Photocatalytic Decomposition of Azo Dyes and Phenols Using Polymer Composites Containing Nanostructured Poly(Titanium Oxide) Doped with Gold or Silver Nanoparticles under Light Irradiation in a Wide Wavelength Range
Abstract
:1. Introduction
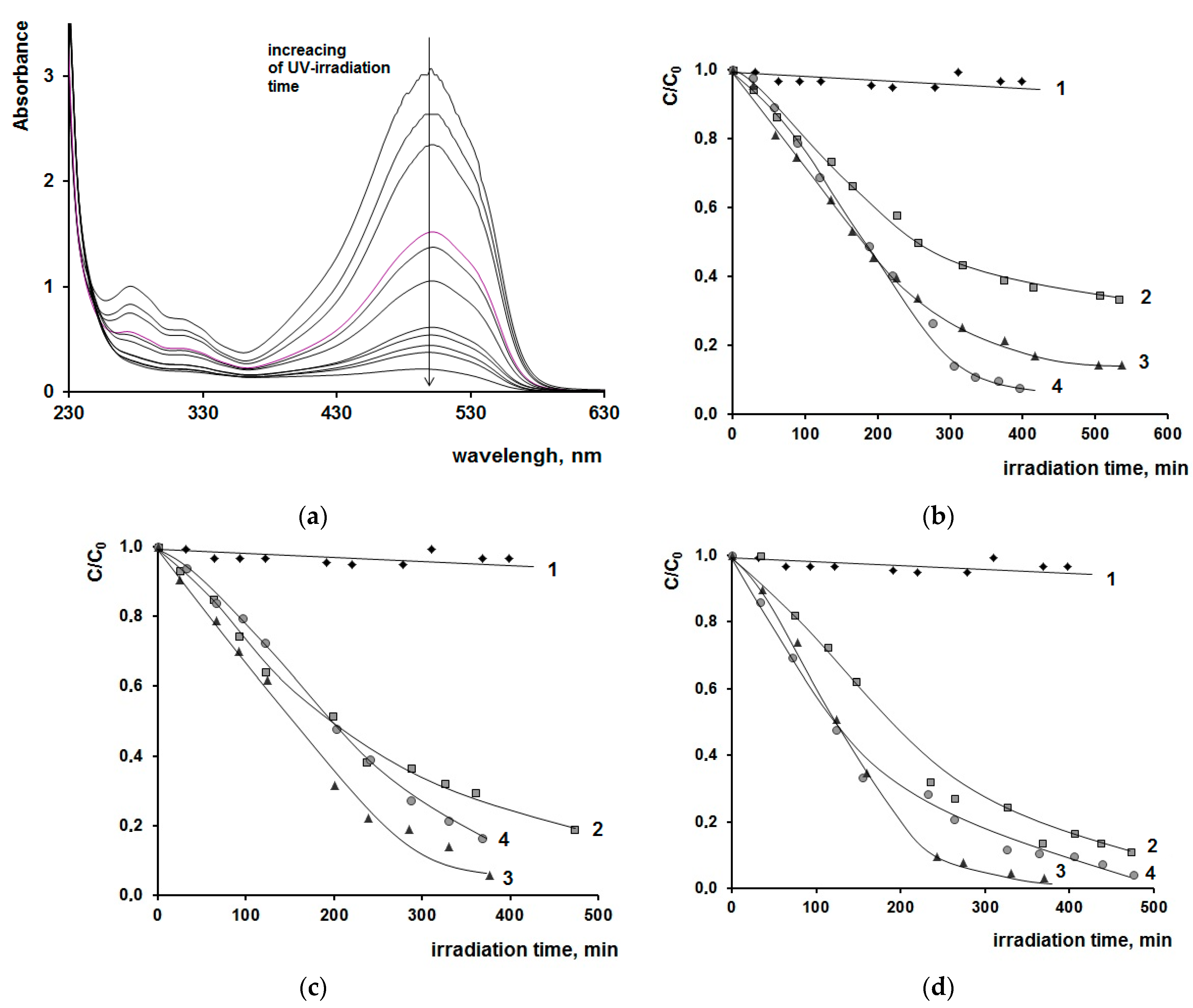
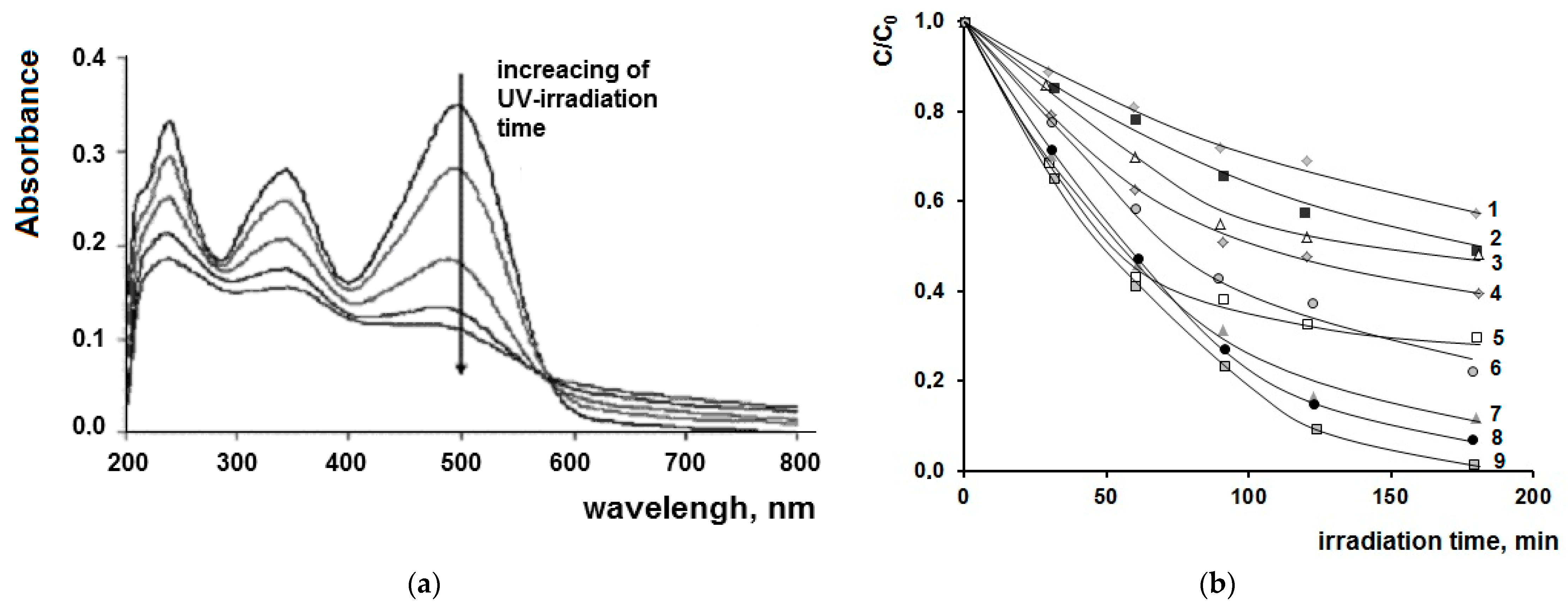
