Hydrogen Production by N-Heterocycle Dehydrogenation over Pd Supported on Aerogel-Prepared Mg-Al Oxides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of MgAlOx Aerogel Supports and Pd Catalysts Based on Them
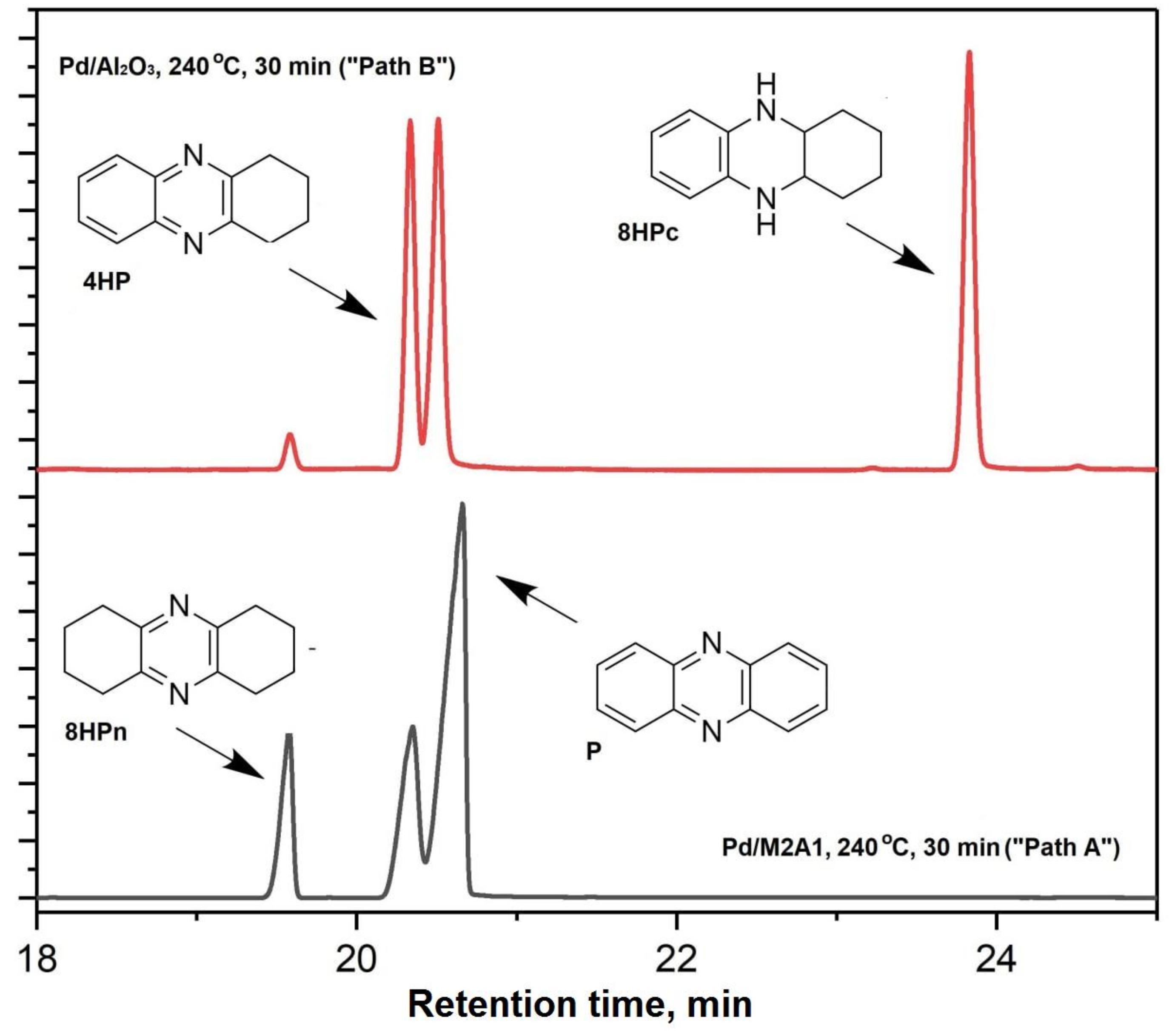
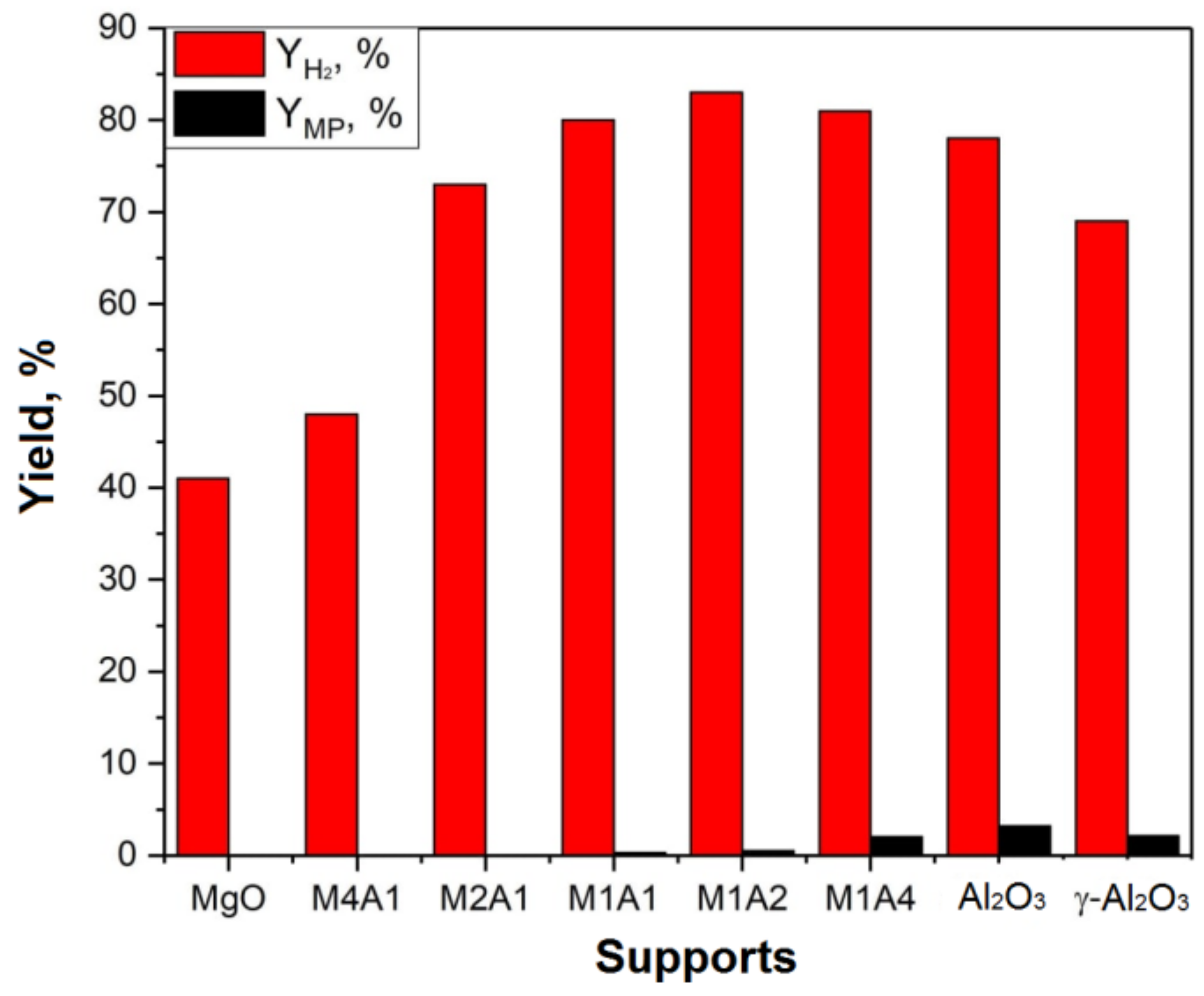
2.2. Investigation of the Catalytic Activity of Aerogel-Supported Pd Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Tetradecahydrophenzine Dehydrogenation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, J. The impact of climate policy on fossil fuel consumption: Evidence from the Regional Greenhouse Gas Initiative (RGGI). Energy Econ. 2021, 100, 105333. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Schlücker, E.; Wasserscheid, P. Transport and Storage of Hydrogen via Liquid Organic Hydrogen Carrier (LOHC) Systems. In Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 811–830. [Google Scholar] [CrossRef]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2017, 118, 372–433. [Google Scholar] [CrossRef]
- Dalebrook, A.F.; Gan, W.; Grasemann, M.; Moret, S.; Laurenczy, G. Hydrogen storage: Beyond conventional methods. Chem. Commun. 2013, 49, 8735–8751. [Google Scholar] [CrossRef]
- Egeland-Eriksen, T.; Hajizadeh, A.; Sartori, S. Hydrogen-based systems for integration of renewable energy in power systems: Achievements and perspectives. Int. J. Hydrogen Energy 2021, 46, 31963–31983. [Google Scholar] [CrossRef]
- Abdin, Z.; Khalilpour, K.R. Single and Polystorage Technologies for Renewable-Based Hybrid Energy Systems. In Polygeneration with Polystorage for Chemical and Energy Hubs; Academic Press: London, UK, 2019; pp. 77–131. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Kumar, A.; Muthukumar, P.; Sharma, P.; Kumar, E.A. Absorption based solid state hydrogen storage system: A review. Sustain. Energy Technol. Assess. 2022, 52, 102204. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid organic hydrogen carriers (LOHCs)—Techno-economic analysis of LOHCs in a defined process chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Byun, M.; Lee, A.; Cheon, S.; Kim, H.; Lim, H. Preliminary feasibility study for hydrogen storage using several promising liquid organic hydrogen carriers: Technical, economic, and environmental perspectives. Energy Convers. Manag. 