Abstract
In this experimental investigation, a procreation approach was used to produce a catalyst based on SnO2@rGO nanocomposite for use in a selective catalytic reduction (SCR) system. Plastic waste oil is one such alternative that helps to ensure the survival of fossil fuels and also lessens the negative impacts of improper waste disposal. The SnO2@rGO nanocomposite was prepared by fine dispersion of SnO2 nanoparticles on monolayer-dispersed reduced graphene oxide (rGO) and carefully investigated for its potential in adsorbing CO, CO2, NOX, and hydrocarbon (HC). The as-synthesized SnO2@rGO nanocomposite was characterized by Fourier transform infrared spectroscopy, high-resolution transmission electron microscopy, scanning electron microscopy, X-ray diffraction spectroscopy, thermogravimetry, and surface area analyses. Then, the impact of catalysts inside the exhaust engine system was evaluated in a realistic setting with a single-cylinder, direct-injection diesel engine. As a result, the catalysts reduced harmful pollution emissions while marginally increasing brake-specific fuel consumption. The nanocomposite was shown to exhibit higher NOX adsorption efficiencies when working with different toxic gases. Maximum reductions in the emission of NOX, hydrocarbons, and CO were achieved at a rate of 78%, 62%, and 15%, respectively. These harmful pollutants were adsorbed on the active sites of catalyst and are converted to useful fuel gases through catalytic reduction thereby hindering the trajectory of global warming.
1. Introduction
The low ecological impact, high efficiency, and rock-solid dependability of diesel engines demand their utilization in a variety of industrial sectors [1]. Agriculture and transportation are two examples of these sectors. However, as fossil fuels continue to deplete at an alarming rate, it is of the utmost need to discover a replacement fuel source in order to keep up with the ever-increasing demand for energy. Despite essentially not requiring any modification to the engines themselves, biofuels incorporating petroleum diesel at different ratios are viable and clean fuels [2]. Although, there are some significant distinctions between petroleum diesel and biodiesel, including greater densities, cetane ratings, and oxygen concentrations [3,4], all of which contribute to the pollutants that diesel engines emit. Increased emissions of nitrogen oxide (NOX) are a common finding when biodiesel is used [5]. One of the regulated pollutants is this kind of emission. At a constant 1500 rpm engine speed, Lahane et al. [6] found that NOX emissions rose by anywhere from 1.4 to 22.8% when using altered biodiesel–diesel mixes (B5 to B100). When subjected to the steady state supplementary emissions test and heavy-duty transient Federal Test Procedure (FTP) cycle, it has been discovered that NOX emissions from B20 to B40 fuels were stable or increased by 4.3% [7]. Several researchers concluded that the increased oxygen concentrations of biodiesel were to blame for the resulting rise in NOX emissions. Complete combustion is facilitated by biodiesel’s greater oxygen concentration, leading to higher combustion temperatures [8].
These pollutants constitute a serious threat to the surroundings because they help produce photochemical smog and acid rain from diesel engines [9]. Because of this, many nations have enacted stricter regulations for NOX emissions from diesel vehicles than those for gasoline-powered vehicles. Thus, there has been an increase in the development of after-treatment systems aimed at reducing NOX emissions. The two most common types of after-treatment available today are lean NOX traps (LNT) and selective catalytic reduction (SCR). In order to conform to the ever-stricter NOX emission regulations, both these technologies are being put to use. However, LNT is rarely employed as it has lower sulfur resistance, requires the usage of expensive metals (Pt/Rh), and uses more fuels than SCR. Regardless of whether the fuels are biodiesel or petroleum diesel, it has been demonstrated that SCR systems employing NH3 in oxygen-rich exhausts may successfully reduce NOX emissions [10].
Using biodiesel in SCR systems has been shown in several research works to achieve higher conversion efficiencies than using petroleum diesel. SCR with B20 fuel reduced NOx and NO contents by 68–93% compared to no exhaust after treatment [11]. The results were measured against those obtained when no therapy was used. The employment of a SCR and a catalytic converter (CC) after the treatment system similarly reduced NOX emissions in comparison to B50 biodiesel. The biodiesel’s NOX levels dropped by 15.2% compared to petroleum-based diesel [12]. Furthermore, Solaimuthu et al. [13] observed that the efficacy of SCR’s NOX conversion enhanced as the biodiesel mix ratio increased, going from 20% for B100 with SCR to 30% for B100 with 20% biodiesel blend [13]. Such assumptions were backed up by the observation that the SCR’s NOX conversion efficiency increased along with the biodiesel blend percentage [14]. Among the different SCR catalyst types, V2O5-WO3/TiO2 SCR catalysts are becoming increasingly prevalent. Yet, there are still some problems that arise when the catalysts used to reduce NOX are degraded through heat and chemical exposure, which often happens after extended durations of use. At higher temperatures, V2O5-WO3/TiO2 SCR catalysts worsen or even stop working because the catalyst’s sintering reduces the surface area and the unfavorable conversion of anatase to rutile TiO2 between 500 and 600 °C [15].
These undesirable events that occur with SCR catalysts lower their ability to store NH3 and oxidizing capability, which makes it harder to convert NOX into oxygen [16]. Furthermore, vanadium and tungsten released upon heating V2O5-WO3/TiO2 SCR wash coat at high temperatures may be harmful to human health [17]. Thus, long-term use of biodiesel necessitates an inquiry into SCR’s ability to maintain compliance with the strict NOX emission requirements. Therefore, numerous studies have focused on exploring the reasons for deterioration of SCR catalysts during their contact with petroleum fuel.
As a potential solution to these problems, diesel plastic blend (DPB) was examined as a potential replacement for SCR. DPB is a potentially renewable and sustainable fuel, but it poses a major detrimental effect on the contaminants emitted by diesel engines and the efficiency of SCR systems. This is despite the fact that DPB itself is clean and renewable. There have been only a few studies conducted on how DPB affects the longevity and effectiveness of SCR catalysts. However, only a small percentage of this research focused on the endurance of DPB-powered engines under hydrothermal aging. This is because extensive durability testing of DPB engines is both time-consuming and costly. It is crucial to conduct engine durability testing under conditions that are as close to real life as possible in order to accurately replicate the usual wear and tear.
This study focuses on the ever-increasing need for petroleum that has compelled scientists to look for viable alternatives. Current automotive engines must be compatible with these fuels if they are to be widely adopted. The oil recovered from plastic wastes is one such alternative, as it helps to ensure the survival of fossil fuels and also lessens the negative impacts of improper waste disposal. The goal of this study is to find an economical way to recycle toxic and non-biodegradable plastic trash into a usable fuel source. Moreover, as NOX emissions have significantly increased due to the use of waste plastic oil blends, the work was directed further to reduce them. In addition, we intended to gain insight into the pollutant adsorption behavior of SnO2@rGO catalysts using a combination of DPB and conventional diesel fuel. The DPB fuel used in the experiments was produced from recycled waste plastic oil, while the standard diesel fuel used for comparison was derived from petroleum. The SnO2@rGO catalyst’s nanostructures were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), thermogravimetry (TGA), and surface area analyses to assess its binding capability with the exhaust pollutants. Furthermore, SnO2@rGO was subjected to an in-depth examination of the catalysts’ NOX-removing capability. The novelty of the present study is highlighted by the selective catalytic reduction of exhaust emission in an internal combustion (IC) engine achieved by a combination of plastic oil and SCR SnO2@rGO catalyst.