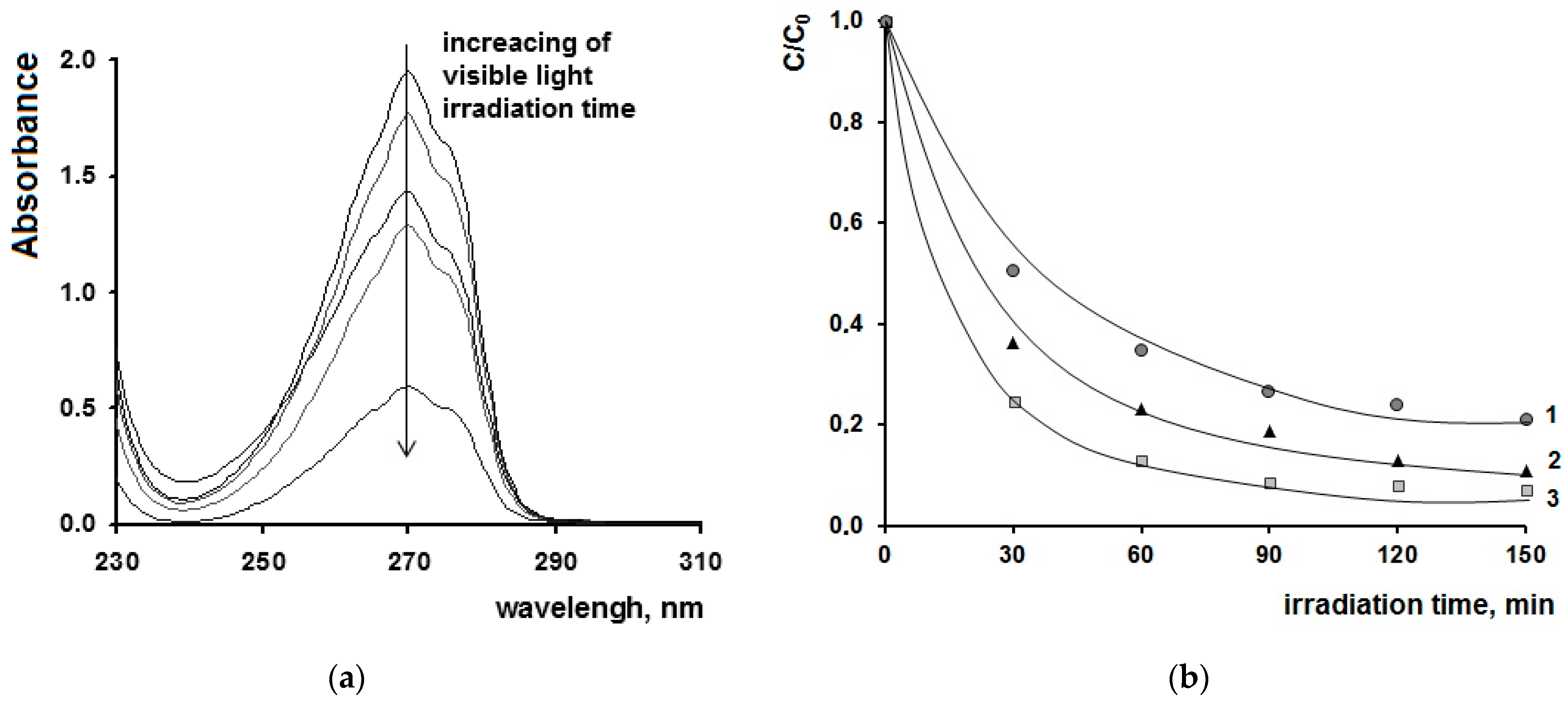
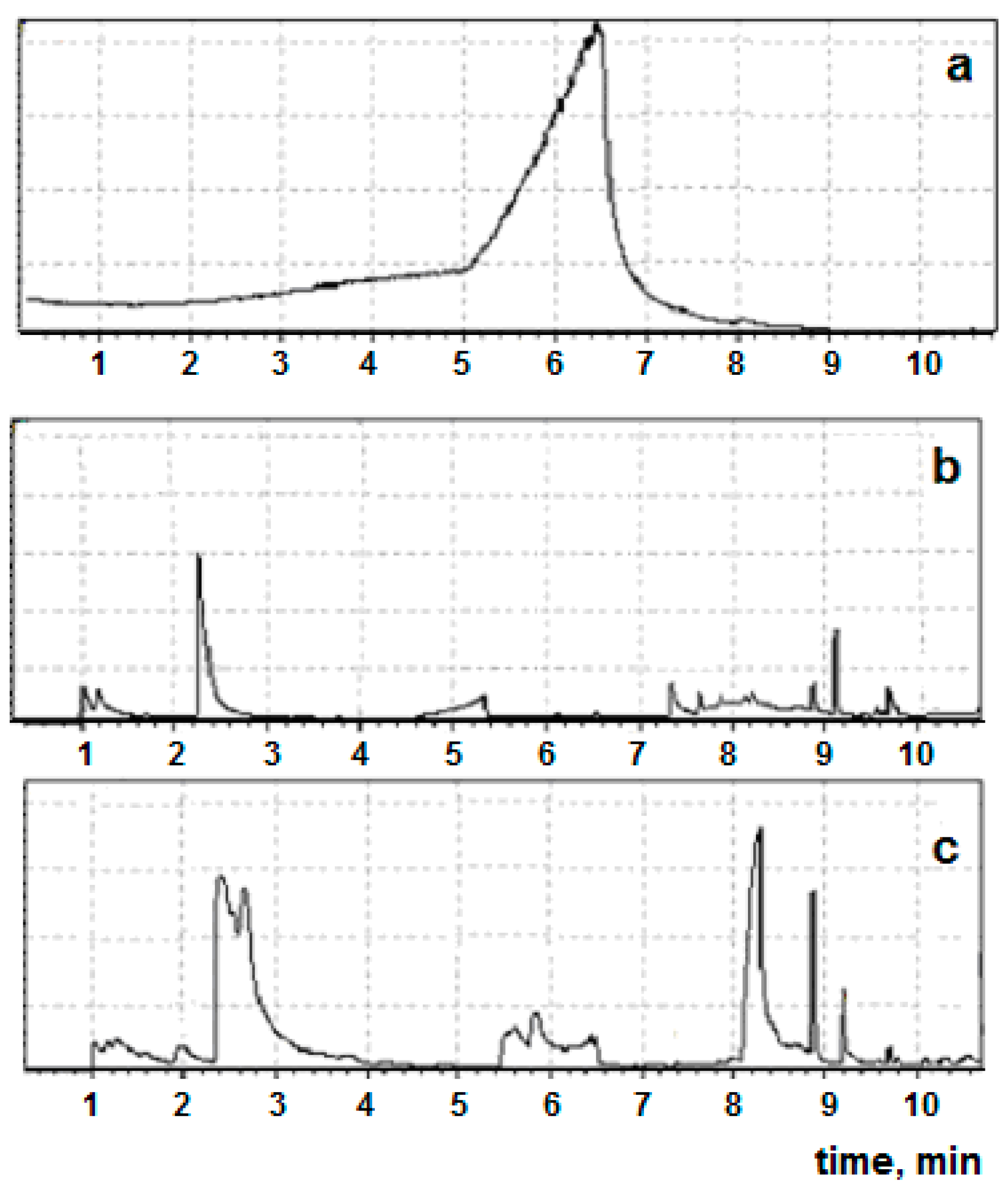
2. Results and Discussions
- In the case of UV light—CO2, 2-butanone, 2-propionic acid;
- In the case of visible light—CO2, acetone, 2-propanol, butanediol—1,4, 2,4-dimethylpentanone-3, butyric acid butyl ester, 2-methyl-2-(2-hydroxyethoxy)acetic acid ethyl ester.
