Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2
Abstract
:1. Introduction
2. Results and Discussion
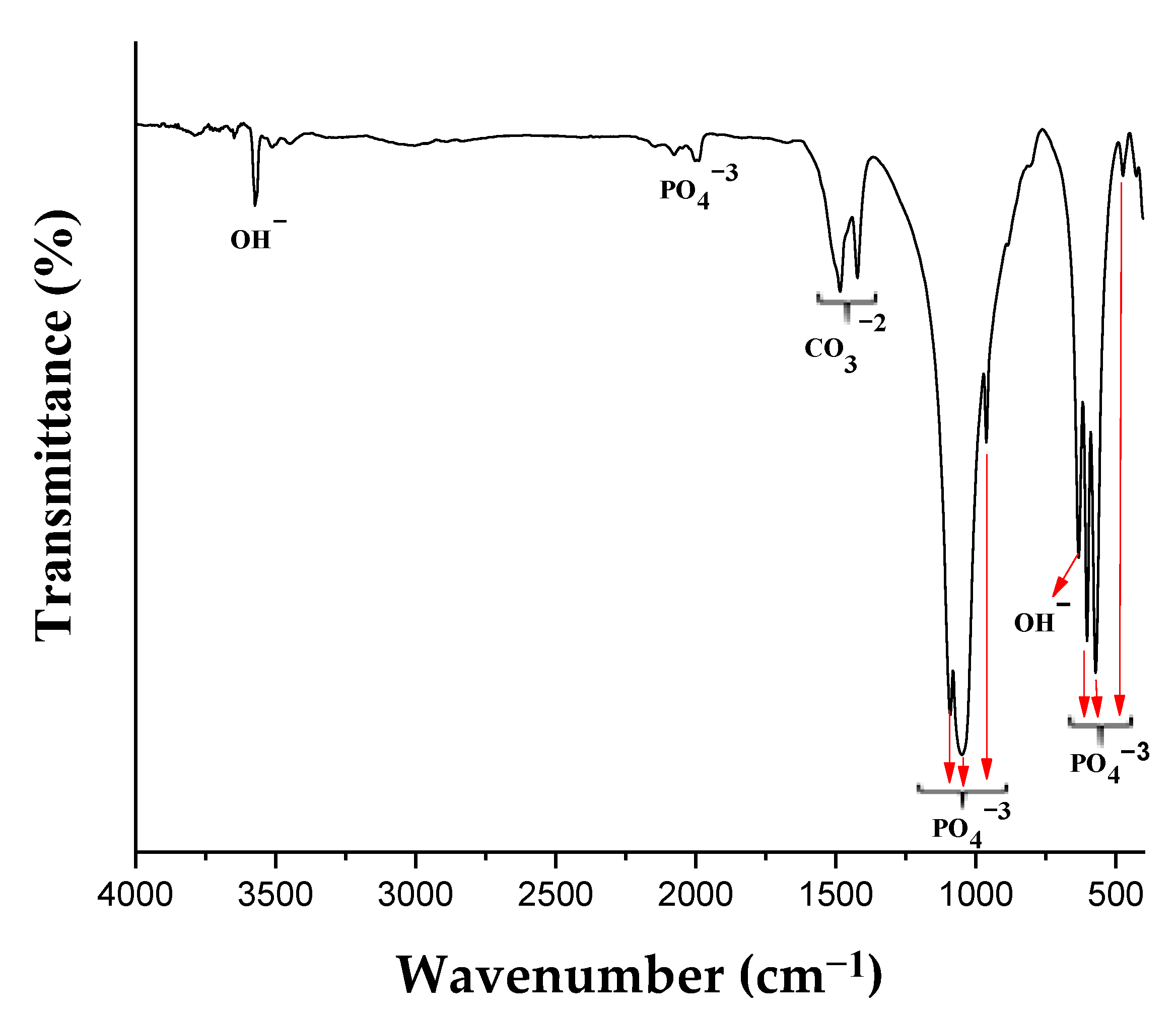
2.1. Characterization
2.2. Adsorption Experiments
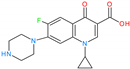
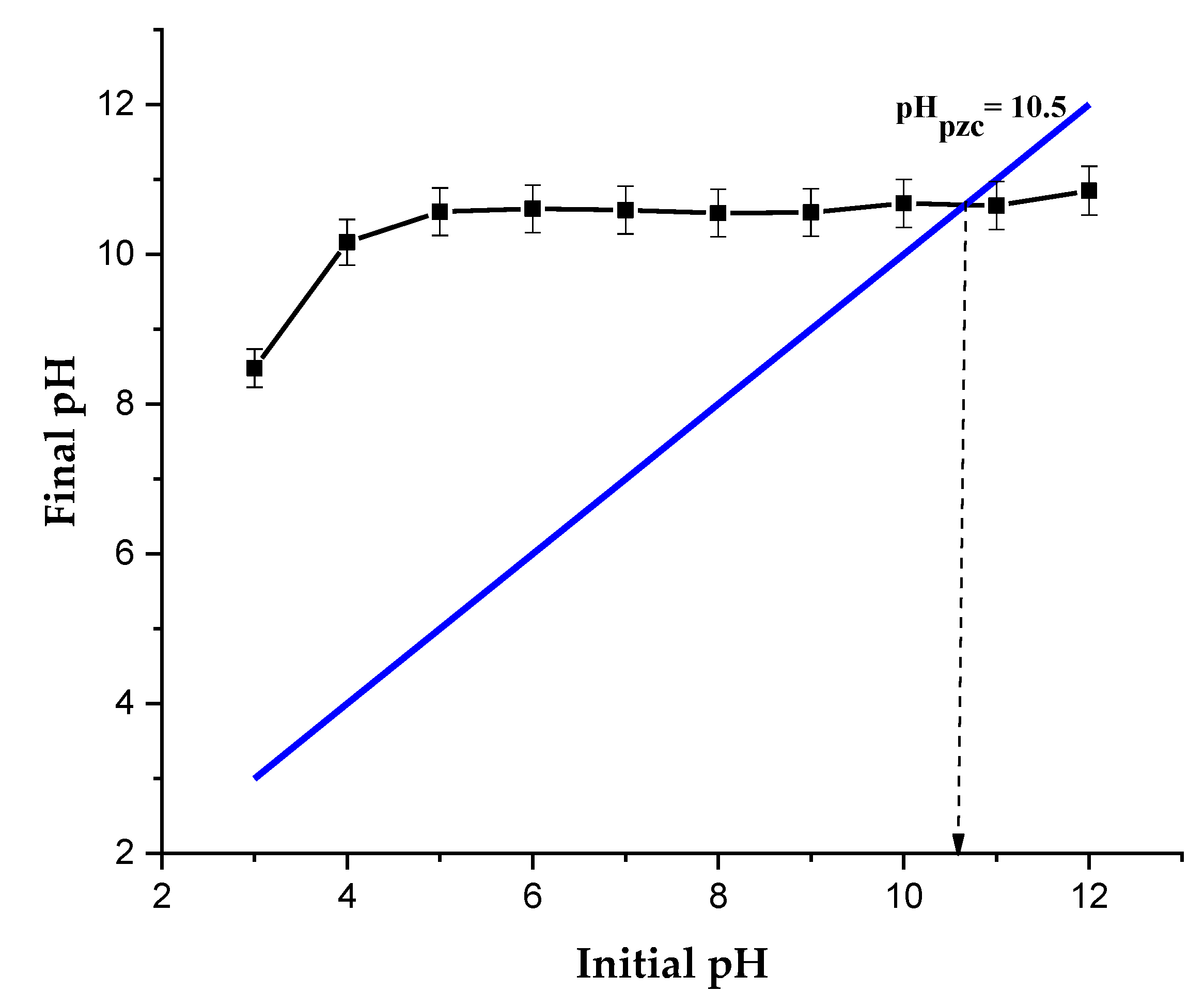
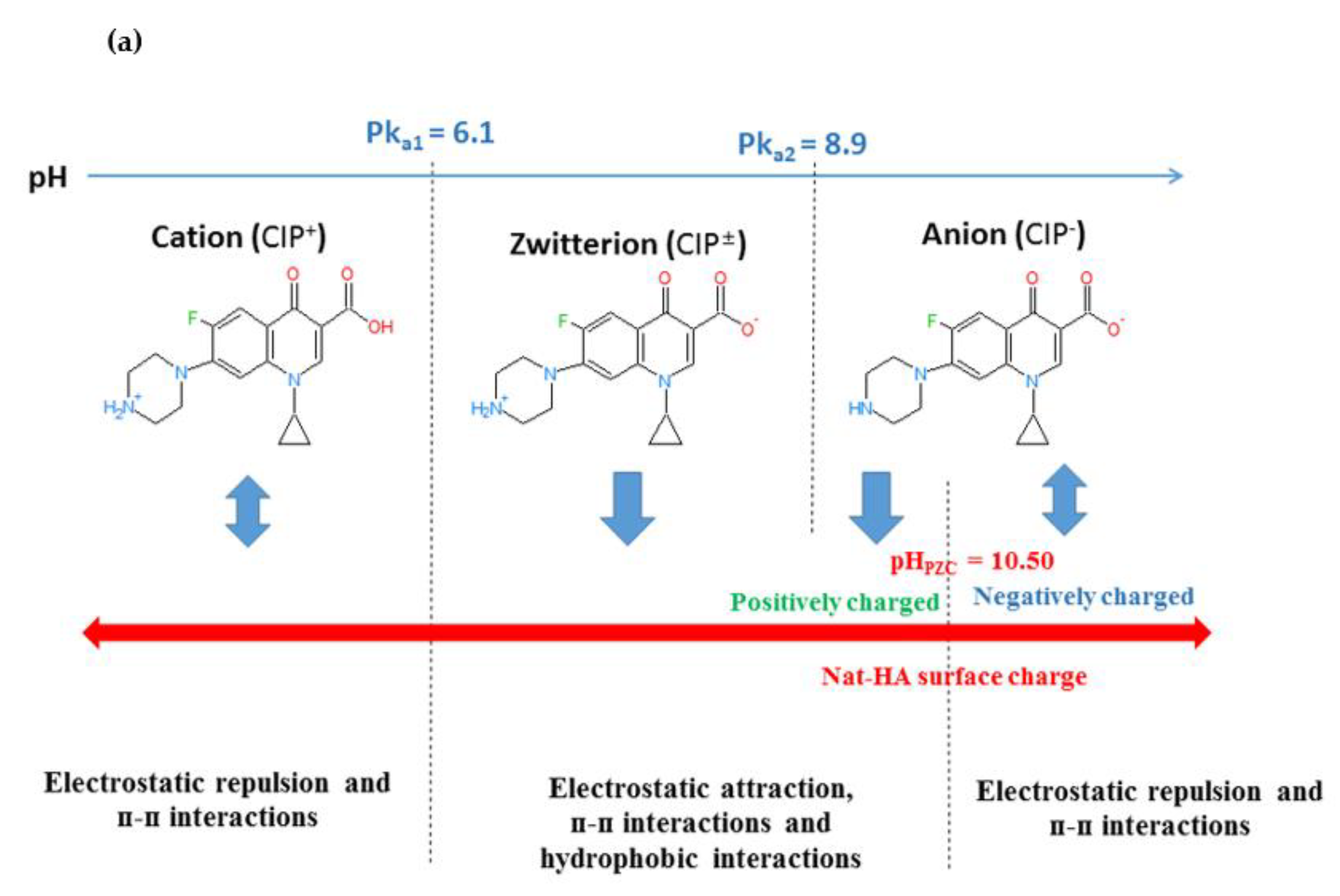
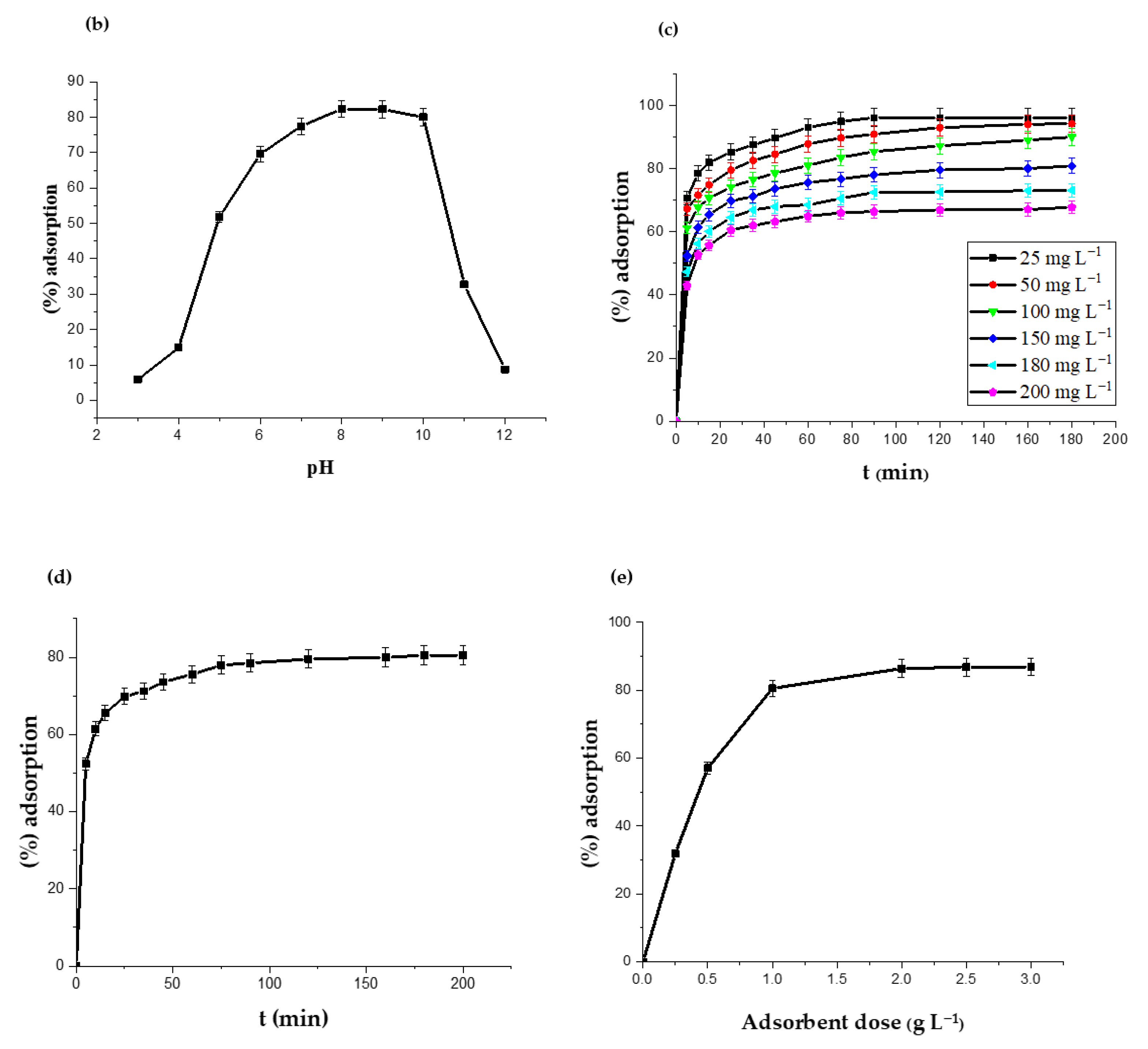
2.2.1. Effect of the Initial pH and the Binding Mechanism
2.2.2. Effect of the Initial CIP Concentration
2.2.3. Effect of the Contact Time
2.2.4. Effect of the Adsorbent Dosage
2.3. Adsorption Kinetics
2.4. Adsorption Isotherms
2.5. Thermodynamic Study
2.6. Photodegradation of CIP on TiO2
2.6.1. Effect of the Catalyst Dose
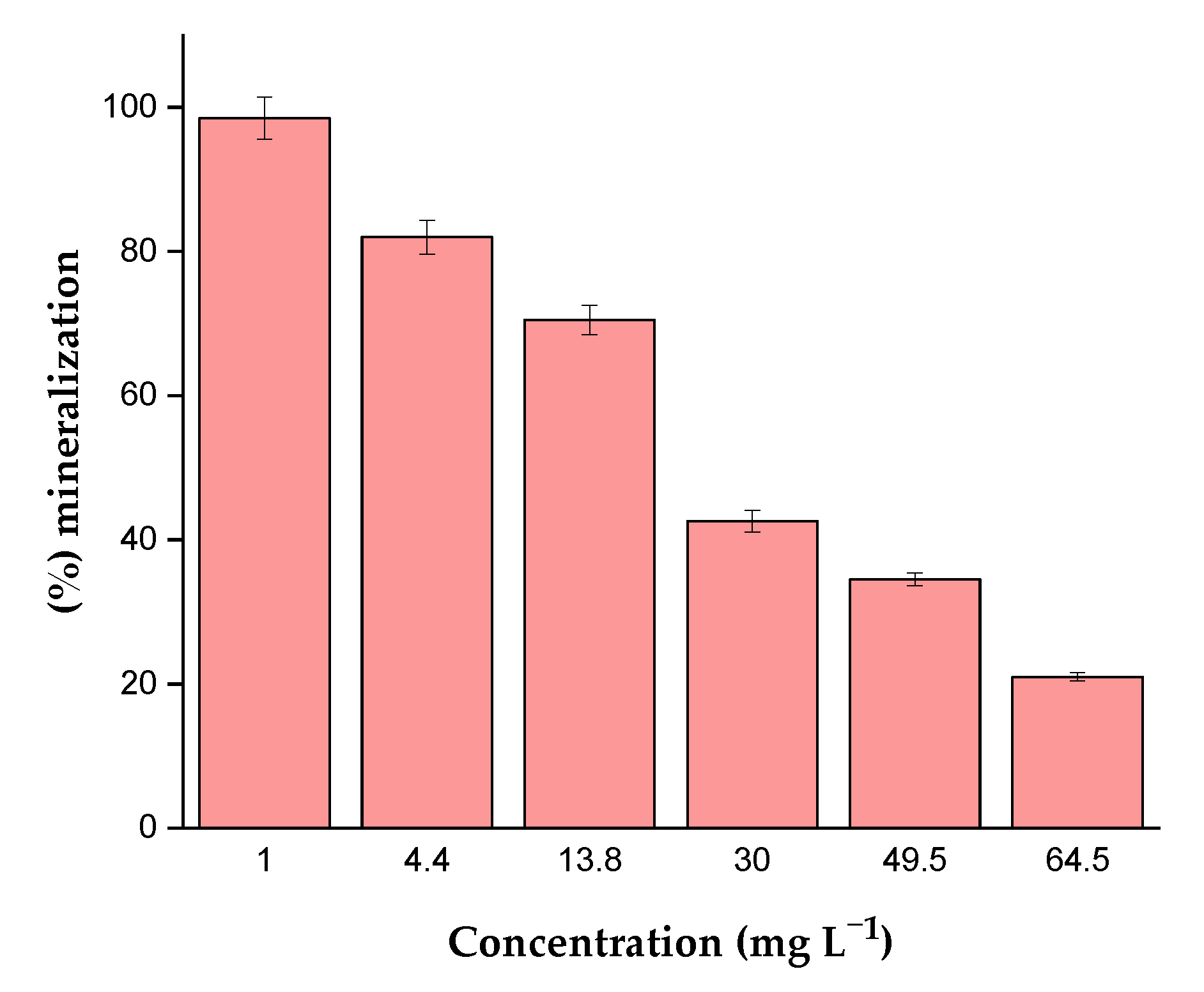
2.6.2. Effect of the Initial CIP Concentration
2.6.3. Proposed Transformation Scheme of CIP Photodegradation
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of nat-HA
3.3. Characterization of nat-HA
3.4. Batch Adsorption and Photoactivity
3.4.1. Adsorption Study
3.4.2. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fobbe, R.; Kuhlmann, B.; Nolte, J.; Preuß, G.; Skark, C.; Zullei-Seibert, N. Organic Pollutants in the Water Cycle: Properties, Occurrence, Analysis and Environmental Relevance of Polar Compounds. In Organic Pollutants in the Water Cycle: Properties, Occurrence, Analysis and Environmental Relevance of Polar Compounds; Wiley: Hoboken, NJ, USA, 2006; pp. 121–153. ISBN 978-3-527-60877-5. [Google Scholar]
- Halling-Sørensen, B.; Lützhøft, H.-C.; Andersen, H.; Ingerslev, F. Environmental Risk Assessment of Antibiotics: Comparison of Mecillinam, Trimethoprim and Ciprofloxacin. J. Antimicrob. Chemother. 2000, 46, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The Presence of Pharmaceuticals in the Environment Due to Human Use—Present Knowledge and Future Challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Jalil, M.; Baschini, M.; Sapag, K. Removal of Ciprofloxacin from Aqueous Solutions Using Pillared Clays. Materials 2017, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Li, Z.; Jiang, W.-T. Adsorption of Ciprofloxacin on 2:1 Dioctahedral Clay Minerals. Appl. Clay Sci. 2011, 53, 723–728. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L. Adsorption of Ciprofloxacin from Aqueous Solutions onto Cationic and Anionic Flax Noil Cellulose. Desalin. Water Treat. 2016, 57, 1–14. [Google Scholar] [CrossRef]
