High Internal Phase Pickering Emulsion Stabilized by Lipase-Coated ZIF-8 Nanoparticles towards Recyclable Biphasic Biocatalyst
Abstract
:1. Introduction
2. Results and Discussion
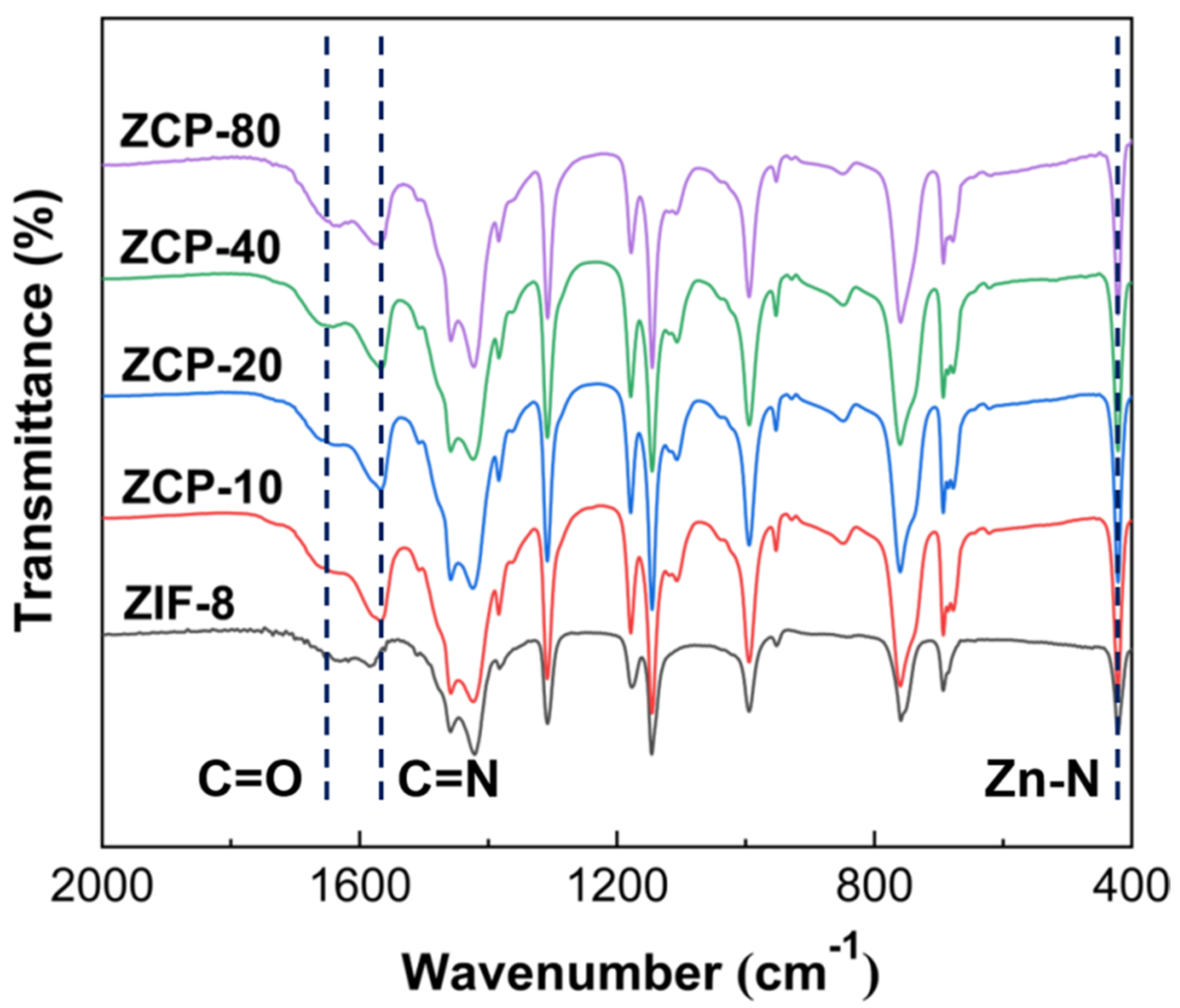
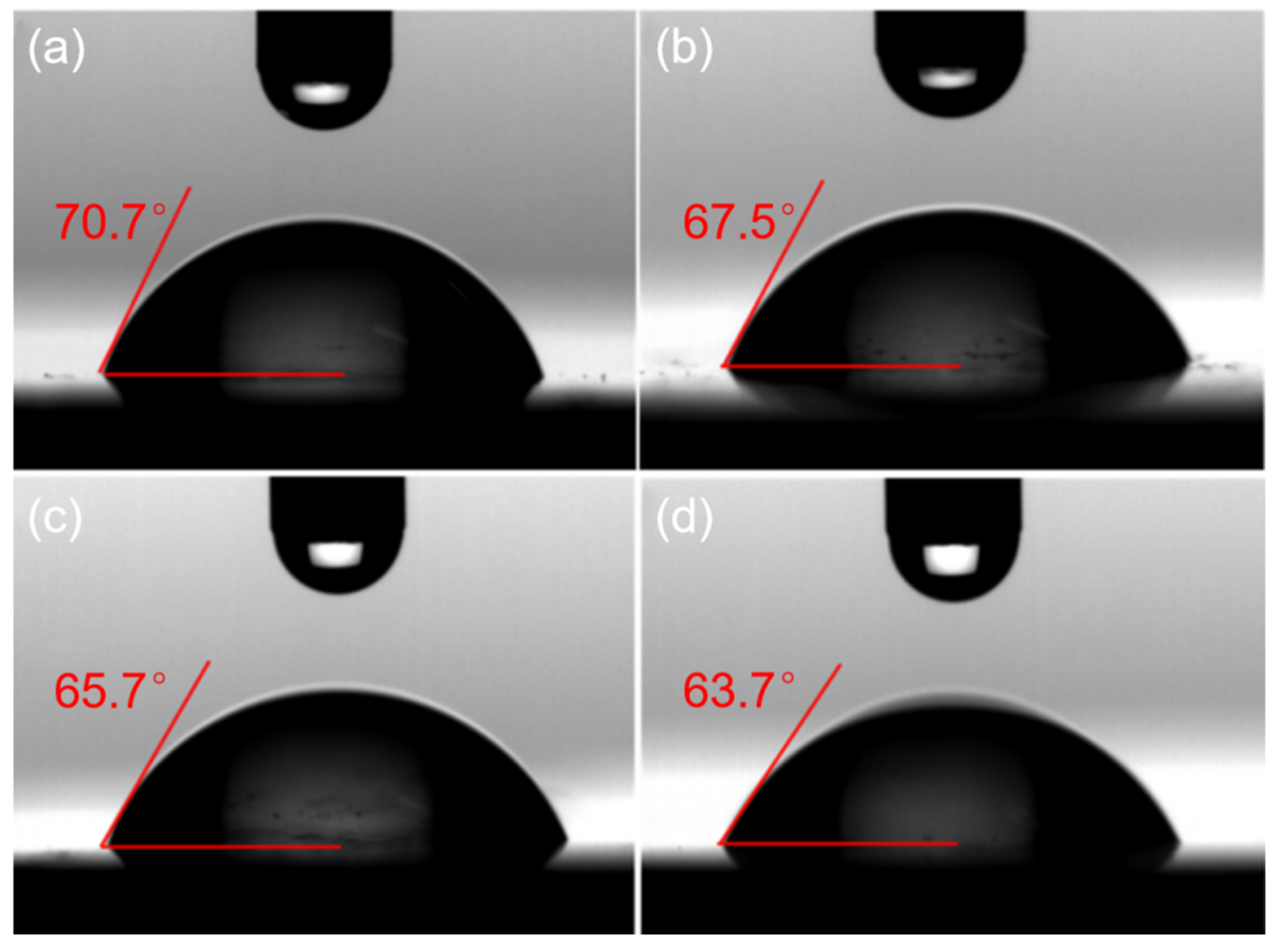
2.1. Preparation and Characterization of ZCPs
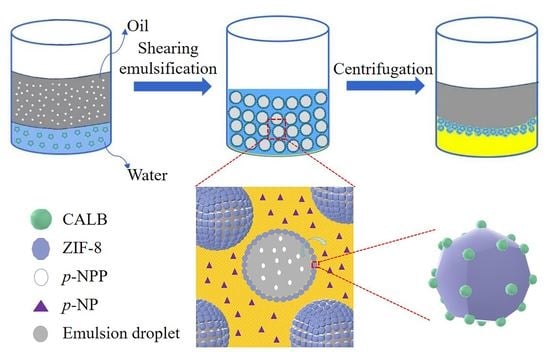
2.2. Preparation of Pickering Emulsion
2.3. Catalytic Activity of Pickering Interfacial Biocatalysts
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of ZCP
3.2.2. Preparation of Pickering HIPE
3.2.3. Biocatalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Qiao, S.; Zheng, Y.; Andaloussi, Y.H.; Li, X.; Zhang, Z.; Li, A.; Cheng, P.; Ma, S.; Chen, Y. Fabricating Covalent Organic Framework Capsules with Commodious Microenvironment for Enzymes. J. Am. Chem. Soc. 2020, 142, 6675–6681. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, X.; Zhang, S.; Tang, L.; Jiang, Z. Enzyme-conjugated ZIF-8 particles as efficient and stable Pickering interfacial biocatalysts for biphasic biocatalysis. J. Mater. Chem. B 2016, 4, 2654–2661. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, J.; Zhou, L.; Ma, L.; He, Y.; Huang, Z.; Jiang, Y. Enzyme nanocapsules armored by metal-organic frameworks: A novel approach for preparing nanobiocatalyst. Chem. Eng. J. 2017, 327, 1192–1197. [Google Scholar] [CrossRef]
- Arana-Pena, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, A.; Alcantara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice... or not? Biotechnol. Adv. 2021, 51, 107584. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, S. Controllable immobilization of enzymes in metal-organic frameworks for biocatalysis. Chem. Catal. 2021, 1, 20–22. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Zhu, Y.; Zhang, S.; Chen, J. Enzyme immobilized millimeter-sized polyHIPE beads with easy separability and recyclability. React. Chem. Eng. 2019, 4, 1136–1144. [Google Scholar] [CrossRef]
- Meng, T.; Bai, R.; Wang, W.; Yang, X.; Guo, T.; Wang, Y. Enzyme-Loaded Mesoporous Silica Particles with Tuning Wettability as a Pickering Catalyst for Enhancing Biocatalysis. Catalysts 2019, 9, 78. [Google Scholar] [CrossRef]
