Synthesis of Hollow Mesoporous Silica Nanospheroids with O/W Emulsion and Al(III) Incorporation and Its Catalytic Activity for the Synthesis of 5-HMF from Carbohydrates
Abstract
1. Introduction
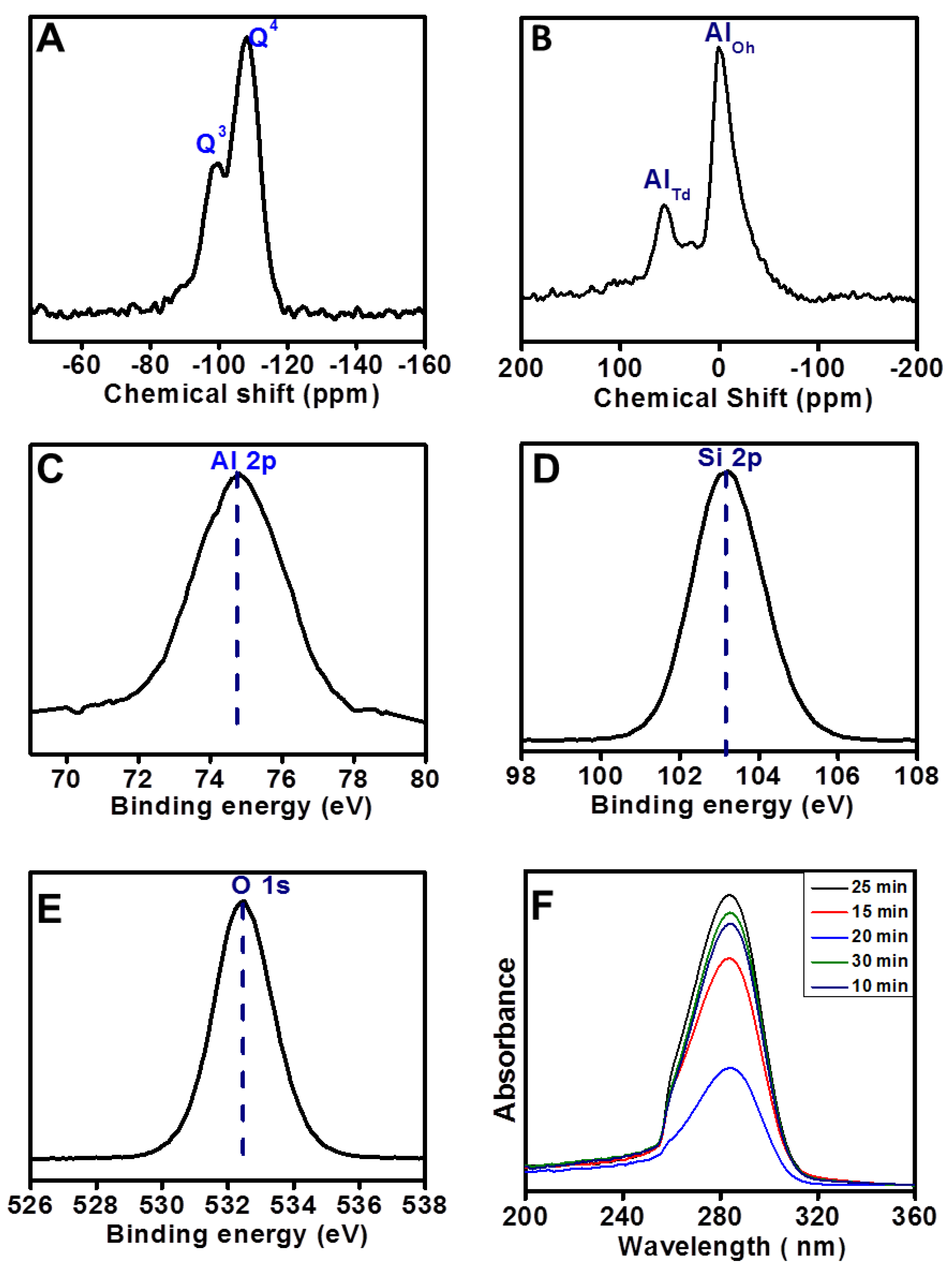
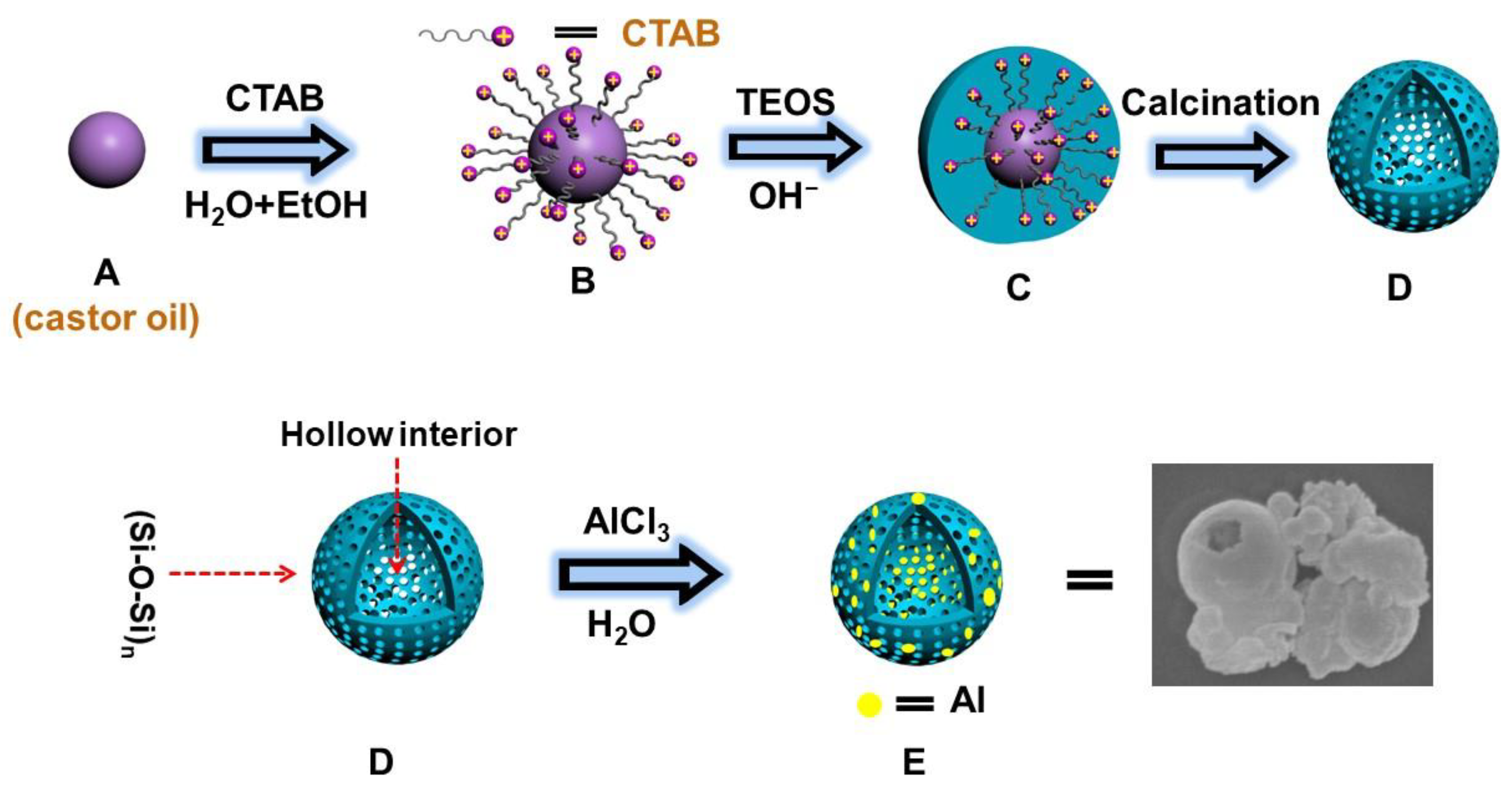
2. Results
3. Discussion
3.1. Proposed Mechanism
3.2. Catalysis
3.3. HMF Conversion Reaction
3.4. Hot Filtration Test
3.5. Catalyst Reusability
| Entry | Substrate | Substrate Amount (mg) | Catalyst Amount (mg) | Solvent ‡ | Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | Fructose | 24 | 5 | DMSO | 160 | 25 | 69.8 |
| 2 | Fructose | 24 | 4 | DMSO | 160 | 25 | 81.8 |
| 3 | Fructose | 24 | 3 | DMSO | 160 | 25 | 83.7 |
| 4 | Fructose | 24 | 2 | DMSO | 160 | 25 | 78.4 |
| 5 | Fructose | 24 | 1 | DMSO | 160 | 25 | 62.8 |
| 6 | Fructose | 24 | 7 | DMSO | 160 | 25 | 61 |
| 7 | Fructose | 24 | 10 | DMSO | 160 | 25 | 58.8 |
| 8 | Fructose | 24 | 3 | DMSO | 170 | 25 | 75.4 |
| 9 | Fructose | 24 | 3 | DMSO | 150 | 25 | 63.6 |
| 10 | Fructose | 24 | 3 | DMSO | 140 | 25 | 50.4 |
| 11 | Fructose | 24 | 3 | DMSO | 160 | 20 | 74.9 |
| 12 | Fructose | 24 | 3 | DMSO | 160 | 15 | 65.3 |
| 13 | Fructose | 24 | 3 | DMSO | 160 | 10 | 33.7 |
| 14 | Fructose | 24 | 3 | DMSO | 160 | 30 | 78.6 |
| 15 | Fructose | 24 | 3 | DMSO + H2O | 160 | 25 | 48.4 |
| 16 | Fructose | 24 | 3 | DMF | 160 | 25 | 23.7 |
| 17 | Fructose | 24 | 3 | NMP | 160 | 25 | 10.7 |
| 18 | Glucose | 24 | 3 | DMSO | 160 | 25 | 26.9 |
| 19 | Mannose | 24 | 3 | DMSO | 160 | 25 | 26.1 |
| 20 | Galactose | 24 | 3 | DMSO | 160 | 25 | 27.3 |
| Catalyst | Temp. (°C) | Solvent | Time (min.) | Substrate | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Al-MCM-41 | 195 | H2O/MIBK | 150 | Glucose | 63 | [60] |
| Al-KCC-1 | 162 | DMSO | 60 | Fructose | 92.9 | [61] |
| Al-KCC-1 | 170 | DMSO | 120 | Glucose | 39 | [61] |
| Al-KIT-5 | 135 | DMSO | 60 | Fructose | 79.5 | [62] |
| Al-MCM-41 | 170 | H2O/MIBK | 75 | Fructose | 42.3 | [63] |
| Al-SBA15 | 120 | DMSO | 120 | Fructose | 74 | [64] |
| Al-TUD-1 | 170 | H2O/Toluene | 360 | Fructose | 16 | [65] |
| PHMS-2 | 160 | DMSO | 25 | Fructose | 83.7 | This work |
4. Materials and Methods
4.1. Chemicals
4.2. Instrumentation
4.3. Synthesis of PHMS-1
4.4. Synthesis of PHMS-2
