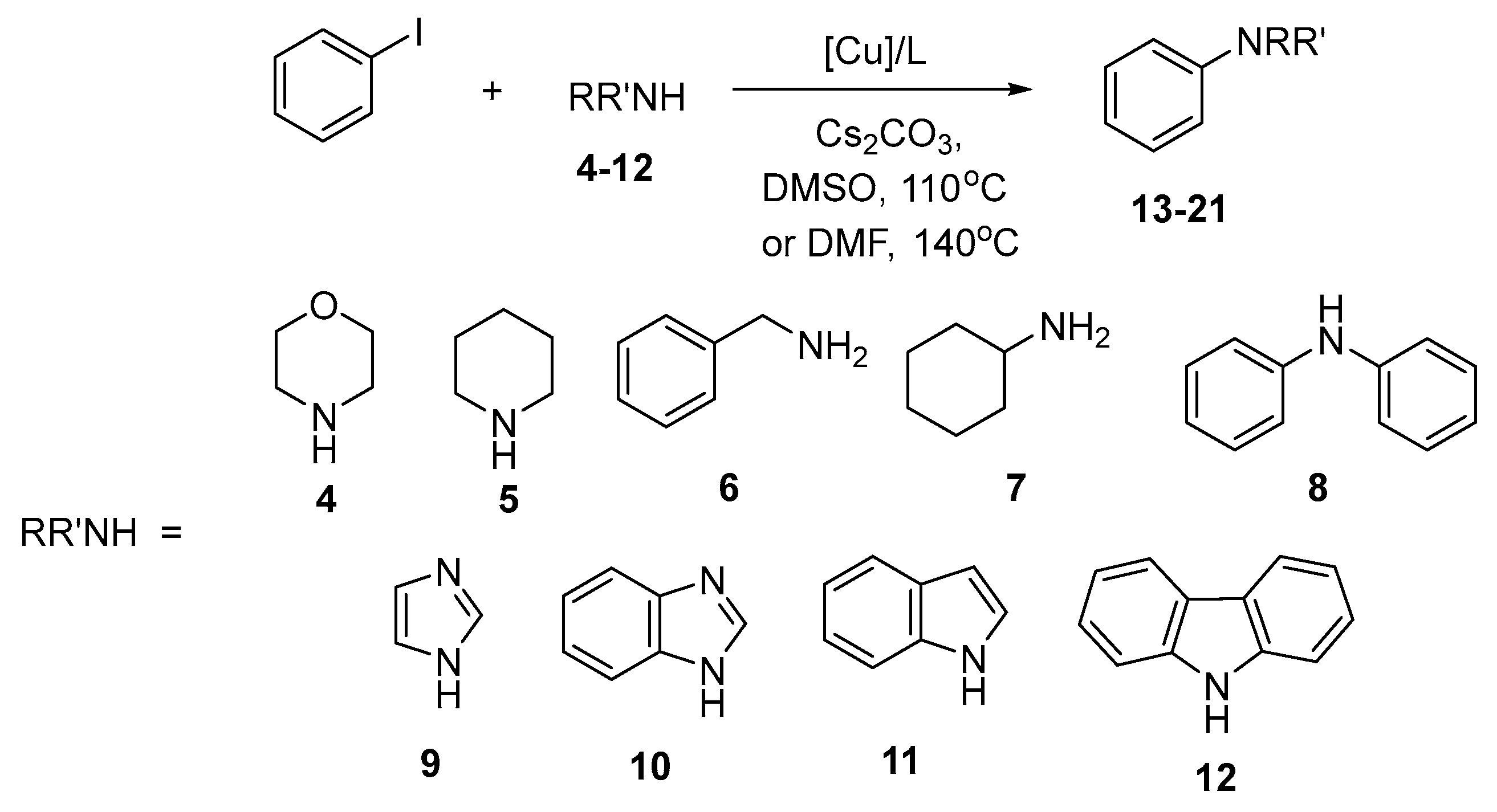
Abstract
Commercially available copper and copper (II) oxide nanoparticles (CuNPs and CuO NPs) were characterized using TEM and electronography methods to elucidate their true average size and composition. The catalytic amine arylation using unsupported copper nanoparticles differing in their size and copper oxidation state was investigated. The reaction of the model iodobenzene with n-octylamine was shown to be successfully catalyzed by CuNPs of average size 25 and 10/80 nm in the presence of the ligands such as 2-isobutyrylcyclohexanone (L1) and rac-1,1′-bi-2-naphthol (BINOL, L2), giving high yields (up to 95%) of the target N-octylaniline. CuO in bulk and nano forms was shown to be almost equally efficient in this process. Studies on the Cu-catalyzed amination of substituted iodobenzenes and 2-iodopyridine, as well as the arylation of different aliphatic amines and NH-heterocycles, verified that CuNPs (25 or 10/80 nm) with L1 and L2 are the most versatile and efficient nanocatalysts for a variety of substrates. Investigation of copper leaching under different conditions was carried out.
1. Introduction
The search for efficient catalysts of C(sp2)-N bond formation is still an important task of the organic synthesis. Though Pd-catalyzed amination of aryl halides can be judged as the most versatile tool able to solve numerous difficult synthetic problems [1,2,3], the application of copper-containing catalysts for this purpose draws constant attention of chemists due to much cheaper catalytic systems which can successfully replace more expensive palladium in many transformations [4,5]. One may speak of a real renaissance of the Ullmann chemistry in the last two decades due to the development of novel catalytic protocols involving copper with various ligands [6]. The copper-catalyzed reactions can be roughly divided into two wide areas, the first considering homogeneous catalytic reactions, based mainly on CuI or other Cu(I) salts with additional ligands, and the second encompassing a great variety of heterogeneous catalysts. The latter are often formed by immobilized copper salts, first of all, using most frequent copper iodide. Immobilized catalysts differ by the nature of supports and linkers which can simultaneously serve as ligands for copper cations. The most often used support is silica gel [7,8,9,10], and others reported include titania [11], polyvinyl acetate [12], polystyrene [13], and polyaniline [14,15]. Many researchers’ efforts are aimed at the reusable magnetic supports for copper catalysts containing magnetite particles [10,16,17,18,19].
Special attention is payed to copper nanoparticles (CuNPs) in catalysis. As for the amination reaction, a vast majority of reported cases deal with the supported nanoparticles, e.g., a dendrimeric system based on 1,3,5-triazine immobilized on silica gel with Fe3O4 particles serves as such support [10]. Described is the amination in the presence of CuNPs immobilized on TiO2 [11], graphene [20], and maghemite-silica magnetic support [21]. Additionally, the C-N bond forming reactions are catalyzed by CuO nanoparticles grafted on silica gel [22] or multiwall carbon nanotubes (MWCNTs) [23]. More sophisticated Cu@Cu2O nanoparticles embedded in reduced graphene oxide were described [24]. Additional ligands are not used for supported CuNPs