- Jiang, W.-T.; Chang, P.-H.; Wang, Y.-S.; Yolin, T.; Jean, J.-S.; Li, Z.; Krukowski, K. Removal of Ciprofloxacin from Water by Birnessite. J. Hazard. Mater. 2013, 250–251, 362–369. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Gao, Y.; Xu, X.; Li, Q.; Wang, Y. Adsorption and Cosorption of Ciprofloxacin and Ni(II) on Activated Carbon-Mechanism Study. J. Taiwan Inst. Chem. Eng. 2014, 45, 681–688. [Google Scholar] [CrossRef]
- Peng, X.; Hu, F.; Huang, J.; Wang, Y.; Dai, H.; Liu, Z. Preparation of a Graphitic Ordered Mesoporous Carbon and Its Application in Sorption of Ciprofloxacin: Kinetics, Isotherm, Adsorption Mechanisms Studies. Microporous Mesoporous Mater. 2016, 228, 196–206. [Google Scholar] [CrossRef]
- Mao, H.; Wang, S.; Lin, J.-Y.; Wang, Z.; Ren, J. Modification of a Magnetic Carbon Composite for Ciprofloxacin Adsorption. J. Environ. Sci. 2016, 49, 179–188. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Feng, P.; Chai, H.; Huang, Y. ZIF-67 Derived Hollow Cobalt Sulfide as Superior Adsorbent for Effective Adsorption Removal of Ciprofloxacin Antibiotics. Chem. Eng. J. 2018, 344, 95–104. [Google Scholar] [CrossRef]
- Reymann, T.; Kerner, M.; Kümmerer, K. Assessment of the Biotic and Abiotic Elimination Processes of Five Micropollutants during Cultivation of the Green Microalgae Acutodesmus Obliquus. Bioresour. Technol. Rep. 2020, 11, 100512. [Google Scholar] [CrossRef]
- Gil, M.; Alvarez, S.; Peres, J.; Ovejero, G. Effective Adsorption of Non-Biodegradable Pharmaceuticals from Hospital Wastewater with Different Carbon Materials. Chem. Eng. J. 2017, 320, 319. [Google Scholar] [CrossRef]
- Gouza, A.; Saoiabi, S.; el Karbane, M.; Masse, S.; Laurent, G.; Rami, A.; Saoiabi, A.; Laghzizil, A.; Coradin, T. Oil Shale Powders and Their Interactions with Ciprofloxacin, Ofloxacin, and Oxytetracycline Antibiotics. Environ. Sci. Pollut. Res. 2017, 24, 25977–25985. [Google Scholar] [CrossRef]