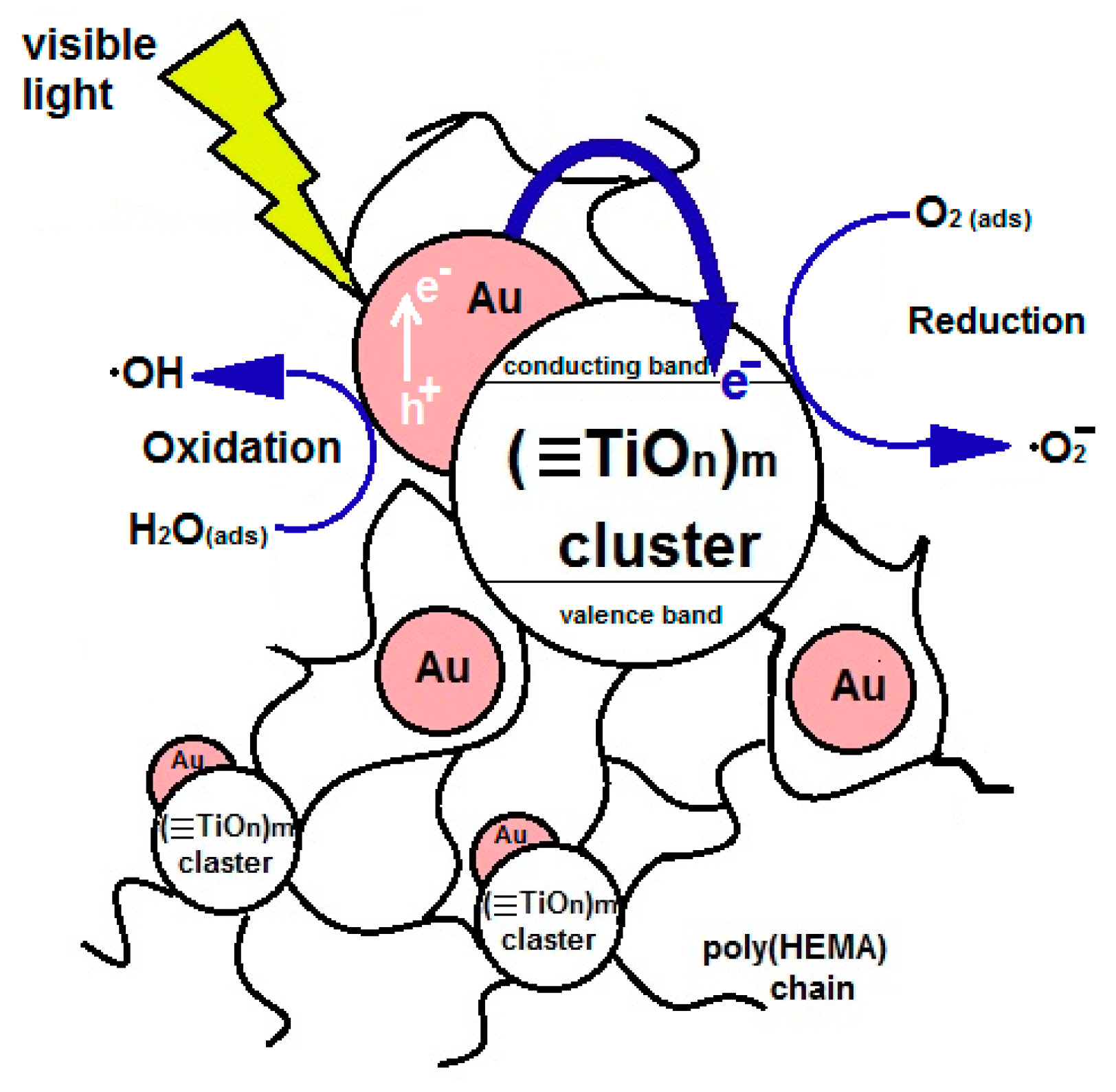
- Charge transfer transitions between the d-electrons of the metal and the conduction band or valence band of the PTO;
- Formation of impurity levels in band gap of the PTO; if these energy levels are located close to the edges of band gap, then they can overlap it, thus its width decreases [77];
- The appearance of allowed energy states in the band gap of TiO2, as a consequence of the presence of segregated clusters of MxOy on its surface [78], which can transfer excited state energy from NPs into the conduction band of the PTO according to the scheme proposed earlier for powdered titanium dioxide [79]. At the same time, the recombination rate of the electron-hole pair will decrease, and its lifetime will increase.
3. Materials and Methods
3.1. Synthesis of Nanocomposites
3.2. Investigation of Nanocomposites’ Optical Properties
3.3. Study of the Structure of Materials Based on Organic-Inorganic Copolymers
3.4. Investigation of Photocatalytic Activity
3.5. Total Organic Carbon Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D. Heterogeneous photocatalysis: From water photolysis to applications in environmental cleanups. Int. J. Hydrog. Ener. 2007, 14, 2664–2672. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations, and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]
- Marcì, G.; Palmisano, L. Heterogeneous Photocatalysis. Relationships with Heterogeneous Catalysis and Perspectives, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–24. [Google Scholar]
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.C.; Huang, H. Titanium oxide-based photocatalytic materials development and their role in air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Reddy, P.V.G.; Reddy, B.R.P.; Reddy, M.V.K.; Reddy, K.R.; Shetti, N.P.; Saleh, T.A.; Aminabhavi, T.M. A review on multicomponent reactions catalyzed by zero-dimensional/one-dimensional titanium dioxide (TiO2) nanomaterials: Promising green methodologies in organic chemistry. J. Environ. Manag. 2021, 279, 111603. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 362–399. [Google Scholar] [CrossRef]
- Prakash, J.; Cho, J.; Mishra, Y.K. Photocatalytic TiO2 nanomaterials as potential antimicrobial and antiviral agents: Scope against blocking the SARS-COV-2 spread. Micro Nano Eng. 2022, 14, 100100. [Google Scholar] [CrossRef]
- Qi, Y.; Xiang, B.; Zhang, J. Effect of titanium dioxide (TiO2) with different crystal forms and surface modifications on cooling property and surface wettability of cool roofing materials. Sol. Energy Mater. Sol. Cells 2017, 172, 34–43. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hsu, Y.L.; Su, H.Y.; Huang, R.C.; Hou, F.Y.; Tu, G.C.; Liu, W.H. A comparative study of amorphous, anatase, rutile, and mixed-phase TiO2 films by mist chemical vapor deposition and ultraviolet photodetectors applications. IEEE Sens. J. 2018, 18, 4022–4029. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile, and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Nair, R.V.; Gummaluri, V.S.; Matham M., V.; Vijayan, C. A review on optical bandgap engineering in TiO2 nanostructures via doping and intrinsic vacancy modulation towards visible light applications. J. Phys. D App. Phys. 2022, 55, 313003. [Google Scholar] [CrossRef]