2. Results and Discussion
2.1. Characterization of rGO, SnO2, and SnO2@rGO Nanomaterials
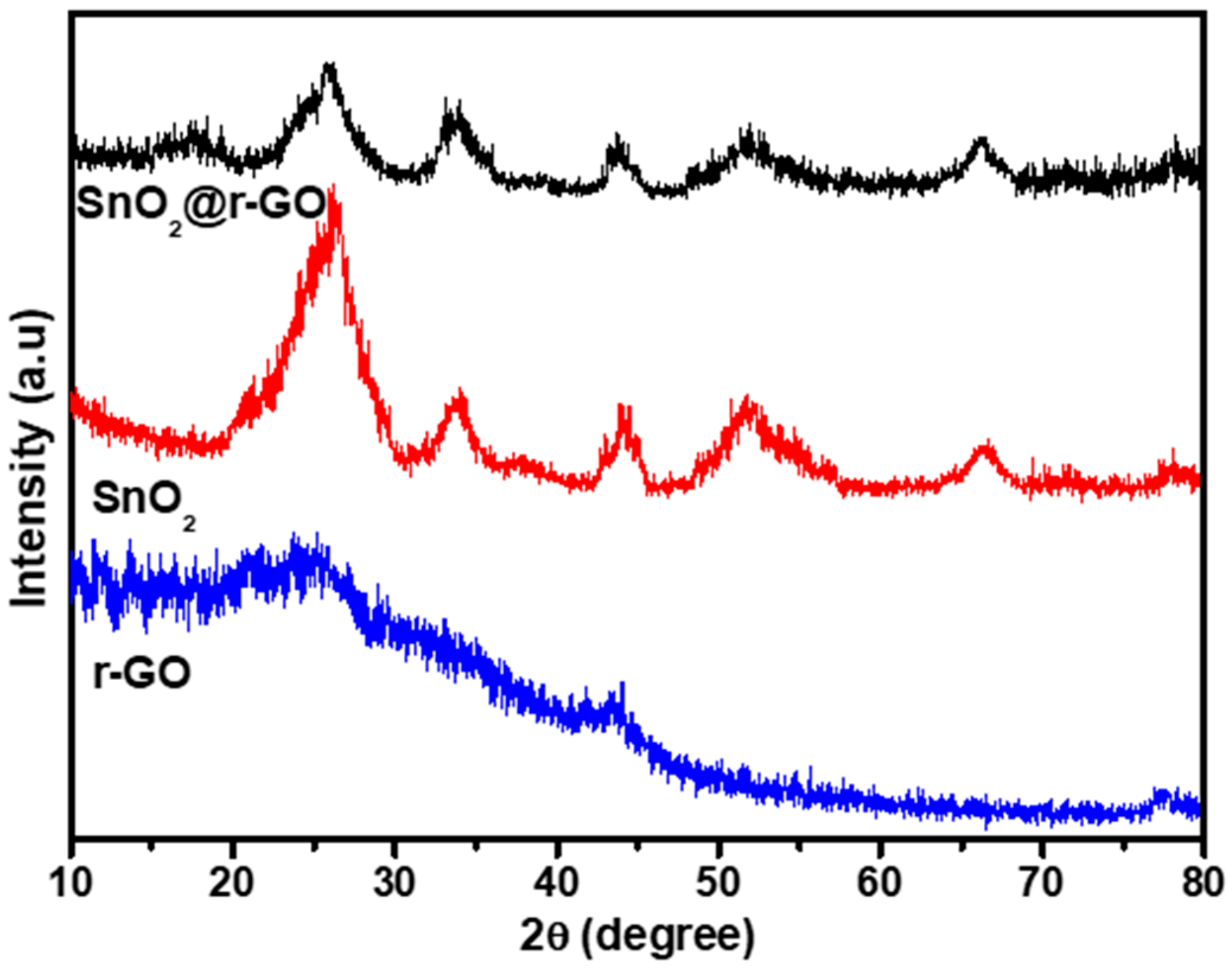
X-ray diffraction spectra were used to examine the crystal structure and phase development of rGO, SnO2 nanoparticles, and SnO2-rGO. It is evident from Figure 1 that two peaks at 24.57° and 43.95° were shown for rGO nanosheets which corresponded to (002) and (100) planes, respectively. The SnO2 nanoparticles exhibited five diffraction peaks at 26.48°, 33.56°, 43.16°, 51.48°, and 66.40° corresponding to the planes (110), (101), (210), (211), and (301), respectively, implying a tetragonal rutile structure based on JCPDS card No. 41-1445. The diffraction peaks of both rGO nanosheets and SnO2 nanoparticles appeared in the XRD spectra of SnO2@rGO nanocomposite confirming its successful synthesis. More specifically, as the two diffraction peaks of rGO (24.57° and 43.95°) overlapped with the closely appeared two diffraction peaks (26.48° and 43.16°) out of five diffraction peaks shown for SnO2 nanoparticles, the five diffraction peaks that appeared for SnO2@rGO should obviously be resulting from the sum of the diffraction peaks of rGO and SnO2. However, the impact of screening of rGO diffraction peaks caused by reduction and exfoliation of the graphene sheet as well as uniform scattering of SnO2 nanoparticles on the rGO surface cannot be ignored.
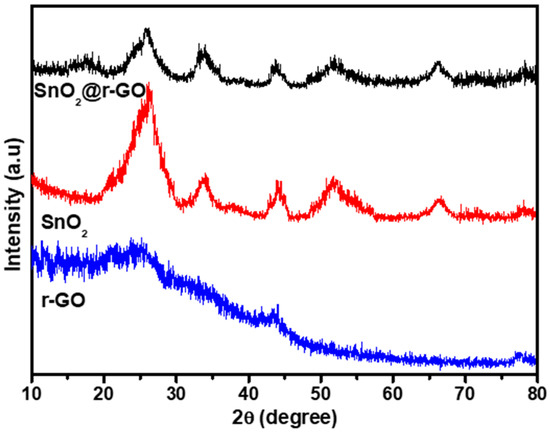
Figure 1.
XRD analysis of rGO, SnO2 nanoparticles, and SnO2@rGO nanocomposite.
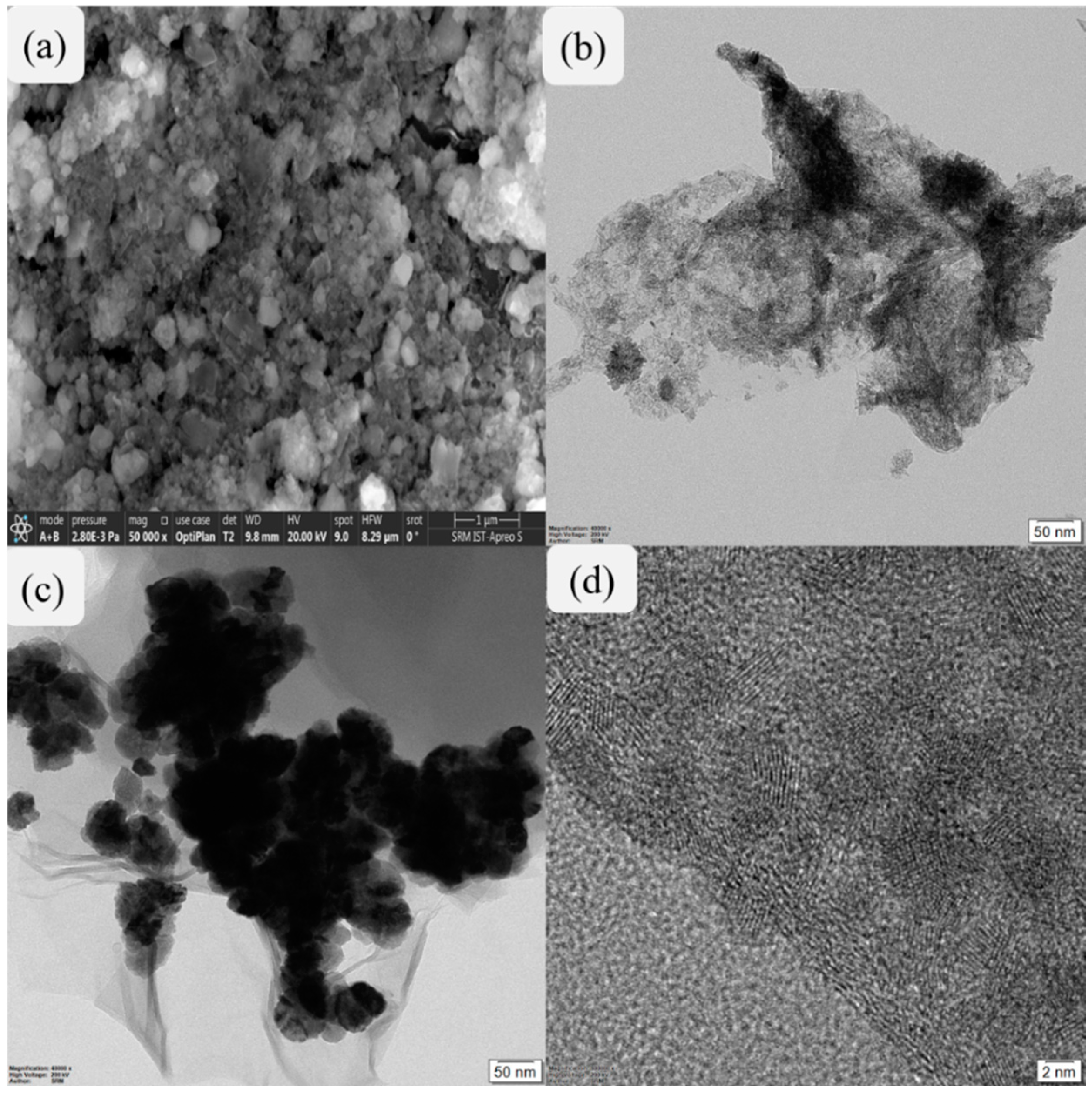
The structural morphology of SnO2 nanoparticles was analyzed by SEM microscopy as shown in Figure 2a. Figure 2b depicts the two-dimensional structure of rGO, while Figure 2c reveals the uniform distribution of SnO2 nanoparticles over the two-dimensional structure of rGO. In addition, an HRTEM image of SnO2@rGO nanocomposite shown in Figure 2d clearly reveals dark-grey colored parallel lines indicating the interplanar distances in SnO2 which can be attributed to the uniform scattering of SnO2 nanoparticles on rGO surface. This structural morphology revealed by electron microscopy images confirms that the SnO2@rGO nanocomposite was successfully prepared with better suitability towards reduction in exhaust emission in IC engines.
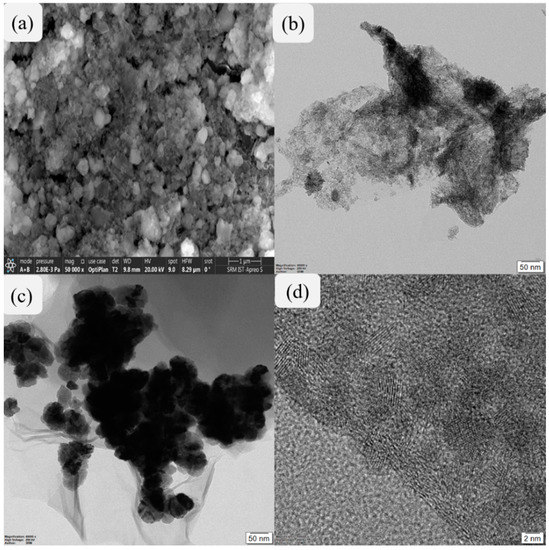
Figure 2.
Electron microscopic images of synthesized nanomaterials representing an SEM image of SnO2 nanoparticles (a), TEM image of rGO (b), TEM image of SnO2@rGO nanocomposite (c), and HRTEM image of SnO2-rGO nanocomposite (d).