2022, 268, 116001. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Singh, R.; Singh, M.; Gautam, S. Hydrogen economy, energy, and liquid organic carriers for its mobility. Mater. Today Proc. 2021, 46, 5420–5427. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Zubar, V.; Borghs, J.C.; Rueping, M. Hydrogenation or Dehydrogenation of N-Containing Heterocycles Catalyzed by a Single Manganese Complex. Org. Lett. 2020, 22, 3974–3978. [Google Scholar] [CrossRef]
- Forberg, D.; Schwob, T.; Zaheer, M.; Friedrich, M.; Miyajima, N.; Kempe, R. Single-catalyst high-weight% hydrogen storage in an N-heterocycle synthesized from lignin hydrogenolysis products and ammonia. Nat. Commun. 2016, 7, 13201. [Google Scholar] [CrossRef]
- Stepanenko, S.A.; Shivtsov, D.M.; Koskin, A.P.; Koskin, I.P.; Kukushkin, R.G.; Yeletsky, P.M.; Yakovlev, V.A. N-Heterocyclic Molecules as Potential Liquid Organic Hydrogen Carriers: Reaction Routes and Dehydrogenation Efficacy. Catalysts 2022, 12, 1260. [Google Scholar] [CrossRef]
- He, T.; Pei, Q.; Chen, P. Liquid organic hydrogen carriers. J. Energy Chem. 2015, 24, 587–594. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Bai, X.; Wu, W. Ultrasonic-assisted preparation of ultrafine Pd nanocatalysts loaded on Cl−-intercalated MgAl layered double hydroxides for the catalytic dehydrogenation of dodecahydro-N-ethylcarbazole. Ultrason. Sonochemistry 2022, 88, 106097. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.; Ke, H.; Cheng, H. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts. Int. J. Hydrogen Energy 2014, 39, 18976–18983. [Google Scholar] [CrossRef]
- Koper, O.B.; Lagadic, I.; Volodin, A.; Klabunde, K.J. Alkaline-Earth Oxide Nanoparticles Obtained by Aerogel Methods. Characterization and Rational for Unexpectedly High Surface Chemical Reactivities. Chem. Mater. 1997, 9, 2468–2480. [Google Scholar] [CrossRef]