4.5. Pyridine Adsorbed FT-IR
4.6. Catalyst Activation
4.7. 5-HMF Catalysis
4.8. HMF Yield Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hyde, E.D.E.R.; Seyfaee, A.; Neville, F.; Moreno-Atanasio, R. Colloidal Silica Particle Synthesis and Future Industrial Manufacturing Pathways: A Review. Ind. Eng. Chem. Res. 2016, 55, 8891–8913. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef]
- Kärger, J.; Valiullin, R. Mass Transfer in Mesoporous Materials: The Benefit of Microscopic Diffusion Measurement. Chem. Soc. Rev. 2013, 42, 4172–4197. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquidcrystal template mechanism. Nature 1992, 359, 710. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Santra, C.; Shah, S.; Mondal, A.; Pandey, J.K.; Panda, A.B.; Maity, S.; Chowdhury, B. Synthesis, Characterization of VPO Catalyst Dispersed on Mesoporous Silica Surface and Catalytic Activity for Cyclohexane Oxidation Reaction. Microporous Mesoporous Mater. 2016, 223, 121–128. [Google Scholar] [CrossRef]
- Arkhireeva, A.; Hay, J.N. Synthesis of Sub-200 nm Silsesquioxane Particles Using a Modified StÖber Sol-Gel route. J. Mater. Chem. 2003, 13, 3122–3127. [Google Scholar] [CrossRef]
- Ghimire, P.P.; Jaroniec, M. Renaissance of StÖber method for synthesis of colloidal particles: New developments and opportunities. J. Colloid Interface Sci. 2021, 584, 838–865. [Google Scholar] [CrossRef]
- Suzuki, K.; Ikari, K.; Imai, H. Synthesis of Silica Nanoparticles Having a Well-Ordered Mesostructure Using a Double Surfactant System. J. Am. Chem. Soc. 2004, 126, 462–463. [Google Scholar] [CrossRef]
- Schmidt-Winkel, P.; Glinka, C.J.; Stucky, G.D. Microemulsion Templates for Mesoporous Silica. Langmuir 2000, 16, 356–361. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Perez-Garnes, M.; Morales, V.; Sanz, R.; Garcia-Munoz, R.A. Cytostatic and Cytotoxic Effects of Hollow-Shell Mesoporous Silica Nanoparticles Containing Magnetic Iron Oxide. Nanomaterials 2021, 11, 2455. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Pan, W.; Yu, Z.; Yang, L.; Tang, B. Hollow mesoporous silica nanoparticles with tunable structures for controlled drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 2123–2129. [Google Scholar] [CrossRef]