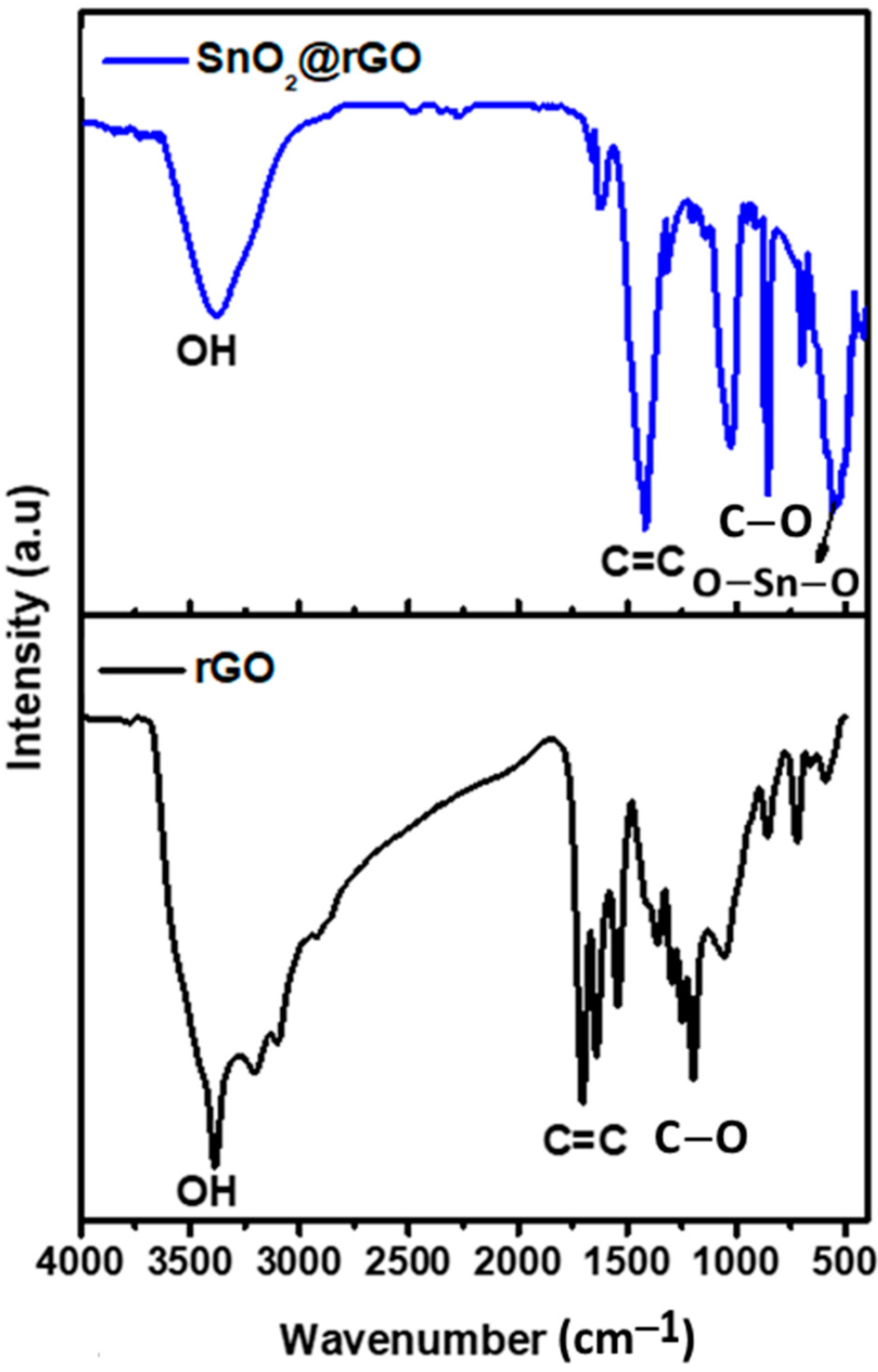
FTIR spectroscopy was utilized in order to ascertain different kinds of functional groups existing in SnO2 nanoparticles, rGO nanosheets, and SnO2@rGO nanocomposite. The FTIR spectra of nanomaterials that were synthesized are shown in Figure 3 in the frequency range of 500–4000 cm−1. The findings demonstrate how the microstructural properties of SnO2 nanoparticles changed in the frequency region 500–600 cm−1. On the other hand, rGO exhibited three stretching vibrations of C–O, C=C, and C=O, respectively, in the frequency of 1084.21, 1636.64, and 1721.65 cm−1. Due to the presence of water molecules, O–H stretching vibrations were observed at 3386.17 and 3437.41 cm−1 in the FTIR spectra of SnO2 nanoparticles and SnO2@rGO nanocomposite, respectively. The band appearing in the frequency of 545.57 cm−1 for SnO2 nanoparticles may be assigned to the O–Sn–O stretching vibration, while that at 681.38 cm−1 may be ascribed to the Sn–O stretching vibration. These functional groups in SnO2@rGO possess a strong tendency to bind toxic chemicals released during vehicle exhaust, facilitating the reduction of harmful automobile emissions released into the environment.
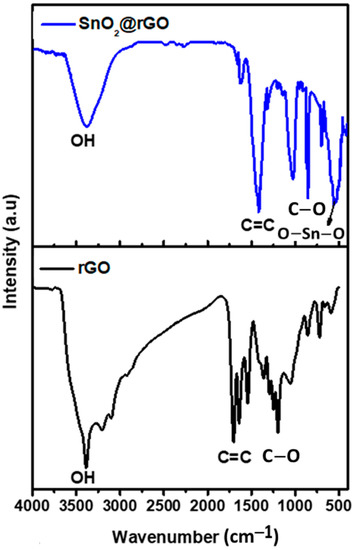
Figure 3.
FTIR analysis of rGO nanosheet and SnO2@rGO nanocomposite.
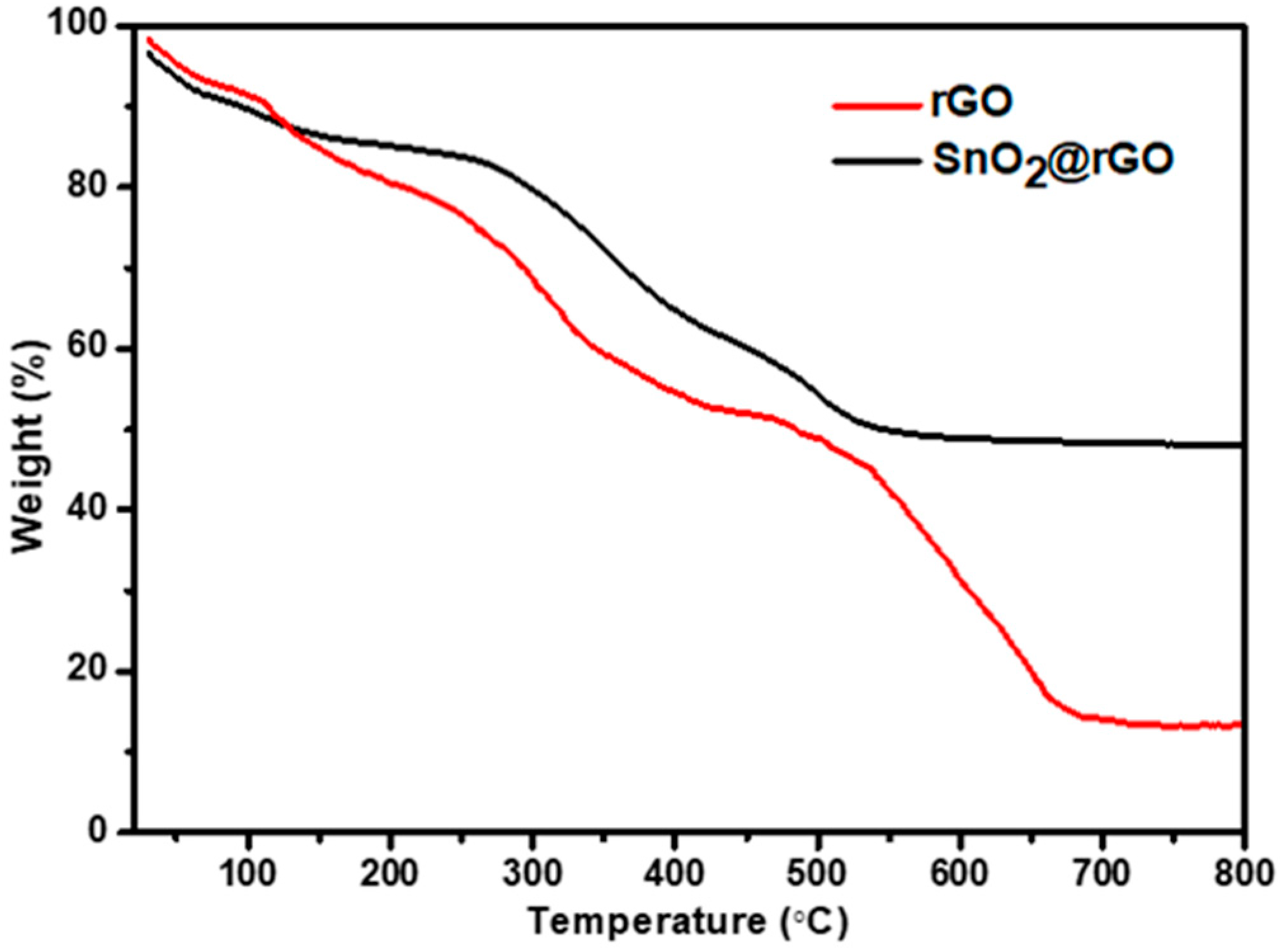
Thermogravimetric analysis was performed to determine the stability and quality attributes of SnO2 contained in SnO2@rGO nanocomposite. It is evident from Figure 4 that both rGO and SnO2@rGO undergo decomposition accompanied by mass loss by adopting two different processes. In the temperature range spanning from 0 to 150 °C, the weight loss in the TGA curve of rGO is caused mostly by evaporation of absorbed water and burning of carbon. However, the loss of mass between 200 and 400 °C may be attributed to the dissociation of oxygen-containing compounds, while the deterioration of carbon skeleton resulted in a weight loss at high temperatures. This significant weight loss caused by decomposition confirms that neither GO or rGO remains in the catalyst sample. A residual mass fraction of 13.36% in the rGO sample at temperatures above 650 °C may be contributed by the contaminants incorporated during the preparation. On the other hand, the TGA curve for SnO2@rGO shows a constant mass loss of 50% from room temperature to 500 °C, suggesting that SnO2 makes up half the weight of SnO2@rGO composites.