- Xiong, L.-B.; Li, J.-L.; Yang, B.; Yu, Y. Ti3+ in the surface of titanium dioxide: Generation, properties, and photocatalytic application. J. Nanomat. 2012, 2012, 831524. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, A.; Kameneva, O.; Alexandrov, A.; Bityurin, N.; Chhor, K.; Kanaev, A. Chemical Activity of Photoinduced Ti3+ Centers in Titanium Oxide Gels. J. Phys. Chem. B 2006, 110, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.; Kameneva, O.; Rozes, L.; Sanchez, C.; Bityurin, N.; Kanaev, A. Extinction of photo-induced Ti3+ centres in titanium oxide gels and gel-based oxo-PHEMA hybrids. Chem. Phys. Let. 2006, 429, 523–527. [Google Scholar] [CrossRef]
- Rozes, L.; Sanchez, C. Titanium oxo-clusters: Precursors for a Lego-like construction of nanostructured hybrid materials. Chem. Soc. Rev. 2011, 40, 1006–1030. [Google Scholar] [CrossRef] [PubMed]
- Museur, L.; Gorbovyi, P.; Traore, M.; Kanaev, A.; Rozes, L.; Sanchez, C. Luminescence properties of pHEMA-TiO2 gels based hybrids materials. J. Lumin. 2012, 132, 1192–1199. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M.G. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattices Microstruct. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Sornalingam, K.; McDonagh, A.; Zhou, J.L.; Johira, M.A.H.; Ahmed, M.B. Photocatalysis of estrone in water and wastewater: Comparison between Au-TiO2 nanocomposite and TiO2, and degradation by-products. Sci. Total Environ. 2018, 610–611, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterization and photonic efficiency of Pt, Au, and Pd deposited on TiO2. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting overdoped-TiO2 nanoparticles. Solar Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Tang, K.Y.; Chen, J.X.; Legaspi, E.D.R.; Owh, C.; Lin, M.; Tee, I.S.Y.; Kai, D.; Loh, X.J.; Li, Z.; Regulacio, M.D.; et al. Gold-decorated TiO2 nanofibrous hybrid for improved solar-driven photocatalytic pollutant degradation. Chemosphere 2021, 265, 129114. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.Y.; Liu, J.B.; Wu, J.M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [Green Version]
- Pandiyaraj, K.N.; Vasu, D.; Ghobeira, R.; Tabaei, P.S.E.; Geyter, N.D.; Morent, R.; Pichumani, M.; Padmanabhanan, P.V.A.; Deshmukh, R.R. Dye wastewater degradation by the synergetic effect of an atmospheric pressure plasma treatment and the photocatalytic activity of plasma-functionalized Cu-TiO2 nanoparticles. J. Hazard. Mat. 2020, 405, 124264. [Google Scholar] [CrossRef]
- Kozlov, D.A.; Lebedev, V.A.; Polyakov, A.Y.; Khazova, K.M.; Garshev, A.V. The microstructure effect on the Au/TiO2 and Ag/TiO2 nanocomposites photocatalytic activity. Nanosyst. Phys. Chem. Math. 2018, 9, 266–278. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, W.; Liu, P. Enhanced Photocatalytic Efficiency of TiO2 Membrane Decorated with Ag and Au Nanoparticles. Appl. Sci. 2018, 8, 945. [Google Scholar] [CrossRef] [Green Version]
