Influence of FeP and Al(H2PO4)3 Nanocatalysts on the Thermolysis of Heavy Oil in N2 Medium
Abstract
:1. Introduction
2. Results and Discussions
3. Experimental Section
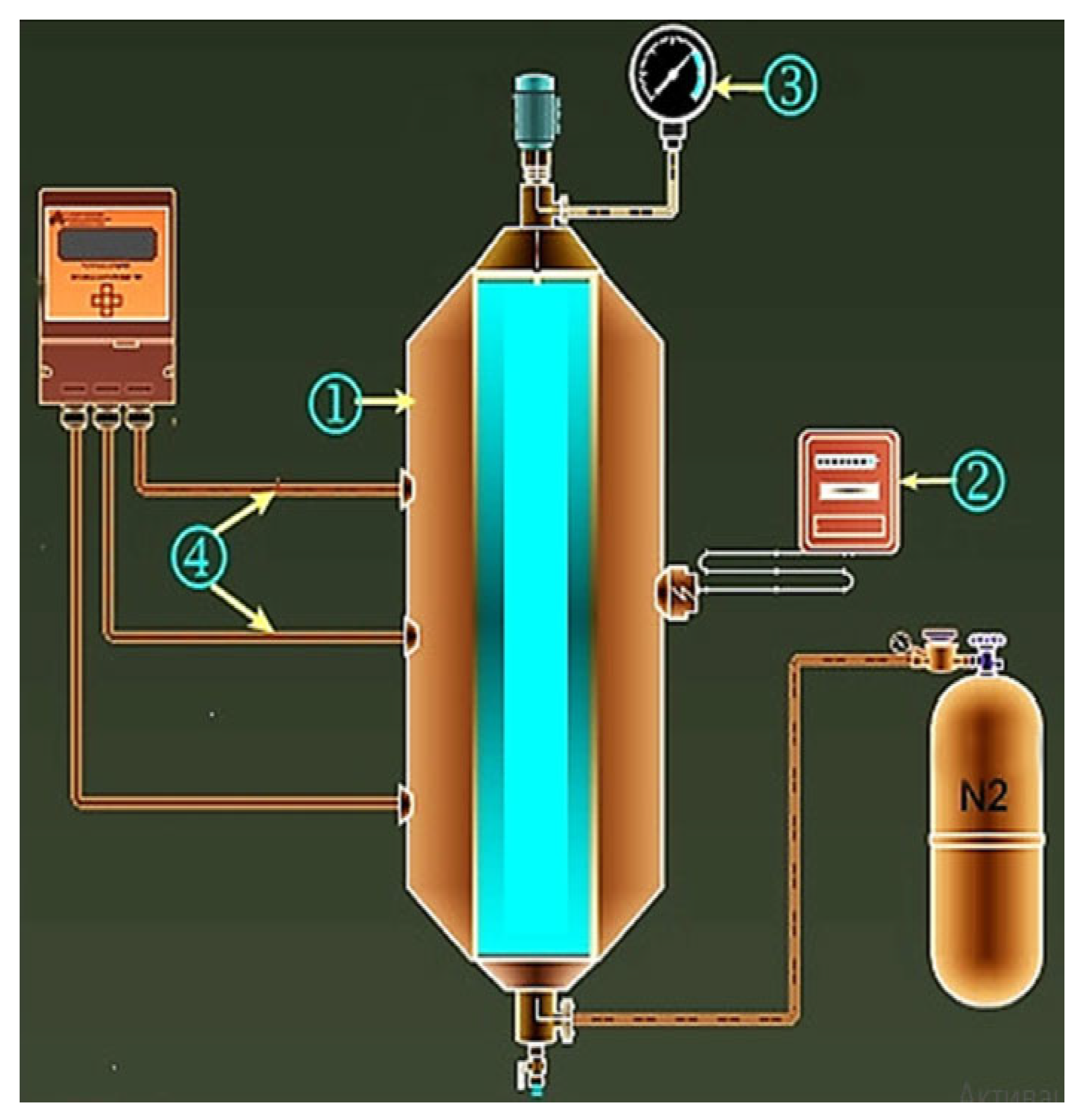
3.1. Experiments in Autoclave
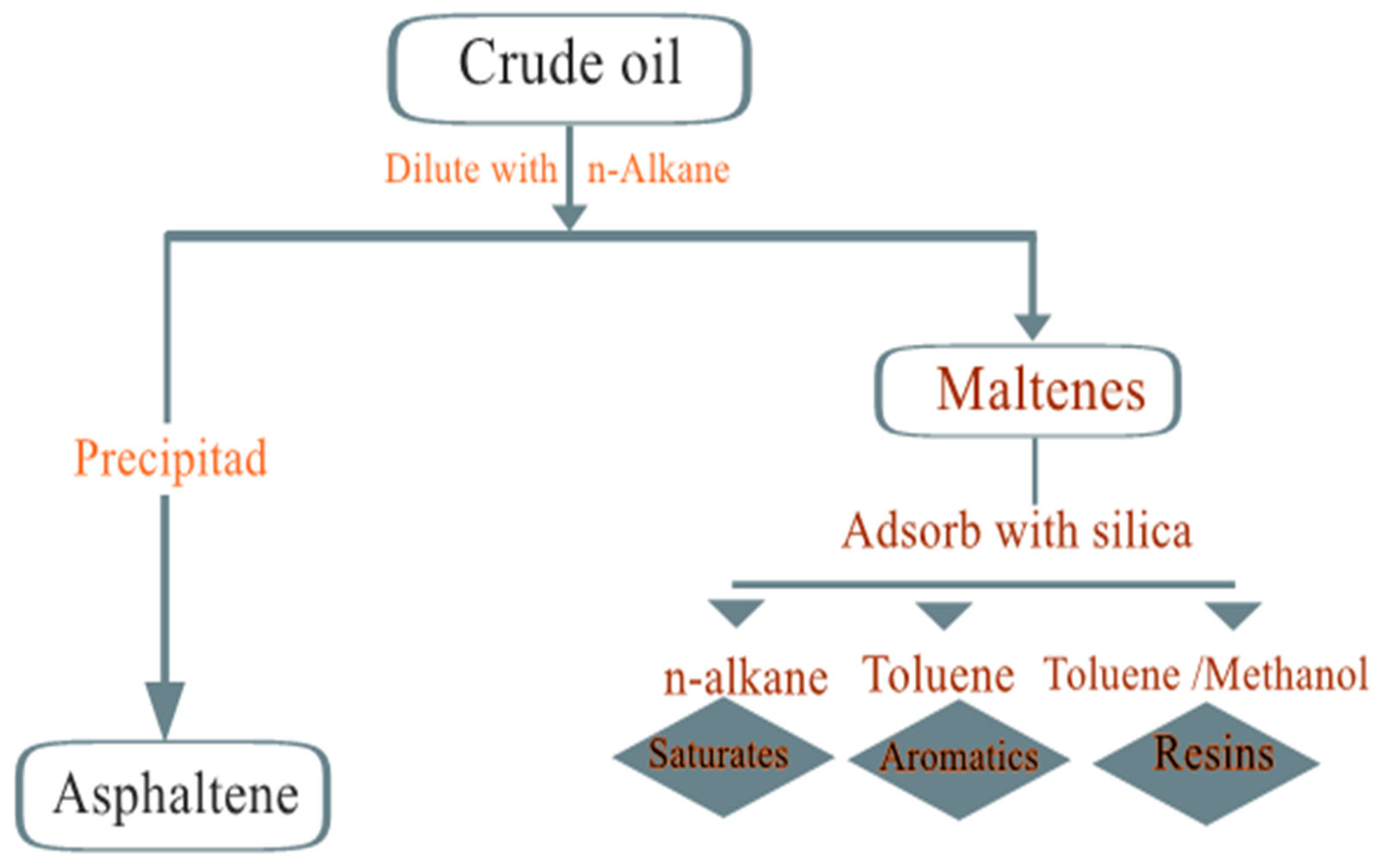
3.2. SARA-Analysis
3.3. Total Sulfur Analysis
3.4. Viscosity Measurements
3.5. Atmospheric Distillation of Crude Oil
3.6. Chromatography-Mass Spectroscopy
3.7. Optical Density Photocolorimeter
4. Conclusions
- The viscosity of the heavy oil sample was reduced by 42%.