- Laabd, M.; Brahmi, Y.; El Ibrahimi, B.; Hsini, A.; Toufik, E.M.; Abdellaoui, Y.; Abou Oualid, H.; Ouardi, M.; Albourine, A. A Novel Mesoporous Hydroxyapatite@Montmorillonite Hybrid Composite for High-Performance Removal of Emerging Ciprofloxacin Antibiotic from Water: Integrated Experimental and Monte Carlo Computational Assessment. J. Mol. Liq. 2021, 338, 116705. [Google Scholar] [CrossRef]
- Xiao, X.; Zeng, X.; Lemley, A. Species-Dependent Degradation of Ciprofloxacin in a Membrane Anodic Fenton System. J. Agric. Food Chem. 2010, 58, 10169–10175. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, S.-P.; Mcbride, M.; Lemley, A. Degradation of Ciprofloxacin by Cryptomelane-Type Manganese(III/IV) Oxides. Environ. Sci. Pollut. Res. Int. 2012, 20, 10–21. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. PH Significantly Affects Removal of Trace Antibiotics in Chlorination of Municipal Wastewater. Water Res. 2012, 46, 3703–3713. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Rodayan, A.; Berk, D.; Yargeau, V. Removal of the Antibiotic Levofloxacin (LEVO) in Water by Ozonation and TiO2 Photocatalysis. Chem. Eng. J. 2012, 189–190, 41–48. [Google Scholar] [CrossRef]
- Li, W.; Su, B.; Xu, J. Photodegradation of Four Fluoroquinolone Compounds by Titanium Dioxide under Simulated Solar Light Irradiation. J. Chem. Technol. Biotechnol. 2012, 87, 643–650. [Google Scholar] [CrossRef]
- Vasquez, M.; Hapeshi, E.; Fatta-Kassinos, D.; Kümmerer, K. Biodegradation Potential of Ofloxacin and Its Resulting Transformation Products during Photolytic and Photocatalytic Treatment. Environ. Sci. Pollut. Res. Int. 2012, 20, 1302–1309. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Dorival García, N.; Zafra Gomez, A.; Navalón, A.; González, J.; Vílchez, J. Removal of Quinolone Antibiotics from Wastewaters by Sorption and Biological Degradation in Laboratory-Scale Membrane Bioreactors. Sci. Total Environ. 2012, 442C, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Genç, N.; Can, E. Adsorption Kinetics of the Antibiotic Ciprofloxacin on Bentonite, Activated Carbon, Zeolite, and Pumice. Desalin. Water Treat. 2015, 53, 785–793. [Google Scholar] [CrossRef]
- Chen, Y.; Lan, T.; Duan, L.; Wang, F.; Zhao, B.; Zhang, S.; Wei, W. Adsorptive Removal and Adsorption Kinetics of Fluoroquinolone by Nano-Hydroxyapatite. PLoS ONE 2015, 10, e0145025. [Google Scholar] [CrossRef]