as the support to some extent plays the role of the ligand. It is notable that the use of unsupported copper nanoparticles is very rare for C-N bond formation. Comprehensive reviews [25,26] describe only a few examples of the use of such catalysts: Cu2O NPs were employed for the amination of some aryl iodides and the process was shown to be limited to compounds without electron withdrawing substituents [27]. Several examples deal with the N-arylation of the amines, mainly NH-heterocycles, catalyzed by free CuO NPs [28,29,30,31,32]; one study is dedicated to the application of nano CuI for C-N bond formation [33]. However, in other catalytic reactions the use of unsupported nanoparticles is well documented. They were employed in the CuAAC reactions [34,35], O-arylation of phenols [36], formation of C-S [37,38], and C-Se/C-Te [39] bonds. Their role in alcohols oxidation [40], Sonogashira coupling [41,42], CH-activation reactions [43,44,45], and multicomponent syntheses [46,47] was described.
Our own interest in this sphere is determined by the possibility of the use of simple, commercially available unsupported copper nanoparticles in the amination of (hetero)aryl halides, elucidation of the regularities of the catalytic processes, dependence of the yields of the amination products on the nature of copper source, solvent, (hetero)aryl halides, and amines which can be introduced in the reaction. It is important to find an area of successful use of such commercially available nanoparticles and to understand their advantages and bottlenecks comparing to homogeneous and immobilized copper catalysts. Recently we have published our preliminary data on this topic [48,49,50], and in the present research we focused at a more comprehensive investigation of the efficiency of Cu, Cu2O, and CuO nanoparticles in the model amination reactions employing various model amines (primary, secondary aliphatic, NH-heterocycles) and aryl iodides with various substituents.
2. Results and Discussion
2.1. TEM Characterization of Copper Nanoparticles
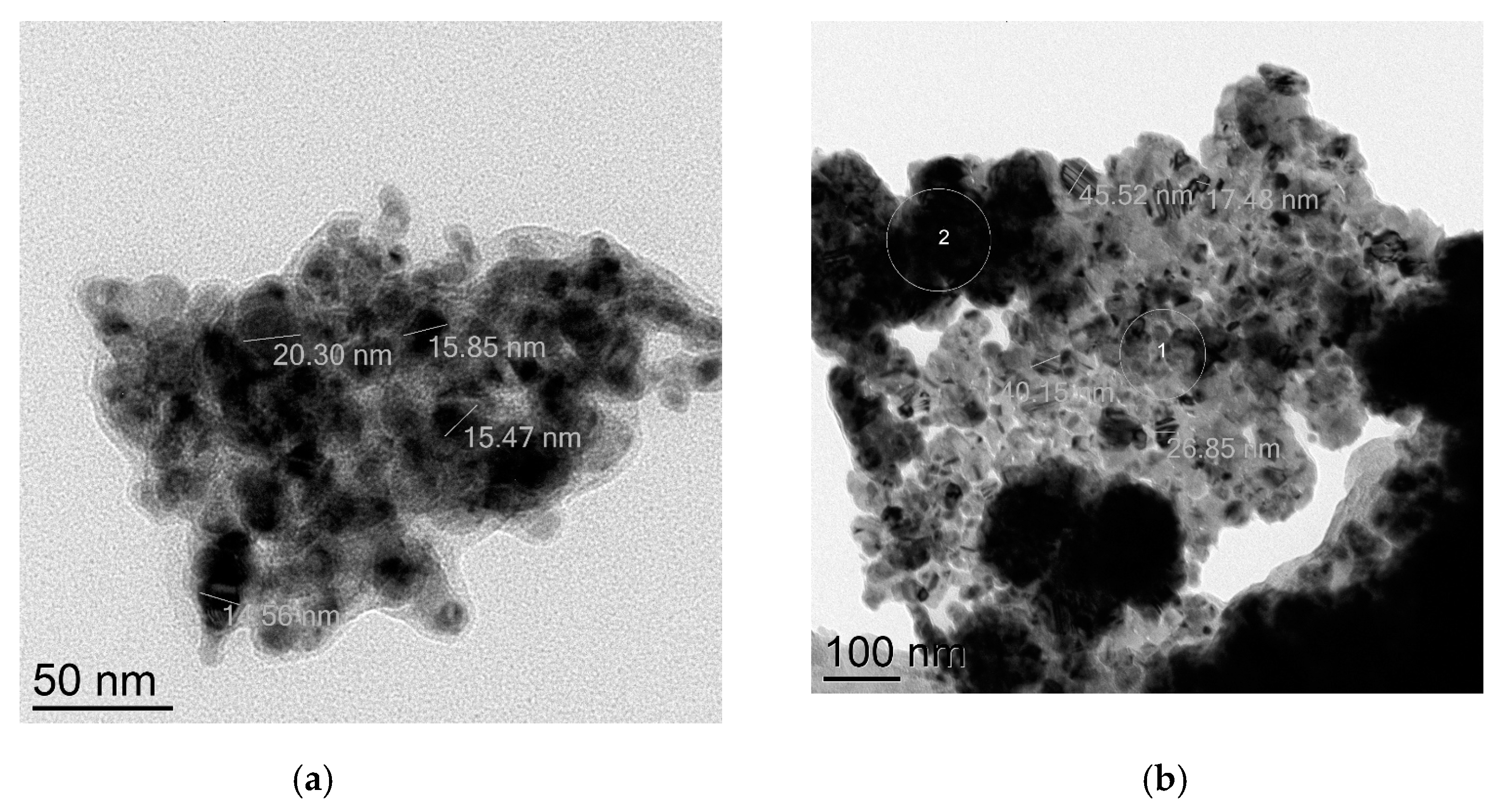
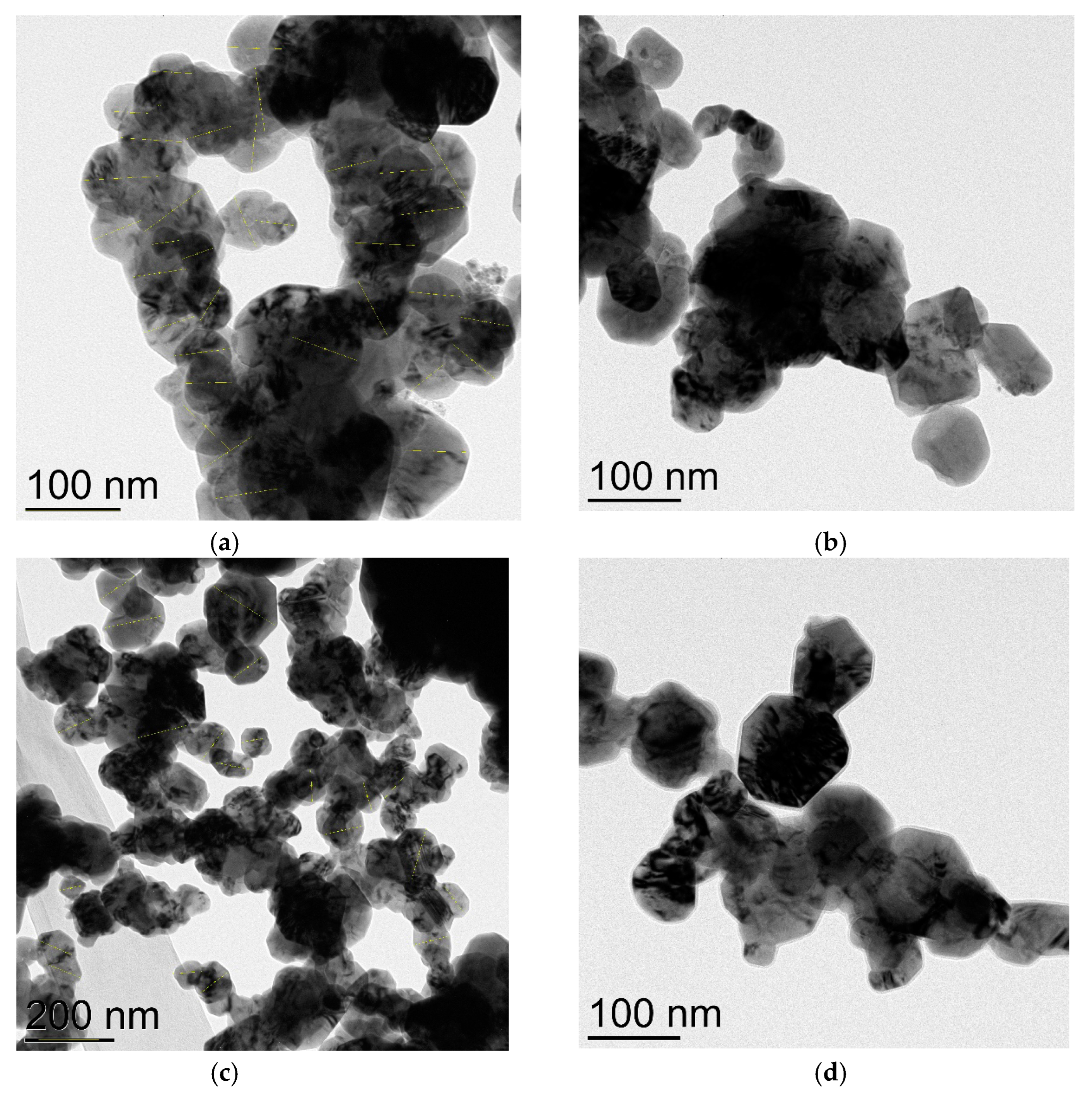
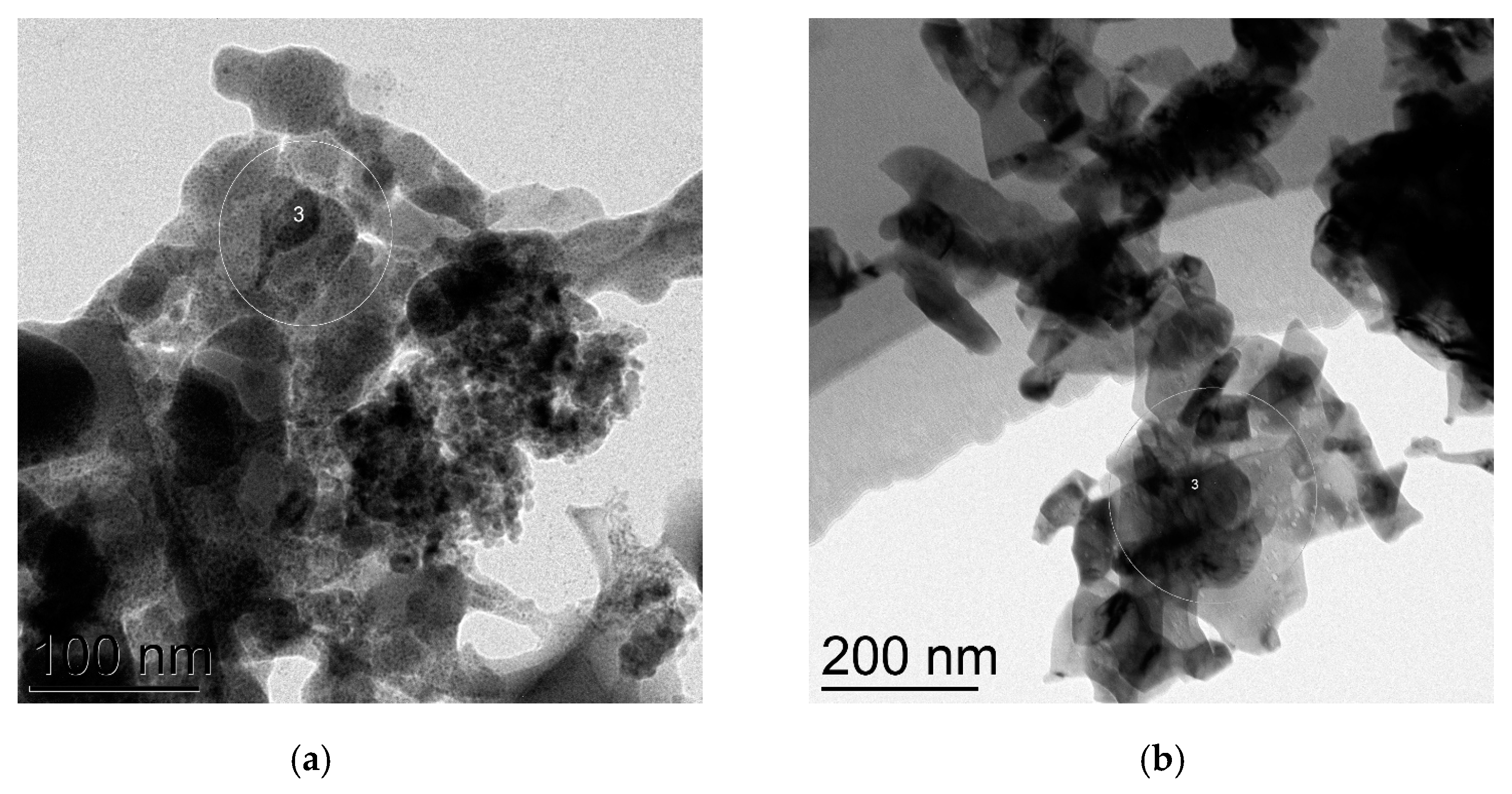
Four commercially available CuNPs were used in the present investigation, as well as commercial CuO NPs, while Cu2O NPs were obtained by a long (20 days) oxidation of CuNPs on air. Transmission electron microscopy (TEM) was employed to verify the true sizes of these nanoparticles. CuNPs labeled “25 nm” in fact consist of isolated nanoparticles of average size ca. 16 nm (Figure 1a) and bigger, possibly agglomerated nanoparticles of sizes 35–52 nm (Figure 1b). Electronogram (Figure S1) disclosed that nanoparticles consist of metallic copper (face-centered cubic lattice) and are covered with a thin layer of Cu2O (primitive cubic lattice) due to oxidation on air.
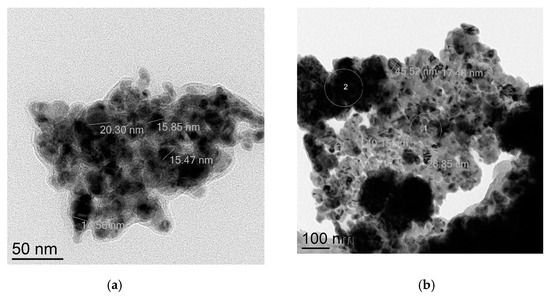
Figure 1.
TEM images of CuNPs of the average particles size 25 nm: (a) 16 nm nanoparticles; (b) 35–52 nm nanoparticles.
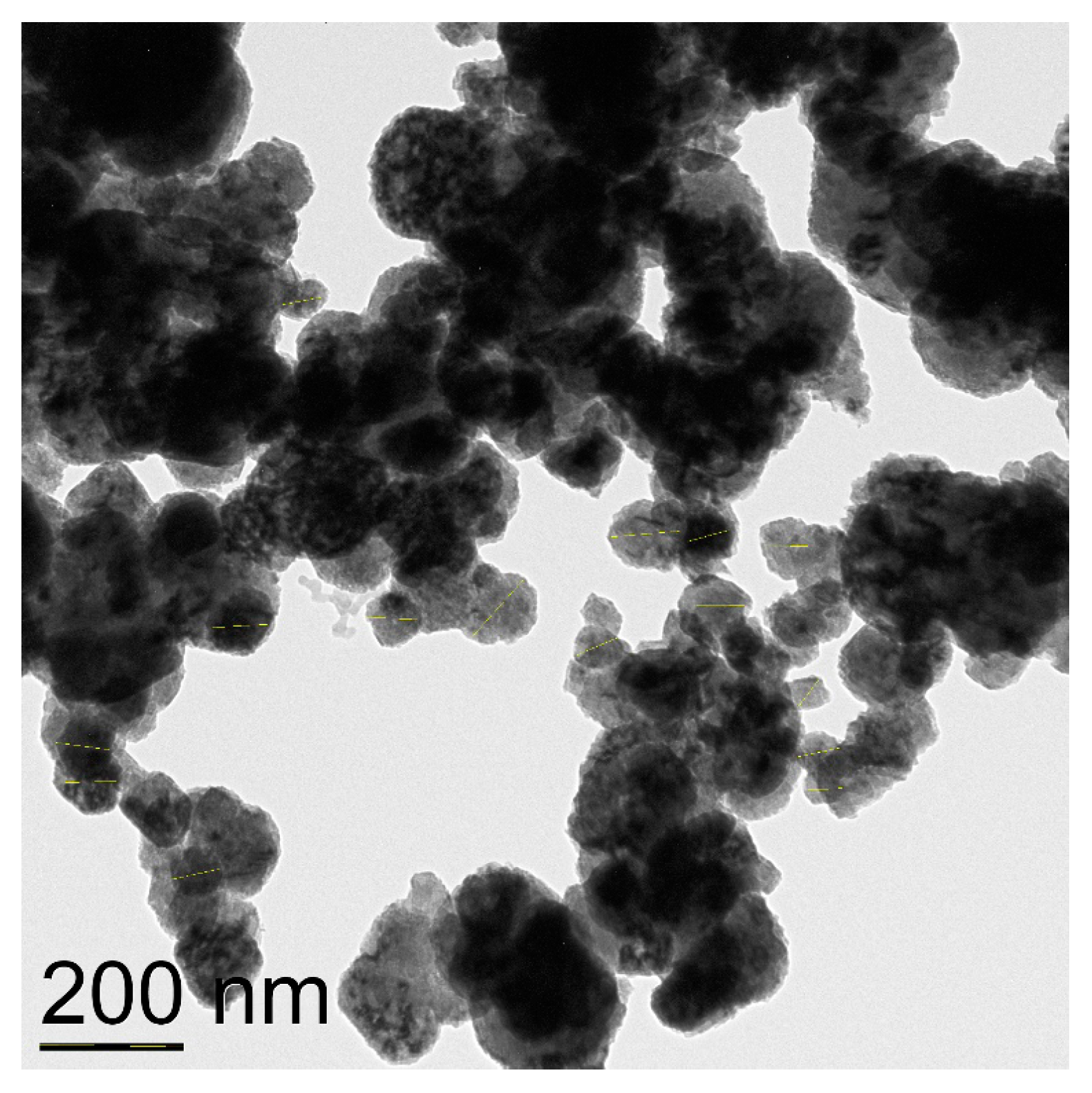
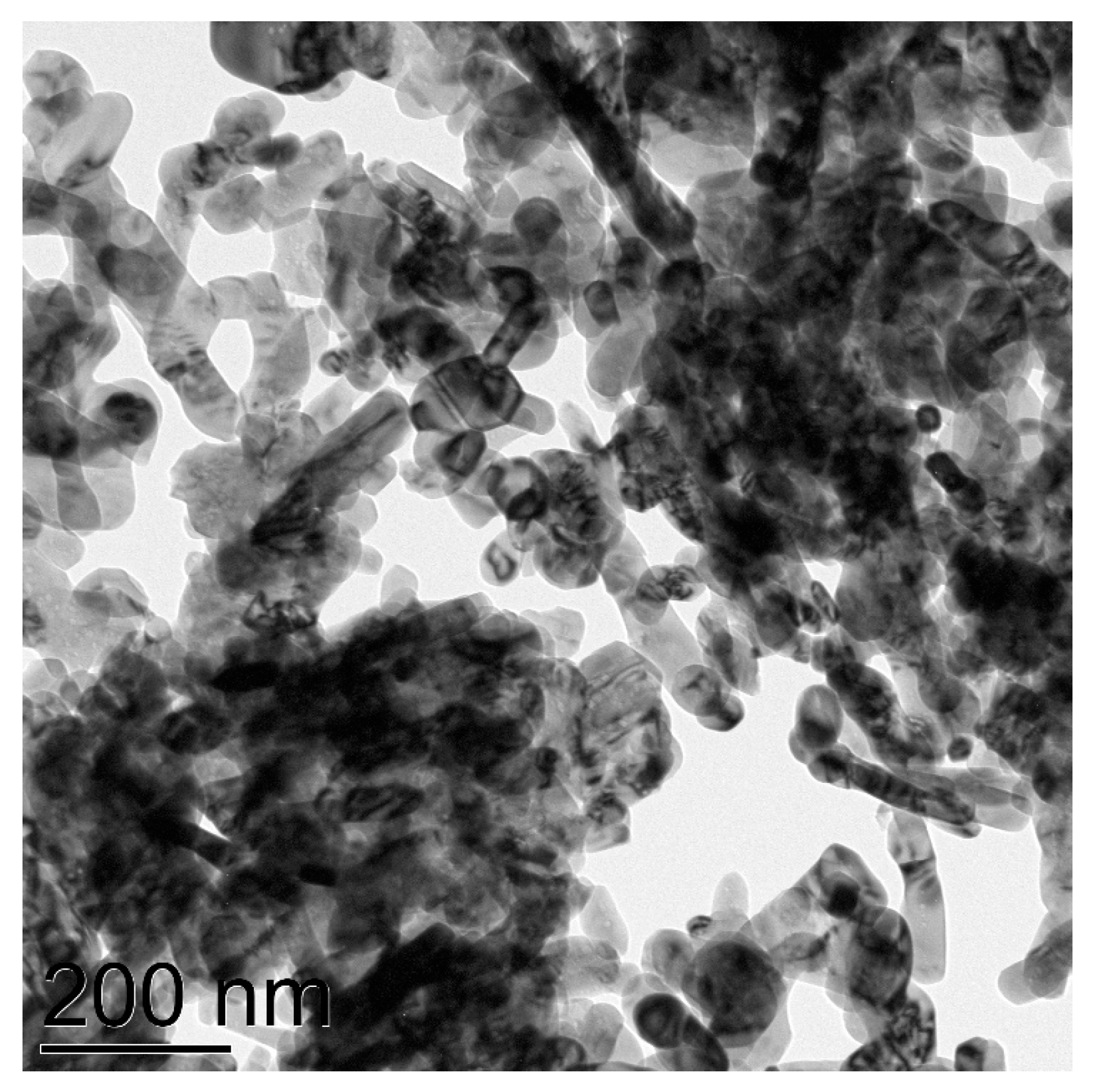
CuNPs labeled “40 nm” were shown to consist of spherical nanoparticles of the average size of 72 nm and also contain agglomerates of size over 90 nm (Figure 2). Electronogram shows that the specimen consists of the metal copper core and is covered with Cu2O and CuO phases (Figure S2).