- The content of asphaltenes was reduced by 59.6%.
- The yield of light fractions increased.
- The boiling point was altered from 170 °C to 75 °C.
- The total content of resins and asphaltenes was reduced from 34%wt. to 14.7%wt.
- The total content of sulfur decreased from 4.72%wt. to 4.09%wt.
- The amount of isoalkanes after catalytic thermolysis increased from 22.26 wt.% to 36.31 wt.%.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.Q. Study on Catalytic Aquathermolysis of Heavy Oil at Relatively Low Temperature; China University of Geosciences: Wuhan, China, 2010. [Google Scholar]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Tomczyk, N.A.; Winans, R.E.; Shinn, J.H.; Robinson, R.C. On the Nature and Origin of Acidic Species in Petroleum. 1. Detailed Acid Type Distribution in a California Crude Oil. Energy Fuels 2001, 15, 1498–1504. [Google Scholar]
- Li, G.; Chen, Y.; An, Y.; Chen, Y. Catalytic aquathermolysis of super-heavy oil: Cleavage of C-S bonds and separation of light organosulfurs. Fuel Process. Technol. 2016, 153, 94–100. [Google Scholar] [CrossRef]
- Rana, M.; Sámano, V.; Ancheyta, J.; Diaz, J.A.I. A review of recent advances on process technologies for upgrading of heavy oils and residual. Fuel 2007, 86, 1216–1231. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Extra-heavy oil aquathermolysis using nickel-based catalyst: Some aspects of in-situ transformation of catalyst precursor. Catalysts 2021, 11, 189. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Fan, H.F.H.F.; Fan, Y.J.; Liu, X.F.; Zhao, L.G. Studies on effect of metal ions on aquathermolysis reaction ofLiaohe heavy oils under steam treatment. J. Fuel Chem. Technol. 2001, 29, 430–433. [Google Scholar]
- Chao, K.; Chen, Y.; Li, J.; Zhang, X.; Dong, B. Upgrading and visbreaking of superheavy oil by catalytic aquathermolysis with aromatic sulfonic copper. Fuel Process. Technol. 2012, 104, 174–180. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Sh S, I.S.; Ismael, M.; Aliev, F.A.; Davletshin, R.R.; Vakhin, A.V. Influence of Naphthenic Hydrocarbons and Polar Solvents on the Composition and Structure of Heavy-Oil Aquathermolysis Products. Ind. Eng. Chem. Res. 2021, 60, 13191–13203. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Li, X.; Zhao, M.; Zhang, Z.; Su, C. Preparation of silica supported nanoscale zero valence iron and its feasibility in viscosity reduction of heavy oil. Micro Nano Lett. 2014, 9, 355–358. [Google Scholar] [CrossRef]