- Luna, C.; Gascón-Pérez, V.; López-Tenllado, F.J.; Bautista, F.M.; Verdugo-Escamilla, C.; Aguado-Deblas, L.; Calero, J.; Romero, A.A.; Luna, D.; Estévez, R. Enzymatic Production of Ecodiesel by Using a Commercial Lipase CALB, Immobilized by Physical Adsorption on Mesoporous Organosilica Materials. Catalysts 2021, 11, 1350. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Shi, J.; Tang, H.; Xiang, X.; Huang, F.; Zheng, M. Carbon Nanoparticle-Stabilized Pickering Emulsion as a Sustainable and High-Performance Interfacial Catalysis Platform for Enzymatic Esterification/Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 7619–7629. [Google Scholar] [CrossRef]
- Qi, L.; Zhou, Y.J.; Luo, Z.G.; Gao, Q.Y.; Shi, Y.C. Facile synthesis of lipase-loaded starch nanoparticles as recyclable biocatalyst in Pickering interfacial systems. Carbohydr. Polym. 2023, 299, 11. [Google Scholar] [CrossRef]
- Wang, M.; Wang, M.; Zhang, S.; Chen, J. Pickering gel emulsion stabilized by enzyme immobilized polymeric nanoparticles: A robust and recyclable biocatalyst system for biphasic catalysis. React. Chem. Eng. 2019, 4, 1459–1465. [Google Scholar] [CrossRef]
- Zhao, Z.; Lin, T.; Liu, W.; Hou, L.; Ye, F.; Zhao, S. Colorimetric detection of blood glucose based on GOx@ZIF-8@Fe-polydopamine cascade reaction. Spectrochim Acta A Mol. Biomol. Spectrosc. 2019, 219, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yan, L.; Chen, H.; Ju, X.; Zhou, Z.; Li, L. Development of functionalized metal–organic frameworks immobilized cellulase with enhanced tolerance of aqueous-ionic liquid media for saccharification of bagasse. Fuel 2021, 304, 121484. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, Y.; Jia, X. Sodium dodecyl sulfate-assisted synthesis of hierarchically porous ZIF-8 particles for removing mercaptan from gasoline. Chem. Eng. J. 2014, 256, 14–22. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Z.; Lu, X. Biomimetic Mineralization Inducing Lipase–Metal–Organic Framework Nanocomposite for Pickering Interfacial Biocatalytic System. ACS Sustain. Chem. Eng. 2019, 7, 7127–7139. [Google Scholar] [CrossRef]
- Fu, H.; Ou, P.; Zhu, J.; Song, P.; Yang, J.; Wu, Y. Enhanced Protein Adsorption in Fibrous Substrates Treated with Zeolitic Imidazolate Framework-8 (ZIF-8) Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 7626–7636. [Google Scholar] [CrossRef]
- Nowroozi-Nejad, Z.; Bahramian, B.; Hosseinkhani, S. Efficient immobilization of firefly luciferase in a metal organic framework: Fe-MIL-88(NH2) as a mighty support for this purpose. Enzyme Microb. Technol. 2019, 121, 59–67. [Google Scholar] [CrossRef]