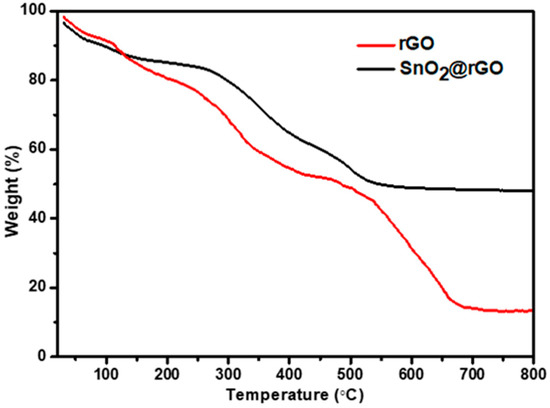
Figure 4.
Thermogravimetric analysis of rGO and SnO2@rGO nanocomposite.
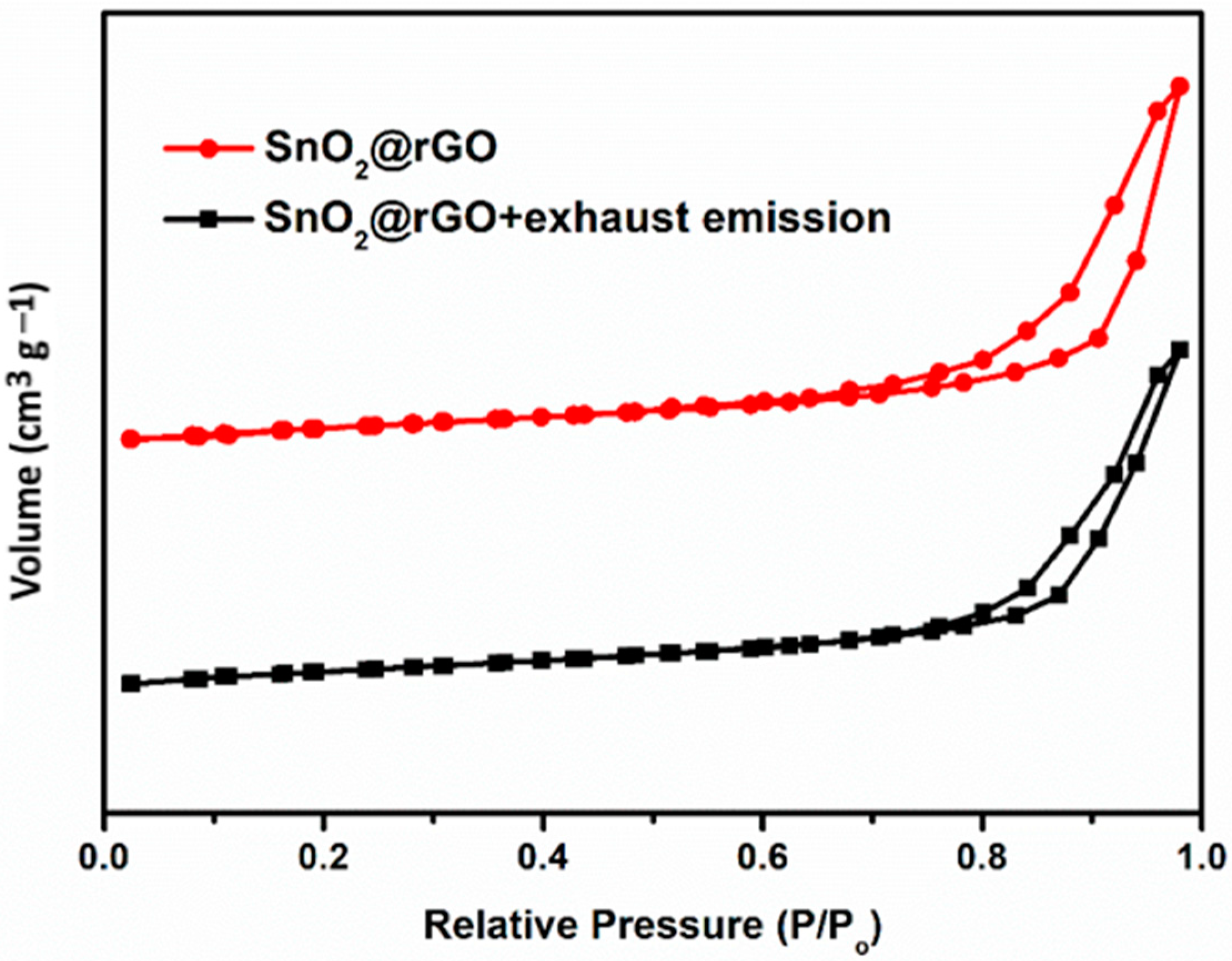
The gas adsorption performance is influenced by both surface area and pore volume due to the interactions between the exhaust gas molecule and the active sensing surface. The SnO2@rGO nanocomposite has demonstrated a type-II hysteresis loop in the N2 adsorption–desorption isotherm as shown in Figure 5. The BET surface area and pore volume diameter of the SnO2@rGO catalyst before (110 m2 g−1 and 0.08 cm3 g−1) and after (114 m2 g−1 and 0.05 cm3 g−1) the exhaust gas could pass through indicated that the SnO2@rGO nanocomposite retained a significant amount of porosity structure even after subjection to heat treatment, demonstrating its strong mechanical stability that is essential for gas adsorption. In other words, the SnO2@rGO nanocomposite possessed many active sites for surface contact reactions and rigid porous structures for facilitating gas adsorption/desorption capabilities toward regeneration and recycling.
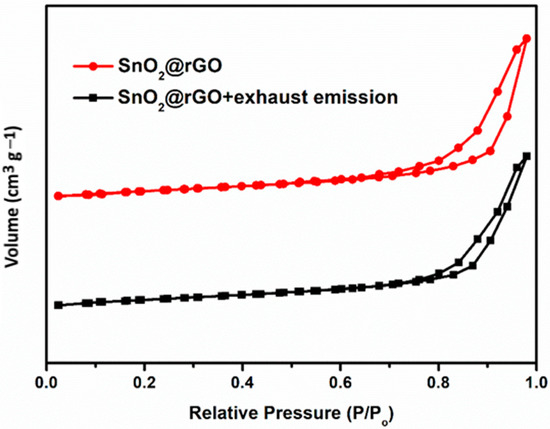
Figure 5.
BET-N2 adsorption-desorption isotherms of SnO2@rGO before and after the exhaust emission gas could pass through.