- Wu, C.; Su, J.; Zhang, R.; Lei, G.; Cao, Y. The use of amphiphilic nickel chelate for catalytic aquathermolysis of extra-heavy oil under steam injection conditions. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 36, 1437–1444. [Google Scholar] [CrossRef]
- Aliev, F.A.; Akhunov, A.; Mirzaev, O.; Vakhin, A.V. Development of New Amphiphilic Catalytic Steam Additives for Hydrothermal Enhanced Oil Recovery Techniques. Catalysts 2022, 12, 921. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, Y.; Liu, Y.; Hu, S. A Study on Catalytic Aquathermolysis of Heavy Crude Oil during Steam Stimulation. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar]
- Clark, P.D.; Hyne, J.B. Steam–oil chemical reactions: Mechanisms for the aquathermolysis of heavy oil. AOSTRA J. Res. 1984, 1, 15–20. [Google Scholar]
- Hyne, J.B. A Synopsis of Work on the Chemical Reactions between Water and Heavy Oil Sands during Stimulated Steam Stimulation; AOSTRA Publication Series; AOSTRA: Calgary, Alberta, 1986. [Google Scholar]
- Li, Y.R.; Li, Q.Y.; Wang, X.D.; Yu, L.G.; Yang, J.J. Aquathermolysis of Heavy crude oil with ferric oleate catalyst. Pet. Sci. 2018, 15, 613–624. [Google Scholar] [CrossRef]
- Elayaraja, M.; Parashu, R.K.; Gavin, L.; Stephanie L, B. Control of Phase in Phosphide Nanoparticles Produced by Metal Nanoparticle Transformation: Fe2P and FeP. ACS Nano 2009, 3, 2383–2393. [Google Scholar] [CrossRef]
- Fei, X.M.; Cheng, Y.X.; Fucong, L.; Shu, C.S.; Yang, Y.L.; Jian, L.; Liang, Z. Construction of FeP Hollow Nanoparticles Densely Encapsulated in Carbon Nanosheet Frameworks for Efficient and Durable Electrocatalytic Hydrogen Production. Adv. Sci. 2019, 6, 1801490. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Musin, R.Z.; Nasyrova, Z.R.; Aliev, F.A.; Vakhin, A.V. Hydrothermal Impact on Hydrocarbon Generation from Low-Permeable Domanic Sedimentary Rocks with Different Lithofacies. Energy Fuels 2021, 35, 11223–11238. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Wang, Y.; Li, P. GC-MS used in study on the mechanism of the viscosity reduction of heavy oil through aquathermolysis catalyzed by aromatic sulfonic H3PMo12O40. Energy 2010, 35, 3454–3460. [Google Scholar] [CrossRef]