- Mishakov, I.; Heroux, D.; Chesnokov, V.; Koscheev, S.; Melgunov, M.; Bedilo, A.; Buyanov, R.; Klabunde, K. Reaction of nanocrystalline MgO with 1-iodobutane. J. Catal. 2005, 229, 344–351. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Gerus, Y.Y.; Cherepanova, S.V.; Bedilo, A.F. Synthesis of C12A7 calcium aluminate aerogels. Mater. Lett. 2021, 293, 129699. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Bedilo, A.F.; Veselov, G.B.; Gerus, Y.Y.; Shuvarakova, E.I.; Stoyanovskii, V.O.; Vedyagin, A.A. Comparative Study of Pd-Mayenite Catalysts Prepared via Aerogel Approaches. Gels 2022, 8, 809. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Mishakov, I.V.; Ilyina, E.V. A step forward in the preparation of V–Mg–O catalysts for oxidative dehydrogenation of propane. J. Sol-Gel Sci. Technol. 2020, 97, 117–125. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A.; Cherepanova, S.V.; Nadeev, A.N.; Bedilo, A.F.; Klabunde, K.J. Synthesis and characterization of mesoporous VOx/MgO aerogels with high surface area. Microporous Mesoporous Mater. 2012, 160, 32–40. [Google Scholar] [CrossRef]
- Anderson, A.M.; Bruno, B.A.; Santos, J.; Barry, P.J.; Carroll, M.K. PGM nanoparticle-based alumina aerogels for three-way catalyst applications. Catal. Commun. 2022, 172, 106547. [Google Scholar] [CrossRef]
- Li, J.; Tong, F.; Li, Y.; Liu, X.; Guo, Y.; Wang, Y. Dehydrogenation of dodecahydro-N-ethylcarbazole over spinel supporting catalyst in a continuous flow fixed bed reactor. Fuel 2022, 321, 124034. [Google Scholar] [CrossRef]
- Song, J.; Wang, S.; Xu, Y.; Liu, Q.; Zhao, Y. LDH derived MgAl2O4 spinel supported Pd catalyst for the low-temperature methane combustion: Roles of interaction between spinel and PdO. Appl. Catal. A Gen. 2021, 621, 118211. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Gao, D.; Sun, T.; Zhang, C.; Wang, S. Influence of metal oxides on the performance of Pd/Al2O3 catalysts for methane combustion under lean-fuel conditions. Fuel Process. Technol. 2013, 111, 55–61. [Google Scholar] [CrossRef]
- Tuo, Y.; Meng, Y.; Chen, C.; Lin, D.; Feng, X.; Pan, Y.; Li, P.; Chen, D.; Liu, Z.; Zhou, Y.; et al. Partial positively charged Pt in Pt/MgAl2O4 for enhanced dehydrogenation activity. Appl. Catal. B Environ. 2021, 288, 119996. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for Preparation of Catalytic Materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Klabunde, K.J. Synthesis of Catalytically Active Sulfated Zirconia Aerogels. J. Catal. 1998, 176, 448–458. [Google Scholar] [CrossRef]