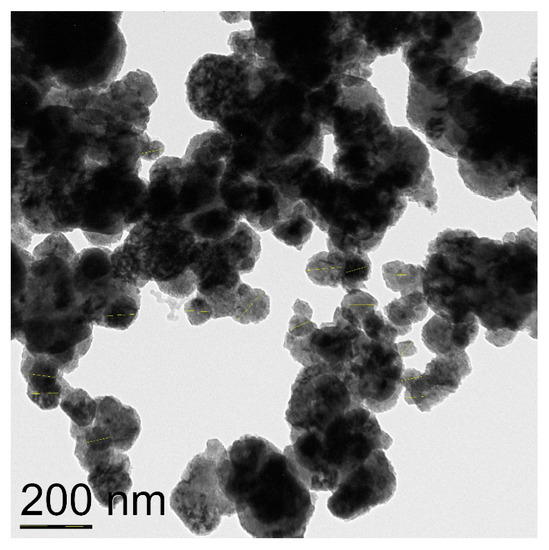
Figure 2.
TEM image of CuNPs of the average particles size 72 nm.
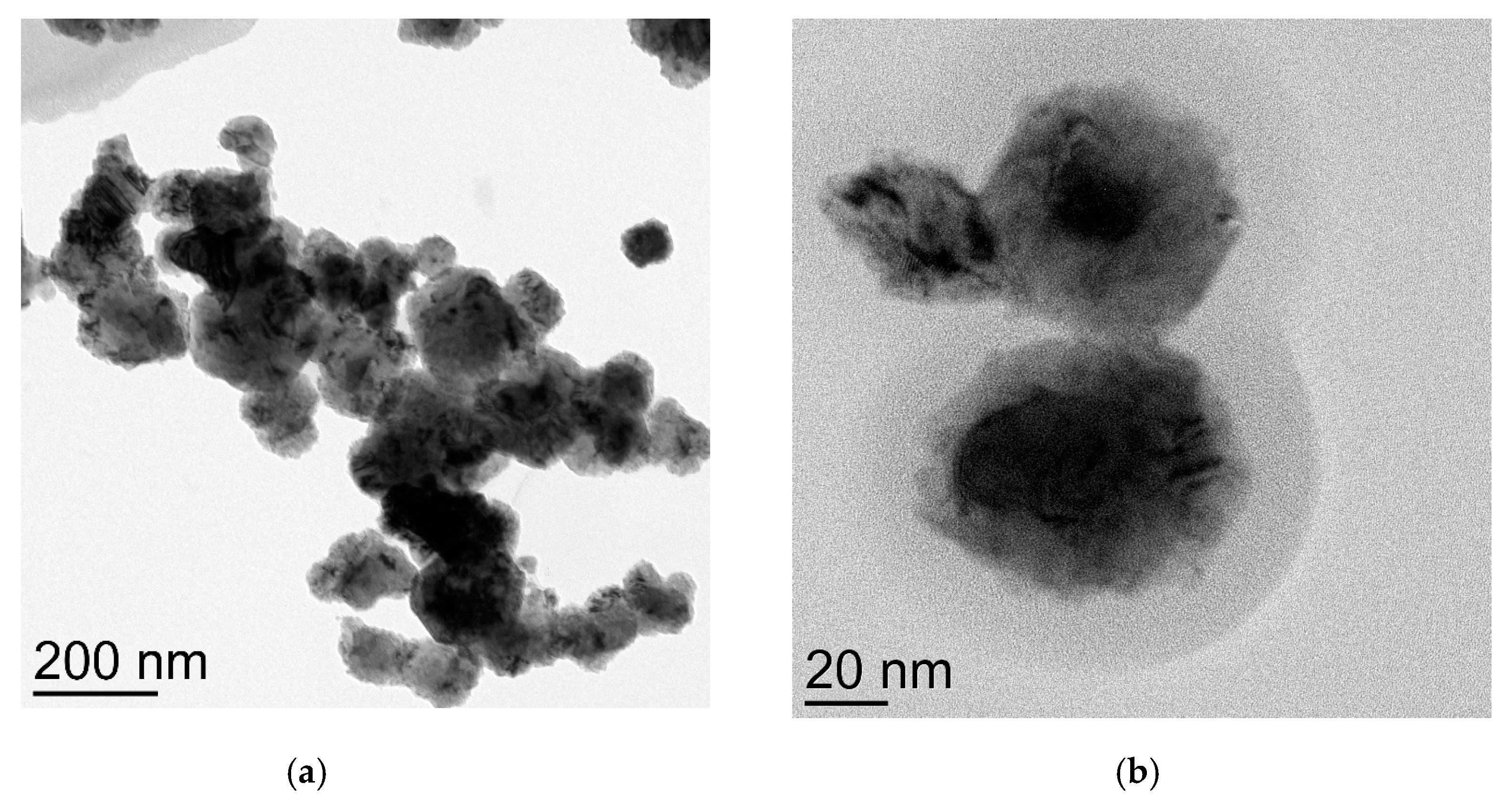
Cu NPs claimed “60 nm” indeed possess isolated nanoparticles of the average size of 86 nm (Figure 3a) which are covered with a substantial layer of copper (I) and (II) oxides (Figure 3b). This was verified by the electronogram (Figure S3).

Figure 3.
TEM images of CuNPs of the average particles size 86 nm: (a) general view of the spatial organization of nanoparticles; (b) image of the single nanoparticle (black) covered with the oxide phase (grey).
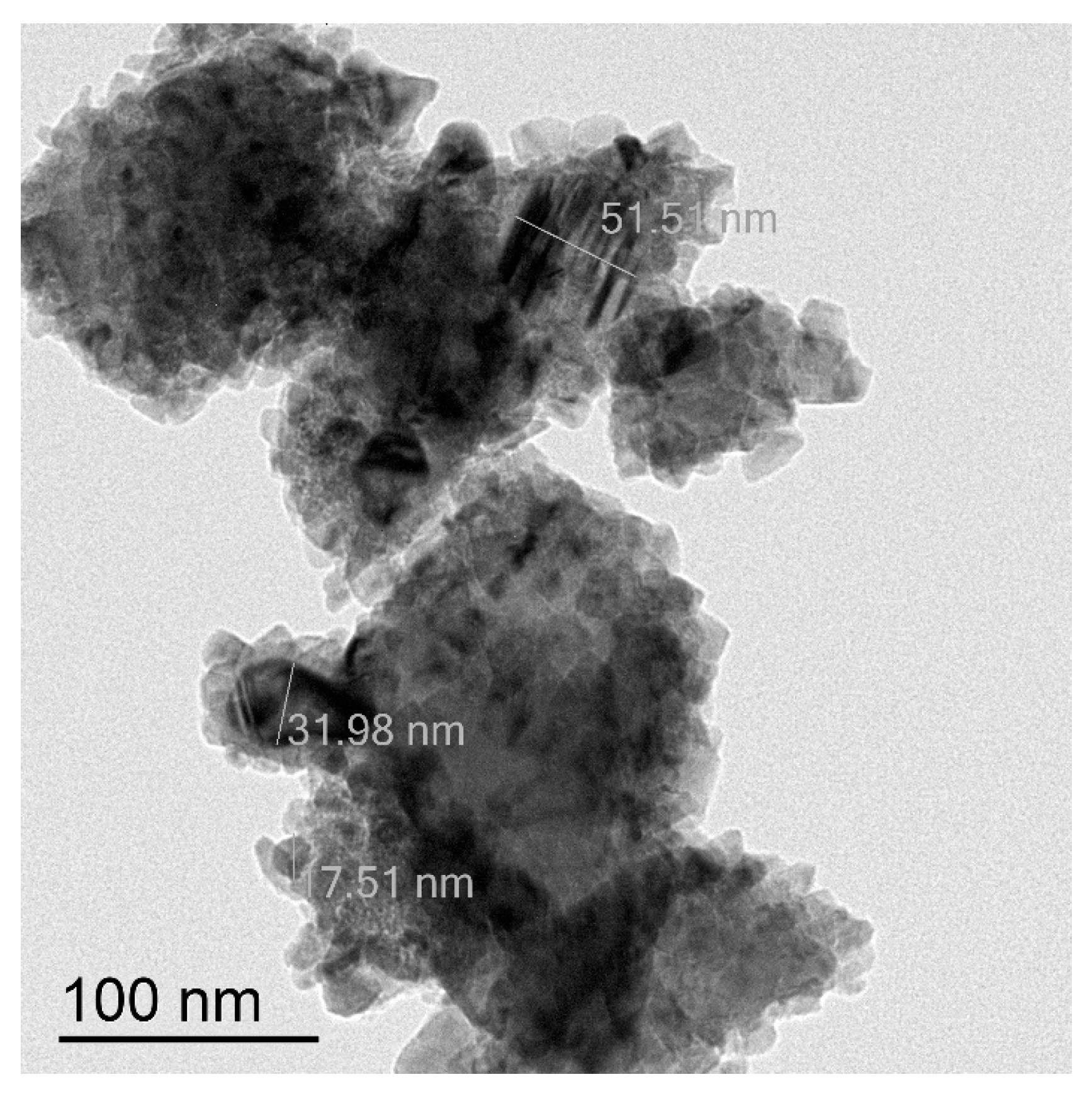
Another type of CuNPs is a mixture of small nanoparticles of the size 8–10 nm and much bigger agglomerates (>80 nm) (Figure 4). Thus, the average size of these species is around 53 nm. Electronogram shows that the metal copper core is covered with a thin surface layer of CuO (Figure S4). Further these CuNPs will be indicated as 10/80 nm.
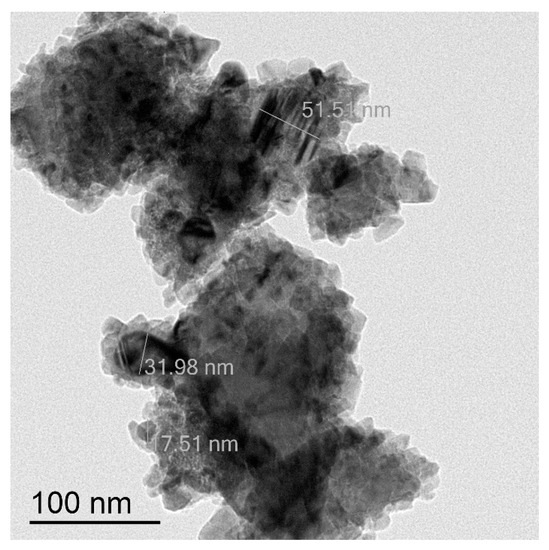
Figure 4.
TEM image of CuNPs of the average particles size 10/80 nm.
As Cu2O nanoparticles were not commercially available, we carried out a prolonged (20 days) oxidation of corresponding CuNPs on air. It was found out that CuNPs 25 nm did not oxidize at all and only a thin layer of this oxide remained on the surface of the metal copper core, though CuNPs 72 and 86 nm were fully transformed into corresponding Cu2O nanoparticles and retained their average size (68 nm, Figure 5a,b; 87 nm, Figure 5c,d). Electronogram verified the presence of the only phase of copper (I) oxide—primitive cubic lattice (Figures S5 and S6).
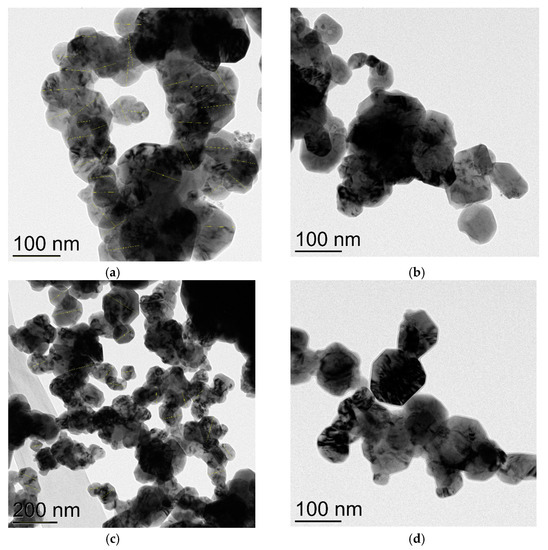
Figure 5.
TEM images of Cu2O NPs of the average particles size 68 nm (two views, (a,b)) and 87 nm (two views, (c,d)).