- Inagaki, M.; Hiratake, J.; Nishioka, T.; Oda, J. One-Pot Synthesis Of Optically-Active Cyanohydrin Acetates from Aldehydes Via Lipase-Catalyzed Kinetic Resolution Coupled with Insitu Formation and Racemization Of Cyanohydrins. J. Org. Chem. 1992, 57, 5643–5649. [Google Scholar] [CrossRef]
- Nobakht, N.; Faramarzi, M.A.; Shafiee, A.; Khoobi, M.; Rafiee, E. Polyoxometalate-metal organic framework-lipase: An efficient green catalyst for synthesis of benzyl cinnamate by enzymatic esterification of cinnamic acid. Int. J. Biol. Macromol. 2018, 113, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.Z.; Xia, L.M. Effect of lipase immobilization on resolution of (R, S)-2-octanol in nonaqueous media using modified ultrastable-Y molecular sieve as support. Appl. Biochem. Biotechnol. 2006, 134, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jia, H.F.; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Ispas, C.; Sokolov, I.; Andreescu, S. Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal. Bioanal. Chem. 2009, 393, 543–554. [Google Scholar] [CrossRef]
- Beh, J.J.; Lim, J.K.; Ng, E.P.; Ooi, B.S. Synthesis and size control of zeolitic imidazolate framework-8 (ZIF-8): From the perspective of reaction kinetics and thermodynamics of nucleation. Mater. Chem. Phys. 2018, 216, 393–401. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Leclercq, L.; Clacens, J.M.; De Campo, F.; Nardello-Rataj, V. Pickering interfacial catalysis for biphasic systems: From emulsion design to green reactions. Angew. Chem. 2015, 54, 2006–2021. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, B.; Wang, S.; Huang, X.; Jiang, H.; Yin, S.; Ngai, T.; Yang, X. Growth of Au nanoparticles on phosphorylated zein protein particles for use as biomimetic catalysts for cascade reactions at the oil–water interface. Chem. Sci. 2021, 12, 3885–3889. [Google Scholar] [CrossRef]
- Chang, F.; Vis, C.M.; Ciptonugroho, W.; Bruijnincx, P.C.A. Recent developments in catalysis with Pickering Emulsions. Green Chem. 2021, 23, 2575–2594. [Google Scholar] [CrossRef]