- Rechberger, F.; Niederberger, M. Synthesis of aerogels: From molecular routes to 3-dimensional nanoparticle assembly. Nanoscale Horiz. 2017, 2, 6–30. [Google Scholar] [CrossRef]
- Yorov, K.E.; Baranchikov, A.E.; Kiskin, M.A.; Sidorov, A.A.; Ivanov, V.K. Functionalization of Aerogels with Coordination Compounds. Russ. J. Coord. Chem. 2022, 48, 89–117. [Google Scholar] [CrossRef]
- Lin, Q.; Ji, Y.; Jiang, Z.-D.; Xiao, W.-D. Effects of Precursors on Preparation of Pd/α-alumina Catalyst for Synthesis of Dimethyl Oxalate. Ind. Eng. Chem. Res. 2007, 46, 7950–7954. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982; pp. 126–132. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bedilo, A.F.; Richards, R.M.; Chesnokov, V.V.; Volodin, A.M.; Zaikovskii, V.I.; Buyanov, R.A.; Klabunde, K.J. Nanocrystalline MgO as a Dehydrogenation Catalyst. J. Catal. 2002, 206, 40–48. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Saraev, A.A.; Matveev, A.V.; Dubinin, Y.V.; Knop-Gericke, A.; Bukhtiyarov, V.I. In Situ NAP-XPS and Mass Spectrometry Study of the Oxidation of Propylene over Palladium. J. Phys. Chem. C 2018, 122, 4315–4323. [Google Scholar] [CrossRef]
- Matveev, A.V.; Kaichev, V.V.; Saraev, A.A.; Gorodetskii, V.V.; Knop-Gericke, A.; Bukhtiyarov, V.I.; Nieuwenhuys, B.E. Oxidation of propylene over Pd(5 5 1): Temperature hysteresis induced by carbon deposition and oxygen adsorption. Catal. Today 2015, 244, 29–35. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Rybinskaya, A.A.; Kenzhin, R.M.; Volodin, A.M.; Bedilo, A.F. Characterization of electron donor sites on Al2O3 surface. Phys. Chem. Chem. Phys. 2012, 14, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Bedilo, A.F.; Shuvarakova, E.I.; Volodin, A.M.; Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A.; Chesnokov, V.V.; Heroux, D.S.; Klabunde, K.J. Effect of Modification with Vanadium or Carbon on Destructive Sorption of Halocarbons over Nanocrystalline MgO: The Role of Active Sites in Initiation of the Solid-State Reaction. J. Phys. Chem. C 2014, 118, 13715–13725. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Shuvarakova, E.I.; Rybinskaya, A.A.; Medvedev, D.A. Characterization of Electron-Donor and Electron-Acceptor Sites on the Surface of Sulfated Alumina Using Spin Probes. J. Phys. Chem. C 2014, 118, 15779–15794. [Google Scholar] [CrossRef]