2.2. Brake Fuel Consumption (BSFC) and Brake Thermal Efficiency (BTE)
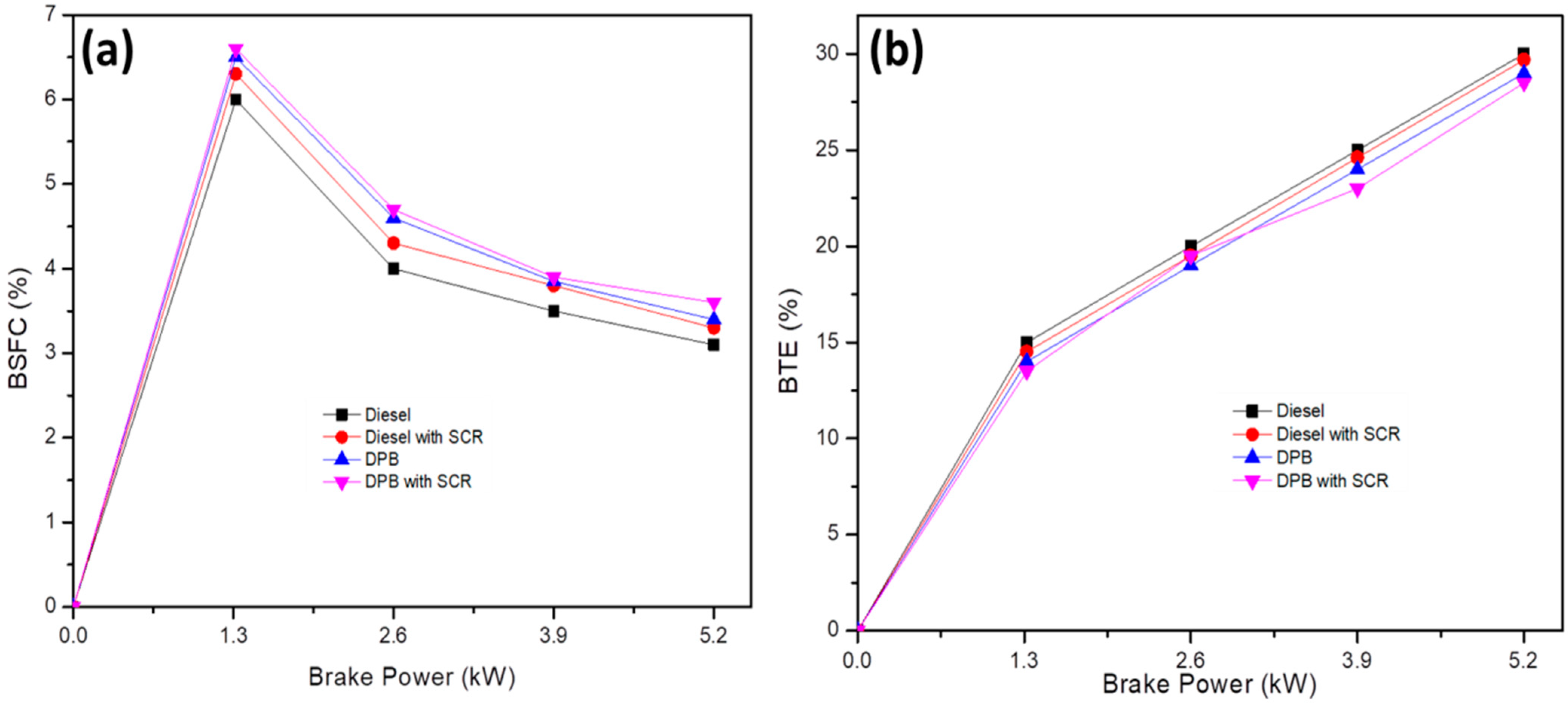
The variance in engine load-related fuel usage for brakes is depicted in Figure 6a. The BSFC was found to increase to ~8% for DPB due to the lower calorific value of plastic oil than regular diesel and the plastic oil’s interference with combustion. By providing an electron to a free radical generated close to the front of the hydrocarbon flame, this kind of addition can prevent oxidative processes. As a result, under the current engine loading state, more fuel is required to operate the system. The usage of an SCR system results in an increase in BSFC because the exhaust backpressure increases and restricts the flow of exhaust gases. When the engine is at full load (5.2 kW), the increase in BSFC was around 7% for DPB with SCR fuel when compared to that carried out without SCR. These results are in good agreement with the previous reports which are attributed to the higher viscosity of DPB compared to diesel that promotes less effective spray atomization leading to higher BSFC. Moreover, the lower calorific value of DPB is the main reason for the rise in consumption of particular fuels [18].
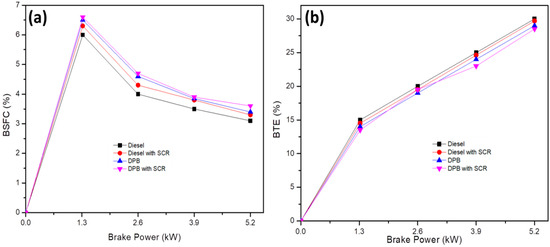
Figure 6.
Variation of BSFC (a) and BTE (b) corresponding with engine load. BSFC, brake fuel consumption; BTE, brake thermal efficiency.
The correlation between engine load and brake thermal efficiency is depicted in Figure 6b. It can be seen that BTE increased with a raise in engine load. The results showed that the thermal efficiency of the brakes was only reduced by 2% when fueled with DPB compared to diesel. The blended fuel is pre-treated with DPB, which disrupts the combustion of air-fuel mixture, leading to the observed effect. Utilizing the SCR system raises exhaust backpressure within the engine, which decreases BTE and increases BSFC.
2.3. Performance of Pollutant Adsorption by SnO2@rGO
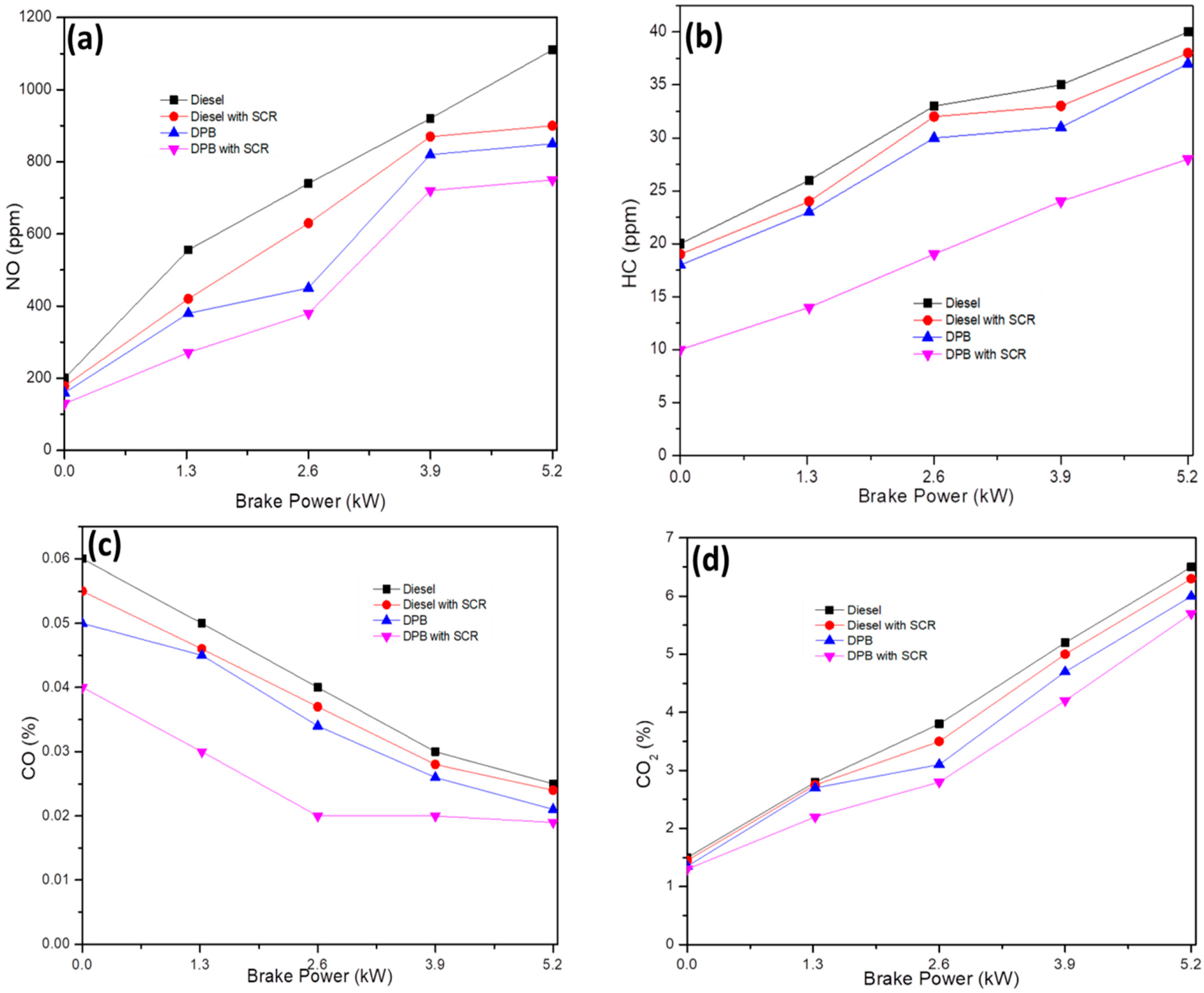
The emission of NOX from the SCR-coated exhaust under different engine loads with DPB and diesel fuel is depicted in Figure 7a. Diesel fuel with a higher cetane number and availability of additional oxygen has contributed to higher NO production compared to DPB [19]. The NOX emission with SCR-coated exhaust and DPB fuel was significantly reduced due to an increase in convective heat movement within the combustion chamber, which resulted in a lower mean temperature for the combustion process [20,21]. Furthermore, the urea spray along with the SnO2@rGO catalyst was responsible for the reduced emission. This as-synthesized catalyst improved the catalytic reaction for adsorption of harmful pollutants. It can also be observed that the NOX concentration at full engine load (5.2 kW) operated by DPB with SCR (750 ppm) was lower than that by diesel without SCR (900 ppm). However, the NOX emission level decreased to a meager level by the SCR catalyst incorporated in the exhaust tailpipe and plastic oil blended with diesel [22]. The higher adsorption ability of the SnO2@rGO catalyst was attributed to its NOX- binding ability with the specific functional groups as shown by the FTIR spectra and eventual fragmentation of harmful NOX to N2 and O2. By utilizing a DPB fuel for engine operation and an SnO2@rGO catalyst at the exhaust outlet NOX emission has indeed been drastically reduced even at full load conditions.
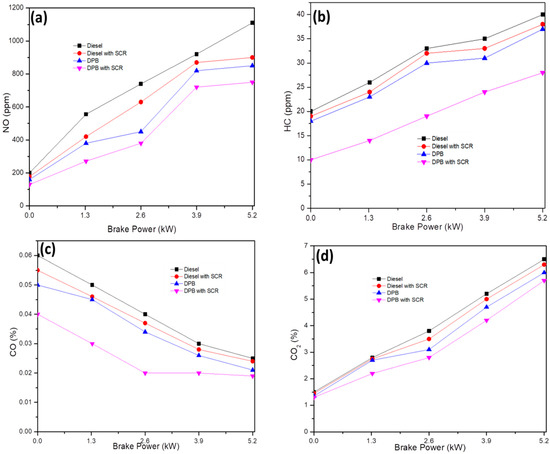
Figure 7.
Dependence of NOX (a), HC (b), CO (c), and CO2 (d) emissions from the SCR-coated exhaust under different engine loads.