- Bago Rodriguez, A.M.; Schober, L.; Hinzmann, A.; Groger, H.; Binks, B.P. Effect of Particle Wettability and Particle Concentration on the Enzymatic Dehydration of n-Octanaloxime in Pickering Emulsions. Angew. Chem. 2021, 60, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Jiang, J.Z.; Cai, C. Palladium nanoparticles anchored on amphiphilic Janus-type cellulose nanocrystals for Pickering interfacial catalysis. Chem. Commun. 2020, 56, 9396–9399. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yu, C.; Wei, Q.B.; Liu, D.M.; Qiu, J.S. Pickering Emulsion Catalysis: Interfacial Chemistry, Catalyst Design, Challenges, and Perspectives. Angew. Chem.-Int. Edit. 2022, 61, 24. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, W.; Ali, D.C.; Zhang, X.; Wang, Z. Interfacial biocatalysis in bacteria-stabilized Pickering emulsions for microbial transformation of hydrophobic chemicals. Catal. Sci. Technol. 2021, 11, 2816–2826. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, X.; Li, Y.; Yang, C.; Ngai, T. Engineering proteinaceous colloidosomes as enzyme carriers for efficient and recyclable Pickering interfacial biocatalysis. Chem. Sci. 2021, 12, 12463–12467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; van Oers, M.C.; Rutjes, F.P.; van Hest, J.C. Polymersome colloidosomes for enzyme catalysis in a biphasic system. Angew. Chem. 2012, 51, 10746–10750. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Glebe, U.; Charan, H.; Boker, A.; Wu, C. Enzyme-Polymer Conjugates as Robust Pickering Interfacial Biocatalysts for Efficient Biotransformations and One-Pot Cascade Reactions. Angew. Chem. 2018, 57, 13810–13814. [Google Scholar] [CrossRef]
- Lima, R.N.; dos Anjos, C.S.; Orozco, E.V.M.; Porto, A.L.M. Versatility of Candida antarctica lipase in the amide bond formation applied in organic synthesis and biotechnological processes. Mol. Catal. 2019, 466, 75–105. [Google Scholar] [CrossRef]
- Zhang, S.M.; Chen, J.D. PMMA based foams made via surfactant-free high internal phase emulsion templates. Chem. Commun. 2009, 16, 2217–2219. [Google Scholar] [CrossRef]
- Bago Rodriguez, A.M.; Binks, B.P. High internal phase Pickering emulsions. Curr. Opin. Colloid Interface Sci. 2022, 57, 101556. [Google Scholar] [CrossRef]
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Cameron, N.R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef]