- Koskin, A.P.; Andreev, R.V.; Primachenko, O.N.; Shuvarakova, E.I.; Bedilo, A.F. Perfluorosulfonic Acid Polymer Composites: Effect of the Support and Synthesis Method on the Acid and Catalytic Properties. Mol. Catal. 2020, 492, 111006. [Google Scholar] [CrossRef]
- Shuvarakova, E.I.; Bedilo, A.F.; Kenzhin, R.M.; Ilyina, E.V.; Gerus, Y.Y. Synthesis and Investigation of Finely Dispersed Calcium Aluminates and Catalysts Based on Them. Russ. J. Phys. Chem. B 2022, 16, 411–420. [Google Scholar] [CrossRef]
- Shuvarakova, E.I.; Ilyina, E.V.; Cherepanova, S.V.; Gerasimov, E.Y.; Bedilo, A.F.; Vedyagin, A.A. Synthesis of Vanadia-Mayenite Nanocomposites and Characterization of Their Structure, Morphology and Surface Sites. J. Compos. Sci. 2022, 6, 254. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Stoyanovskii, V.O.; Rogov, V.A.; Kriventsov, V.V.; Mishakov, I.V. The role of chemisorbed water in formation and stabilization of active sites on Pd/Alumina oxidation catalysts. Catal. Today 2018, 307, 102–110. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Bedilo, A.F.; Cherepanova, S.V.; Gerus, Y.Y.; Shuvarakova, E.I.; Vedyagin, A.A. Aerogel synthesis of calcium aluminates with varied stoichiometry. J. Sol-Gel Sci. Technol. 2022, 104, 259–266. [Google Scholar] [CrossRef]
- Ye, T.-N.; Li, J.; Kitano, M.; Hosono, H. Unique nanocages of 12CaO·7Al2O3 boost heterolytic hydrogen activation and selective hydrogenation of heteroarenes over ruthenium catalyst. Green Chem. 2017, 19, 749–756. [Google Scholar] [CrossRef]
- Fang, M.; Machalaba, N.; Sánchez-Delgado, R.A. Hydrogenation of arenes and N-heteroaromatic compounds over ruthenium nanoparticles on poly(4-vinylpyridine): A versatile catalyst operating by a substrate-dependent dual site mechanism. Dalton Trans. 2011, 40, 10621–10632. [Google Scholar] [CrossRef]
- Fang, M.; Sánchez-Delgado, R.A. Ruthenium nanoparticles supported on magnesium oxide: A versatile and recyclable dual-site catalyst for hydrogenation of mono- and poly-cyclic arenes, N-heteroaromatics, and S-heteroaromatics. J. Catal. 2014, 311, 357–368. [Google Scholar] [CrossRef]