- Hacıosmanoğlu, G.G.; Mejías, C.; Martín Bueno, J.; Santos, J.; Aparicio, I.; Alonso, E. Antibiotic Adsorption by Natural and Modified Clay Minerals as Designer Adsorbents for Wastewater Treatment: A Comprehensive Review. J. Environ. Manag. 2022, 317, 115397. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zeng, G.; Liu, N.; Liu, S. Graphene and Graphene-Based Nanocomposites Used for Antibiotics Removal in Water Treatment: A Review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef]
- Khanday, W.; Ahmed, M.; Okoye, U.; Hummadi, E.; Hameed, B. Single-Step Pyrolysis of Phosphoric Acid-Activated Chitin for Efficient Adsorption of Cephalexin Antibiotic. Bioresour. Technol. 2019, 280, 255–259. [Google Scholar] [CrossRef]
- Magesh, N.; Renita, A.; Ponnusamy, S.K. Practice on Treating Pharmaceutical Compounds (Antibiotics) Present in Waste Water Using Biosorption Technique with Different Bio Waste Compounds. A Review. Environ. Prog. Sustain. Energy 2020, 39, e13429. [Google Scholar] [CrossRef]
- Benedini, L.; Placente, D.; Ruso, J.; Messina, P. Adsorption/Desorption Study of Antibiotic and Anti-Inflammatory Drugs onto Bioactive Hydroxyapatite Nano-Rods. Mater. Sci. Eng. C 2019, 99, 180–190. [Google Scholar] [CrossRef]
- Ragab, A.; Ahmed, I.; Bader, D. The Removal of Brilliant Green Dye from Aqueous Solution Using Nano Hydroxyapatite/Chitosan Composite as a Sorbent. Molecules 2019, 24, 847. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Ampiaw, R.; Lee, W. Adsorptive Removal of Dyes from Wastewater Using a Metal-Organic Framework: A Review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef]
- Mondal, S.; Dorozhkin, S.; Pal, U. Recent Progress on Fabrication and Drug Delivery Applications of Nanostructured Hydroxyapatite. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 10, e1504. [Google Scholar] [CrossRef]
- Pai, S.; Kini, S.; Selvaraj, R.; Pugazhendhi, A. A Review on the Synthesis of Hydroxyapatite, Its Composites and Adsorptive Removal of Pollutants from Wastewater. J. Water Process Eng. 2020, 38, 101574. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, X.; Jiang, X.; Brody, H.; Rowe, D.; Wei, M. Fabrication and Characterization of Biomimetic Collagen-Apatite Scaffolds with Tunable Structures for Bone Tissue Engineering. Acta Biomater. 2013, 9, 7308–7319. [Google Scholar] [CrossRef]