- Mohammed, A.T.A.; Wang, L.J.; Jin, R.H.; Liu, G.H.; Tan, C.X. Hollow-Shell-Structured Mesoporous Silica-Supported Palladium Catalyst for an Efficient Suzuki-Miyaura Cross-Coupling Reaction. Catalysts 2021, 11, 582. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, X.; Fang, W.; Chen, C.; Zheng, N. Self-templating synthesis of hollow mesoporous silica and their applications in catalysis and drug delivery. Nanoscale 2013, 5, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, M.K.; Nguyen, T.L.; Kim, J. Hollow Mesoporous Silica Nanoparticles with Extra-Large Mesopores for Enhanced Cancer Vaccine. ACS Appl. Mater. Interfaces 2020, 12, 34658–34666. [Google Scholar] [CrossRef] [PubMed]
- Marinheiro, D.; Ferreira, B.J.M.L.; Oskoei, P.; Oliveira, H.; Daniel-da-Silva, A.L. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Zhang, W. Yolk−Shell Catalyst of Single Au Nanoparticle Encapsulated within Hollow Mesoporous Silica Microspheres. ACS Catal. 2011, 1, 207–211. [Google Scholar] [CrossRef]
- Lucassen-Reynders, E.H.; van den Tempel, M. Stabilization of Water-in-Oil Emulsions by Solid Particles. J. Phys. Chem. 1963, 67, 731–734. [Google Scholar] [CrossRef]
- Moulik, S.P.; Paul, B.K. Structure, Dynamics and Transport Properties of Microemulsions. Adv. Colloid Interface Sci. 1998, 78, 99–195. [Google Scholar] [CrossRef]
- Tchakalova, V.; Testard, F.; Wong, K.; Parker, A.; Benczédi, D.; Zemb, T. Solubilization and interfacial curvature in microemulsions: I. Interfacial expansion and co-extraction of oil. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 31–39. [Google Scholar] [CrossRef]
- Santra, S.; Bagwe, R.; Dutta, D.; Stanley, J.T.; Walter, G.A.; Tan, W.; Moudgil, B.M.; Mericle, R.A. Synthesis and Characterization of Fluorescent, Radio-Opaque, and Paramagnetic Silica Nanoparticles for Multimodal Bioimaging Applications. Adv. Mater. 2005, 17, 2165–2169. [Google Scholar] [CrossRef]
- Zhao, X.; Bagwe, R.P.; Tan, W. Development of Organic-Dye-Doped Silica Nanoparticles in a Reverse Microemulsion. Adv. Mater. 2004, 16, 173–176. [Google Scholar] [CrossRef]
- Arriagada, F.J.; Osseo-Asare, K. Controlled Hydrolysis of Tetraethoxysilane in a Non-ionic Water-in-oil Microemulsion: A Statistical Model of Silica Nucleation. Colloids Surf. A 1999, 154, 311–326. [Google Scholar] [CrossRef]
