Bioinspired Green Synthesis of Bimetallic Iron and Zinc Oxide Nanoparticles Using Mushroom Extract and Use against Aspergillus niger; The Most Devastating Fungi of the Green World
Abstract
:1. Introduction
2. Results
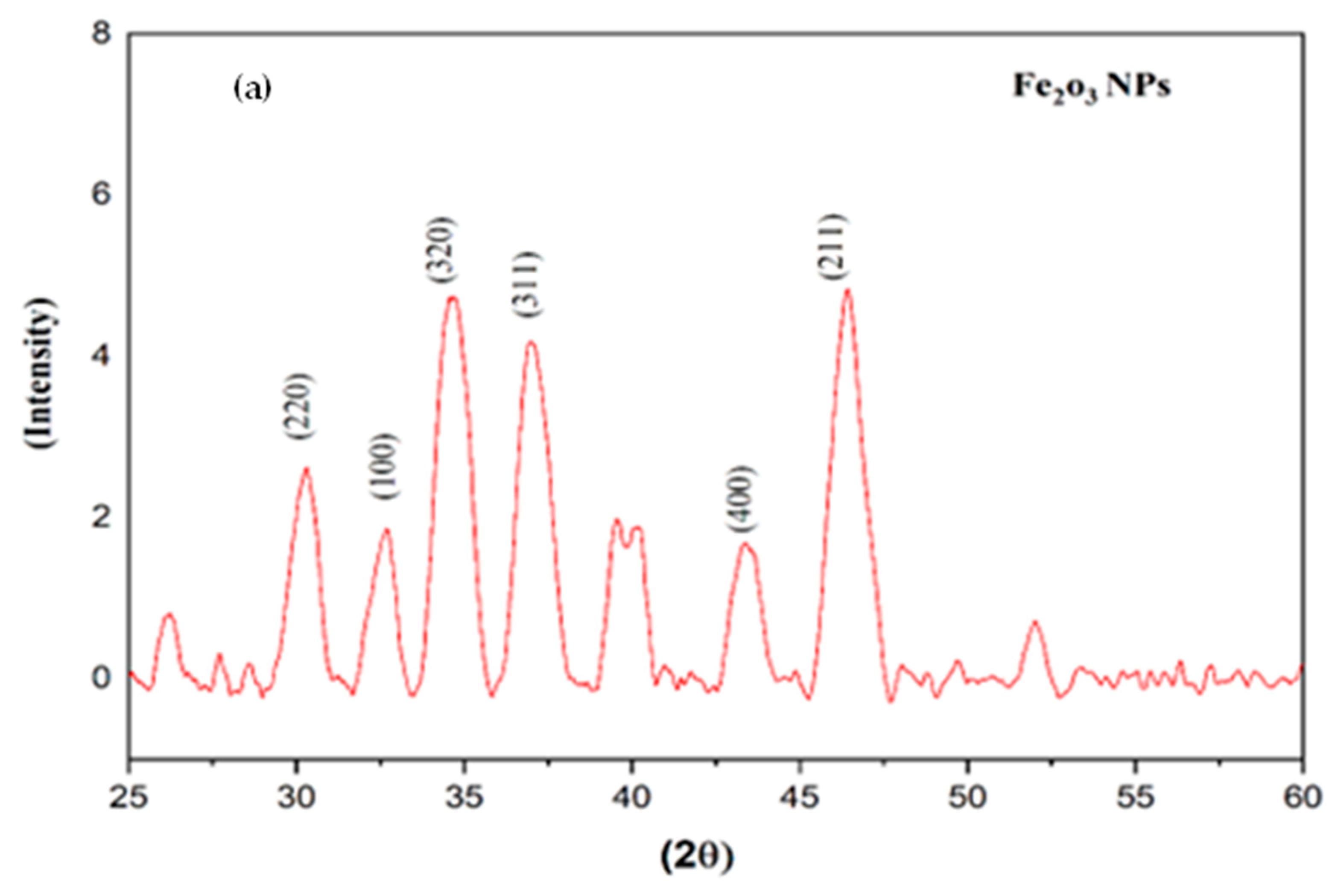
2.1. X-ray Diffraction of Iron and Zinc NPs
2.2. FITR Analysis of Iron and Zinc Nanoparticles
2.3. SEM Analysis of Iron and Zinc NPs
2.4. EDX Analysis of Iron and Zinc NPs
2.5. Ultraviolet-Visible Spectrum of Iron and Zinc NPs
2.6. Antifungal Activity of Iron and Zinc oxide NPs
3. Discussion
4. Materials and Methods
4.1. Collection of Mushroom Material
4.2. Preparation of Zinc and Iron Myconanoparticles
4.3. Characterization of Iron and Zinc NPs
4.4. In Vitro Antifungal Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afsah-Hejri, L.; Hajeb, P.; Ara, P.; Ehsani, R.J. A comprehensive review on food applications of terahertz spectroscopy and imaging. CRFSFS 2019, 18, 1563–1621. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Stanczyk, F.Z.; Chang, L.; Miles, R.A.; Lobo, R.A. The ratio of androstenedione: 11β-hydroxyandrostenedione is an important marker of adrenal androgen excess in women. Int. J. Fertil Steril. 1992, 58, 148–152. [Google Scholar] [CrossRef]
- Feynman, R.P. An invitation to enter a new field of physics. Int. J. Eng. Sci. 1960, 23, 8. [Google Scholar]
- Fischer, M.W.; Money, N.P. Why mushrooms form gills: Efficiency of the lamellate morphology. Fungal. Genet. Biol. 2010, 114, 57–63. [Google Scholar] [CrossRef]
- Gianola, D.; de los Campos, G.; Toro, M.A.; Naya, H.; Schön, C.-C.; Sorensen, D.J.G. Do molecular markers inform about pleiotropy? J. Genet. 2015, 201, 23–29. [Google Scholar] [CrossRef]
- Imran Din, M.; Rani, A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: A green adeptness. Int. J. Anal. Chem. 2016, 28, 33–48. [Google Scholar] [CrossRef]
- Kamal, A.; Saba, M.; Farooq, M. Biocompatible formulations based on mycosynthesized iron oxide nanoparticles: Fabrication, characterization, and biological investigation. J. Basic Microbiol. 2022, 11, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Ganapathy, M.; Sharmila, S.; Shankar, M.; Vimalan, M.; Potheher, I.V. ZnO/Ni(OH)2 core-shell nanoparticles: Synthesis, optical, electrical and photoacoustic property analysis. J. Alloys Compd. 2017, 12, 33–55. [Google Scholar]
- Bahadoran, A.; Jabarabadi, M.K.; Mahmood, Z.H.; Bokov, D.; Janani, B.J.; Fakhri, A. Quick and sensitive colorimetric detection of amino acid with functionalized-silver/copper nanoparticles in the presence of cross linker, and bacteria detection by using DNA-template nanoparticles as peroxidase activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120636. [Google Scholar] [CrossRef]
- Lai, Y.; Fakhri, A.; Janani, B.J. Synergistic activities of silver indium sulfide/nickel molybdenum sulfide nanostructures anchored on clay mineral for light-driven bactericidal performance, and detection of uric acid from gout patient serum. J. Photochem. Photobiol. B Biol. 2022, 234, 112526. [Google Scholar] [CrossRef]
- Marchiol, L. Synthesis of metal nanoparticles in living plants. Ital. J. Agron. 2012, 7, e37. [Google Scholar] [CrossRef]
- Mueller, G.M.; Schmit, J.P. Fungal biodiversity: What do we know? What can we predict. Anim. Biodivers. Conserv. 2017, 16, 1–5. [Google Scholar] [CrossRef]