- Lyu, F.J.; Zhang, Y.F.; Zare, R.N.; Ge, J.; Liu, Z. One-Pot Synthesis of Protein-Embedded Metal-Organic Frameworks with Enhanced Biological Activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, Y.; Zhang, S.; Binks, B.P. Fabrication of Hierarchical Macroporous ZIF-8 Monoliths Using High Internal Phase Pickering Emulsion Templates. Langmuir ACS J. Surf. Colloids 2021, 37, 8435–8444. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, S.; Zhu, X.; Li, Y.; Liu, H.; Hu, J. In situ interfacial growth of zeolitic imidazolate framework (ZIF-8) nanoparticles induced by a graphene oxide Pickering emulsion. RSC Adv. 2015, 5, 31502–31505. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.D.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Xing, X.; Jia, J.Q.; Zhang, J.F.; Zhou, Z.W.; Li, J.; Wang, N.; Yu, X.Q. CALB Immobilized onto Magnetic Nanoparticles for Efficient Kinetic Resolution of Racemic Secondary Alcohols: Long-Term Stability and Reusability. Molecules 2019, 24, 490. [Google Scholar] [CrossRef]
- Chiaradia, V.; Soares, N.S.; Valerio, A.; de Oliveira, D.; Araujo, P.H.; Sayer, C. Immobilization of Candida antarctica Lipase B on Magnetic Poly(Urea-Urethane) Nanoparticles. Appl. Biochem. Biotechnol. 2016, 180, 558–575. [Google Scholar] [CrossRef]
- Kaptay, G. On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 387–401. [Google Scholar] [CrossRef]
- Horozov, T. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, M.; Zhang, S. Preparation of Macroporous Polymers from Microcapsule-Stabilized Pickering High Internal Phase Emulsions. Langmuir ACS J. Surf. Colloids 2019, 35, 9504–9512. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Lumsdon, S.O. Influence of Particle Wettability on the Type and Stability of Surfactant-Free Emulsions. Langmuir ACS J. Surf. Colloids 2000, 16, 8622–8631. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Z.; Nandakumar, K.; Xu, Z.; Masliyah, J.H. Effect of charged colloidal particles on adsorption of surfactants at oil-water interface. J. Colloid Interface Sci. 2004, 274, 625–630. [Google Scholar] [CrossRef]
- Ma, H.; Luo, M.; Dai, L.L. Influences of surfactant and nanoparticle assembly on effective interfacial tensions. Phys. Chem. Chem. Phys. 2008, 10, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Wang, L.; Zhou, L.; Huang, Z.; Ma, L.; He, Y.; Shi, L.; Gao, J. Virus-like organosilica nanoparticles for lipase immobilization: Characterization and biocatalytic applications. Biochem. Eng. J. 2019, 144, 125–134. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, S.M.; Zhu, Y.; Chu, Y.Q.; Chen, J.D. Hydrophilic polymer foams with well-defined open-cell structure prepared from pickering high internal phase emulsions. J. Polym. Sci. Pol. Chem. 2013, 51, 2181–2187. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhao, M.; Lin, Y.S. Stability of ZIF-8 in water under ambient conditions. Microporous Mesoporous Mat. 2019, 279, 201–210. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Z.; Lee, Y.; Wang, X.; Zhao, B.; Jung, Y.M. Quantitative evaluation of proteins with bicinchoninic acid (BCA): Resonance Raman and surface-enhanced resonance Raman scattering-based methods. Analyst 2012, 137, 5834–5838. [Google Scholar] [CrossRef]
- Cortes-Rios, J.; Zarate, A.M.; Figueroa, J.D.; Medina, J.; Fuentes-Lemus, E.; Rodriguez-Fernandez, M.; Aliaga, M.; Lopez-Alarcon, C. Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Anal. Biochem. 2020, 608, 113904. [Google Scholar] [CrossRef]
- Kowalczykiewicz, D.; Szymańska, K.; Gillner, D.; Jarzębski, A.B. Rotating bed reactor packed with heterofunctional structured silica-supported lipase. Developing an effective system for the organic solvent and aqueous phase reactions. Microporous Mesoporous Mat. 2021, 312. [Google Scholar] [CrossRef]





| Sample | Enzyme Loading Capacity (μg∙mg−1) | Immobilization Rate (%) |
|---|---|---|
| ZCP-10 | 31.6 | 31.6 |
| ZCP-20 | 60.5 | 30.3 |
| ZCP-40 | 97.4 | 24.4 |
| ZCP-80 | 98.9 | 12.4 |
| Sample | fi (vol%) 1 | ZCP Concentration (wt%) 2 | ZCP | The Mean Droplet Diameter (μm) |
|---|---|---|---|---|
| H-4-ZCP-10 | 75 | 4 | ZCP-10 | 55 ± 15 |
| H-4-ZCP-20 | 75 | 4 | ZCP-20 | 76 ± 25 |
| H-4-ZCP-40 | 75 | 4 | ZCP-40 | 113 ± 46 |
| H-4-ZCP-80 | 75 | 4 | ZCP-80 | 117 ± 50 |
| H-2-ZCP-20 | 75 | 2 | ZCP-20 | 110 ± 39 |
| H-8-ZCP-20 | 75 | 8 | ZCP-20 | 38 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Sun, Y.; Sun, Y.; Cai, R.; Zhang, S. High Internal Phase Pickering Emulsion Stabilized by Lipase-Coated ZIF-8 Nanoparticles towards Recyclable Biphasic Biocatalyst. Catalysts 2023, 13, 383. https://doi.org/10.3390/catal13020383
Xu C, Sun Y, Sun Y, Cai R, Zhang S. High Internal Phase Pickering Emulsion Stabilized by Lipase-Coated ZIF-8 Nanoparticles towards Recyclable Biphasic Biocatalyst. Catalysts. 2023; 13(2):383. https://doi.org/10.3390/catal13020383
Chicago/Turabian StyleXu, Chuanbang, Yan Sun, Yuanyuan Sun, Ruiyun Cai, and Shengmiao Zhang. 2023. "High Internal Phase Pickering Emulsion Stabilized by Lipase-Coated ZIF-8 Nanoparticles towards Recyclable Biphasic Biocatalyst" Catalysts 13, no. 2: 383. https://doi.org/10.3390/catal13020383