- Moslah, C.; Kandyla, M.; Mousdis, G.A.; Petropoulou, G.; Ksibi, M. Photocatalytic Properties of Titanium Dioxide Thin Films Doped with Noble Metals (Ag, Au, Pd, and Pt). Phys. Status Solidi (A) 2018, 215, 1800023. [Google Scholar] [CrossRef]
- Malik, A.S.; Liu, T.; Rittiruam, M.; Saelee, T.; Da Silva, J.L.; Praserthdam, S.; Praserthdam, P. On a high photocatalytic activity of high-noble alloys Au–Ag/TiO2 catalysts during oxygen evolution reaction of water oxidation. Sci. Rep. 2022, 12, 2604. [Google Scholar] [CrossRef] [PubMed]
- Yoshiiri, K.; Wang, K.; Kowalska, E. TiO2/Au/TiO2 Plasmonic Photocatalysts: The Influence of Titania Matrix and Gold Properties. Inventions 2022, 7, 54. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhang, L.; Fu, H.; He, P.; Han, D.; Lawson, T.; An, X. The Use of Tunable Optical Absorption Plasmonic Au and Ag Decorated TiO2 Structures as Efficient Visible Light Photocatalysts. Catalysts 2020, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Varapragasam, S.J.P.; Mia, S.; Wieting, C.; Balasanthiran, C.; Hossan, M.Y.; Baride, A.; Rioux, R.M.; Hoefelmeyer, J.D. Ag–TiO2 Hybrid Nanocrystal Photocatalyst: Hydrogen Evolution under UV Irradiation but Not under Visible-Light Irradiation. ACS Appl. Energy Mater. 2019, 2, 8274–8282. [Google Scholar] [CrossRef]
- Salomatina, E.; Bityurin, N.; Gulenova, M.; Gracheva, T.; Drozdov, M.; Knyazev, A.; Kir’yanov, K.; Markin, A.; Smirnova, L. Synthesis, structure, and properties of organic-inorganic (co)polymers containing poly(titanium oxide). J. Mater. Chem. C. 2013, 1, 6375–6385. [Google Scholar] [CrossRef]
- Salomatina, E.V.; Moskvichev, A.N.; Knyazev, A.V.; Smirnova, L.A. Effect of kinetic features in synthesis of hybrid copolymers based on Ti(OPri)4 and hydroxyethyl methacrylate on their structure and properties. Rus. J. App. Chem. 2015, 88, 197–207. [Google Scholar] [CrossRef]
- Loginova, A.S.; Ignatov, S.K.; Chukhmanov, E.P.; Salomatina, E.V.; Smirnova, L.A. Structure, spectra, and photoinduced electron-redistribution properties of TiO2/organic copolymers with gold nanoparticles. A DFT study. Comput. Theor. Chem. 2017, 1118, 1–15. [Google Scholar] [CrossRef]
- Howe, R.F.; Gratzel, M. EPR observation of trapped electrons in colloidal titanium dioxide. J. Phys. Chem. 1985, 89, 4495–4499. [Google Scholar] [CrossRef]
- Gratzel, M.; Howe, R.F. Electron paramagnetic resonance studies of doped titanium dioxide colloids. J. Phys. Chem. 1990, 94, 2566–2572. [Google Scholar] [CrossRef]
- Anpo, M.; Aikawa, N.; Kubokawa, Y. Photocatalytic hydrogenation of alkynes and alkenes with water over titanium dioxide. Platinum loading effect on the primary processes. J. Phys. Chem. 1984, 88, 3998–4000. [Google Scholar] [CrossRef]