Similar behavior was observed for HC emission where a full load engine operated by DPB with SCR was significantly lower compared to all other fuels and SCR without the SnO2@rGO catalyst (Figure 7b). HC emissions are essentially unburned raw fuels released as fuel vapors or by-products of gasoline after thermal breakdown. In general, with an increase in the load, the HC emissions often exhibit higher activity for subsequent adsorption and activation. The synthesized SnO2@rGO catalyst possessing large quantities of surface acid sites can facilitate reduction in the influence of HC emission.
The relationship between the amount of load on the engine and the CO emissions is shown in Figure 7c. CO is produced when combustion is insufficiently complete or efficient. According to the emission values, CO is drastically reduced in DPB with SCR compared to diesel without SCR. With higher oxygen content in DPB and the presence of oxygen functional groups in SnO2@rGO, the CO emission is decreased compared to diesel fuel without SCR. Moreover, the incorporation of the SnO2@rGO catalyst in the exhaust tailpipe caused splitting of CO emission into carbon and oxygen. Ultimately, the CO emission with DPB test fuel and SCR-coated exhaust reduced to 15% compared to diesel without SCR at maximum load condition.
The fluctuation in CO2 emissions that occurs in relation to the engine load is shown in Figure 7d. When using DPB with SCR as a test fuel, there is no change in the CO2 level when the engine is in idle condition [23]. However, when the engine load was 0%, 25%, 50%, 75%, or 100% under working condition, the CO2 emission was reduced by 3%, 7.5%, 7%, 7.8%, and 8.4%, respectively. The enhanced availability of oxygen in blended fuel caused the oxidation of CO to CO2, resulting in good combustion. In addition, the SnO2@rGO catalyst possesses higher efficiency in the reduction of CO2 emission, which may be attributed to its crystalline and anodic nature facilitating higher binding and reaction with CO2 emission through the active sites.
2.4. Smoke Opacity
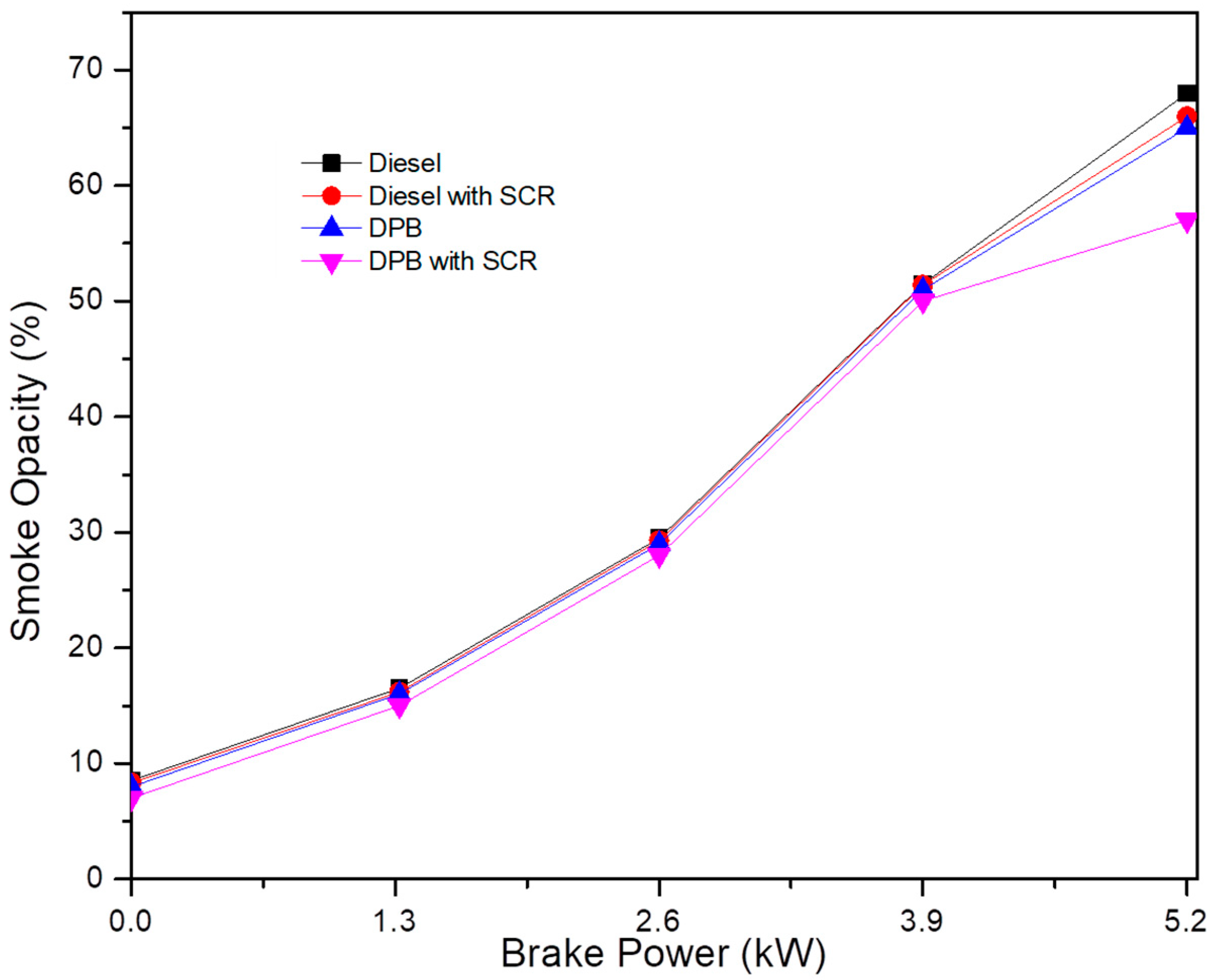
Figure 8 illustrates the possible range of smoke opacity as a function of engine load. When operating with DPB as the test fuel, smoke opacity was reduced by 3% compared to clean diesel fuel. The composition of lower sulfur and higher oxygen levels in DPB was responsible for a lower smoke opacity. Better combustion occurs due to a higher oxygen level being present in the fuel, leading to less soot being formed. Even at full capacity, there is only a 3% increase in opacity. These data demonstrate that the combustion process is disrupted by the incorporation of antioxidant chemicals, which in turn increases soot levels, but only slightly. The smoke opacity at full load is reduced from 70% using the SCR system with DPB compared to the diesel fuel operation that did not use SCR. The installation of an after-treatment system may have caused backpressure in the system, which may explain the observed rise in smoke concentration [24]. If more fuel is used, then there will be only a slight rise in soot.
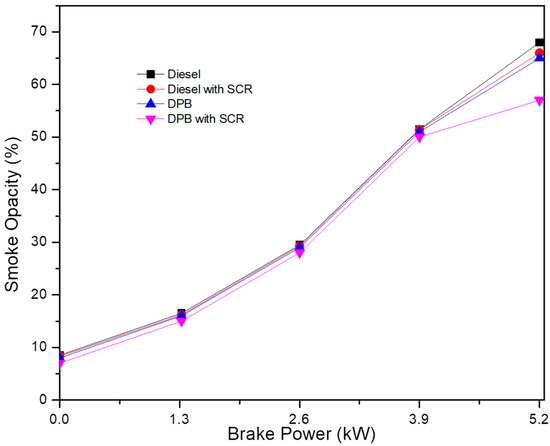
Figure 8.
Variation in smoke opacity as a function of engine load.
3. Materials and Methods
3.1. Materials
All reagents and solvents used in this study were of analytical grade and were obtained from Southern India Scientific Co., Ltd. (Chennai, India). Sigma Aldrich (St. Louis, MO, USA) provided the hydrazine monohydrate (NH2NH2.H2O), graphite, tin chloride (SnCl4.4H2O), sodium nitrate (NaNO3), hydrogen peroxide (H2O2), sulfuric acid (H2SO4), and potassium permanganate (KMnO4). Double-distilled water was used for nanoparticle manufacturing process. Table 1 shows the properties of diesel derived from petroleum and recycled plastic waste for comparison and both of them served as test subjects for the newly synthesized SCR.

Table 1.
Properties of oil.
3.2. Synthesis of SnO2 Nanoparticles
The SnO2 nanoparticles were synthesized by a hydrothermal method by mixing a 4:1 ratio of 2-propanol and distilled water with SnCl4.5H2O, followed by adding NaOH to bring the solution mixture to pH 12 and heated to 150 °C in a stainless-steel autoclave for 24 h.
3.3. Synthesis of Reduced Graphene Oxide/Tin Oxide (SnO2@rGO)
Initially, the Hummers method was employed for synthesis of graphene oxide (GO), and then a reduction strategy was adopted to convert GO to reduced GO (rGO). Briefly, 200 mg of GO was first dispersed evenly in 30 mL of distilled water. In the next step, 0.5 mL of hydrazine monohydrate was added to GO dispersion and thoroughly mixed with simultaneous heating at 80 °C for 3 h. Once the desired rGO nanosheets were obtained, they were washed and centrifuged several times in ethanol and distilled water to ensure complete removal of excess reagents. After drying the rGO nanosheets at 70 °C for 18 h, they were mixed with SnO2 and ultrasonicated to obtain the SnO2@rGO nanocomposite.