At last, the investigation of the commercial CuO nanoparticles with claimed size “<50 nm” revealed that their average size is in fact ca. 65 nm (Figure 6), they are well isolated, possess stretched shape, and electronogram verified the composition CuO with monoclinic C2/c lattice (Figure S7).
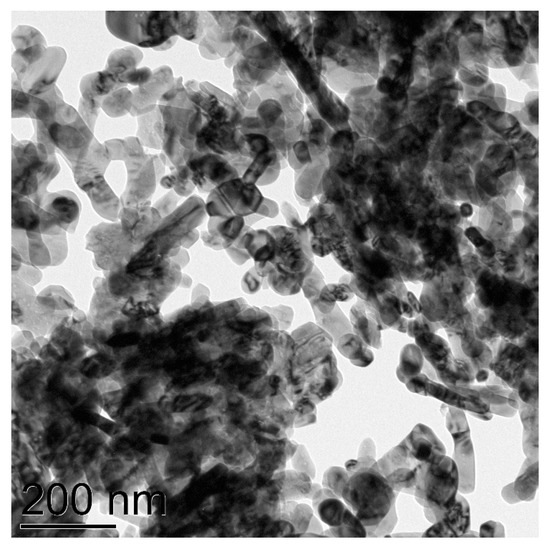
Figure 6.
TEM image of CuO NPs of the average particles size 65 nm.
2.2. Amination of Iodobenzene with n-Octylamine: Comparison of Different CuNPs and Copper Oxides
At the first step of our research, we compared the efficiency of four commercially available CuNPs with Cu2O and CuO (bulk and nanoparticles) in a model reaction of the simplest iodobenzene with n-octylamine (1). The choice of this primary aliphatic amine was determined by its boiling point (175 °C) to be substantially higher than the maximal reaction temperature (140 °C). The reactions were run in anhydrous DMSO at 110 °C with 0.5 M concentration of the amine and normally using a slight excess of PhI (1.25 equiv.) to ensure better conversion of the amine (Scheme 1). 10 Mol% of CuNPs and appropriate ligand were generally employed, Cs2CO3 (1.25 equiv.) was used as a base. Then, 2-acetylcyclohexanone (L0), 2-isobutyrylcyclohexanone (L1), rac-1,1′-bi-2-naphthol (rac-BINOL, L2), L-proline (L3) and N,N-dimethylglycine (L4) were chosen as ligands for testing as they were shown to be the most efficient in the copper-catalyzed amination reactions studied by us earlier. In the absence of any ligand the product yields were noted to be very poor. The reactions were carried out in vials (loading: 1 mL of the reaction mixture in 4 mL vials) with screw caps, under air, though some experiments were repeated under argon and no significant changes in the product yield were noted. After the reactions were over (in 24 h) they were analyzed by 1H and 13C NMR spectroscopy. The results of the experiments with CuNPs are presented in Table 1, and those obtained with copper oxides in Table 2.
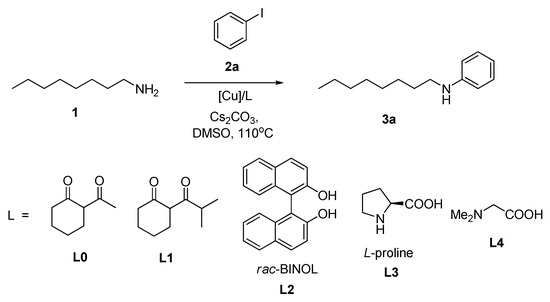
Scheme 1.
Model reaction of copper-catalyzed amination of n-octylamine (1) with iodobenzene.

Table 1.
Arylation of n-octylamine (1) with iodobenzene (2a) in the presence of CuNPs 1.

Table 2.
Arylation of n-octylamine (1) with iodobenzene (2a) in the presence of Cu2O and CuO 1.
As can be seen from Table 1, L2 ligand turned out to be the most efficient with all CuNPs providing the best yields of the N-octylaniline (3a) (entries 5, 8, 13, 16) which ranged from 86 to 95%. L1 and L0 were also efficient, while L3 provided a comparable yield of 3a only with CuNPs (10/80 nm) (entry 18), and L4 was shown to be much less efficient (entry 19). All CuNPs tested can provide yields of the N-arylation product over 80%; nevertheless, the most efficient catalyst is CuNPs 10/80 nm probably because it contains the smallest nanoparticles of size 8–10 nm. We have also demonstrated that the excess PhI was helpful to increase the yield of the product, though such improvement was quite different (entries 2 and 3, 5 and 6, 10 and 11). The attempt to apply 5 mol% catalyst in the best case (CuNPs 10/80 nm/L2) led to a certain decrease in the yield of 3a (entry 17).
The same ligands L1–L4 were tested in the reactions catalyzed with copper oxides (Table 2). We compared the efficiency of bulk Cu2O and CuO with corresponding nanoparticles. The use of these copper oxides for amine and amide arylation is well documented in the literature [51,52,53,54], and our own practice showed their performance in the arylation of adamantane-containing amines [55]. In the present investigation Cu2O NPs demonstrated no advantages over CuNPs in terms of the yield of 3a (entries 2, 4, 8, 9). The use of 20 mol% [Cu] (i.e., 10 mol% Cu2O) provided a slightly better yield of the target product in the case of L1 and L2 (entries 1, 3) and with L3 it was crucial to employ 10 mol% Cu2O, as with 5 mol% (10 mol% [Cu]) the yield diminished seriously (entries 5 and 6). L4 was useless in this reaction (entry 7). CuO bulk and CuO NPs were equally efficient in the reaction in the presence of L1 and L2 ligands affording 87–89% yields of 3a (entries 11–13, 18, 19), while L4 again was useless (entry 22). In the case of L1 the application of 5 mol% catalyst did not diminish the yield and even slightly improved it (entries 10 and 11, 17 and 18), and with L3, on the contrary, it led to a significant decrease (entries 14 and 15). Thus, one may conclude that no difference in the reaction outcome with various copper oxides (bulk and nanosized) was noted provided a proper ligand (L2 or both L1 and L2) was employed. Moreover, in general their efficiency was quite comparable with that of the above described CuNPs. As for other ligands which were reported to be efficient for certain Cu-catalyzed amination reactions, including N,N′-dimethylethylenediamine (DMEDA), N,N,N′,N′-tetramethylethylenediamine (TMEDA), 1,10-phenanthroline and 2,2′-bipyridine, in our reactions with copper nanoparticles they were totally useless and led to very poor yields of 3a (<10%). To note, we did not observe the formation of N,N-diarylated by-products; this is natural for copper-catalyzed amination reactions.
2.3. Amination of Iodoarenes with n-Octylamine in the Presence of CuNPs
At the next step, we studied the dependence of the outcome of the amination reaction on the substituents in the benzene ring (Scheme 2). The reactions of n-octylamine with iodoarenes 2b–m were run in DMSO at 110 °C as well as in DMF at 140 °C to compare the two most universal solvents for copper-catalyzed amination. Only CuNPs of different sizes were compared in these reactions.
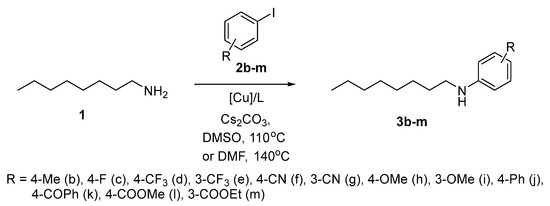
Scheme 2.
Amination of iodoarenes with n-octylamine.
The data are collected in Table 3. In the course of our previous research [48,49], we established that CuNps 25 nm/L1 (5/5 mol%) were most efficient for the arylation of n-octylamine with a model iodobenzene. Here, this catalytic system provided high yields of the products (>80%) only in some cases, i.e., in the reactions with 4-fluroiodobenzene (2c) (entry 4), 4- and 3-iodoanisoles (2h,i) (entries 12, 14), while with iodoarenes 2f,g,j,k bearing electron withdrawing substituents the yields of corresponding products 3f,g,j,k ranged from 57 to 76% (entries 8, 10, 16, 18). A quite moderate yield of 3b (45%) was observed also with 4-iodotoluene (2b) (entry 1). Similar yields of the products were obtained in the reactions with methyl 4-iodobenzoate (2l) and ethyl 3-iodobenzoate (2m) (entries 20, 22), also probably due to partial saponification of the esters in the presence of base and traces of water from the solvent. From the results obtained with CuNPs 25 nm/L1 catalytic system one cannot derive veritable regularities of the influence of substituents on the process.

Table 3.
Copper-catalyzed arylation of n-octylamine (1) with iodoarenes(2b–m) 1.
Better results were obtained when using CuNPs 10/80 nm in the presence of L2 ligand (10/10 mol%). With iodoarenes 2f,g,j,k the yields of the corresponding arylation products increased to 84–87% (entries 9, 11, 17, 19), and the yield of 3b raised from 45 to 66% (entry 2). Helpful was also running the reaction in DMF at 140 °C, as in several cases the yields of the products of arylation reached 90% and more (entries 5, 7, 13, 15). Only with the esters 2l,m the results were poor (entries 21, 23), which gives additional evidence to the problems with saponification. Thus, the application of the CuNPs 10/80 nm/L2 (10/10 mol%) catalytic system is favorable for the amination of aryl iodides with both electron donor and electron withdrawing substituents in both DMSO and DMF.
2.4. Arylation of Aliphatic Amines and NH-Heterocycles with Iodobenzene in the Presence of Copper Nanoparticles
To investigate further the efficiency of unsupported copper nanoparticles, we studied the arylation of several aliphatic amines 4–7, diphenylamine (8), and four NH-heterocycles 9–12 (Scheme 3). The reactions were generally run in DMSO at 110 °C, 10 mol% catalyst and ligand were employed. Table 4 contains data for the arylation of the amines 4–8. The reactivity of these amines turned to be quite different; moreover, in each case a peculiar dependence on the catalytic system was noted. Arylation of morpholine (4) in the presence of CuNPs 25 nm/L1 system gave 71% yield of the N-phenyl derivative 13 (entry 1). The use of a 1.5-fold excess of either amine or PhI resulted in near quantitative yields of 13 (entries 2, 3), but the attempt to use ligand L3, which worked efficiently in the arylation of n-octylamine, was unsuccessful (entry 4). CuO/L2 system was much less efficient for the arylation of morpholine, either in DMSO or in DMF (at 140 °C), as well as in bulk and nano forms of the copper oxide (entries 5–7). The arylation of piperidine (5) in the presence of CuNPs 25 nm/L1 system provided 67% yield of the product 14 (entry 8), it could be increased to 85% when using 1.5 equiv. amine (entry 9), and CuO/L2 in DMSO was equally efficient (entry 10), but running the reaction in DMF at 140 °C did not improve the result (entries 11, 12). From the reactions of benzylamine (6) and cyclohexylamine (7) one may note that CuO in bulk and nano forms provides quite similar results of the arylation (entries 14–17); it is true for other reactions catalyzed with CuO (entries 6, 7, 11, 12). As for diphenylamine (8), the only catalytic system efficient to produce triphenylamine was CuNPs 25 nm/L1 (entries 18, 19) while CuNPs and CuO NPs with L2 ligand gave much poorer results (entries 20–22). Thus, one may conclude about the superiority of copper nanoparticles in the presence of L1 for the arylation of different amines.
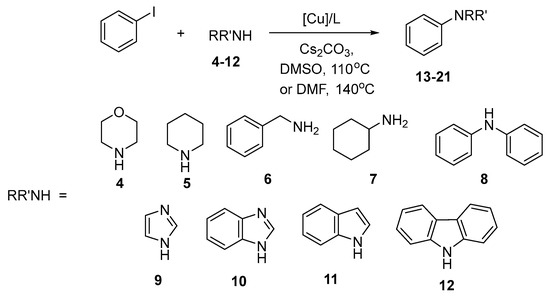
Scheme 3.
Arylation of aliphatic amines and NH-heterocycles with iodobenzene.

Table 4.
Copper-catalyzed arylation of the amines 4–8 with iodobenzene 1.
The results of the studies of the arylation of some NH-heterocycles 9–12 are summarized in Table 5. The following catalytic systems were studied: CuNPs 25 nm/L1, CuNPs 25 nm/L2, CuNPs 53 nm/L2, and CuO NPs/L2. At 1:1 ratio of the reagents the reaction of imidazole (9) provided ca. 80% yield of the product 18 with all catalytic systems tested (entries 1, 3, 4, 6), an increase to 90% was due to the introduction of 1.5 equiv. imidazole (entry 2), and carrying out the reaction in DMF at 140 °C resulted in a tiny decrease in the yield of the arylation product 18 (entry 5). For benzimidazole (10) CuO NPs with L2 was more efficient (entry 9), while the arylation of indole (11) ran smoothly in the presence of CuNPs 25 mn/L1 catalytic system (entries 10, 11) and under other conditions yield of 20 was substantially lower (entries 12–15). To note, in the reactions of 9–11 almost full conversion of starting compounds was observed; however, the yields of the target products were often in the range of 70–80% due to side reactions. Carbazole turned out to be less reactive in the presence of CuNPs 25mn/L1 (entries 16, 17), but the yield of 21 could be increased by the employment of the catalytic systems CuNPs 53 nm/L2 and CuO NPs/L2 (entries 18, 19). In general, the arylation of NH-heterocycles turned out to be a more complicated task than that of aliphatic amines and in each case the adjustment of the catalytic system was needed.

Table 5.
Copper-catalyzed arylation of the amines 9–12 with iodobenzene 1.