- Gustafsson, H.; Isaksson, S.; Altskär, A.; Holmberg, K. Mesoporous silica nanoparticles with controllable morphology prepared from oil-in-water emulsions. J. Colloid Interface Sci. 2016, 467, 253–260. [Google Scholar] [CrossRef]
- Horikoshi, S.; Akao, Y.; Ogura, T.; Sakai, H.; Abe, M.; Serpone, N. On the stability of surfactant-free water-in-oil emulsions and synthesis of hollow SiO2 nanospheres. Colloids Surfaces Physicochem. Eng. Asp. 2010, 372, 55–60. [Google Scholar] [CrossRef]
- Li, W.J.; Sha, X.X.; Dong, W.J.; Wang, Z.C. Synthesis of stable hollow silica microspheres with mesoporous shell in nonionic W/O emulsion. Chem. Commun. 2002, 2434–2435. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Gucci, F.; Cairns, G.; Chianella, I.; Leighton, G.J.T. Hollow Silica Nano and Micro Spheres with Polystyrene Templating: A Mini-Review. Materials 2022, 15, 8578. [Google Scholar] [CrossRef] [PubMed]
- Nyalosaso, J.L.; Derrien, G.; Charnay, C.; de Menorval, L.-C.; Zajac, J. Aluminium-derivatized silica monodisperse nanospheres by a one-step synthesis-functionalization method and application as acid catalysts in liquid phase. J. Mater. Chem. 2012, 22, 1459–1468. [Google Scholar] [CrossRef]
- Shylesh, S.; Kapoor, M.P.; Juneja, L.R.; Samuel, P.R.; Srilakshmi, C.; Singh, A.R. Catalytic Meerwein-Ponndorf-Verley reductions over mesoporous silica supports: Rational design of hydrophobic mesoporous silica for enhanced stability of aluminum doped mesoporous catalysts. J. Mol. Catal. A Chem. 2009, 301, 118–126. [Google Scholar] [CrossRef]
- Lin, H.; Gan, T.; Wu, K. Sensitive and rapid determination of catechol in tea samples using mesoporous Al-doped silica modified electrode. Food Chem. 2009, 113, 701. [Google Scholar] [CrossRef]
- Kosari, M.; Seayad, A.M.; Xi, S.; Kozlov, S.M.; Borgna, A.; Zeng, H.C. Synthesis of Mesoporous Copper Aluminosilicate Hollow Spheres for Oxidation Reactions. ACS Appl. Mater. Interfaces 2020, 12, 23060–23075. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, D.; Gao, Q.; Xu, Y.; Wu, D.; Sun, Y.; Xu, J.; Deng, F. Hollow mesoporous aluminosilicate spheres with acidic shell. Mater. Chem. Phys. 2011, 125, 286–292. [Google Scholar] [CrossRef]