3.4. Characterization
The morphology of SnO2 nanoparticles was examined by capturing an SEM image, while that of rGO and SnO2@rGO nanocomposite was visualized by recording TEM images. The sample specimens for recording TEM and HRTEM images were prepared by ultrasonically dispersing the samples first, followed by drop-casting on carbon-coated copper grids, drying overnight in a vacuum oven, and mounting on a JEOL JEM 2100 electron microscope (Tokyo, Japan) for collecting images at an accelerating voltage of 200 kV. The XRD patterns of rGO, SnO2 nanoparticles, and SnO2@rGO were analyzed with a PANalytical X’Pert diffractometer (Malvern, UK) at 40 kV and 30 mA using CuKα radiation (1.5418 Å) with a scanning speed of 3 °C/min. To elucidate the functional groups associated with rGO and SnO2@rGO, the FTIR spectra were recorded in the frequency ranging from 500–4000 cm−1 in a Cary 660 FTIR spectrometer from Agilent Technologies (Santa Clara, CA, USA). Both stability and quality attributes of SnO2 in SnO2@rGO were determined by performing thermogravimetric analysis of rGO and SnO2@rGO using a STA 449 F1 Jupiter® Simultaneous TGA/DSC analyzer from Netzsch (Selb, Germany), while both surface area and pore diameter of SnO2@rGO catalyst before and after the exhaust gas could pass through were measured by BET-N2 analysis (Nova 4200, Anton Paar QuantaTec Inc. - Quantachrome Instruments, Boynton Beach, FL, USA) involving N2 adsorption–desorption isotherms.
3.5. Experimental Setup of Engine
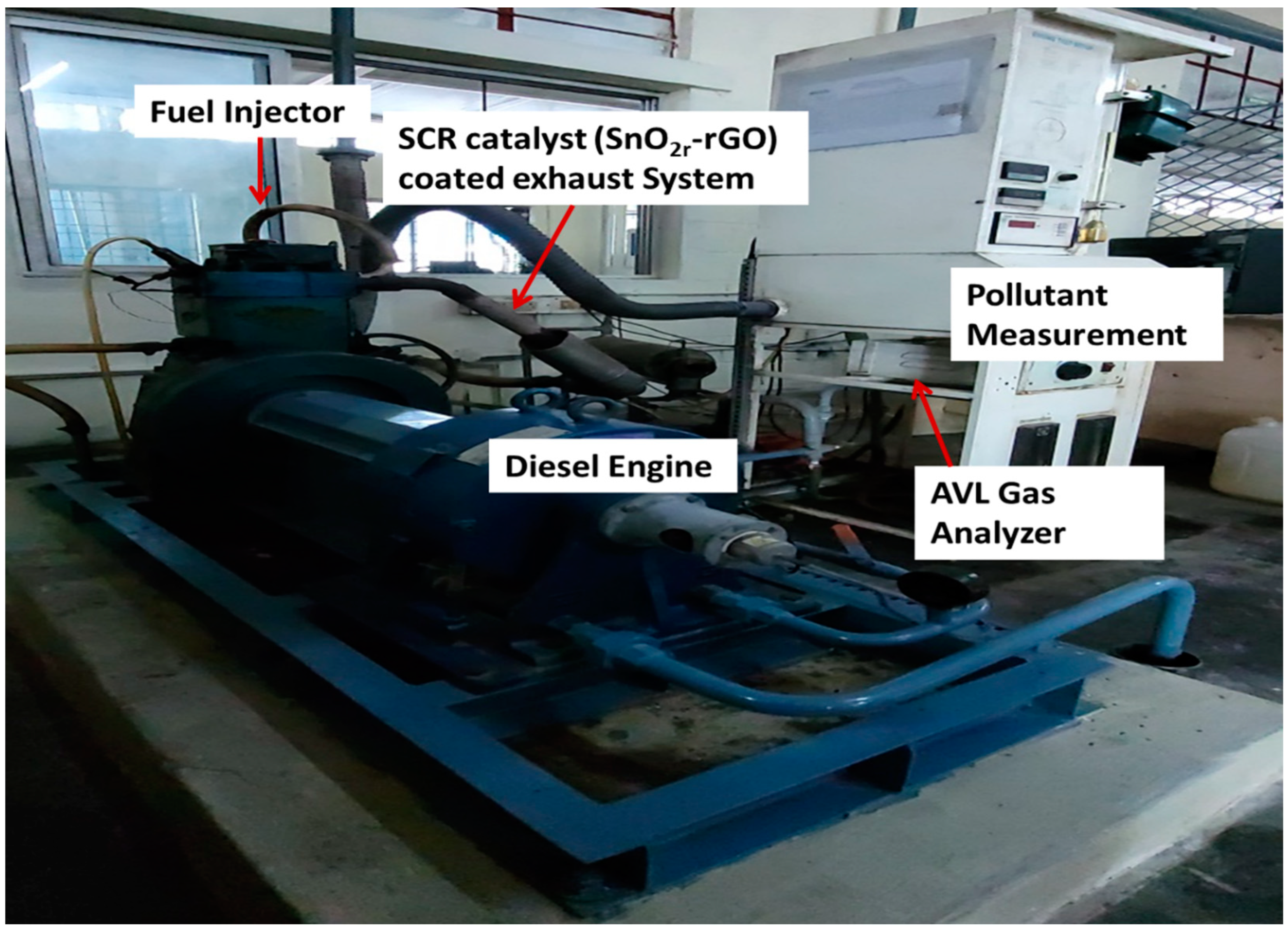
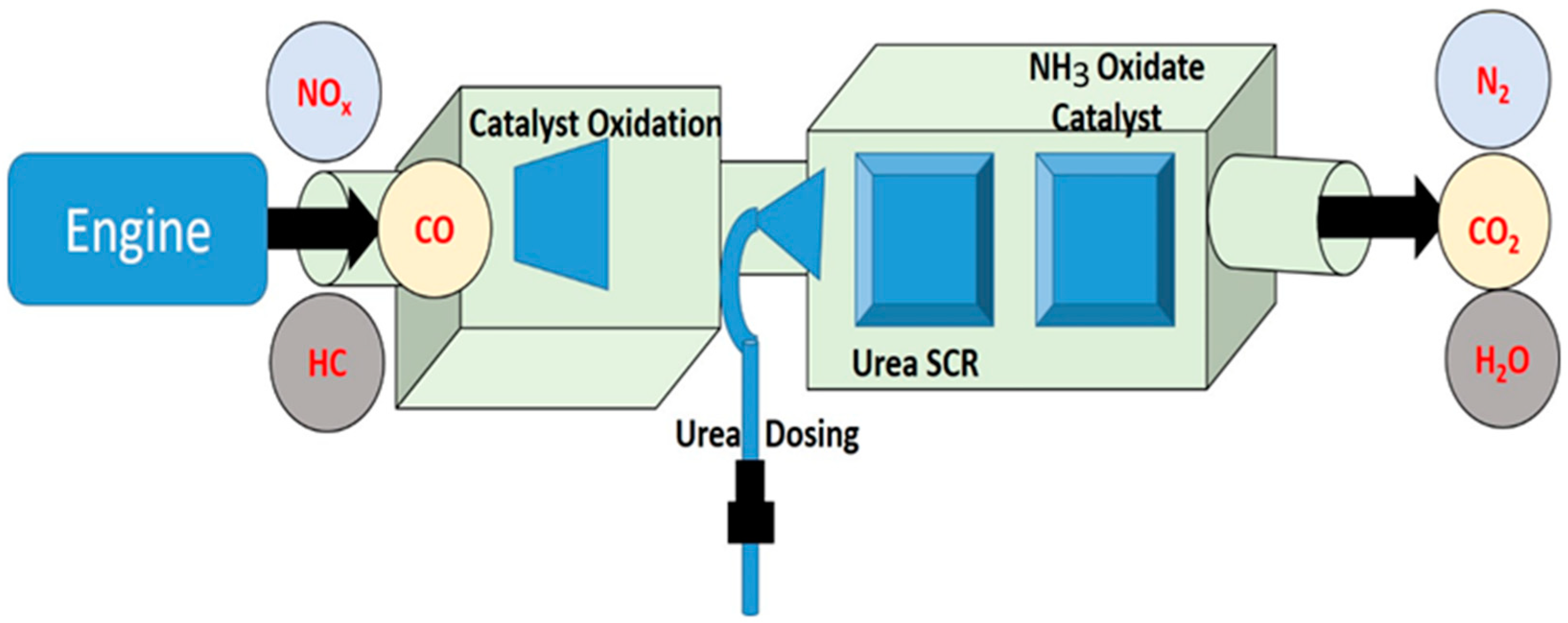
Exhaust gas studies were performed on a real diesel vehicle fitted with SCR. A 5.2-L displacement single-cylinder off-road engine with turbocharger air supply was used as the test vehicle. In spite of the fact that the after-treatment system, which includes SCR, can be set up in various ways [6,10], for the purposes of this study, they were set up in the sequence of SCR starting at the diesel exhaust manifold (Figure 9). It is worth pointing out that the after-treatment equipment that is arranged in a linear fashion in this vehicle may require updating before it can be used in other cars. For instance, the linear arrangement may need to be changed so that it uses two rows. It is anticipated that even with the sequential arrangement linking the SCR catalyst system, there would not be a major change in the qualities of the exhaust gas (Figure 10). The findings of this investigation might also be applicable to other research employing post-treatment equipment that is very similar to what is used in this study. A mixer has been positioned in front of the SCR catalyst, and it is in this mixer that the urea spray and the exhaust gas are mixed together. In front of the SCR catalysts, a urea injection system was fitted, and stainless-steel tubing was inserted in front of and behind the catalysts to collect exhaust gas samples. SCR catalysts were also added. An AVL 444 gas analyzer was used to measure the accurate readings of O2, HC, CO, and NOX. Both SnO2 and rGO used for synthesis of SnO2@rGO catalyst are widely utilized in the industrial sector and were reported as SCR catalysts in the investigation of NO emission in the real engine [25]. Application of different engine loads was achieved by altering the brake power (kW) and the corresponding pollutant measurement was performed in the presence and absence of SCR for diesel and DPB.