2.5. Amination of 2-Iodopyridine with n-Octylamine in the Presence of Copper Nanoparticles
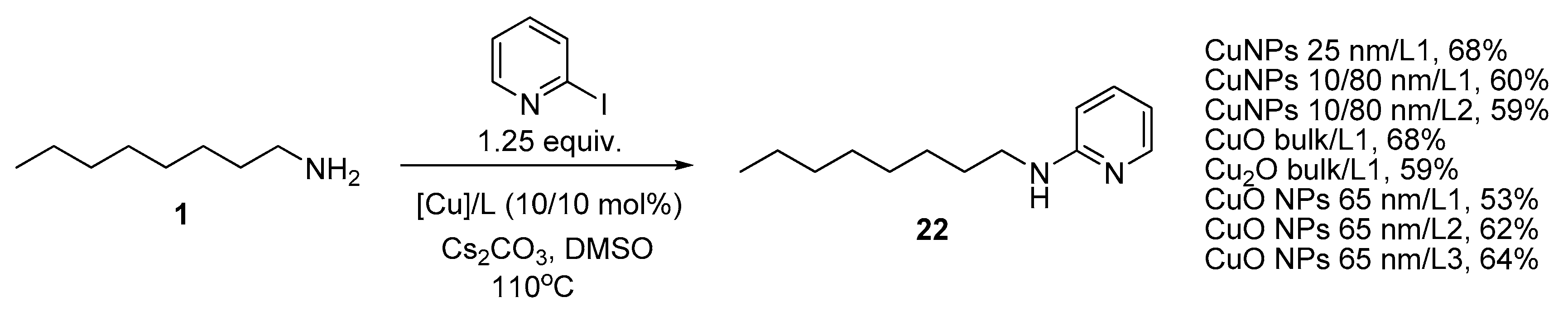
The amination of 2-iodopyridine was carried out using a series of catalytic systems with CuNPs 25 nm, 10/80 nm, Cu2O and CuO bulk and CuO NPs 65 nm (Scheme 4). The reactions were run in DMSO at 110 °C using 1.25 equiv. 2-iodopyridine, and it was found out that the yield of the target compound 22 ranged from 53 to 68%, the best result was obtained with CuNPs 25 nm/L1 and CuO bulk/L1. Ligand L3 was efficient with CuO NPs as it was shown to provide the best yield in the same reaction catalyzed with CuNPs 25 nm [50]. However, though 2-iodopyridine is undoubtedly more reactive than iodobenzene, the results of its copper-catalyzed amination were less impressive due to various side reactions including C-C coupling leading to 2,2′-bipyridyl.
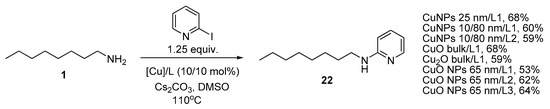
Scheme 4.
Amination of 2-iodopyridine with n-octylamine.
2.6. Investigations on the Leaching of Copper Nanocatalysts
One of the most important questions when using metal nanoparticles as catalysts is to verify the possibility of the metal leaching to the solution and to assess homogeneity/heterogeneity of the catalytic process. Here, we decided to compare CuNPs 10/80 nm, which demonstrated the best efficiency with previously studied CuNPs 25 nm and copper oxides, both in bulk and nano forms. For this purpose, at first the experiments were carried out to assess the rate of the catalytic process in the presence of various catalysts. The reaction of n-octylamine with iodobenzene (1.25 equiv.) was run under standard conditions using the following catalytic systems which were shown to provide the best yields of the arylation product 3a: CuNPs 25 nm/L1 (10/10 mol%), CuNPs 10/80 nm/L2 (10/10 mol%), Cu2O bulk/L2 (10/10 mol%), CuO bulk/L2 (10/10 mol%), and CuO NPs 65 nm/L1 (10/10 mol%) (Table 6). The yield of the 3a was monitored by the 1H NMR spectra of the reaction mixture after 1.5, 3, 6, 12, and 24 h.

Table 6.
Copper-catalyzed arylation of n-octylamine (1) with iodobenzene (2a) at time intervals 1.
It is clearly seen from Table 6 that for the catalytic systems with L2 ligand the reaction occurs mainly between 6 and 12 h. It suggests the induction period which is important to form a sufficient amount of the reactive catalytic species, very probably by leaching of the copper particles from the solid catalyst. For the catalytic systems involving L1 ligand one may suppose a shorter induction period which also can be less pronounced.
To confirm leaching which may be decisive for the catalytic process, we studied it deliberately by treating various copper catalysts solely with the solvent (DMSO), then adding ligands and/or n-octylamine to find out possible regularities. The experiments were carried out for 6 h at 110 °C. After treating the catalysts, the solutions were centrifuged, diluted with plenty of water, and subjected to ICP-MS analysis to determine the amount of copper in a solution. The results are given in Table 7.

Table 7.
Studies on leaching of copper from copper catalysts 1.
The data obtained demonstrate that for all CuNPs leaching is quite notable, the increase in the average size of the nanoparticles results in a more intensive leaching (from 5.6% for CuNPs 25 nm to 13% for CuNPs 86 nm), the addition of the ligand or amine also favors leaching. On the other hand, leaching is weaker by an order for copper oxides (in bulk or nano forms, 0.5–0.6%) but the addition of ligands substantially accelerates the process. Though the dependence of the reaction rate and the product outcome on the amount of the catalyst undergoing leaching, as well as the state of copper in solution, is still to be established, the principal fact that in all reactions leaching is quite notable is evidenced by these experiments. Further, the role of ligand is crucial as it not only increases leaching of copper to solution but participates in the catalytic process; without ligand no reaction occurs and the result is strongly dependent on the ligand nature. Note, however, that L2 being the best choice for the amination catalyzed with nanoparticles provides only moderate yield of the arylation product in the reactions catalyzed with convenient CuI [49].
Three additional experiments were carried out to verify an important role of leaching for our reactions to take place. After the treatments described above of CuNPs 25 nm (i) with DMSO, (ii) DMSO and L1, and (iii) DMSO and n-octylamine, the residual copper nanoparticles were removed by centrifugation and solutions were charged with all needed ingredients (iodobenzene (i, ii, iii), optionally n-octylamine (i, ii) or ligand (i, iii), Cs2CO3 (i, ii, iii)), except for copper catalyst, to carry out the arylation of amine under standard conditions (10 mol% catalyst). After 24 h at 110 °C all three reactions produced the product 3a in similar yields of 72–77% (the analogous normal reaction described in paragraph 2.2 gave 86% yield). This fact gives additional evidence to a crucial role of leaching in described catalytic reactions. Nanoparticles may serve as a reservoir of active catalytic species to feed the homogeneous catalytic process (this assumption, however, does not rule out the possibility of the simultaneous heterogeneous catalytic reaction). As homogeneous catalytic process is evidenced by leaching, great importance of a proper ligand can be easily understood because it is the ligand which contributes much to the stability of copper complexes in the catalytic cycle with multiple oxidation/reduction steps. On the other hand, the role of ligands in the described reactions with nanoparticles is not the same as in purely homogeneous amination reactions catalyzed by CuI. In ref. [49] we demonstrated that CuI gave high yields of the amination products only in the presence of L1 ligand, while with L2 and especially L3 the yields dramatically diminished.
The obtained results of the studies on copper leaching made hot test experiments unnecessary in our case. Two additional experiments were done to elucidate the reactivity of the copper resting in the solution after the reaction was over. The residue was removed by centrifugation of the reaction mixtures which proceeded in the presence of CuNPs 25 nm/L1 and CuNPs 10/80 nm/L2 catalytic systems (10 mol%). Then, appropriate amounts of n-octylamine, iodobenzene, and Cs2CO3 were added and the reaction mixtures were stirred at 110 °C for 24 h. The analysis of the reaction mixtures revealed that the conversion of a new portion of n-octylamine into the arylation product 3a was 58% in the first experiment and 54% in the second one. The yields are somewhat lower than those described above; however, these data demonstrate that after the reaction is over there is still enough active copper in the solution to catalyze the second cycle. These data are quite different from that obtained in the course of the investigation of unsupported CuO and CuI nanoparticles which catalyzed C-N coupling without additional ligands [29,33]. The reason is likely that the authors of the cited papers conducted the amination reactions using different nanoparticles under different conditions.
The investigation of the nanocatalysts microstructures after the model amination reaction of n-octylamine with PhI was carried out for CuNPs 25 nm and CuNPs 10/80 nm using TEM. It was found out that both copper nanoparticles underwent full oxidation into CuO; in the first case the size of majority of nanoparticles is ca. 50 nm, though there are a number of smaller particles (10–30 nm) (Figure 7a). Electronogram of the specimen shows predominance of the amorphous phase of CuO (Figure S8). CuNPs 10/80 nm was also fully oxidized into CuO and average particle size increased to 86 nm (Figure 7b and Figure S9). The changes of the particles size and of their oxidation state are highly likely to occur in the course of dissolution and precipitation processes, such complex behavior of nanoparticles was thoroughly studied in [56] in regard of PdNPs. The questions associated with different reusability of the nanocatalysts in the presence of different ligands are to be elucidated in the course of further special studies.
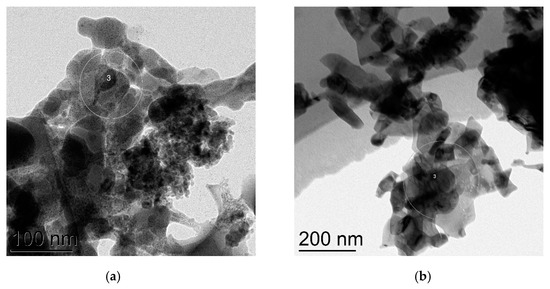
Figure 7.
TEM images of the copper nanocatalysts after the amination reaction: (a) initial CuNPs 25 nm converted into CuO NPs; (b) initial CuNPs 10/80 nm converted into CuO NPs.
3. Materials and Methods
All copper-catalyzed amination reactions were run in screw-cap vials using anhydrous DMSO or DMF. 1H and 13C NMR spectra were recorded on Bruker Avance-400 spectrometer in CDCl3 using residual peaks of the solvent as standards (δ 7.26 ppm and 77.0 ppm, respectively). MALDI-TOF mass spectra were obtained with Bruker Autoflex II spectrometer using dithranol as matrix and (polyethylene) glycols as internal standard for precise calibration. TEM experiments were carried out using JEM 2100 (JEOL, Tokyo, Japan) device with an accelerating voltage of 200 kV. The analyzed powder was suspended in isopropanol and dispersed for 5 min in an ultrasonic bath. The resulting suspension was dripped onto a copper mesh coated with an amorphous carbon film. Electronography was carried out with INCA Energy (Oxford Instruments, Abington, UK) instrument. ICP MS analyses of dissolved copper was done using Agilent 7700 spectrometer. For this purpose, 0.5 mL of the DMSO solution containing copper was dissolved in 10 mL of distilled water which was subjected to analysis. Copper nanoparticles, CuO nanoparticles, bulk Cu2O and CuO, amines 1, 4–8, NH-heterocycles 9–12 and aryl iodides 2a–m, ligands L0–L4, and Cs2CO3 were purchased from Sigma-Aldrich and Fluka and were used without special purification. Cu2O nanoparticles (average sizes 68 and 87 nm) were obtained by a long (3 weeks) oxidation of a thin layer of the powder of Cu nanoparticles (average sizes 72 and 86 nm, respectively) on air.
3.1. Typical Procedure for the Copper-Catalyzed Amination of Aryl Iodides
A screw-cap vial (volume 4 mL) equipped with a magnetic bar was charged with the copper catalyst (0.05 mmol [Cu], 10 mol% 3.2 mg in the case of CuNPs, 4 mg in the case of CuO, 3.6 mg in the case of Cu2O), corresponding ligand (0.05 mmol, 10 mol%), aryl iodide (0.625 mmol), amine (0.5 mmol), cesium carbonate (0.625 mmol, 204 mg) and solvent (DMSO or DMF, 1 mL). The vial was tightly closed and the reaction mixture was stirred at 110 °C (in the case of DMSO) or at 140 °C (in the case of DMF) for 24 h. In certain cases 0.025 mmol CuNPs (5 mol%, 1.6 mg) and 0.025 mmol ligand (5 mol%) were used to find out the dependence of the product yield on the catalyst loading. In the case of Cu2O bulk 0.1 mmol [Cu] (20 mol%, 0.05 mmol, 7.2 mg Cu2O) were also used. After cooling down the reaction mixture to ambient temperature, and sedimentation of the residue 30 μL of the reaction mixture was dissolved in 0.5 mL CDCl3 and analyzed by 1H NMR to establish the yield of the arylation product in the reaction mixture. In some cases, to isolate the arylation product, the reaction mixture was diluted with dichloromethane (1 mL) and extracted with water (10 mL). The organic layer was evaporated and subjected to column chromatography on silica gel (Fluka, 40–63 nm) using hexanes-EtOAc as eluent.
3.2. Spectral Data for the Products of N-Arylation
N-octylaniline (3a) [57]. 1H-NMR (400 MHz, CDCl3): δ 0.79–0.83 (m, 3H, CH3), 1.15–1.35 (m, 10H, CH2), 1.54 (quintet, 2H, 3J = 7.2 Hz, CH2CN), 3.02 (t, 2H, 3J = 7.1 Hz, CH2N), 3.62 (br. s, 1H, NH), 6.52 (d, 2H, 3Jobs = 7.8 Hz, H2,2′(Ph)), 6.59 (t, 1H, 3J = 7.3 Hz, H4(Ph)), 7.06–7.10 (m, 2H, H3,3′(Ph)).
4-Methyl-N-octylaniline (3b) [58]. 1H-NMR (400 MHz, CDCl3): δ 0.78–0.81 (m, 3H, CH3), 1.16–1.35 (m, 10H, CH2), 1.52 (quintet, 2H, 3J = 7.1 Hz, CH2CN), 2.99 (t, 2H, 3J = 7.0 Hz, CH2N), 3.47 (br. s, 1H, NH), 6.45 (d, 2H, 3Jobs = 8.2 Hz, H2,2′ (Ph)), 6.89 (d, 2H, 3Jobs = 8.2 Hz, H3,3′(Ph)).