- Ogwal Engola, A.P. Ecology and nutraceutical value of edible indigenous mushrooms. A case study of Kyebe Subcounty in Rakai District, Uganda. M.R.T. 2018, 15, 184–245. [Google Scholar]
- Hu, B.; Cui, Y.; Yang, X.; Xu, X.; Janani, B.J.; Fakhri, A. Fabrication of novel rational Ti-Sn doped Cu-ferrite nanoparticles for robust photocatalysis reaction, magnetic resonance imaging, and chemo-magneto-photo-thermal therapy. Surf. Interfaces 2022, 33, 102226. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. STAM 2008, 9, 035004. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 18, 71–80. [Google Scholar] [CrossRef]
- Rajabi, H.; Stamm, K.; Appel, E.; Gorb, S. Micro-morphological adaptations of the wing nodus to flight behaviour in four dragonfly species from the family Libellulidae (Odonata: Anisoptera). Arthropod Struct. Dev. 2018, 47, 442–448. [Google Scholar] [CrossRef]
- Sharmila, G.; Fathima, M.F.; Haries, S.; Geetha, S.; Kumar, N.M.; Muthukumaran, C. Green synthesis, characterization and antibacterial efficacy of palladium nanoparticles synthesized using Filicium decipiens leaf extract. J. Mol. Struct. 2017, 1138, 35–40. [Google Scholar] [CrossRef]
- Souto, E.B.; Dias-Ferreira, J.; Craveiro, S.A.; Severino, P.; Sanchez-Lopez, E.; Garcia, M.L.; Mahant, S. Therapeutic interventions for countering leishmaniasis and Chagas’s disease: From traditional sources to nanotechnological systems. J. Pathog. 2019, 8, 119. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Maaza, M. Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev. 2017, 10, 186–201. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2021, 166, 207–215. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [PubMed]
- Kamal, A.; Haroon, U.; Manghwar, H.; Alamer, K.H.; Alsudays, I.M.; Althobaiti, A.T.; Iqbal, A.; Akbar, M.; Farhana; Anar, M.; et al. Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using Zea mays L. Molecules 2022, 27, 5333. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.S.; Munis, M.F.H.; Alsudays, I.M.; Alamer, K.H.; Haroon, U.; Kamal, A.; Ali, M.; Ahmed, J.; Ahmad, Z.; Attia, H. First Report of Fruit Rot of Cherry and Its Control Using Fe2O3 Nanoparticles Synthesized in Calotropis procera. Molecules 2022, 27, 4461. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Munis, M.F.H.; Zaman, W.; Ullah, F.; Shah, S.N.; Ayaz, A.; Farooq, M.; Bahadur, S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc. Res. Tech. 2019, 82, 415–420. [Google Scholar] [CrossRef]
- Ali, M.; Wang, X.; Haroon, U.; Chaudhary, H.J.; Kamal, A.; Ali, Q.; Saleem, M.H.; Usman, K.; Alatawi, A.; Ali, S.; et al. Antifungal activity of Zinc nitrate derived nano Zno fungicide synthesized from Trachyspermum ammi to control fruit rot disease of grapefruit. Ecotoxicol. Environ. Saf. 2022, 233, 113311. [Google Scholar] [CrossRef]
- Kamal, A.; Saba, M.; Ullah, K.; Almutairi, S.M.; AlMunqedhi, B.M.; Ragab abdelGawwad, M. Mycosynthesis, Characterization of Zinc Oxide Nanoparticles, and Its Assessment in Various Biological Activities. Crystals 2023, 13, 171. [Google Scholar] [CrossRef]
- Sandhya, J.; Kalaiselvam, S. Biogenic synthesis of magnetic iron oxide nanoparticles using inedible borassus flabellifer seed coat: Characterization, antimicrobial, antioxidant activity and in vitro cytotoxicity analysis. Mater. Res. Express 2020, 7, 015045. [Google Scholar] [CrossRef]