- Mekatel, E.H.; Samira, A.; Bellal, B.; Trari, M.; Djamel, N. Photocatalytic Reduction of Cr(VI) on Nanosized Fe2O3 Supported on Natural Algerian Clay: Characteristics, Kinetic and Thermodynamic Study. Chem. Eng. J. 2012, 200–202, 611–618. [Google Scholar] [CrossRef]
- Belbachir, I.; Makhoukhi, B. Adsorption of Bezathren Dyes onto Sodic Bentonite from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 75, 105–111. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Çalışkan, Y.; Yatmaz, H.; Bektaş, N. Photocatalytic Oxidation of High Concentrated Dye Solutions Enhanced by Hydrodynamic Cavitation in a Pilot Reactor. Process Saf. Environ. Prot. 2017, 111, 428–438. [Google Scholar] [CrossRef]
- Mekatel, E.H.; Samira, A.; Trari, M.; Djamel, N.; Dahdouh, N.; Ladjali, S. Combined Adsorption/Photocatalysis Process for the Decolorization of Acid Orange 61. Arab. J. Sci. Eng. 2018, 44, 5311–5322. [Google Scholar] [CrossRef]
- Silva, T.; Silva, M.; Cunha-Queda, C.; Fonseca, A.; Saraiva, I.; Boaventura, R.; Vilar, V. Sanitary Landfill Leachate Treatment Using Combined Solar Photo-Fenton and Biological Oxidation Processes at Pre-Industrial Scale. Chem. Eng. J. 2013, 228, 850–866. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Abd Shukor, M.; Singh, R. Properties of Hydroxyapatite Produced by Annealing of Bovine Bone. Ceram. Int. 2007, 33, 1171–1177. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Fan, H.; Xiao, Y.; Zhang, X. Fabrication of Porous Hydroxyapatite Ceramics by Microwave Sintering Method. Key Eng. Mater. 2005, 288–289, 529–532. [Google Scholar] [CrossRef]
- Harja, M.; Ciobanu, G. Studies on Adsorption of Oxytetracycline from Aqueous Solutions onto Hydroxyapatite. Sci. Total Environ. 2018, 628–629, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Agougui, H.; Jabli, M.; Majdoub, H. Synthesis, Characterization of Hydroxyapatite-Lambda Carrageenan and Evaluation of Its Performance for the Adsorption of Methylene Blue from Aqueous Suspension. J. Appl. Polym. Sci. 2017, 134, 45385. [Google Scholar] [CrossRef]