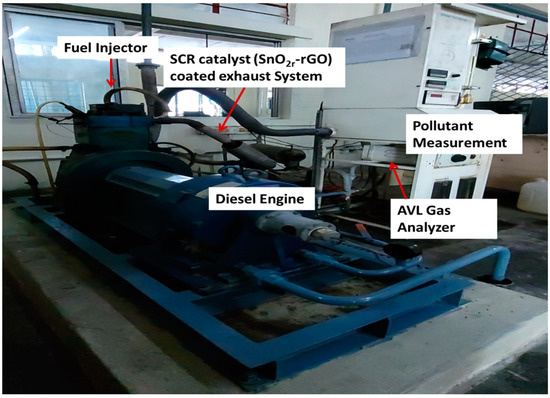
Figure 9.
Engine experimental setup.
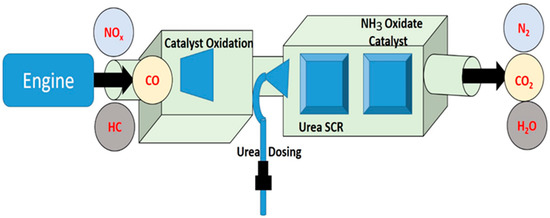
Figure 10.
Schematic setup for selective catalytic reduction of exhaust emission in an internal combustion engine.
4. Conclusions
The current research investigated whether SnO2@rGO nanocomposite can be used as SCR catalysts for heavy-duty diesel engines using clean and blended diesel fuel. The material characterization indicated the formation of SnO2@rGO with a suitable morphology and functional groups capable of adsorbing toxic pollutants from vehicle exhaust. The fuel consumption decreased for plastic oil blended diesel, and along with an SnO2@rGO- coated exhaust tailpipe decreased significantly. Similarly, the harmful pollutants were adsorbed by the catalyst, eventually decreasing their emission concentration by catalytic reduction. Finally, the tested smoke opacity was found to be reduced when DPB was used as the test fuel.
Author Contributions
Conceptualization, K.R. and B.S.I.; Methodology, S.P., K.R. and R.K.; Software, S.P., R.K. and J.V.K.; Validation, S.P., K.R., J.V.K., N.A. and B.S.I.; Formal analysis, S.P., K.R., R.K. and J.V.K.; Investigation, S.P., K.R. and R.K.; Resources, S.P., N.A. and B.S.I.; Data curation, S.P., K.R., J.V.K. and B.S.I.; Writing—original draft, S.P., K.R., R.K. and N.A.; Writing—review and editing, R.K., J.V.K., N.A. and B.S.I.; Visualization, S.P., R.K., J.V.K., N.A. and B.S.I.; Project administration, K.R., J.V.K., N.A. and B.S.I.; Supervision, K.R., N.A. and B.S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mwangi, J.K.; Lee, W.-J.; Chang, Y.-C.; Chen, C.-Y.; Wang, L.-C. An Overview: Energy Saving and Pollution Reduction by Using Green Fuel Blends in Diesel Engines. Appl. Energy 2015, 159, 214–236. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. High Quality Biodiesel and Its Diesel Engine Application: A Review. Renew. Sustain. Energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; Jaramillo-Jacob, A.d.R. Predicting Cetane Number, Kinematic Viscosity, Density and Higher Heating Value of Biodiesel from Its Fatty Acid Methyl Ester Composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Cordiner, S.; Mulone, V.; Nobile, M.; Rocco, V. Impact of Biodiesel Fuel on Engine Emissions and Aftertreatment System Operation. Appl. Energy 2016, 164, 972–983. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Robbins, C. Review of the Effects of Biodiesel on NOX Emissions. Fuel Process. Technol. 2012, 96, 237–249. [Google Scholar] [CrossRef]
- Yoon, S.H.; Suh, H.K.; Lee, C.S. Effect of Spray and EGR Rate on the Combustion and Emission Characteristics of Biodiesel Fuel in a Compression Ignition Engine. Energy Fuels 2009, 23, 1486–1493. [Google Scholar] [CrossRef]
- Vedaraman, N.; Puhan, S.; Nagarajan, G.; Velappan, K.C. Preparation of Palm Oil Biodiesel and Effect of Various Additives on NOX Emission Reduction in B20: An Experimental Study. Int. J. Green Energy 2011, 8, 383–397. [Google Scholar] [CrossRef]
- Wan Ghazali, W.N.M.; Mamat, R.; Masjuki, H.H.; Najafi, G. Effects of Biodiesel from Different Feedstocks on Engine Performance and Emissions: A Review. Renew. Sustain. Energy Rev. 2015, 51, 585–602. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, J.T.; Park, S. Nitrogen Compounds (NO, NO2, N2O, and NH3) in NOX Emissions from Commercial EURO VI Type Heavy-Duty Diesel Engines with a Urea-Selective Catalytic Reduction System. Energy Fuels 2016, 30, 6828–6834. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Marti, T. NO x -Reduction in Diesel Exhaust Gas with Urea and Selective Catalytic Reduction. Combust. Sci. Technol. 1996, 121, 85–102. [Google Scholar] [CrossRef]
- Tadano, Y.S.; Borillo, G.C.; Godoi, A.F.L.; Cichon, A.; Silva, T.O.B.; Valebona, F.B.; Errera, M.R.; Penteado Neto, R.A.; Rempel, D.; Martin, L.; et al. Gaseous Emissions from a Heavy-Duty Engine Equipped with SCR Aftertreatment System and Fuelled with Diesel and Biodiesel: Assessment of Pollutant Dispersion and Health Risk. Sci. Total Environ. 2014, 500–501, 64–71. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Saravanan, C.G.; Lee, P.S.; Chua, K.J.E.; Chou, S.K. Emission Reduction from a Diesel Engine Fueled by Pine Oil Biofuel Using SCR and Catalytic Converter. Atmos. Environ. 2013, 80, 190–197. [Google Scholar] [CrossRef]
- Solaimuthu, C.; Ganesan, V.; Senthilkumar, D.; Ramasamy, K.K. Emission Reductions Studies of a Biodiesel Engine Using EGR and SCR for Agriculture Operations in Developing Countries. Appl. Energy 2015, 138, 91–98. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Si, W.; Luo, J.; Wang, Y.; Fu, J.; Li, X.; Crittenden, J.; Hao, J. Deactivation and Regeneration of a Commercial SCR Catalyst: Comparison with Alkali Metals and Arsenic. Appl. Catal. B: Environ. 2015, 168–169, 195–202. [Google Scholar] [CrossRef]
- Wetchakun, N.; Incessungvorn, B.; Wetchakun, K.; Phanichphant, S. Influence of Calcination Temperature on Anatase to Rutile Phase Transformation in TiO2 Nanoparticles Synthesized by the Modified Sol–Gel Method. Mater. Lett. 2012, 82, 195–198. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Mnichowicz, B.; Harinath, A.V.; Li, H.; Bahrami, B. Chemical Deactivation of Commercial Vanadium SCR Catalysts in Diesel Emission Control Application. Chem. Eng. J. 2016, 287, 680–690. [Google Scholar] [CrossRef]
- Liu, Z.G.; Ottinger, N.A.; Cremeens, C.M. Vanadium and Tungsten Release from V-Based Selective Catalytic Reduction Diesel after treatment. Atmos. Environ. 2015, 104, 154–161. [Google Scholar] [CrossRef]
- Piqiang, T.; Zhiyuan, H.; Diming, L. Effects of key operating parameters on SCR performance of diesel engines. Comput. Math. Appl. 2014, 65, 4063–4070. [Google Scholar]
- Premkumar, S.; Balaji, G. Exploration of Zeolite 5A as a Catalyst in the after-Treatment System of a CI Engine Powered by Plastic Oil Blend. IOP Conf. Ser. Mater. Sci. Eng. 2020, 912, 042028. [Google Scholar] [CrossRef]
- Sutjiono, R.; Tayal, P.; Zhou, K.; Meckl, P. Real-Time On-Board Indirect Light-off Temperature Estimation as a Detection Technique of Diesel Oxidation Catalyst Effectiveness Level. In Proceedings of the SAE 2013 World Congress & Exhibition, Detroit Michigan, MI, USA, 16–18 April 2013; Technical Paper 2013-01-1517. SAE International: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Premkumar, S.; Balaji, G. Experimental Investigation of HC and CO Emission Reduction from a Diesel Engine Powered by Plastic Oil Blend Using Fly Ash as Catalyst. J. Therm. Anal. Calorim. 2022, 147, 1535–1545. [Google Scholar] [CrossRef]
- De-La-Torre, U.; Pereda-Ayo, B.; Gutiérrez-Ortiz, M.A.; González-Marcos, J.A.; González-Velasco, J.R. Steady-State NH3-SCR Global Model and Kinetic Parameter Estimation for NOx Removal in Diesel Engine Exhaust Aftertreatment with Cu/Chabazite. Catal. Today 2017, 296, 95–104. [Google Scholar] [CrossRef]
- Xiong, S.; Xiao, X.; Liao, Y.; Dang, H.; Shan, W.; Yang, S. Global Kinetic Study of NO Reduction by NH3 over V2O5–WO3/TiO2: Relationship between the SCR Performance and the Key Factors. Ind. Eng. Chem. Res. 2015, 54, 11011–11023. [Google Scholar] [CrossRef]
- Iida, Y.; Ozaki, S. Grain Growth and Phase Transformation of Titanium Oxide during Calcination. J. Am. Ceram. Soc. 1961, 44, 120–127. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A Comprehensive Review on Structures and Gas Sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).