- Micic, O.I.; Zhang, Y.; Cromack, K.R.; Trifunac, A.D.; Thurnauer, M.C. Photoinduced hole transfer from titanium dioxide to methanol molecules in aqueous solution studied by electron paramagnetic resonance. J. Phys. Chem. 1993, 97, 13284–13288. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Kameneva, O.; Bityurin, N.; Rozes, L.; Sanchez, C.; Kanaev, A. Laser-induced photopatterning of organic-inorganic TiO2-based hybrid materials with tunable interfacial electron transfer. Phys. Chem. Chem. Phys. 2009, 11, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Kameneva, O.; Kuznestov, A.I.; Smirnova, L.A.; Rozes, L.; Sanchez, C.; Aleksandrov, A.; Bityurin, N.; Chhor, K.; Kanaev, A. New photoactive hybrid organic-inorganic materials based on titanium-oxo-PHEMA nanocomposites exhibiting mixed valence properties. J. Mater. Chem. 2005, 15, 3380–3383. [Google Scholar] [CrossRef]
- Uklein, A.; Gorbovyi, P.; Traore, M.; Museur, L.; Kanaev, A. Photo-induced refraction of nanoparticulate organic-inorganic TiO2-pHEMA hybrids. Opt. Mater. Express 2013, 3, 533–545. [Google Scholar] [CrossRef]
- Hamed, M.M.; Ahmed, I.M.; Metwally, S.S. Adsorptive removal of methylene blue as an organic pollutant by marble dust as eco-friendly sorbent. J. Ind. Eng. Chem. 2014, 20, 2370–2377. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current {ISO} tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Krishnan, S.; Shriwastav, A. Application of TiO2 nanoparticles sensitized with natural chlorophyll pigments as catalyst for visible light photocatalytic degradation of methylene blue. J. Environ. Chem. Eng. 2021, 9, 104699. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Petrella, A.; Spasiano, D.; Cosma, P.; Rizzi, V.; Race, M.; Mascolo, M.C.; Ranieri, E. Methyl Orange Photo-Degradation by TiO2 in a Pilot Unit under Different Chemical, Physical, and Hydraulic Conditions. Processes 2021, 9, 205. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Regraguy, B.; Rahmani, M.; Mabrouki, J.; Drhimer, F.; Ellouzi, I.; Mahmou, C.; Dahchour, A.; El Mrabet, M.; El Hajjaji, S. Photocatalytic degradation of methyl orange in the presence of nanoparticles NiSO4/TiO2. Nanotechnol. Environ. Eng. 2022, 7, 157–171. [Google Scholar] [CrossRef]
- He, Y.; Grieser, F.; Ashokkumar, M. The mechanism of sonophotocatalytic degradation of methyl orange and its products in aqueous solutions. Ultrason. Sonochem. 2011, 18, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Oyama, T.; Horikoshi, S.; Hidaka, H.; Zhao, J.; Serpone, N. Photocatalyzed N-demethylation and degradation of methylene blue in titania dispersions exposed to concentrated sunlight. Solar Ener. Mater. Solar Cells 2002, 73, 287–303. [Google Scholar] [CrossRef]
- Dai, K.; Chen, H.; Peng, T.; Ke, D.; Yi, H. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere 2007, 69, 1361–1367. [Google Scholar] [CrossRef]
- Wang, S.; Luo, C.; Tan, F.; Cheng, X.; Ma, Q.; Wu, D.; Li, P.; Zhang, F.; Ma, J. Degradation of Congo red by UV photolysis of nitrate: Kinetics and degradation mechanism. Sep. Purif. Technol. 2021, 262, 118276. [Google Scholar] [CrossRef]
- Ullah, I.; Haider, A.; Khalid, N.; Ali, S.; Ahmed, S.; Khan, Y.; Ahmed, N.; Zubair, M. Tuning the band gap of TiO2 by tungsten doping for efficient UV and visible photodegradation of Congo red dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 150–157. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Yang, L.; Luo, S.; Li, Y.; Xiao, Y.; Kang, Q.; Cai, Q. High Efficient Photocatalytic Degradation of p-Nitrophenol on a Unique Cu2O/TiO2 p-n Heterojunction Network Catalyst. Environ. Sci. Technol. 2010, 44, 7641–7646. [Google Scholar] [CrossRef]