- Yuan, L.; Yan, M.; Huang, Z.; He, K.; Zeng, G.; Chen, A.; Hu, L.; Hui, L.; Peng, M.; Huang, T.; et al. Influences of PH and Metal Ions on the Interactions of Oxytetracycline onto Nano-Hydroxyapatite and Their Co-Adsorption Behavior in Aqueous Solution. J. Colloid Interface Sci. 2019, 541, 101–113. [Google Scholar] [CrossRef]
- Sapawe, N.; Abdul Jalil, A.; Triwahyono, S.; Shah, M.; Jusoh, R.; Salleh, N.F.; Hameed, B.; Karim, A.; Bahru, J.; Malaysia, J. Cost-Effective Microwave Rapid Synthesis of Zeolite NaA for Removal of Methylene Blue. Chem. Eng. J. 2013, 229, 388–398. [Google Scholar] [CrossRef]
- Hameed, B. Removal of Cationic Dye from Aqueous Solution Using Jackfruit Peel as Non-Conventional Low-Cost Adsorbent. J. Hazard. Mater. 2008, 162, 344–350. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B. Modified Mesoporous Clay Adsorbent for Adsorption Isotherm and Kinetics of Methylene Blue. Chem. Eng. J. 2012, 198–199, 219–227. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Tiri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from Aqueous Solutions by Adsorption on Kaolin: Kinetic and Equilibrium Studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Salvestrini, S.; Leone, V.; Iovino, P.; Canzano, S.; Capasso, S. Considerations about the Correct Evaluation of Sorption Thermodynamic Parameters from Equilibrium Isotherms. J. Chem. Thermodyn. 2014, 68, 310–316. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.-C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box–Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Karoui, S.; Abou Saoud, W.; Ghorbal, A.; Fourcade, F.; Amrane, A.; Assadi, A.A. Intensification of non-thermal plasma for aqueous Ciprofloxacin degradation: Optimization study, mechanisms, and combined plasma with photocatalysis. J. Water Process Eng. 2022, 50, 103207. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Abou Oualid, H.; Hsini, A.; El Ibrahimi, B.; Laabd, M.; Ouardi, M.; Giacoman Vallejos, G.; Melo, P. Synthesis of Zirconium-Modified Merlinoite from Fly Ash for Enhanced Removal of Phosphate in Aqueous Medium: Experimental Studies Supported by Monte Carlo/SA Simulations. Chem. Eng. J. 2021, 404, 126600. [Google Scholar] [CrossRef]