- Wei, Z.; Shao, F.; Wang, J. Recent advances in heterogeneous catalytic hydrogenation and dehydrogenation of N-heterocycles. Chin. J. Catal. 2019, 40, 980–1002. [Google Scholar] [CrossRef]
- Rahi, R.; Fang, M.; Ahmed, A.; Sánchez-Delgado, R.A. Hydrogenation of quinolines, alkenes, and biodiesel by palladium nanoparticles supported on magnesium oxide. Dalton Trans. 2012, 41, 14490. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Tanaka, Y.; Kobayashi, M.; Yamaguchi, R. Homogeneous Perdehydrogenation and Perhydrogenation of Fused Bicyclic N-Heterocycles Catalyzed by Iridium Complexes Bearing a Functional Bipyridonate Ligand. J. Am. Chem. Soc. 2014, 136, 4829–4832. [Google Scholar] [CrossRef]
- Oh, J.; Bathula, H.B.; Park, J.H.; Suh, Y.-W. A sustainable mesoporous palladium-alumina catalyst for efficient hydrogen release from N-heterocyclic liquid organic hydrogen carriers. Commun. Chem. 2019, 2, 68. [Google Scholar] [CrossRef]
- Oh, J.; Jeong, K.; Kim, T.W.; Kwon, H.; Han, J.W.; Park, J.H.; Suh, Y.-W. 2-(N-Methylbenzyl)pyridine: A Potential Liquid Organic Hydrogen Carrier with Fast H2 Release and Stable Activity in Consecutive Cycles. ChemSusChem 2018, 11, 661–665. [Google Scholar] [CrossRef]
- Oh, J.; Kim, T.W.; Jeong, K.; Park, J.H.; Suh, Y.-W. Enhanced Activity and Stability of a Carbon-Coated Alumina-Supported Pd Catalyst in the Dehydrogenation of a Liquid Organic Hydrogen Carrier, Perhydro 2-(n-methylbenzyl)Pyridine. ChemCatChem 2018, 10, 3892–3900. [Google Scholar] [CrossRef]
- Kim, Y.; Song, Y.; Choi, Y.; Jeong, K.; Park, J.H.; Ko, K.C.; Na, K. Catalytic Consequences of Supported Pd Catalysts on Dehydrogenative H2 Evolution from 2-[(n-Methylcyclohexyl)methyl]piperidine as the Liquid Organic Hydrogen Carrier. ACS Sustain. Chem. Eng. 2020, 9, 809–821. [Google Scholar] [CrossRef]
- Bathula, H.B.; Oh, J.; Jo, Y.; Suh, Y.-W. Dehydrogenation of 2-[(n-Methylcyclohexyl)Methyl]Piperidine over Mesoporous Pd-Al2O3 Catalysts Prepared by Solvent Deficient Precipitation: Influence of Calcination Conditions. Catalysts 2019, 9, 719. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Duan, P.; Xu, D.; Luque, R. Catalytic Hydrodenitrogenation of Pyridine under Hydrothermal Conditions: A Comprehensive Study. ACS Sustain. Chem. Eng. 2020, 9, 362–374. [Google Scholar] [CrossRef]