- Yadav, V.; Verma, P.; Sharma, H.; Tripathy, S.; Saini, V.K. Photodegradation of 4-nitrophenol over B-doped TiO2 nanostructure: Effect of dopant concentration, kinetics, and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 10966–10980. [Google Scholar] [CrossRef] [PubMed]
- Moctezuma, E.; Leyva, E.; Aguilar, C.A.; Luna, R.A.; Montalvo, C. Photocatalytic degradation of paracetamol: Intermediates and total reaction mechanism. J. Hazard. Mater. 2012, 243, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.-Y.; Sheeley, S.A.; Rajh, T.; Cropek, D.M. Photocatalytic reduction of 4-nitrophenol with arginine-modified titanium dioxide nanoparticles. Appl. Catal. B Environ. 2007, 74, 103–110. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, H.; Wang, M.; Cao, C.; Yao, S. Study on the role of hydroperoxyl radical in degradation of p-nitrophenol attacked by hydroxyl radical using photolytical technique. J. Photochem. Photobiol. A Chem. 2013, 259, 17–24. [Google Scholar] [CrossRef]
- Di Paola, A.; Augugliaro, V.; Palmisano, L.; Pantaleo, G.; Savinov, E. Heterogeneous photocatalytic degradation of nitrophenols. J. Photochem. Photobiol. A Chem. 2003, 155, 207–214. [Google Scholar] [CrossRef]
- Kalvert, G.; Pitts, G. Photochemistry, 1st ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1966; 899p. [Google Scholar]
- Nakata, A.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; El Hamidi, A.; Rodríguez-Lorenzo, L.; Santaballa, J.A.; Canle, M. Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2. Nanomaterials 2020, 10, 996. [Google Scholar] [CrossRef]
- Gadirova, E.M. Photochemical degradation of phenol in the presence of titanium dioxide nanoparticles. Proceed. Univ. Appl. Chem. Biotechnol. 2019, 9, 179–182. [Google Scholar]
- Dang, T.T.T.; Le, S.T.T.; Channei, D.; Khanitchaidecha, W.; Nakaruk, A. Photodegradation mechanisms of phenol in the photocatalytic process. Res. Chem. Intermed. 2016, 42, 5961–5974. [Google Scholar] [CrossRef]
- Grabowska, E.; Reszczyńska, J.; Zaleska, A. RETRACTED: Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: A review. Water Res. 2012, 46, 5453–5471. [Google Scholar] [CrossRef]
- Dong, X.; Li, D.; Li, Y.; Sakiyama, H.; Muddassir, M.; Pan, Y.; Srivastava, D.; Kumar, A. A 3,8-connected Cd(II)-based metal–organic framework as an appropriate luminescent sensor for the antibiotic sulfasalazine. CrystEngComm 2022, 24, 7157–7165. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Dobrosz-Gómez, I.; Gómez-García, M.Á.; Zamora, S.M.L.; GilPavas, E.; Bojarska, J.; Kozanecki, M.; Rynkowski, J.M. Transition metal loaded TiO2 for phenol photo-degradation. Comptes Rendus Chim. 2015, 18, 1170–1182. [Google Scholar] [CrossRef]
- Chowdhury, P.; Moreira, J.; Gomaa, H.; Ray, A.K. Visible-Solar-Light-Driven Photocatalytic Degradation of Phenol with Dye-Sensitized TiO2: Parametric and Kinetic Study. Ind. Eng. Chem. Res. 2012, 51, 4523–4532. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mat. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Saih, Y.; Segawa, K. Ultradeep hydrodesulfurization of dibenzothiophene (DBT) derivatives over Mo-sulfide catalysts supported on TiO2-coated alumina composite (Review). Catal. Surv. Asia 2003, 7, 235–249. [Google Scholar] [CrossRef]