4-Fluoro-N-octylaniline (3c). 1H-NMR (400 MHz, CDCl3): δ 0.72–0.76 (m, 3H, CH3), 1.10–1.30 (m, 10H, CH2), 1.46 (quintet, 2H, 3J = 7.1 Hz, CH2CN), 2.90 (t, 2H, 3J = 7.1 Hz, CH2N), 3.55 (br. s, 1H, NH), 6.39 (dd, 2H, 3JHHobs = 8.7 Hz, 4JHF = 4.3 Hz, H2,2′ (Ph)), 6.72 (t, 2H, 3Jobs = 8.7 Hz, 3JHF = 8.7 Hz, H3,3′(Ph)). 13C-NMR (100.6 MHz, CDCl3): δ 12.7 (CH3), 21.1 (CH2), 25.7 (CH2), 27.7 (CH2), 27.8 (CH2), 27.9 (CH2), 30.2 (CH2), 42.7 (CH2N), 111.5 (d, 3JCF = 6.8 Hz, C2,2′(Ph)), 113.8 (d, 2JCF = 21.9 Hz, C3,3′(Ph)), 144.0 (d, 4JCF = 6.5 Hz, C1(Ph)), 153.4 (d, 1JCF = 232.2 Hz, C4(Ph)). MALDI-TOF calcd for C14H23FN [M + H]+ 224.1815, found: 224.1743.
N-octyl-4-(trifluoromethyl)aniline (3d) [59]. 1H-NMR (400 MHz, CDCl3): δ 0.77–0.80 (m, 3H, CH3), 1.12–1.34 (m, 10H, CH2), 1.53 (quintet, 2H, 3J = 7.1 Hz, CH2CN), 3.04 (q, 2H, 3J = 6.4 Hz, CH2N), 4.20 (br. s, 1H, NH), 6.50 (d, 2H, 3Jobs = 8.4 Hz, H2,2′ (Ph)), 7.27 (d, 2H, 3Jobs = 8.4 Hz, H3,3′(Ph)).
N-octyl-3-(trifluoromethyl)aniline (3e) [59]. 1H-NMR (400 MHz, CDCl3): δ 0.77–0.80 (m, 3H, CH3), 1.10–1.35 (m, 10H, CH2), 1.53 (quintet, 2H, 3J = 7.2 Hz, CH2CN), 3.02 (q, 2H, 3J = 6.3 Hz, CH2N), 4.01 (br. s, 1H, NH), 6.64 (d, 1H, 3J = 8.5 Hz, H6(Ph)), 6.68 (br. s, 1H, H2(Ph)), 6.78 (d, 1H, 3J = 7.2 Hz, H4(Ph)), 7.14 (t, 1H, 3Jobs = 7.9 Hz, H5(Ph)).
4-(Octylamino)benzonitrile (3f) [59]. 1H-NMR (400 MHz, CDCl3): δ 0.74–0.77 (m, 3H, CH3), 1.08–1.32 (m, 10H, CH2), 1.49 (quintet, 2H, 3J = 7.1 Hz, CH2CN), 2.99 (q, 2H, 3J = 6.3 Hz, CH2N), 4.69 (br. s, 1H, NH), 6.43 (d, 2H, 3Jobs = 8.7 Hz, H2,2′ (Ph)), 7.26 (d, 2H, 3Jobs = 8.7 Hz, H3,3′(Ph)).
3-(Octylamino)benzonitrile (3g) [60]. 1H-NMR (400 MHz, CDCl3): δ 0.75–0.78 (m, 3H, CH3), 1.08–1.31 (m, 10H, CH2), 1.49 (quintet, 2H, 3J = 7.2 Hz, CH2CN), 2.95 (t, 2H, 3J = 6.2 Hz, CH2N), 4.20 (br. s, 1H, NH), 6.84–6.89 (m, 2H, H2,6(Ph)), 6.78 (d, 1H, 3J = 7.5 Hz, H4(Ph)), 7.06–7.10 (m, 1H, H5(Ph)).
4-Methoxy-N-octylaniline (3h) [59]. 1H-NMR (400 MHz, CDCl3): δ 0.75–0.78 (m, 3H, CH3), 1.10–1.32 (m, 10H, CH2), 1.48 (quintet, 2H, 3J = 7.1 Hz, CH2CN), 2.93 (t, 2H, 3J = 6.8 Hz, CH2N), 3.63 (s, 3H, OCH3), 6.46 (d, 2H, 3Jobs = 8.8 Hz, H2,2′ (Ph)), 6.65 (d, 2H, 3Jobs = 8.8 Hz, H3,3′(Ph)), NH proton was not assigned.
3-Methoxy-N-octylaniline (3i) [60]. 1H-NMR (400 MHz, CDCl3): δ 0.75–0.78 (m, 3H, CH3), 1.06–1.30 (m, 10H, CH2), 1.49 (quintet, 2H, 3J = 6.9 Hz, CH2CN), 2.96 (t, 2H, 3J = 6.7 Hz, CH2N), 3.65 (s, 3H, OCH3), 6.03 (br. s, 1H, H2(Ph)), 6.08–6.12 (m, 2H, H4,6(Ph)), 6.93 (t, 1H, 3Jobs = 7.6 Hz, H5(Ph)), NH proton was not assigned.
N-octyl-[1,1′-biphenyl]-4-amine (3j) [58]. 1H-NMR (400 MHz, CDCl3): δ 0.76–0.79 (m, 3H, CH3), 1.10–1.35 (m, 10H, CH2), 1.53 (quintet, 2H, 3J = 6.8 Hz, CH2CN), 3.03 (br. t, 2H, 3Jobs = 5.9 Hz, CH2N), 3.78 (br. s, 1H, NH), 6.56 (d, 2H, 3Jobs = 6.9 Hz, H2,2′ (Ph)), 7.13 (t, 1H, 3J = 6.8 Hz, H4(Ph’)), 7.26–7.34 (m, 4H, H3,3′(Ph, Ph’)), 7.42 (d, 2H, 3Jobs = 8.0 Hz, H2,2′(Ph’)).
(4-(Octylamino)phenyl)(phenyl)methanone (3k). 1H-NMR (400 MHz, CDCl3): δ 0.87–0.90 (m, 3H, CH3), 1.30–1.45 (m, 10H, CH2), 1.84 (quintet, 2H, 3J = 7.3 Hz, CH2CN), 3.18 (t, 2H, 3J = 7.2 Hz, CH2N), 4.51 (br. s, 1H, NH), 6.58–6.60 (m, 2H, H2,2′ (Ph)), 7.43–7.46 (m, 2H, H3,3′(Ph’)), 7.52 (tt, 1H, 3J = 7.3 Hz, 4J = 1.2 Hz, H4(Ph’)), 7.70–7.75 (m, 4H, H3,3′(Ph), H2,2′(Ph’)). 13C-NMR (100.6 MHz, CDCl3): δ 14.1 (CH3), 22.6 (CH2), 27.0 (CH2), 29.2 (2CH2), 29.3 (CH2), 31.8 (CH2), 43.4 (CH2N), 111.3 (C2,2′(Ph)), 125.9 (C4(Ph)), 128.0 (2CH(Ph)), 129.4 (2CH(Ph)), 131.1 (C4(Ph’)), 133.0 (2CH(Ph)), 139.1 (C1(Ph’)), 152.0 (C1(Ph)), 195.1 (C=O). MALDI-TOF calcd for C21H28NO [M + H]+ 310.2171, found: 310.2129.
Methyl 4-(octylamino)benzoate (3l) [59]. 1H-NMR (400 MHz, CDCl3): δ 0.71–0.74 (m, 3H, CH3), 1.00–1.25 (m, 10H, CH2), 1.47 (quintet, 2H, 3J = 7.3 Hz, CH2CN), 3.10 (br. t, 2H, 3Jobs = 5.6 Hz, CH2N), 3.68 (s, 3H, OCH3), 4.51 (br. s, 1H, NH), 6.40 (d, 2H, 3Jobs = 8.2 Hz, H2,2′ (Ph)), 7.67 (d, 2H, 3Jobs = 8.2 Hz, H3,3′(Ph)).
Ethyl 3-(octylamino)benzoate (3m). 1H-NMR (400 MHz, CDCl3): δ 0.86–0.90 (m, 3H, CH3), 1.20–1.41 (m, 10H, CH2), 1.38 (t, 3H, 3J = 7.2 Hz, CH3), 1.62 (quintet, 2H, 3J = 7.2 Hz, CH2CN), 3.13 (q, 2H, 3J = 7.0 Hz, CH2N), 4.35 (q, 2H, 3J = 7.2 Hz, CH2O), 6.76 (ddd, 1H, 3J = 8.1 Hz, 4J = 2.4 Hz, 4J = 0.7 Hz H6(Ph)), 7.21 (t, 1H, 3Jobs = 7.9 Hz, H5(Ph)), 7.25 (br. t, 1H, 4Jobs = 1.8 Hz H2(Ph)), 7.35 (d, 1H, 3J = 7.6 Hz, H4(Ph)), NH proton was not assigned. 13C-NMR (100.6 MHz, CDCl3): δ 13.9 (CH3), 14.1 (CH3), 22.4 (CH2), 26.9 (CH2), 29.0 (CH2), 29.2 (2CH2), 31.6 (CH2), 43.6 (CH2N), 60.5 (CH2O), 112.9 (CH(Ph)), 116.8 (CH(Ph)), 117.8 (CH(Ph)), 128.8 (C5(Ph)), 131.0 (C3(Ph)), 148.3 (C1(Ph)), 166.9 (C=O). MALDI-TOF calcd for C17H28NO2 [M + H]+ 278.2120, found: 278.2076.
4-Phenylmorpholine (13) [61]. 1H-NMR (400 MHz, CDCl3): δ 3.08–3.10 (m, 4H, CH2N), 3.78–3.80 (m, 4H, CH2O), 6.81 (t, 1H, 3J = 7.4 Hz, H4(Ph)), 6.85 (d, 2H, 3Jobs = 8.6 Hz, H2,2′(Ph)), 7.19–7.23 (m, 2H, H3,3′(Ph)).
1-Phenylpiperidine (14) [62]. 1H-NMR (400 MHz, CDCl3): δ 1.46–1.52 (m, 2H, CH2), 1.58–1.66 (m, 4H, CH2), 3.06–3.08 (m, 4H, CH2N), 6.72(t, 1H, 3J = 7.2 Hz, H4(Ph)), 6.85 (d, 2H, 3Jobs = 8.4 Hz, H2,2′(Ph)), 7.13–7.17 (m, 2H, H3,3′(Ph)).
N-benzylaniline (15) [63]. 1H-NMR (400 MHz, CDCl3): δ 4.27 (s, 2H, CH2), 6.55–6.57 (m, 2H, H2,2′(Ph)), 6.62 (tt, 1H, 3J = 7.3 Hz, 4J = 1.1 Hz, H4(Ph)), 7.06–7.10 (m, 2H, H3,3′(Ph)), 7.17–7.32 (m, 5H, Bn), NH proton was not assigned.
N-cyclohexylaniline (16) [63]. 1H-NMR (400 MHz, CDCl3): δ 1.01–1.20 (m, 3H, CH2), 1.22–1.35 (m, 2H, CH2), 1.54–1.61 (m, 1H, CH2), 1.63–1.73 (m, 2H, CH2), 1.93–2.02 (m, 2H, CH2), 3.13–3.20 (m, 1H, CH2N), 6.49–6.52 (m, 2H, H2,2′(Ph)), 6.56 (tt, 1H, 3J = 7.3 Hz, 4J = 1.0 Hz, H4(Ph)), 7.04–7.08 (m, 2H, H3,3′(Ph)), NH proton was not assigned.
Triphenylamine (17) [61]. 1H-NMR (400 MHz, CDCl3): δ 6.95 (t, 1H, 3J = 7.3 Hz, H4(Ph)), 7.10 (d, 2H, 3Jobs = 8.0 Hz, H2,2′(Ph)), 7.25–7.30 (m, 2H, H3,3′(Ph)).
1-Phenyl-1H-imidazole (18) [64]. 1H-NMR (400 MHz, CDCl3): δ 7.29 (br. s, 2H, H4,5(Imid)), 7.33–7.37 (m, 3H, H2,2′,4(Ph)), 7.45 (t, 2H, 3Jobs = 7.6 Hz, H3,3′(Ph)), 7.98 (br. s, 1H, H2(Imid)).
1-Phenyl-1H-benzo[d]imidazole (19) [64]. 1H-NMR (400 MHz, CDCl3): δ 7.17 (t, 1H, 3J = 7.5 Hz, H4(Ph)), 7.30–7.33 (m, 1H, H(Benzimid)), 7.49 (t, 1H, 3Jobs = 7.0 Hz H(Benzimid)), 7.60–7.64 (m, 2H, H3,3′(Ph)), 7.68 (d, 2H, 3Jobs = 7.7 Hz, H2,2′(Ph)), 7.73 (d, 1H, 3J = 7.9 Hz, H(Benzimid)), 7.77 (d, 1H, 3J = 7.3 Hz, H(Benzimid)), 8.56 (s, 1H, H2(Benzimid)).
1-Phenyl-1H-indole (20) [64]. 1H-NMR (400 MHz, CDCl3): δ 6.74 (d, 1H, 3J = 3.3 Hz, H3(Ind)), 7.20–7.29 (m, 2H, H5,6(Ind)), 7.37–7.41 (m, 2H, H2(Ind), H4(Ph)), 7.53–7.55 (m, 4H, H2,2′,3,3′(Ph)), 7.62 (d, 1H, 3J = 8.1 Hz, H4(Ind)), 7.74 (d, 1H, 3J = 7.5 Hz, H7(Ind)).