- You, C.; Yu, C.; Yang, X.; Li, Y.; Huo, H.; Wang, Z.; Lin, K. Double-Shelled Hollow Mesoporous Silica Nanospheres as an Acid−Base Bifunctional Catalyst for Cascade Reactions. New J. Chem. 2018, 42, 4095–4101. [Google Scholar] [CrossRef]
- Roper, H. Renewable Raw Materials in Europe—Industrial Utilisation of Starch and Sugar. Starch-Starke 2002, 54, 89–99. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, J.; Bhaumik, A. Sulfonated Porous Polymeric Nanofibers as an Efficient Solid Acid Catalyst for the Production of 5-Hydroxymethylfurfural from Biomass. ChemCatChem 2015, 7, 3570–3578. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates-the US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- He, O.W.; Zhang, Y.F.; Wang, P.; Liu, L.N.; Wang, Q.; Yang, N.; Li, W.J.; Champagne, P.; Yu, H.B. Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose. Catalysts 2021, 11, 11. [Google Scholar] [CrossRef]
- Bicker, M.; Hirth, J.; Vogel, H. Dehydration of fructose to 5- hydroxymethylfurfural in sub- and supercritical acetone. Green Chem. 2003, 5, 280–284. [Google Scholar] [CrossRef]
- Modak, A.; Mankar, A.R.; Pant, K.K.; Bhaumik, A. Mesoporous porphyrin-silica nanocomposite as solid acid catalyst for high yield synthesis of HMF in water. Molecules 2021, 26, 2519. [Google Scholar] [CrossRef]
- Tempelman, C.H.L.; Oozeerally, R.; Degirmenci, V. Heterogeneous Catalysts for the Conversion of Glucose into 5-Hydroxymethyl Furfural. Catalysts 2021, 11, 861. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef]
- Han, L.; Zhou, Y.; He, T.; Song, G.; Wu, F.; Jiang, F.; Hu, J. One-Pot Morphology-Controlled Synthesis of Various Shaped Mesoporous Silica Nanoparticles. J. Mater. Sci. 2013, 48, 5718–5726. [Google Scholar] [CrossRef]
- Xiao, Z.; Bao, H.; Jia, S.; Bao, Y.; Niu, Y.; Kou, X. Organic Hollow Mesoporous Silica as a Promising Sandalwood Essential Oil Carrier. Molecules 2021, 26, 2744. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Magi, M.; Lippmaa, E.; Samoson, A.; Engelhardt, G.; Grimmer, A.R. Solid-State High-Resolution Silicon-29 Chemical Shifts in Silicates. J. Phys. Chem. A. 1984, 88, 1518–1522. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q.; Li, S.; Deng, F. Solid-State NMR Characterization of Framework Structure of Zeolites and Zeotype Materials. In Solid-State NMR in Zeolite Catalysis; Xu, J., Ed.; Springer: Singapore, 2019; Volume 103, pp. 99–107. [Google Scholar]