- Guo, Y.; He, H.; Liu, X.; Chen, Z.; Rioux, R.M.; Janik, M.J.; Savage, P.E. Ring-opening and hydrodenitrogenation of indole under hydrothermal conditions over Ni, Pt, Ru, and Ni-Ru bimetallic catalysts. Chem. Eng. J. 2021, 406, 126853. [Google Scholar] [CrossRef]
- Klabunde, K.J.; Stark, J.; Koper, O.; Mohs, C.; Park, D.G.; Decker, S.; Jiang, Y.; Lagadic, I.; Zhang, D.J. Nanocrystals as Stoichiometric Reagents with Unique Surface Chemistry. J. Phys. Chem. 1996, 100, 12142–12153. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A. Nanocrystalline Aerogels of Metal Oxides as Destructive Sorbents and Catalysts. Chem. Sustain. Dev. 2011, 19, 25–32. [Google Scholar]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Fairley, N. CasaXPS VAMAS Processing Software. 2010. Available online: http://www.casaxps.com (accessed on 20 January 2022).

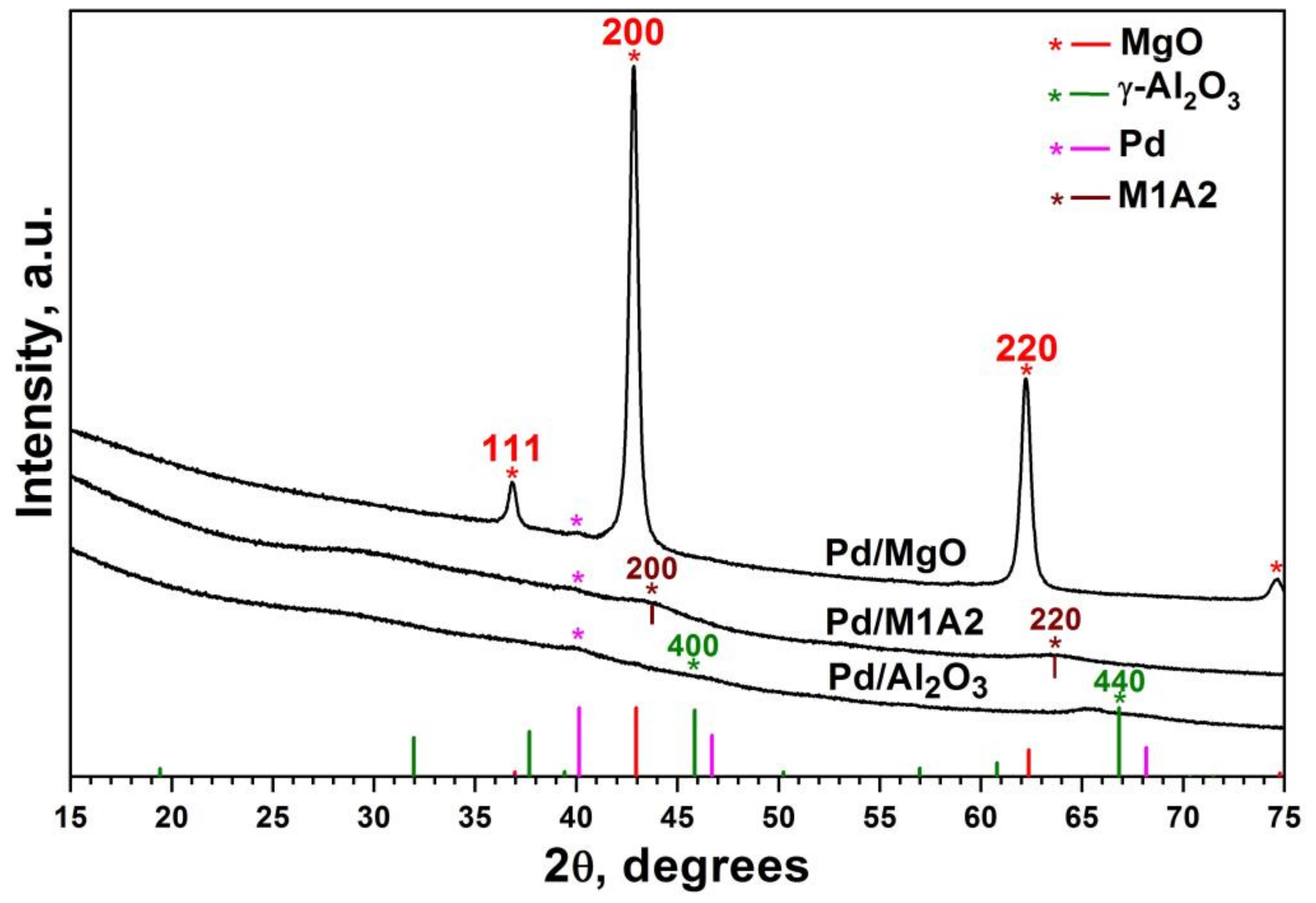
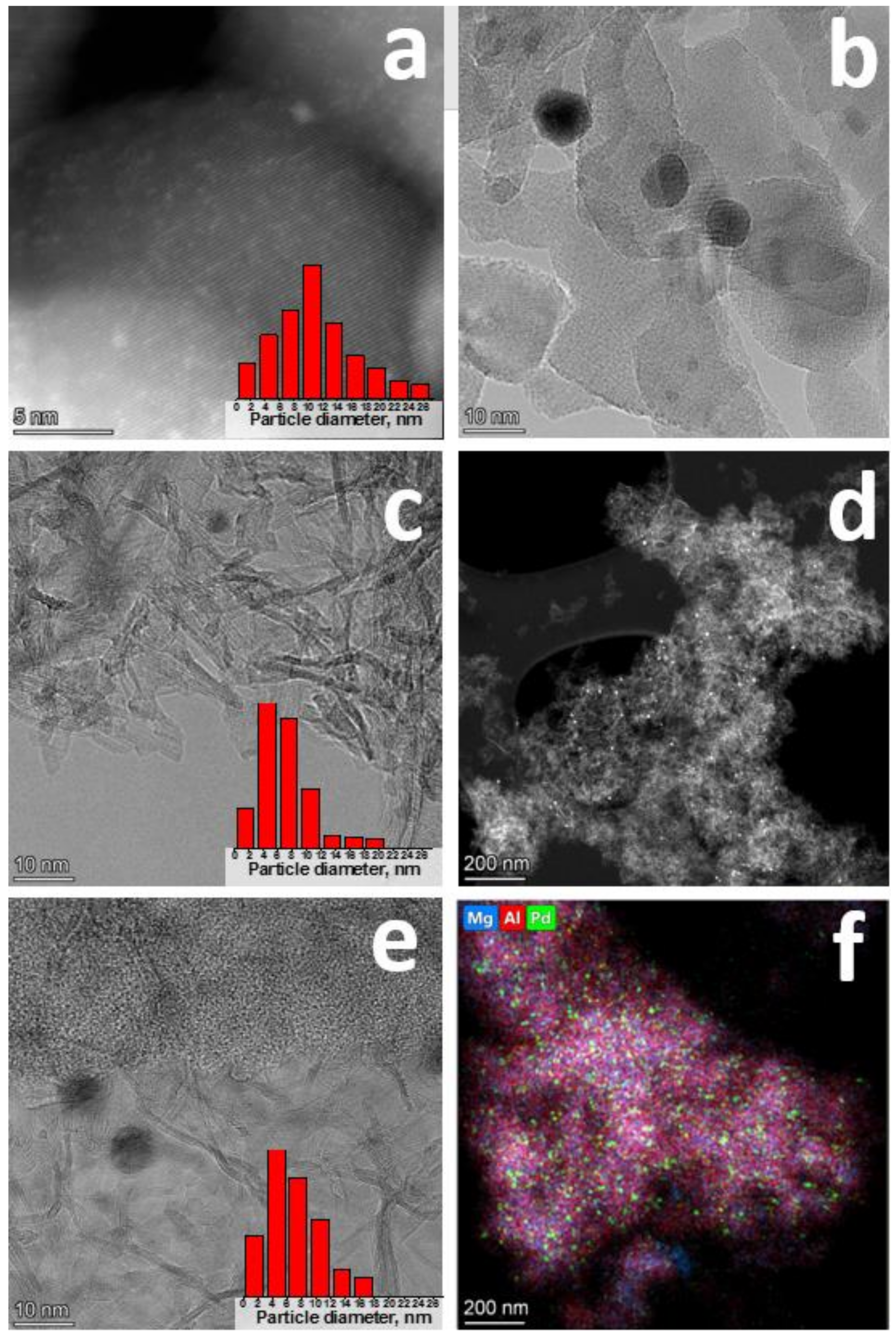
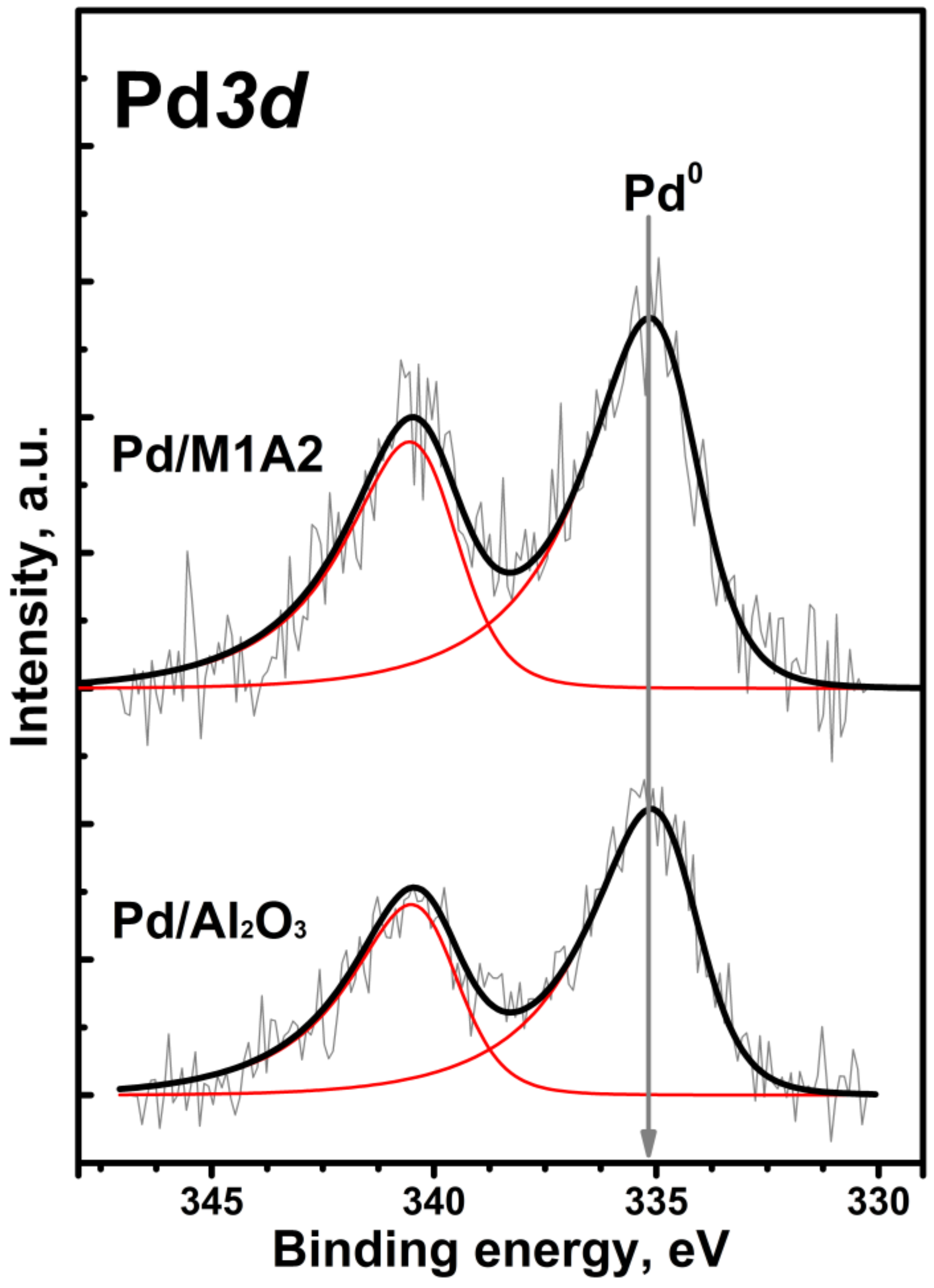
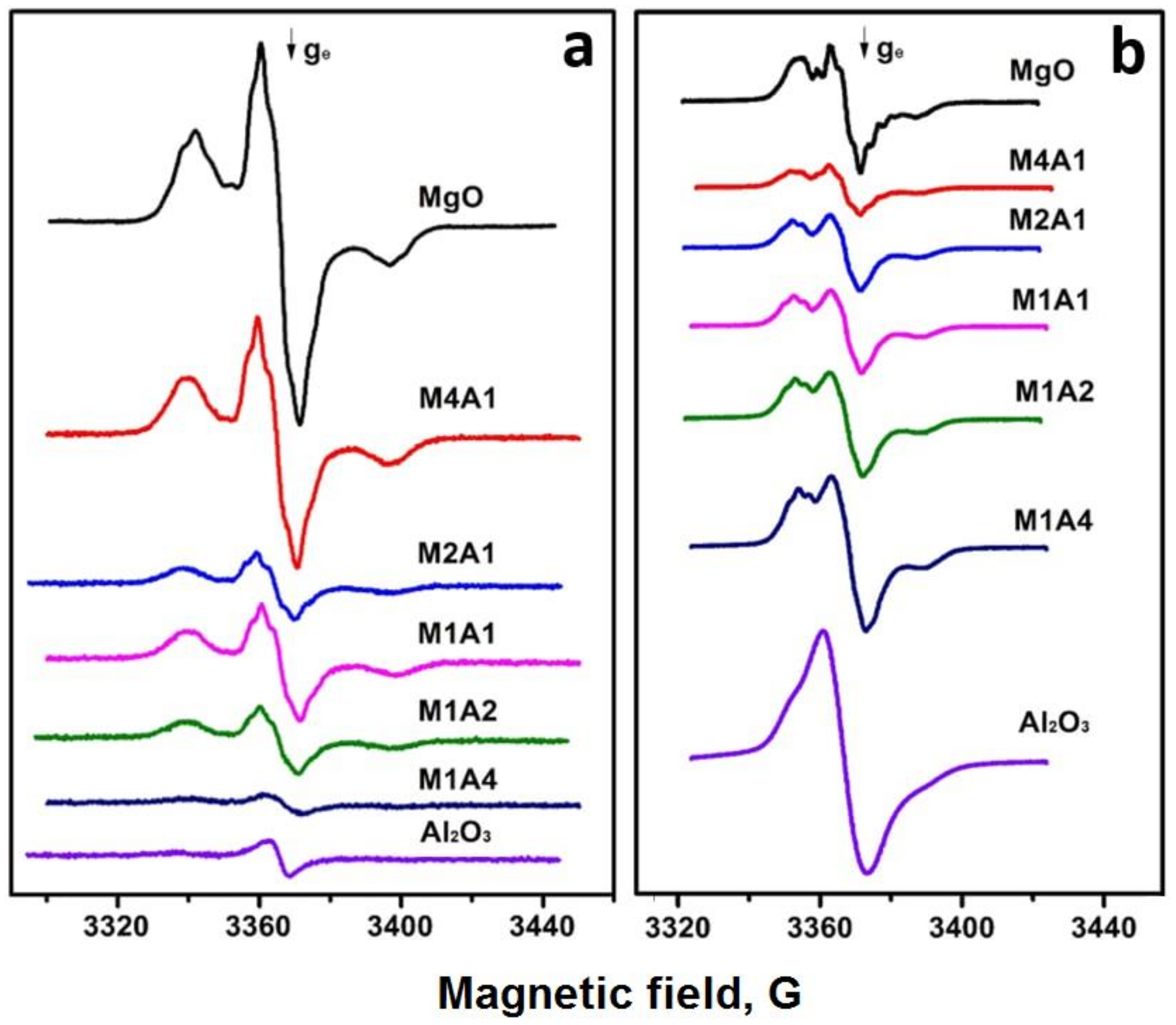

| Sample | SSA, m2/g | Vpore, cm3/g | Pore Size 1, nm | Dav, nm |
|---|---|---|---|---|
| Supports | ||||
| MgO | 220 | 1.2 | 9.4 2 | 21 |
| M4A1 | 415 | 1.6 | 12 3 | 15 |
| M2A1 | 495 | 1.5 | 8.0 3 | 12 |
| M1A1 | 535 | 1.4 | 3.3 2 | 11 |
| M1A2 | 570 | 2.0 | 4.5 2 | 14 |
| M1A4 | 600 | 2.3 | 5.2 2 | 15 |
| Al2O3 | 545 | 2.3 | 3.8 2 | 17 |
| Catalysts 1 wt% Pd | ||||
| Pd/MgO | 70 | 0.7 | 34 2 | 40 |
| Pd/M4A1 | 310 | 1.6 | 16 3 | 20 |
| Pd/M2A1 | 345 | 1.2 | 9.6 3 | 14 |
| Pd/M1A1 | 340 | 1.4 | 5.1 2 | 17 |
| Pd/M1A2 | 410 | 2.1 | 7.1 2 | 21 |
| Pd/M1A4 | 445 | 1.9 | 14 3 | 17 |
| Pd/Al2O3 | 435 | 2.1 | 17 3 | 19 |
| Aerogel Supports | Pd Dispersion by CO Chemisorption (DCO), % | Active Site Concentration, 1018 g−1 | |
|---|---|---|---|
| 1,3,5-Trinitrobenzene (Electron-Donor Sites) | Phenothiazine (Electron-Acceptor Sites) | ||
| MgO | 46 | 2.4 | 3.2 |
| M4A1 | 50 | 2.4 | 2.4 |
| M2A1 | 54 | 1.8 | 3.7 |
| M1A1 | 54 | 1.1 | 4.4 |
| M1A2 | 55 | 1.2 | 7.5 |
| M1A4 | 49 | 0.7 | 9.6 |
| Al2O3 | 33 | 0.6 | 16.8 |