- Umar, L.; Hamzah, Y.; Setiadi, R.N. Biosensor signal improvement using current mirror topology for dissolved oxygen measurement. Meas. Sci. Technol. 2019, 30, 065102. [Google Scholar] [CrossRef]
- Vanathi, P.; Rajiv, P.; Narendhran, S.; Rajeshwari, S.; Rahman, P.K.; Venckatesh, R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater. Lett. 2014, 134, 13–15. [Google Scholar] [CrossRef]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic synthesis of extracellular zinc oxide nanoparticles from Xylaria acuta and its nanoantibiotic potential. Int. J. Nanomed. 2020, 15, 8519–8536. [Google Scholar] [CrossRef]
- Oussou-Azo, A.F.; Nakama, T.; Nakamura, M.; Futagami, T.; Vestergaard, M.C.M. Antifungal potential of nanostructured crystalline copper and its oxide forms. Nanomater 2020, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.S.; Joshi, S.R. Ultrastructures of silver nanoparticles biosynthesized using endophytic fungi. J. Microsc. Ultrastruct. 2015, 3, 29–37. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Katata-Seru, L.; Moremedi, T.; Aremu, O.S.; Bahadur, I. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq. 2018, 256, 296–304. [Google Scholar] [CrossRef]
- Anthony, K.J.P.; Murugan, M.; Jeyaraj, M.; Rathinam, N.K.; Sangiliyandi, G. Synthesis of silver nanoparticles using pine mushroom extract: A potential antimicrobial agent against E. coli and B. subtilis. J. Ind. Eng. Chem. 2014, 20, 2325–2331. [Google Scholar] [CrossRef]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Chi, N.T.L.; Duc, P.A.; Pugazhendhi, A. A review on prospective production of biofuel from microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef]







| NPs | Source | Antifungal Potential | Reference |
|---|---|---|---|
| MB-ZnO | Plants | 60% | Zubair, M.S et al., 2022 [24] |
| Fe2O3 | Plants | 65% | Saqib’s et al., 2019 [25] |
| IONPs | Metal | 62% | Ali, M et al., 2020 [26] |
| ZnO | plant | 60% | Kamal, A et al., 2022 [27] |
| ZnONPs | Mushroom | 70% | Sandhya, J et al., 2020 [28] |
| IONPs | Fungi | 56% | Umar, L et al., 2021 [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, A.; Saba, M.; Kamal, A.; Batool, M.; Asif, M.; Al-Mohaimeed, A.M.; Al Farraj, D.A.; Habib, D.; Ahmad, S. Bioinspired Green Synthesis of Bimetallic Iron and Zinc Oxide Nanoparticles Using Mushroom Extract and Use against Aspergillus niger; The Most Devastating Fungi of the Green World. Catalysts 2023, 13, 400. https://doi.org/10.3390/catal13020400
Kamal A, Saba M, Kamal A, Batool M, Asif M, Al-Mohaimeed AM, Al Farraj DA, Habib D, Ahmad S. Bioinspired Green Synthesis of Bimetallic Iron and Zinc Oxide Nanoparticles Using Mushroom Extract and Use against Aspergillus niger; The Most Devastating Fungi of the Green World. Catalysts. 2023; 13(2):400. https://doi.org/10.3390/catal13020400
Chicago/Turabian StyleKamal, Asif, Malka Saba, Asif Kamal, Momal Batool, Muhammad Asif, Amal M. Al-Mohaimeed, Dunia A. Al Farraj, Darima Habib, and Shabir Ahmad. 2023. "Bioinspired Green Synthesis of Bimetallic Iron and Zinc Oxide Nanoparticles Using Mushroom Extract and Use against Aspergillus niger; The Most Devastating Fungi of the Green World" Catalysts 13, no. 2: 400. https://doi.org/10.3390/catal13020400