- Walkley, B.; Provis, J.L. Solid-state nuclear magnetic resonance spectroscopy of cements. Mater. Today Adv. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Das, S.K.; Bhunia, M.K.; Sinha, A.K.; Bhaumik, A. Synthesis, characterization, and biofuel application of mesoporous zirconium oxophosphates. ACS Catal. 2011, 1, 493–501. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ponce, C.P.; Ortillo, D.O. X-ray Photoelectron Spectroscopic Study of Some Organic and Inorganic Modified Clay Minerals. Materials 2021, 14, 7115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bandosz, T.J.; Akins, D.L. Template-free synthesis of silica ellipsoids. Chem. Commun. 2011, 47, 7791–7793. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Y.; Yan, P.; Xia, Z.; Liu, X.; Zhang, Z.C. Mechanistic Understanding of Humin Formation in the Conversion of Glucose and Fructose to 5-Hydroxymethylfurfural in [BMIM]Cl Ionic Liquid. RSC Adv. 2020, 10, 34732–34737. [Google Scholar] [CrossRef] [PubMed]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustainable Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, Y.; Dai, J.; Long, Y.; Hu, Z.-T. Synthesis of 5- hydroxymethylfurfural from dehydration of biomass-derived glucose and fructose using supported metal catalysts. Green Synth. Catal. 2021, 2, 187–197. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Glucose to Fructose Isomerization in Aqueous Media over Homogeneous and Heterogeneous Catalysts. ChemCatChem 2016, 8, 1100–1110. [Google Scholar] [CrossRef]
- Carraher, J.M.; Fleitman, C.N.; Tessonnier, J.-P. Kinetic and Mechanistic Study of Glucose Isomerization Using Homogeneous Organic Brønsted Base Catalysts in Water. ACS Catal. 2015, 5, 3162–3173. [Google Scholar] [CrossRef]
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. The mechanism of glucose isomerization to fructose over Sn-BEA zeolite: A periodic density functional theory study. ChemSusChem 2013, 6, 1688–1696. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst. Appl. Catal. B 2015, 164, 70–76. [Google Scholar] [CrossRef]