- Ouahiba, E.; Chabani, M.; Assadi, A.A.; Abdeltif, A.; Florence, F.; Souad, B. Mineralization and photodegradation of oxytetracycline by UV/H2O2/Fe2+ and UV/PS/Fe2+ process: Quantification of radicals. Res. Chem. Intermed. 2023, 49, 1–21. [Google Scholar] [CrossRef]
- Ayoub, H.; Kassir, M.; Raad, M.; Bazzi, H.; Hijazi, A. Effect of Dye Structure on the Photodegradation Kinetic Using TiO2 Nanoparticles. J. Mater. Sci. Chem. Eng. 2017, 5, 31–45. [Google Scholar] [CrossRef]
- Gaya, U.; Abdullah, A. Heterogeneous Photocatalytic Degradation of Organic Contaminants Over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Zeghioud, H.; Assadi, A.A.; Khellaf, N.; Djelal, H.; Amrane, A.; Rtimi, S. Reactive species monitoring and their contribution for removal of textile effluent with photocatalysis under UV and visible lights: Dynamics and mechanism. J. Photochem. Photobiol. A Chem. 2018, 365, 94–102. [Google Scholar] [CrossRef]
- Katsumata, H.; Sakai, T.; Suzuki, T.; Knaeco, S. Highly Efficient Photocatalytic Activity of G-C3N4/Ag3PO4 Hybrid Photocatalysts through Z-Scheme Photocatalytic Mechanism under Visible Light. Ind. Eng. Chem. Res. 2014, 53, 8018–8025. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of Antibiotic Ciprofloxacin on Carbon Nanotubes: PH Dependence and Thermodynamics. Chemosphere 2013, 95, 150–155. [Google Scholar] [CrossRef]
- Kaouah, F.; Boumaza, S.; Berrama, T.; Trari, M.; Bendjama, Z. Preparation and Characterization of Activated Carbon from Wild Olive Cores (Oleaster) by H3PO4 for the Removal of Basic Red 46. J. Clean. Prod. 2013, 54, 296–306. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Kebir, M.; Khezami, L.; Amrane, A.; Assadi, A.A. A comparative study of ceramic nanoparticles synthesized for antibiotic removal: Catalysis characterization and photocatalytic performance modeling. Environ. Sci. Pollut. Res. 2021, 28, 13900–13912. [Google Scholar] [CrossRef] [PubMed]
- Kenfoud, H.; Baaloudj, O.; Nasrallah, N.; Bagtache, R.; Assadi, A.A.; Trari, M. Structural and electrochemical characterizations of Bi12CoO20 sillenite crystals: Degradation and reduction of organic and inorganic pollutants. J. Mater. Sci. Mater. Electron. 2021, 32, 16411–16420. [Google Scholar] [CrossRef]





| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||||
|---|---|---|---|---|---|---|---|