9-Phenyl-9H-carbazole (21) [64]. 1H-NMR (400 MHz, CDCl3): δ 7.16–7.21 (m, 2H, H(Carb)), 7.29–7.33 (m, 4H, H(Carb)), 7.38 (t, 1H, 3J = 8.0 Hz, H4(Ph)), 7.46 (d, 2H, 3Jobs = 8.90 Hz, H2,2′(Ph)), 7.52 (t, 2H, 3Jobs = 7.7 Hz, H3,3′(Ph)), 8.05 (d, 2H, 3J = 7.7 Hz, H(Carb)).
N-octylpyridin-2-amine (22) [57]. 1H-NMR (400 MHz, CDCl3): δ 1H-NMR (400 MHz, CDCl3): δ 0.77–0.80 (m, 3H, CH3), 1.10–1.35 (m, 10H, CH2), 1.53 (quintet, 2H, 3J = 7.3 Hz, CH2CN), 3.15 (q, 2H, 3J = 6.4 Hz, CH2N), 4.51 (br. s, 1H, NH), 6.29 (d, 1H, 3J = 8.4 Hz, H3(Py)), 6.45 (dd, 1H, 3J = 6.7 Hz, 3J = 5.2 Hz, H5(Py)), 7.30–7.34 (m, 1H, H4(Py)), 7.97 (d, 1H, 3J = 5.2 Hz, H6(Py)).
3.3. Studies on the Copper Leaching into Solution
A screw-cap vial (volume 4 mL) equipped with a magnetic bar was charged with the copper catalyst (3.2 mg (0.05 mmol) in the case of CuNPs, 4 mg in the case of CuO, 3.6 mg in the case of Cu2O), optionally with the ligand L1 or L2 (0.05 mmol), optionally with n-octylamine (0.5 mmol), and 1 mL DMSO. The vial was tightly closed and the mixture was stirred at 110 °C for 6 h. After cooling down the reaction mixture to ambient temperature it was centrifuged, 0.5 mL solution was taken and dissolved in 10 mL distilled water. The water solution was subjected to ICP MS analysis to establish the amount of copper in the solution.
3.4. Investigation of the Activity of the Leached Copper from CuNPs 25 nm
Three screw-cap vials (each a volume of 4 mL) equipped with magnetic bars were charged with (i) copper catalyst (3.2 mg CuNPs 25 nm, 0.05 mmol) and 1 mL DMSO; (ii) copper catalyst (3.2 mg CuNPs 25 nm, 0.05 mmol), ligand L1 (8.5 mg, 0.05 mmol), 1 mL DMSO; (iii) copper catalyst (3.2 mg CuNPs 25 nm, 0.05 mmol), ligand L1 (8.5 mg, 0.05 mmol), n-octylamine (65 mg, 0.5 mmol), 1 mL DMSO. The vials were tightly closed and the reaction mixtures were stirred at 110 °C for 24 h. After cooling down the reaction mixtures to ambient temperature the residues were centrifuged, 0.5 mL of each reaction mixture was taken and involved in the following amination reaction: (i) with the addition of iodobenzene (64 mg, 0.313 mmol), ligand L1 (4.2 mg, 10 mol%), n-octylamine (32 mg, 0.25 mmol), cesium carbonate (0.313 mmol, 102 mg); (ii) with the addition of iodobenzene (64 mg, 0.313 mmol), n-octylamine (32 mg, 0.25 mmol), cesium carbonate (0.313 mmol, 102 mg); (iii) with the addition of iodobenzene (64 mg, 0.313 mmol), cesium carbonate (0.313 mmol, 102 mg). The reactions were run for 24 h at 110 °C, after cooling down the reaction mixture to ambient temperature and sedimentation of the residue 30 μL of each reaction mixture was dissolved in 0.5 mL CDCl3 and analyzed by 1H NMR to establish the yields of 3a. It was found to be (i) 75%, (ii) 72%, (iii) 77%.
3.5. Investigation of the Activity of the Residual Copper Catalyst in Solution after the Amination Reaction
A screw-cap vial (volume 4 mL) equipped with a magnetic bar was charged with the copper catalyst (3.2 mg CuNPs 25 nm or 10/80 nm, 10 mol%), corresponding ligand L1 or L2 (0.05 mmol, 10 mol%), iodobenzene (128 mg, 0.625 mmol), n-octylamine (65 mg, 0.5 mmol), cesium carbonate (0.625 mmol, 204 mg) and 1 mL DMSO. The vial was tightly closed and the reaction mixture was stirred at 110 °C for 24 h. After cooling down the reaction mixture to ambient temperature and sedimentation of the residue 30 μL of the reaction mixture was dissolved in 0.5 mL CDCl3 and analyzed by 1H NMR to establish the yield of the arylation product in the reaction mixture. The reaction mixture was centrifuged, 0.5 mL of the solution was taken and again charged with corresponding ligand L1 or L2 (0.025 mmol, 10 mol%), iodobenzene (64 mg, 0.313 mmol), n-octylamine (32 mg, 0.25 mmol), cesium carbonate (0.313 mmol, 102 mg). The vial was tightly closed and the reaction mixture was stirred at 110 °C for 24 h. After cooling down the reaction mixture to ambient temperature and sedimentation of the residue 30 μL of the reaction mixture was dissolved in 0.5 mL CDCl3 and analyzed by 1H NMR to establish the resulting amount of the arylation product in the reaction mixture and calculate the conversion of n-octylamine into 3a in the second run. It was 58% when using CuNPs 25 nm/L1 and 54% in the case of CuNPs 10/80 nm/L2 catalytic systems.
4. Conclusions
To sum up, we have studied the possibilities of employing commercially available unsupported copper nanoparticles (CuNPs) in the amines arylation. At first, using transmission electron microscopy (TEM) and electronography, the true sizes of nanoparticles were established. The reaction of the model iodobenzene with the primary aliphatic sterically unhindered n-octylamine was shown to be efficiently catalyzed with CuNPs of average sizes 25 and 10/80 nm in the presence of such ligands as 2-acetylcyclohexanone (L0), 2-isobutyryl cyclohexanone (L1), rac-BINOL (L2) and L-proline (L3). The yields of the target N-octylaniline (3a) exceeded 80% and reached 95% in the best case (CuNPs 10/80 nm/L2); CuO in bulk and nano forms was almost equally efficient in the presence of ligands L1 and L2. Investigation of a wider scope of starting aryl iodides and amines showed that in general the catalytic systems employing CuNPs 25 and 10/80 nm with L1 and L2 can be proposed as the most universal, providing the best yields of the target products (up to 93%). Arylation of NH-heterocycles was found to be most demanding and a diligent search for the best catalytic system in each case was needed. DMSO was used as a solvent in the majority of reactions; DMF was shown to be suitable in these reactions and provided high yields of the arylation products especially in the reactions with substituted aryl iodides. No significant dependence of the product yield on the electronic nature of substituents was observed. In any case, the application of appropriate ligands is crucial to obtain high yields of the arylation products under described conditions.
Additional experiments demonstrated that the leaching of copper into solution takes place and strongly depends on the size of CuNPs, the nature of the solvent, and ligands added. It was also shown that various catalytic systems are characterized by different induction times needed to achieve the reaction maximal rate, probably after the formation of a sufficient amount of reactive catalytic species. Thus, nanoparticles may serve as a reservoir of active catalytic species and feed the homogeneous catalytic process. TEM and electronography disclosed that after the reaction was over CuNPs were fully transformed into CuO nanoparticles with notable agglomeration. However, they continue to be catalytically active; moreover, their activity may differ from that of initially taken CuO NPs. All these interdependent facts (leaching, induction time, reusability of the catalyst) will be studied during further research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13020331/s1, Figures S1–S9: Electronograms of the nanoparticles, Figures S10–S15: 1H and 13C spectra of new compounds 3c,k,m.
Author Contributions
Conceptualization, A.D.A. and I.P.B.; methodology, A.D.A.; investigation, V.I.F., A.V.M. and A.A.S.; resources, A.D.A. and I.P.B.; data curation, A.D.A.; writing—original draft preparation, V.I.F. and A.V.M.; writing—review and editing, A.D.A. and I.P.B.; visualization, A.A.S.; supervision, I.P.B.; project administration, A.D.A.; funding acquisition, I.P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the RSF, grant number 19-13-00223P.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Heravi, M.M.; Kheilkordi, Z.; Zadsirjan, V.; Heydari, M.; Malmir, M. Buchwald-Hartwig reaction: An overview. J. Organomet. Chem. 2018, 861, 17–104. [Google Scholar] [CrossRef]
- Dorel, R.; Grugel, C.P.; Haydl, A.M. The Buchwald–Hartwig Amination After 25 Years. Angew. Chem. Int. Ed. 2019, 58, 17118–17129. [Google Scholar] [CrossRef]
- Shaughnessy, K.H.; Ciganek, E.; De Vasher, R.B.; Denmark, S.E. Copper-Catalyzed Amination of Aryl and Alkenyl Electrophiles; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Junge, K.; Wienhöfer, G.; Beller, M.; Tlili, A.; Evano, G.; Taillefer, M.; Kempe, R.; Malbertz, C.; Klankermayer, J. New Trends in Organometallic Catalysts. In Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Three Volumes, 3rd ed.; Cornils, B., Herrmann, W.A., Beller, M., Paciello, R., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Neetha, M.; Saranya, S.; Harry, N.A.; Anilkumar, G. Recent Advances and Perspectives in the Copper-Catalysed Amination of Aryl and Heteroaryl Halides. ChemistrySelect 2020, 5, 736–753. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Aghili, N.; Tajbakhsh, M. SBA-15 Immobilized Phenanthroline–Copper(I) Complex as a Recyclable Efficient atalyst for N-Arylation of Amides and N–H Heterocycles with Aryl Halides. Catal. Lett. 2016, 146, 193–203. [Google Scholar] [CrossRef]
- Niakan, M.; Asadi, Z.; Zare, S. Preparation and Characterization of PANI@NiO Visible Light Photocatalyst for Wastewater Treatment. ChemistrySelect 2020, 5, 12618–12623. [Google Scholar]
- Veisi, H.; Hamelian, M.; Hemmati, S.; Dalvand, A. CuI catalyst heterogenized on melamine-pyridines immobilized SBA-15: Heterogeneous and recyclable nanocatalyst for Ullmann-type CN coupling reactions. Tetrahedron Lett. 2017, 58, 4440–4446. [Google Scholar] [CrossRef]
- Esmaeilpour, M.; Sardarian, A.R.; Firouzabadi, H. Dendrimer-encapsulated Cu(Π) nanoparticles immobilized on superparamagnetic Fe3O4@SiO2 nanoparticles as a novel recyclable catalyst for N-arylation of nitrogen heterocycles and green synthesis of 5-substituted 1H-tetrazoles. Appl. Organomet. Chem. 2018, 32, e4300. [Google Scholar] [CrossRef]
- Mitrofanov, A.Y.; Murashkina, A.V.; Martín-García, I.; Alonso, F.; Beletskaya, I.P. Formation of C-C, C-S and C-N bonds catalysed by supported copper nanoparticles. Catal. Sci. Technol. 2017, 7, 4401–4412. [Google Scholar] [CrossRef]
- Hemmati, S.; Kamangar, S.A.; Yousefi, M.; Salehi, M.H.; Hekmati, M. Cu(I)-anchored polyvinyl alcohol coated-magnetic nanoparticles as heterogeneous nanocatalyst in Ullmann-type C–N coupling reactions. Appl. Organomet. Chem. 2020, 34, e5611. [Google Scholar] [CrossRef]
- Islam, M.; Mondal, S.; Mondal, P.; Roy, A.S.; Tuhina, K.; Mobarok, M.; Paul, S.; Salam, N.; Hossain, D. An Efficient Recyclable Polymer Supported Copper(II) Catalyst for C–N Bond Formation by N-Arylation. Catal. Lett. 2011, 141, 1171–1181. [Google Scholar] [CrossRef]
- Arundhathi, R.; Kumar, D.C.; Sreedhar, B. C-N bond formation catalysed by CuI Bonded to polyaniline nanofiber. Eur. J. Org. Chem. 2010, 2010, 3621–3630. [Google Scholar] [CrossRef]
- Islam, S.M.; Salam, N.; Mondal, P.; Roy, A.S.; Ghosh, K.; Tuhina, K. A highly active reusable polymer anchored copper catalyst for C-O, C-N and C-S cross coupling reactions. J. Mol. Catal. A Chem. 2014, 387, 7–19. [Google Scholar] [CrossRef]
- Chouhan, G.; Wang, D.; Alper, H. Magnetic nanoparticle-supported proline as a recyclable and recoverable ligand for the CuI catalyzed arylation of nitrogen nucleophiles. Chem. Commun. 2007, 2007, 4809–4811. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Esmaeilpour, M.; Javidi, J. 1,4-Dihydroxyanthraquinone-copper(II) supported on superparamagnetic Fe3O4@SiO2: An efficient catalyst for N -arylation of nitrogen heterocycles and alkylamines with aryl halides and click synthesis of 1-aryl-1,2,3-triazole derivatives. RSC Adv. 2016, 6, 90154–90164. [Google Scholar] [CrossRef]