- Shahangi, F.; Chermahini, A.N.; Saraji, M. Dehydration of fructose and glucose to 5-hydroxymethylfurfural over Al-KCC-1 silica. J. Energy Chem. 2018, 27, 769–780. [Google Scholar] [CrossRef]
- Chermahini, A.N.; Hafizi, H.; Andisheh, N.; Saraji, M.; Shahvar, A. The catalytic effect of Al-KIT-5 and KIT-5-SO3H on the conversion of fructose to 5-hydroxymethylfurfural. Res. Chem. Intermed. 2017, 43, 5507–5521. [Google Scholar] [CrossRef]
- Jiang, C.W.; Su, A.X.; Li, X.M. Preparation of Aluminosilicate Mesoporous Catalyst and its Application for Production 5-Hydroxymethyl Furfural Dehydration from Fructose. Adv. Res. Mater. 2011, 396–398, 1190–1193. [Google Scholar] [CrossRef]
- Qiao, Y.; Theyssen, N.; Hou, Z. Acid-catalyzed dehydration of fructose to 5-(hydroxymethyl) furfural. Recycl. Catal. 2015, 2, 36–60. [Google Scholar] [CrossRef]
- Lima, S.; Antunes, M.M.; Fernandes, A.; Pillinger, M.; Ribeiro, M.F.; Valente, A.A. Acid-Catalysed Conversion of Saccharides into Furanic Aldehydes in the Presence of Three-Dimensional Mesoporous Al-TUD-1. Molecules 2010, 15, 3863–3877. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, A.; Chowdhury, B.; Bhaumik, A. Synthesis of Hollow Mesoporous Silica Nanospheroids with O/W Emulsion and Al(III) Incorporation and Its Catalytic Activity for the Synthesis of 5-HMF from Carbohydrates. Catalysts 2023, 13, 354. https://doi.org/10.3390/catal13020354
Ghosh A, Chowdhury B, Bhaumik A. Synthesis of Hollow Mesoporous Silica Nanospheroids with O/W Emulsion and Al(III) Incorporation and Its Catalytic Activity for the Synthesis of 5-HMF from Carbohydrates. Catalysts. 2023; 13(2):354. https://doi.org/10.3390/catal13020354
Chicago/Turabian StyleGhosh, Anirban, Biswajit Chowdhury, and Asim Bhaumik. 2023. "Synthesis of Hollow Mesoporous Silica Nanospheroids with O/W Emulsion and Al(III) Incorporation and Its Catalytic Activity for the Synthesis of 5-HMF from Carbohydrates" Catalysts 13, no. 2: 354. https://doi.org/10.3390/catal13020354
APA StyleGhosh, A., Chowdhury, B., & Bhaumik, A. (2023). Synthesis of Hollow Mesoporous Silica Nanospheroids with O/W Emulsion and Al(III) Incorporation and Its Catalytic Activity for the Synthesis of 5-HMF from Carbohydrates. Catalysts, 13(2), 354. https://doi.org/10.3390/catal13020354









