 2019, 53, 9926–9936. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Man, J.H.K.; Lo, I.M.C. Integrating reactive chlorine species generation with H(2) evolution in a multifunctional photoelectrochemical system for low operational carbon emissions saline sewage treatment. Environ. Sci. Technol. 2022, 56, 16156–16166. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Song, R.; Ren, F.; Wang, H.; Gao, W.; Li, Z.; Li, C. Photoelectrocatalytic degradation of refractory pollutants over WO3/W network photoelectrode with heterophase junction for enhancing mass transportation and charge separation. Appl. Catal. B-Environ. 2022, 309, 121292. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, G.; Zhang, Y.; Liu, J.; Zhang, Y.-n.; Shi, H. A solar-driven photocatalytic fuel cell with dual photoelectrode for simultaneous wastewater treatment and hydrogen production. J. Mater. Chem. A 2015, 3, 3416–3424. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, B.; Ni, C.; Qi, Y.; Bi, X. Enhanced reduction of nitrate by noble metal-free electrocatalysis on P doped three-dimensional Co3O4 cathode: Mechanism exploration from both experimental and DFT studies. Chem. Eng. J. 2020, 382, 123034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).