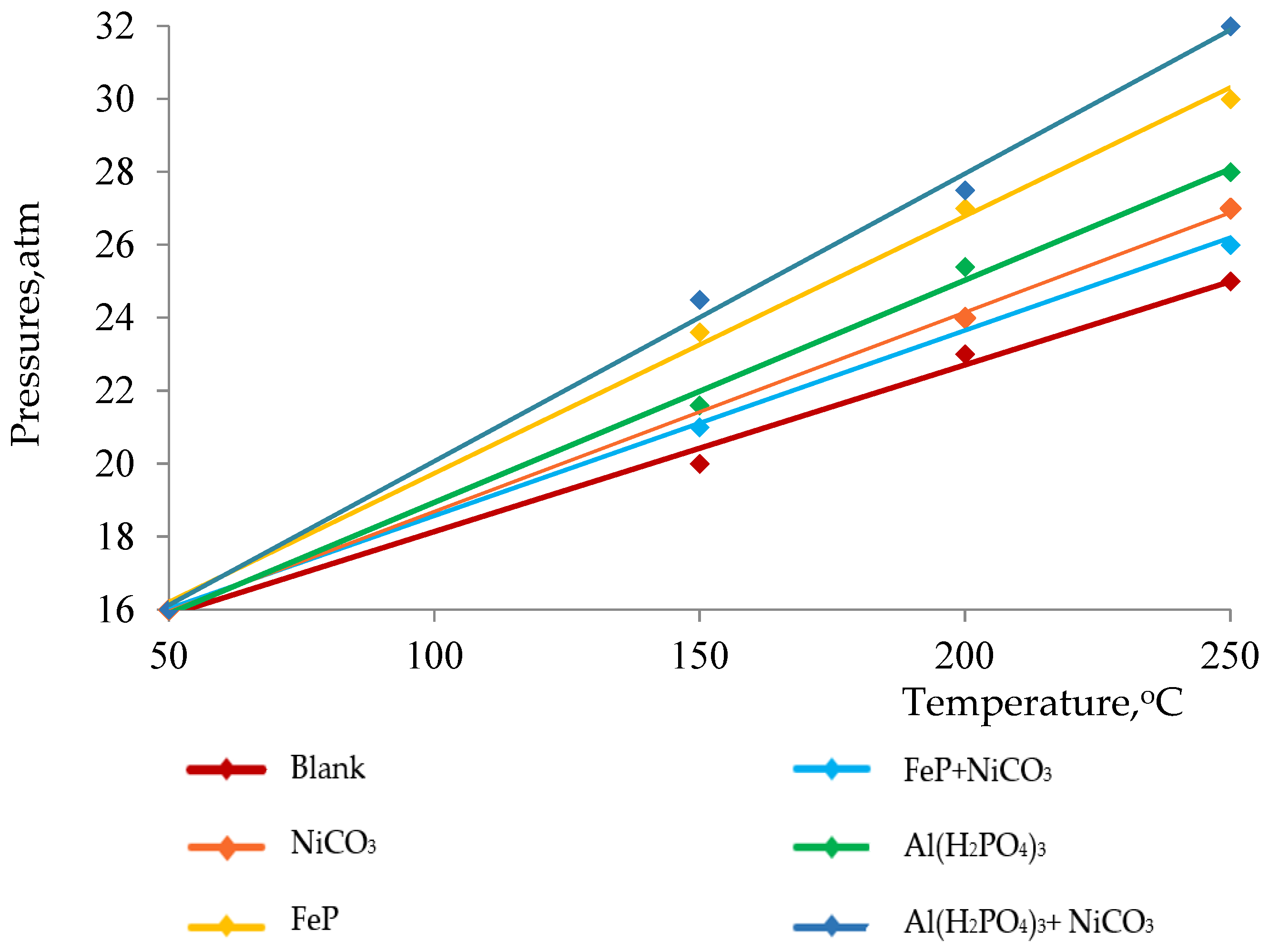

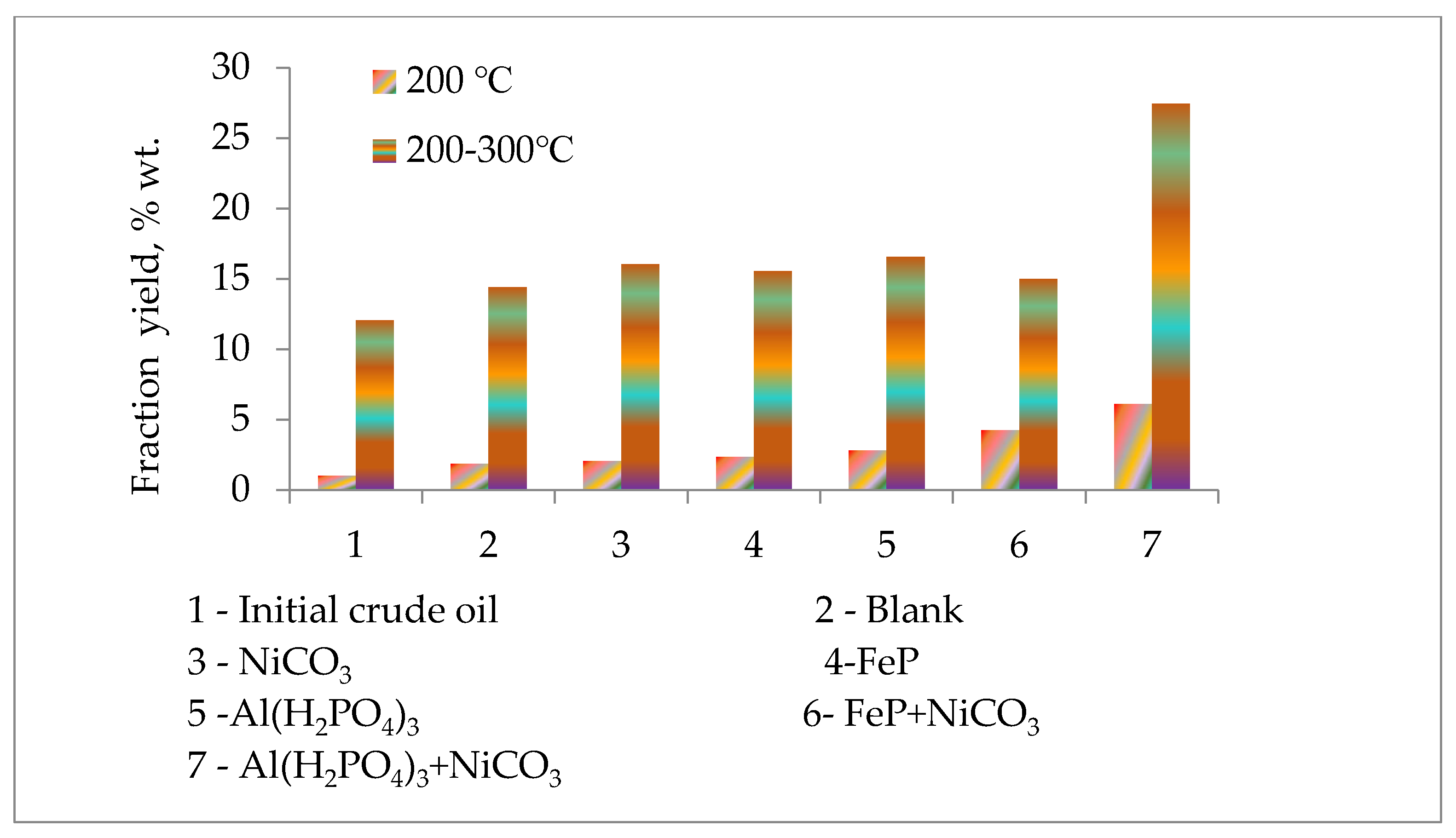
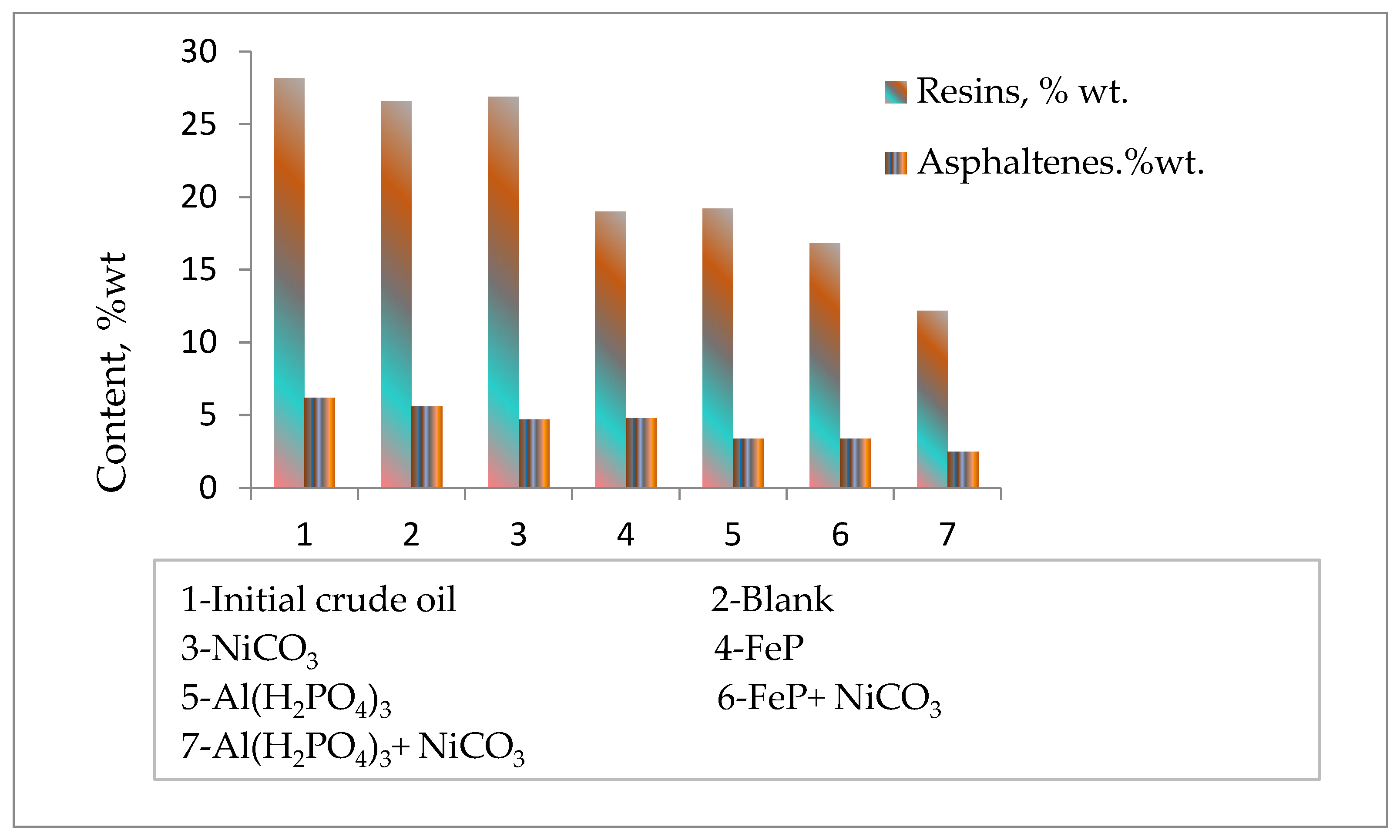
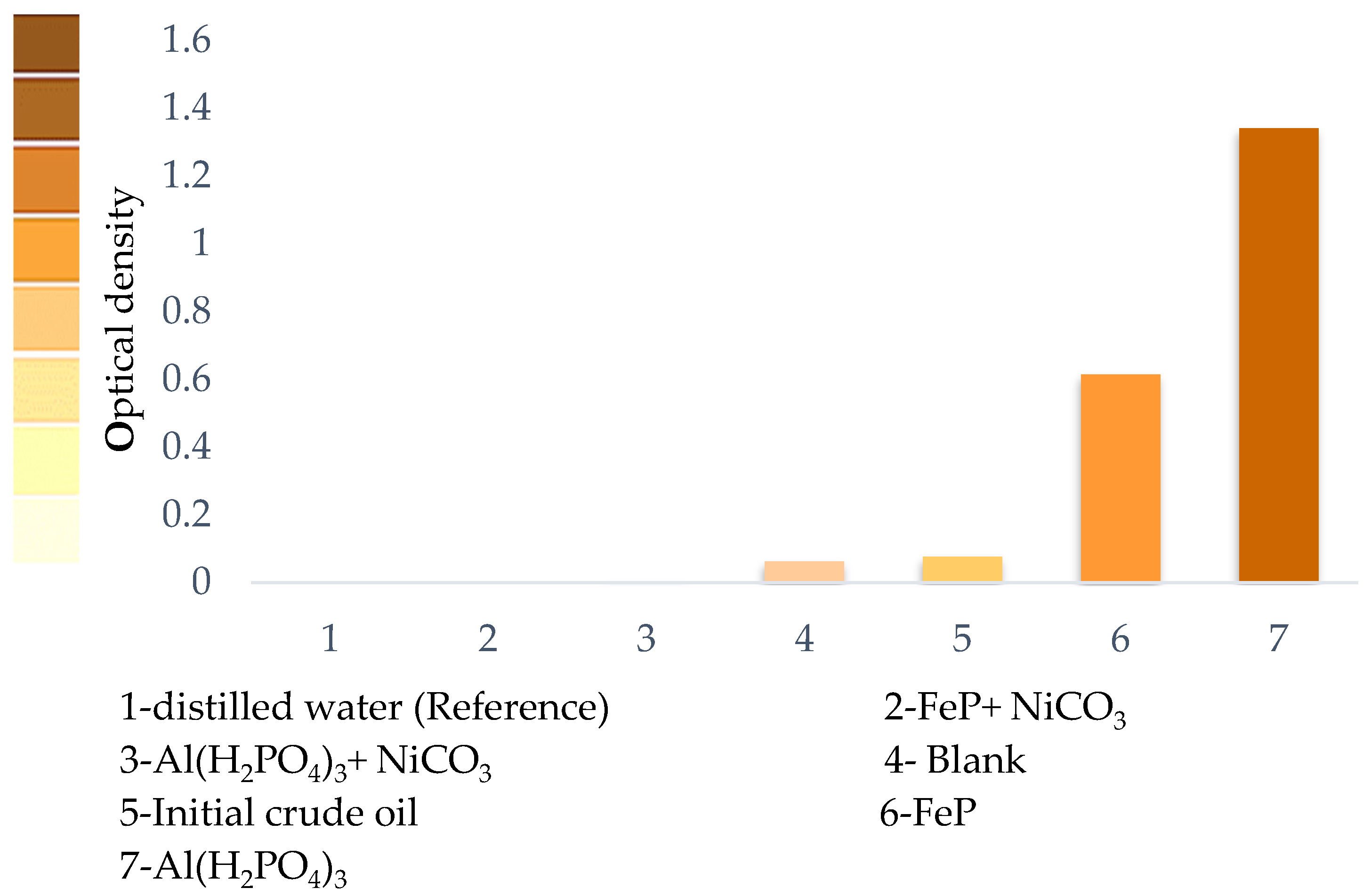

| Fraction Yield, %wt. | SARA Analysis, %wt. | ||||||
|---|---|---|---|---|---|---|---|
| Samples | Boiling Points, °C | 200 °C | 200–300 °C | 300 °C | Resins | Asphaltene | Sulfur %wt. |
| Initial oil | 170 | 1.02 | 12.07 | 13.09 | 28.2 | 6.2 | 4.72 |
| Blank | 140 | 1.85 | 14.40 | 16.25 | 26.6 | 5.6 | 4.27 |
| NiCO3 | 140 | 2.06 | 16.05 | 18.11 | 26.9 | 4.7 | 4.56 |
| FeP | 150 | 2.35 | 15.55 | 17.90 | 19.0 | 4.8 | 4.31 |
| Al(H2PO4)3 | 98 | 2.80 | 16.55 | 19.35 | 19.2 | 3.4 | 4.25 |
| FeP + NiCO3 | 75 | 4.25 | 15.00 | 19.25 | 16.8 | 3.4 | 4.26 |
| Al(H2PO4)3 + NiCO3 | 75 | 6.11 | 27.45 | 33.55 | 12.2 | 2.5 | 4.09 |
| Components | Initial Crude Oil | Crude Oil after Thermolysis | |
|---|---|---|---|
| Blank | Al(H2PO4)3 + NiCO3 | ||
| Alkenes | 8.01 | 1.94 | 1.30 |
| Alkanes | 30.39 | 35.31 | 38.63 |
| n-alkanes | 8.13 | 6.52 | 2.32 |
| isoalkanes | 22.26 | 28.79 | 36.31 |
| Naphthenes | 54.89 | 57.04 | 58.76 |
| Arenas | 2.39 | 4.83 | 0.79 |