| Catalyst | Reactant | Reaction Conditions | H2 Evolution Rate (mmol gMet−1 min−1) | Ref. |
|---|---|---|---|---|
| Pd2Ru@SiCN | 14HP | 2 mmol 14HP, 70 mg Pd2Ru@SiCN (0.36 mol. % active metal), 190 °C, 0.75 mL diglyme, 24 h. | 39.31 | [19] |
| Ir complexes (Homog.) | 2,6-dimethyldecahydro-1,5-naphthyridine | 0.25 mmol of reactant and the catalyst under reflux in p-xylene for 20 h, 138 °C, 5 mol% of Ir. | 0.43 | [57] |
| 1 wt% Pd/γ-Al2O3 | decahydroquinoline | 10.83 mmol of reactant, 230 °C, 4 h, M/R = 0.1 mol%. | 62 | [58] |
| 1 wt% Pd/C | 2-[(n-methylcyclohexyl)-methyl]piperidine (MBP) | 7.732 mmol of H12-MBP, M/R = 0.1 mol%, 270 °C, 4 h. | 237 | [59] |
| Pd/3.3CCA | 7.732 mmol of H12-MBP, M/R = 0.1 mol%, 250 °C, 4 h. | 168 | [60] | |
| 3PdA | 7.732 mmol of H12-MBP, 270 °C, 4 h, M/R = 0.59 mol%. | 27.5 | [61] | |
| MPdA600_5h | 7.3 mmol of reactant, M/R ratio of 0.1 mol%, and 250 °C for 4 h. | 16.69 | [62] | |
| 1 wt% Pd/Mg-Al-Ox | 14HP | 1 mmol 14HP in tetraglyme (3 mL), 240 °C, 45 min, 50 mg of catalyst. | 252 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivtsov, D.M.; Koskin, A.P.; Stepanenko, S.A.; Ilyina, E.V.; Ayupov, A.B.; Bedilo, A.F.; Yakovlev, V.A. Hydrogen Production by N-Heterocycle Dehydrogenation over Pd Supported on Aerogel-Prepared Mg-Al Oxides. Catalysts 2023, 13, 334. https://doi.org/10.3390/catal13020334
Shivtsov DM, Koskin AP, Stepanenko SA, Ilyina EV, Ayupov AB, Bedilo AF, Yakovlev VA. Hydrogen Production by N-Heterocycle Dehydrogenation over Pd Supported on Aerogel-Prepared Mg-Al Oxides. Catalysts. 2023; 13(2):334. https://doi.org/10.3390/catal13020334
Chicago/Turabian StyleShivtsov, Danil M., Anton P. Koskin, Sergey A. Stepanenko, Ekaterina V. Ilyina, Artem B. Ayupov, Alexander F. Bedilo, and Vadim A. Yakovlev. 2023. "Hydrogen Production by N-Heterocycle Dehydrogenation over Pd Supported on Aerogel-Prepared Mg-Al Oxides" Catalysts 13, no. 2: 334. https://doi.org/10.3390/catal13020334
APA StyleShivtsov, D. M., Koskin, A. P., Stepanenko, S. A., Ilyina, E. V., Ayupov, A. B., Bedilo, A. F., & Yakovlev, V. A. (2023). Hydrogen Production by N-Heterocycle Dehydrogenation over Pd Supported on Aerogel-Prepared Mg-Al Oxides. Catalysts, 13(2), 334. https://doi.org/10.3390/catal13020334