- Sardarian, A.R.; Eslahi, H.; Esmaeilpour, M. Copper(II) Complex Supported on Fe3O4@SiO2 Coated by Polyvinyl Alcohol as Reusable Nanocatalyst in N-Arylation of Amines and N(H)-Heterocycles and Green Synthesis of 1H-Tetrazoles. ChemistrySelect 2018, 3, 1499–1511. [Google Scholar] [CrossRef]
- Bagheri, S.; Pazoki, F.; Radfar, I.; Heydari, A. Copper(I)–creatine complex on magnetic nanoparticles as a green catalyst for N- and O-arylation in deep eutectic solvent. Appl. Organomet. Chem. 2020, 34, e5447. [Google Scholar] [CrossRef]
- Mondal, P.; Sinha, A.; Salam, N.; Roy, A.S.; Jana, N.R.; Islam, S.M. Enhanced Catalytic Performance by Copper Nanoparticle-Graphene Based Composite. RSC Adv. 2013, 3, 5615–5623. [Google Scholar] [CrossRef]
- Nador, F.; Volpe, M.A.; Alonso, F.; Radivoy, G. Synthesis of N-Aryl Imidazoles Catalyzed by Copper Nanoparticles on Nanosized Silica-Coated Maghemite. Tetrahedron 2014, 70, 6082–6087. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Dordahan, F.; Rafiee, F.; Mahdavi, M. C−N Cross-Coupling Reaction Catalysed by Efficient and Reusable CuO/SiO2 Nanoparticles under Ligand-Free Conditions. Appl. Organomet. Chem. 2014, 28, 809–813. [Google Scholar] [CrossRef]
- Gopiraman, M.; Ganesh Babu, S.; Khatri, Z.; Kai, W.; Kim, Y.A.; Endo, M.; Karvembu, R.; Kim, I.S. An Efficient, Reusable Copper-Oxide/Carbon-Nanotube Catalyst for N-Arylation of Imidazole. Carbon 2013, 62, 135–148. [Google Scholar] [CrossRef]
- Movahed, S.K.; Dabiri, M.; Bazgir, A. A One-Step Method for Preparation of Cu@Cu2O Nanoparticles on Reduced Graphene Oxide and Their Catalytic Activities in N-Arylation of N-Heterocycles. Appl. Catal. A 2014, 481, 79–88. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.J. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.T.; Robke, S.; Sahler, S.; Prechtl, M.H.G. Ligand- Free Copper(I) Oxide Nanoparticle-Catalysed Amination of Aryl Halides in Ionic Liquids. Catal. Sci. Technol. 2014, 4, 102–108. [Google Scholar] [CrossRef]
- Khalil, A.; Jouiad, M.; Khraisheh, M.; Hashaikeh, R. Facile Synthesis of Copper Oxide Nanoparticles via Electrospinning. J. Nanomater. 2014, 2014, 438407. [Google Scholar] [CrossRef]
- Jammi, S.; Sakthivel, S.; Rout, L.; Mukherjee, N.; Mandal, S.; Mitra, R.; Saha, P.; Punniyamurthy, T. CuO Nanoparticles Catalyzed C−N, C−O, and C−S Cross-Coupling Reactions: Scope and Mechanism. J. Org. Chem. 2009, 74, 1971–1976. [Google Scholar] [CrossRef]
- Suramwar, N.V.; Thakare, S.R.; Karade, N.N.; Khaty, N.T. Green synthesis of predominant (1 1 1) facet CuO nanoparticles: Heterogeneous and recyclable catalyst for N-arylation of indoles. J. Mol. Catal. A 2012, 359, 28–34. [Google Scholar] [CrossRef]
- Rout, L.; Jammi, S.; Punniyamurthy, T. Novel CuO Nanoparticle Catalyzed C−N Cross Coupling of Amines with Iodobenzene. Org. Lett. 2007, 9, 3397–3399. [Google Scholar] [CrossRef]
- Reddy, K.H.V.; Satish, G.; Ramesh, K.; Karnakar, K.; Nageswar, Y.V.D. An efficient synthesis of N-substituted indoles from indoline/indoline carboxylic acid via aromatization followed by C–N cross-coupling reaction by using nano copper oxide as a recyclable catalyst. Tetrahedron Lett. 2012, 53, 3061–3065. [Google Scholar] [CrossRef]
- Sreedhar, B.; Arundhathi, R.; Reddy, P.L.; Kantam, M.L. CuI Nanoparticles for C−N and C−O Cross Coupling of Heterocyclic Amines and Phenols with Chlorobenzenes. J. Org. Chem. 2009, 74, 7951–7954. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Copper Nanoparticles in Click Chemistry: An Alternative Catalytic System for the Cycloaddition of Terminal Alkynes and Azides. Tetrahedron Lett. 2009, 50, 2358–2362. [Google Scholar] [CrossRef]
- Chanda, K.; Rej, S.; Huang, M.H. Facet-Dependent Catalytic Activity of Cu2O Nanocrystals in the One-Pot Synthesis of 1,2,3- Triazoles by Multicomponent Click Reactions. Chem. Eur. J. 2013, 19, 16036–16043. [Google Scholar] [CrossRef] [PubMed]
- Isomura, Y.; Narushima, T.; Kawasaki, H.; Yonezawa, T.; Obora, Y. Surfactant-Free Single-Nano-Sized Colloidal Cu Nanoparticles for Use as an Active Catalyst in Ullmann-Coupling Reaction. Chem. Commun. 2012, 48, 3784–3786. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Saha, A.; Ranu, B.C. One-Pot Copper Nanoparticle-Catalyzed Synthesis of S-Aryl- and S-Vinyl Dithiocarbamates in Water: High Diastereoselectivity Achieved for Vinyl Dithiocarbamates. Green Chem. 2008, 10, 1224–1230. [Google Scholar] [CrossRef]
- Yavari, I.; Sodagar, E.; Nematpour, M. CuO Nanoparticles as Effective Reusable Heterogeneous Catalyst for S-Arylation Reactions. Helv. Chim. Acta 2014, 97, 420–425. [Google Scholar] [CrossRef]
- Botteselle, G.V.; Godoi, M.; Galetto, F.Z.; Bettanin, L.; Singh, D.; Rodrigues, O.E.D.; Braga, A.L. Microwave-Assisted One-Pot Synthesis of Symmetrical Diselenides, Ditellurides and Disulfides from Organoyl Iodides and Elemental Chalcogen Catalyzed by CuO Nanoparticles. J. Mol. Catal. A Chem. 2012, 365, 186–193. [Google Scholar] [CrossRef]
- Wang, F.; Shi, R.; Liu, Z.-Q.; Shang, P.-J.; Pang, X.; Shen, S.; Feng, Z.; Li, C.; Shen, W. Highly Efficient Dehydrogenation of Primary Aliphatic Alcohols Catalyzed by Cu Nanoparticles Dispersed on Rod-Shaped La2O2CO3. ACS Catal. 2013, 3, 890–894. [Google Scholar] [CrossRef]
- Kou, J.H.; Saha, A.; Bennett-Stamper, C.; Varma, R.S. Inside- Out Core-Shell Architecture: Controllable Fabrication of Cu2O@Cu with High Activity for the Sonogashira Coupling Reaction. Chem. Commun. 2012, 48, 5862–5864. [Google Scholar] [CrossRef]
- Bhosale, M.A.; Sasaki, T.; Bhanage, B.M. A Facile and Rapid Route for the Synthesis of Cu/Cu2O Nanoparticles and Their Application in the Sonogashira Coupling Reaction of Acyl Chlorides with Terminal Alkynes. Catal. Sci. Technol. 2014, 4, 4274–4280. [Google Scholar] [CrossRef]
- Honraedt, A.; Le Callonnec, F.; Le Grognec, E.; Fernandez, V.; Felpin, F.-X. C−H Arylation of Benzoquinone in Water through Aniline Activation: Synergistic Effect of Graphite-Supported Copper Oxide Nanoparticles. J. Org. Chem. 2013, 78, 4604–4609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, F.; Wang, F.; Zhao, N.; Liu, L.; Li, J.; Wang, Z. Synthesis of Quinazolines via CuO Nanoparticles Catalyzed Aerobic Oxidative Coupling of Aromatic Alcohols and Amidines. Org. Biomol. Chem. 2014, 12, 5752–5756. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Ali, W.; Mohanta, P.R.; Khatun, N.; Patel, B.K. CuO Nanoparticle Catalyzed Synthesis of 2,3-Disubstituted Quinazolinones via Sequential N-Arylation and Oxidative C−H Amidation. ACS Sustain. Chem. Eng. 2015, 3, 2582–2590. [Google Scholar] [CrossRef]
- Sharghi, H.; Shiri, P.; Aberi, M. A Solvent-Free and One-Pot Strategy for Ecocompatible Synthesis of Substituted Benzofurans from Various Salicylaldehydes, Secondary Amines, and Nonactivated Alkynes Catalyzed by Copper(I) Oxide Nanoparticles. Synthesis 2014, 46, 2489–2498. [Google Scholar] [CrossRef]
- Sarkar, S.; Chatterjee, N.; Roy, M.; Pal, R.; Sarkar, S.; Sen, A.K. Nanodomain Cubic Cuprous Oxide as Reusable Catalyst in One-Pot Synthesis of 3-Alkyl/Aryl-3-(Pyrrole-2-yl/Indole-3-yl)-2-Phenyl-2,3- Dihydro-Isoindolinones in Aqueous Medium. RSC Adv. 2014, 4, 7024–7029. [Google Scholar] [CrossRef]
- Murashkina, A.V.; Kuliukhina, D.S.; Averin, A.D.; Abel, A.S.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Correia, C.R.D.; Beletskaya, I.P. A comparison of homogeneous and heterogeneous copper catalyzed arylation of amines. Mendeleev Commun. 2022, 32, 91–93. [Google Scholar] [CrossRef]
- Murashkina, A.V.; Averin, A.D.; Panchenko, S.P.; Abel, A.S.; Maloshitskaya, O.A.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Correia, C.R.D.; Beletskaya, I.P. Comparison of the Catalytic Activities of Copper(I) Iodide and Copper Nanoparticles in the N-Arylation of Adamantane-Containing Amines. Russ. J. Org. Chem. 2022, 58, 15–24. [Google Scholar] [CrossRef]
- Kuliukhina, D.S.; Averin, A.D.; Panchenko, S.P.; Abel, A.S.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Correia, C.R.D.; Beletskaya, I.P. CuI and Copper Nanoparticles in the Catalytic Amination of 2-Halopyridines. Russ. J. Org. Chem. 2022, 58, 167–174. [Google Scholar] [CrossRef]
- Anderson, C.A.; Taylor, P.G.; Zeller, M.A.; Zimmerman, S.C. Room Temperature, Copper-Catalyzed Amination of Bromonaphthyridines with Aqueous Ammonia. J. Org. Chem. 2010, 75, 4848–4851. [Google Scholar] [CrossRef]
- Gao, J.; Bhunia, S.; Wang, K.; Gan, L.; Xia, S.; Ma, D. Discovery of N-(Naphthalen-1-yl)-N′-alkyl Oxalamide Ligands Enables Cu-Catalyzed Aryl Amination with High Turnovers. Org. Lett. 2017, 19, 2809–2812. [Google Scholar] [CrossRef]
- Pawar, G.G.; Wu, H.; De, S.; Ma, D. Copper(I) Oxide/N,N′-Bis[(2-furyl)methyl]oxalamide-Catalyzed Coupling of (Hetero)aryl Halides and Nitrogen Heterocycles at Low Catalytic Loading. Adv. Synth. Catal. 2017, 359, 1631–1636. [Google Scholar] [CrossRef]
- Larsson, P.-F.; Correa, A.; Carril, M.; Norrby, P.-O.; Bolm, C. Copper-catalyzed cross-couplings with part-per-million catalyst loadings. Angew. Chem. Int. Ed. 2009, 48, 5691–5693. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, S.P.; Abel, A.S.; Averin, A.D.; Maloshitskaya, O.A.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; Beletskaya, I.P. Arylation of Adamantanamines: VIII.1 Optimization of the Catalytic System for Copper-Catalyzed Arylation of Adamantane-Containing Amines. Russ. J. Org. Chem. 2017, 53, 1497–1504. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Beletskaya, I.P. Toward the Ideal Catalyst: From Atomic Centers to a “Cocktail” of Catalysts. Organometallics 2012, 31, 1595–1604. [Google Scholar] [CrossRef]
- Zeng, H.; Cao, D.; Qiu, Z.; Li, C.-J. Palladium-Catalyzed Formal Cross-Coupling of Diaryl Ethers with Amines: Slicing the 4-O-5 Linkage in Lignin Models. Angew. Chem. Int. Ed. 2018, 57, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Ueda, Y.; Iwai, T.; Sawamura, M. Nickel-catalyzed amination of aryl fluorides with primary amines. Chem. Commun. 2018, 54, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Rucker, R.P.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M.J.; Froese, R.D.J.; Organ, M.G. Selective Monoarylation of Primary Amines Using the Pd-PEPPSI-IPentCl Precatalyst. Angew. Chem. Int. Ed. 2015, 54, 9507–9511. [Google Scholar] [CrossRef]
- Ge, S.; Green, R.A.; Hartwig, J.F. Controlling first-row catalysts: Amination of aryl and heteroaryl chlorides and bromides with primary aliphatic amines catalyzed by a BINAP-ligated single-component Ni(0) complex. J. Am. Chem. Soc. 2014, 136, 1617–1627. [Google Scholar] [CrossRef]
- Topchiy, M.A.; Asachenko, A.F.; Nechaev, M.S. Solvent-Free Buchwald–Hartwig Reaction of Aryl and Heteroaryl Halides with Secondary Amines. Eur. J. Org. Chem. 2014, 2014, 3319–3322. [Google Scholar] [CrossRef]
- Alavi, S.A.G.; Nasseri, M.A.; Kazemnejadi, M.; Allahresani, A.; Hussainzadeh, M. NiFe2O4@SiO2@ZrO2/SO42-/Cu/Co nanoparticles: A novel, efficient, magnetically recyclable and bimetallic catalyst for Pd-free Suzuki, Heck and C-N cross-coupling reactions in aqueous media. New J. Chem. 2021, 45, 7741–7757. [Google Scholar] [CrossRef]
- Vidal, F.; McQuade, J.; Lalancette, R.; Jäkle, F. ROMP-Boranes as Moisture-Tolerant and Recyclable Lewis Acid Organocatalysts. J. Am. Chem. Soc. 2020, 142, 14427–14431. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, H.; Sepehri, S.; Aberi, M. Cu(II) complex of pyridine-based polydentate as a novel, efficient, and highly reusable catalyst in C–N bond-forming reaction. Mol. Divers. 2017, 21, 855–864. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).