| Others | 4.32 | 0.88 | 0.52 |
| Total | 100.0 | 100.0 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsalam, Y.I.I.; Khamidullin, R.F.; Katnov, V.E.; Dengaev, A.V.; Aliev, F.A.; Vakhin, A.V. Influence of FeP and Al(H2PO4)3 Nanocatalysts on the Thermolysis of Heavy Oil in N2 Medium. Catalysts 2023, 13, 390. https://doi.org/10.3390/catal13020390
Abdelsalam YII, Khamidullin RF, Katnov VE, Dengaev AV, Aliev FA, Vakhin AV. Influence of FeP and Al(H2PO4)3 Nanocatalysts on the Thermolysis of Heavy Oil in N2 Medium. Catalysts. 2023; 13(2):390. https://doi.org/10.3390/catal13020390
Chicago/Turabian StyleAbdelsalam, Yasser I. I., Renat F. Khamidullin, Vladimir E. Katnov, Aleksey V. Dengaev, Firdavs A. Aliev, and Alexey V. Vakhin. 2023. "Influence of FeP and Al(H2PO4)3 Nanocatalysts on the Thermolysis of Heavy Oil in N2 Medium" Catalysts 13, no. 2: 390. https://doi.org/10.3390/catal13020390
APA StyleAbdelsalam, Y. I. I., Khamidullin, R. F., Katnov, V. E., Dengaev, A. V., Aliev, F. A., & Vakhin, A. V. (2023). Influence of FeP and Al(H2PO4)3 Nanocatalysts on the Thermolysis of Heavy Oil in N2 Medium. Catalysts, 13(2), 390. https://doi.org/10.3390/catal13020390