| C0 (mg L–1) | qe,exp (mg g–1) | k1 (min–1) | qe.cal (mg g–1) | R2 | k2 × 10–2 (g mg–1 min–1) | qe.cal (mg g–1) | R2 |
| 25 | 24.00 | 0.251 | 23.17 | 0.984 | 2.154 | 24.10 | 0.999 |
| 50 | 45.05 | 0.226 | 44.09 | 0.981 | 0.956 | 46.09 | 0.999 |
| 100 | 87.35 | 0.209 | 82.89 | 0.973 | 0.462 | 87.04 | 0.998 |
| 150 | 120.00 | 0.193 | 114.40 | 0.976 | 0.291 | 120.49 | 0.998 |
| 180 | 132.65 | 0.187 | 125.53 | 0.978 | 0.256 | 132.27 | 0.999 |
| 200 | 136.00 | 0.184 | 129.34 | 0.978 | 0.241 | 136.47 | 0.999 |
| Isotherm | Parameters | Values |
|---|---|---|
| Langmuir | qm (mg g−1) | 147.70 |
| KL (L mg−1) | 0.163 | |
| R2 | 0.999 | |
| RL | 0.197 | |
| Freundlich | KF | 36.50 |
| N | 3.02 | |
| R2 | 0.954 | |
| Temkin | A (L mg−1) | 2.25 |
| B (mg g−1) | 28.0 | |
| R2 | 0.996 |
| T (K) | KL (L mg−1 ) | KL°(× 105) (Dimensionless) | Ln KL° | ∆G° (kJ mol−1) | ∆H° (kJ mol−1) | ∆S° (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| 298 | 0.1004 | 3.3272 | 10.41 | −25.77 | –21.09 | 15.72 |
| 308 | 0.0742 | 2.4589 | 10.11 | −25.93 | ||
| 318 | 0.0588 | 1.9486 | 9.87 | –26.09 | ||
| 328 | 0.0457 | 1.5144 | 9.62 | –26.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Hadadi, A.; Manseri, A.; Bouzaza, A.; Assadi, A.; Amrane, A.; Zamouche, M.; El Jery, A.; et al. Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts 2023, 13, 336. https://doi.org/10.3390/catal13020336
Cheikh S, Imessaoudene A, Bollinger J-C, Hadadi A, Manseri A, Bouzaza A, Assadi A, Amrane A, Zamouche M, El Jery A, et al. Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts. 2023; 13(2):336. https://doi.org/10.3390/catal13020336
Chicago/Turabian StyleCheikh, Sabrina, Ali Imessaoudene, Jean-Claude Bollinger, Amina Hadadi, Amar Manseri, Abdelkrim Bouzaza, Aymen Assadi, Abdeltif Amrane, Meriem Zamouche, Atef El Jery, and et al. 2023. "Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2" Catalysts 13, no. 2: 336. https://doi.org/10.3390/catal13020336
APA StyleCheikh, S., Imessaoudene, A., Bollinger, J. -C., Hadadi, A., Manseri, A., Bouzaza, A., Assadi, A., Amrane, A., Zamouche, M., El Jery, A., & Mouni, L. (2023). Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts, 13(2), 336. https://doi.org/10.3390/catal13020336