- Suyoulema, L.J.; Sarina, W.W. A study of photodegradation of sulforhodamine B on Au–TiO2/bentonite under UV and visible light irradiation. Solid State Sci. 2009, 11, 2037–2043. [Google Scholar]
- Kortum, G.F.A. Reflectance Spectroscopy: Principles, Methods, Applications; Springer Science & Business Media: New York, NY, USA, 1969. [Google Scholar]
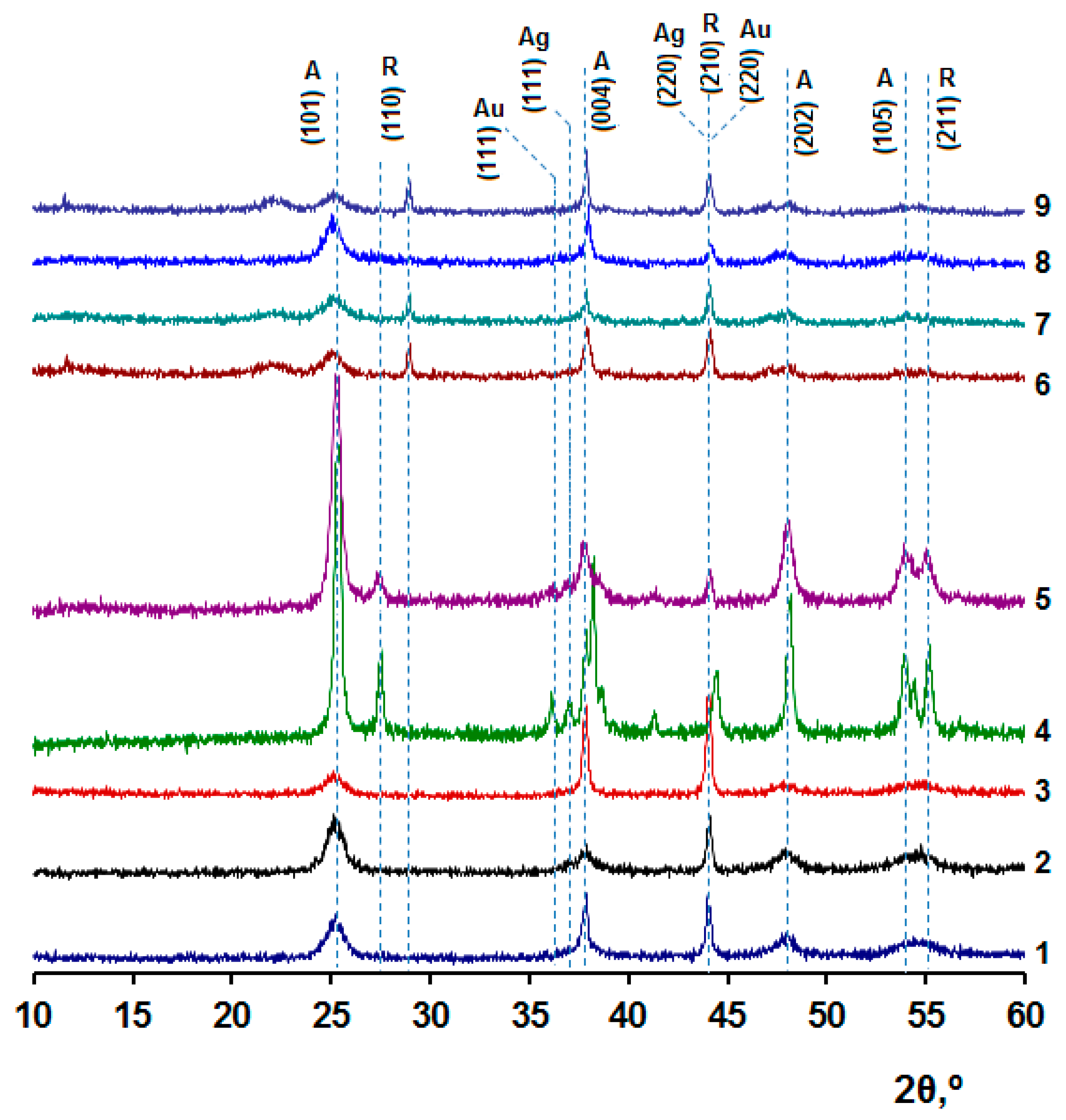
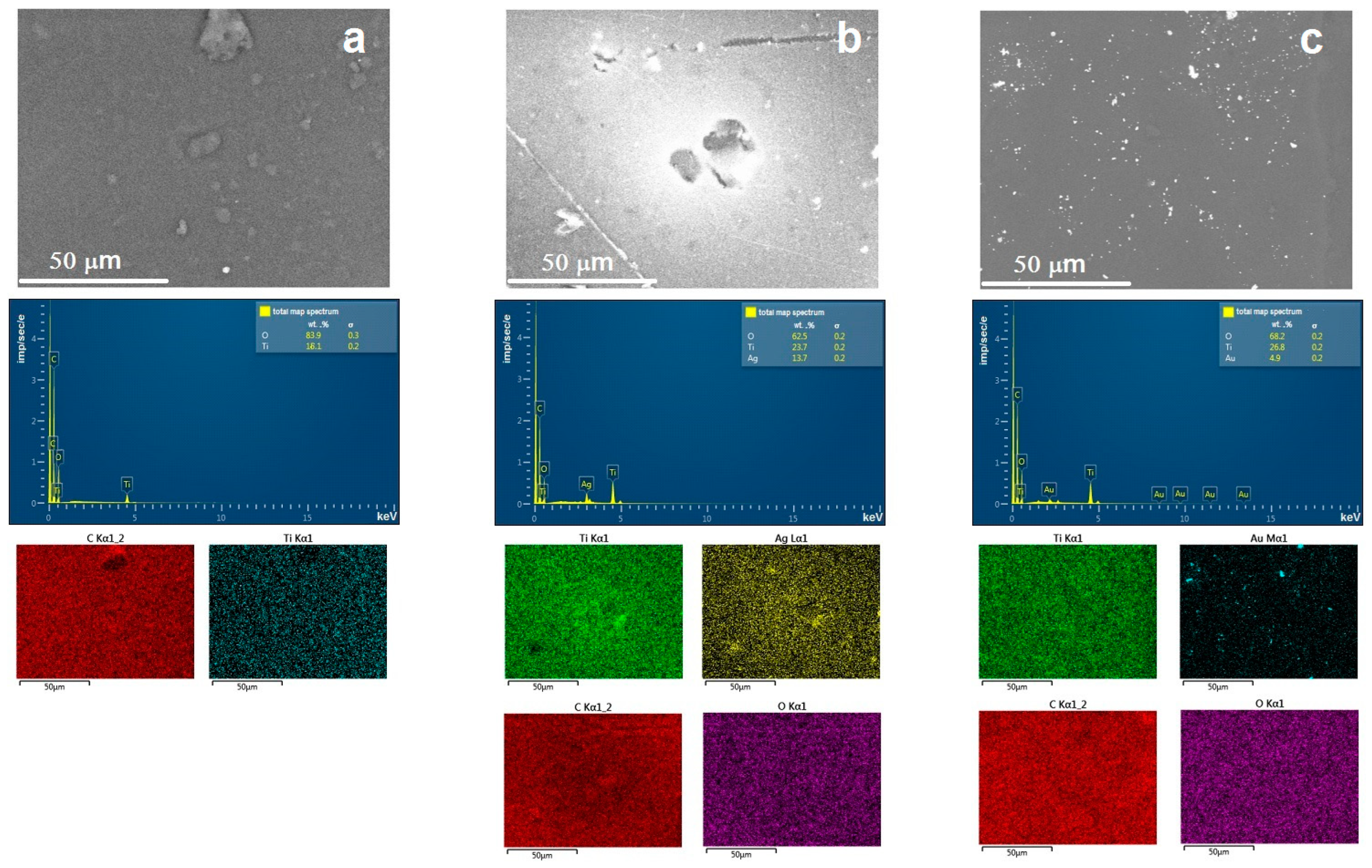


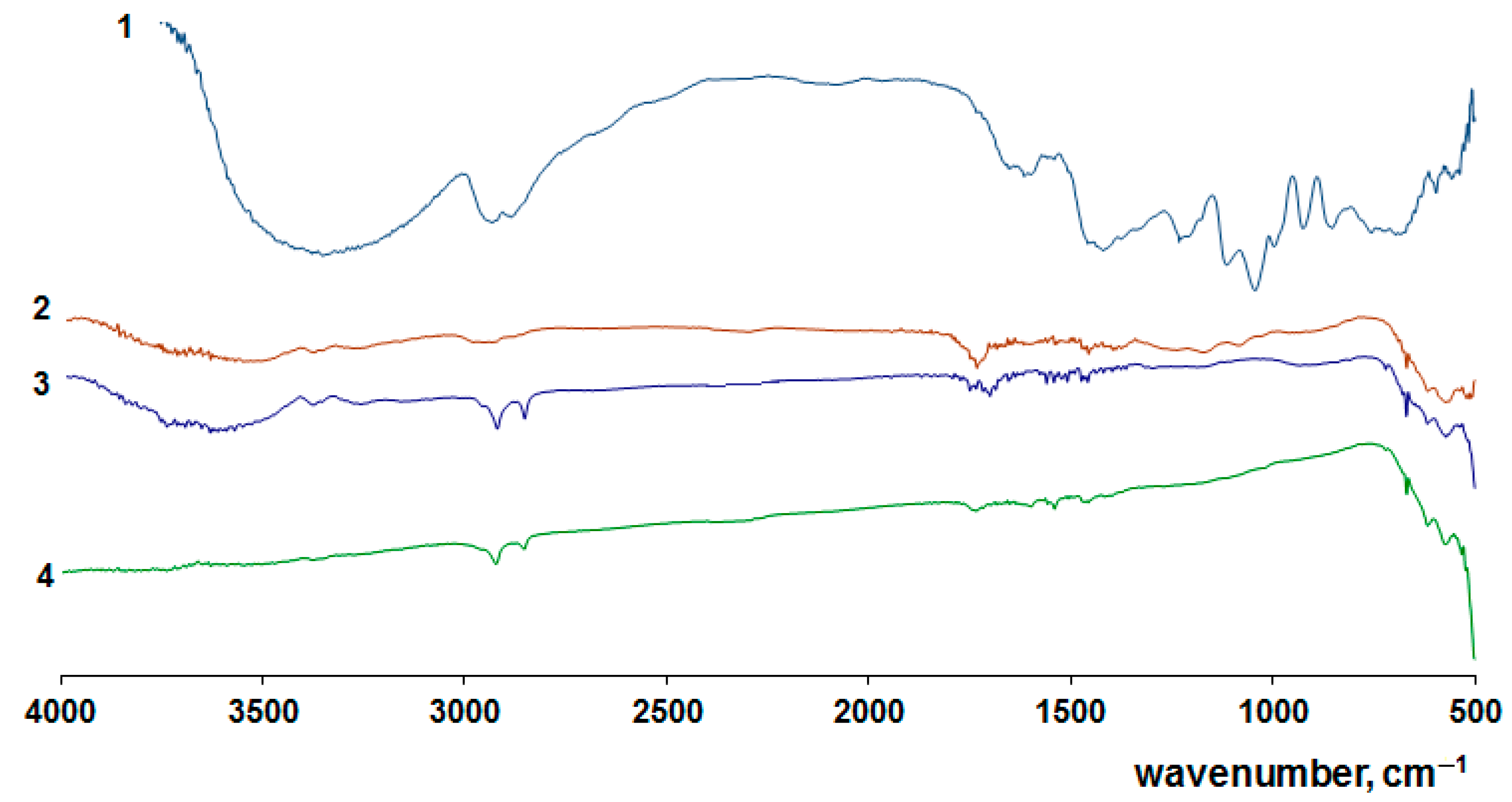
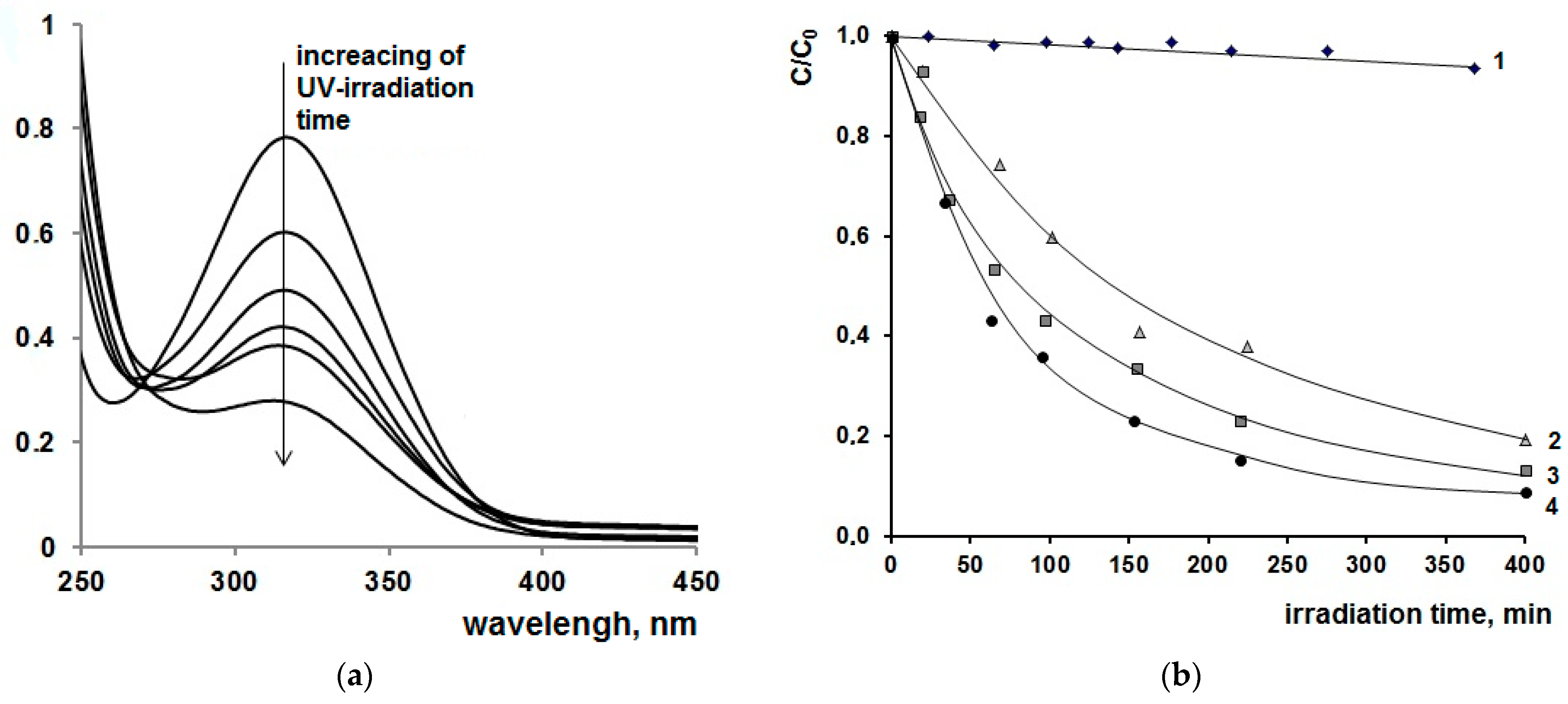
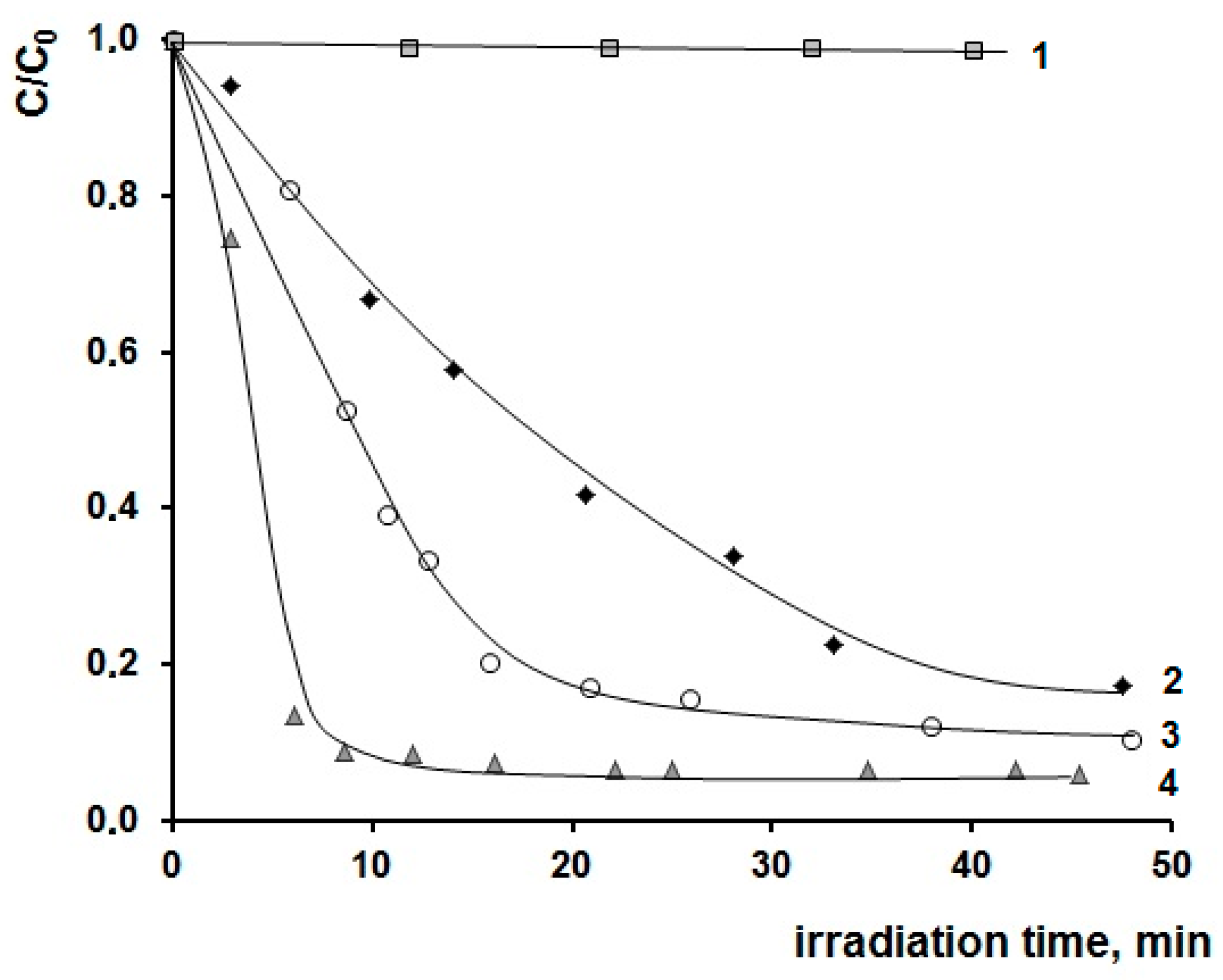

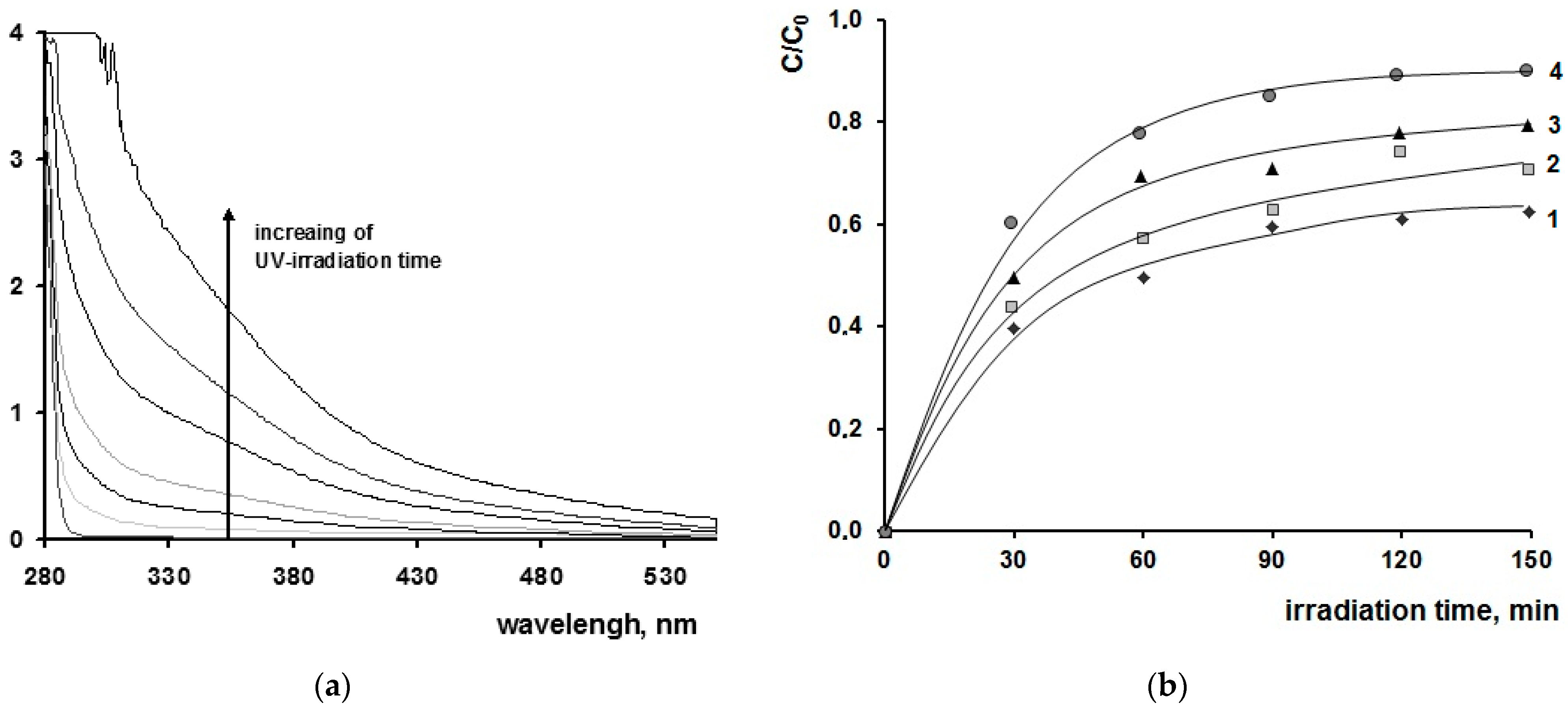


| [M] | Type of NPs | Particle Diameter in the Powder after Degradation of the Organic Part of the Composites, nm | Anatase Content, % | Eg | ||
|---|---|---|---|---|---|---|
| TiO2 | Au | Ag | ||||
| HEMA | without NPs | 7 | - | - | 100.0 | 3.35 |
| Au | 6 | - | 9 | 95.0 | 2.42 | |
| Ag | 6 | 12 | - | 81.2 | 2.75 | |
| BMA | without NPs | 6 | - | - | 100.0 | 3.11 |
| Au | 9 | - | 20 | 87.1 | 2.11 | |
| Ag | 12 | 27 | - | 75.1 | 2.49 | |
| AN | without NPs | 8 | - | - | 100.0 | 3.20 |
| Au | 11 | - | 23 | 93.8 | 2.48 | |
| Ag | 10 | 31 | - | 85.3 | 2.65 | |
| Experiment Conditions | TOC *, mg/L | NPOC **, mg/L | TC ***, mg/L | IC ****, mg/L |
|---|---|---|---|---|
| MO + UV light, without nanocomposite | 80.5 ± 1.9 | 78.5 ± 1.9 | 91 ± 2.2 | 10.5 ± 0.2 |
| MO + UV light, with nanocomposite | 14.5 ± 1.5 | 14.8 ± 1.5 | 14.7 ± 1.5 | 0.12 ± 0.1 |
| Phenol + UV light, without nanocomposite | 95.5 ± 2.3 | 99.5± 2.4 | 101.0 ± 2.4 | 5.5 ± 0.1 |
| Phenol + UV light, with nanocomposite | 7.5 ± 0.7 | 7.4 ± 0.7 | 7.9 ± 0.7 | 0.4 ± 0.1 |
| Phenol + visible light, with nanocomposite | 9.9 ± 1.0 | 10.0 ± 1.0 | 10.9 ± 1.0 | 1.0 ± 0.2 |
| Type of Water Pollutant | Characteristic Wavelengths in the Absorption Spectrum, nm | Concentration of Pollutant, mmol/L | Content of Nanocomposite in Cleaning Solution, g/L |
|---|---|---|---|
| MO | 470 | 3.06 | 0.5 * |
| CR | 500 | 14.00 | |
| p-NP | 318 and 400 | 0.07 | |
| phenol | 350 and 270 | 19.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomatina, E.; Shelud’ko, P.; Kuz’michev, V.; Smirnova, L. Photocatalytic Decomposition of Azo Dyes and Phenols Using Polymer Composites Containing Nanostructured Poly(Titanium Oxide) Doped with Gold or Silver Nanoparticles under Light Irradiation in a Wide Wavelength Range. Catalysts 2023, 13, 423. https://doi.org/10.3390/catal13020423
Salomatina E, Shelud’ko P, Kuz’michev V, Smirnova L. Photocatalytic Decomposition of Azo Dyes and Phenols Using Polymer Composites Containing Nanostructured Poly(Titanium Oxide) Doped with Gold or Silver Nanoparticles under Light Irradiation in a Wide Wavelength Range. Catalysts. 2023; 13(2):423. https://doi.org/10.3390/catal13020423
Chicago/Turabian StyleSalomatina, Evgeniia, Pavel Shelud’ko, Vsevolod Kuz’michev, and Larisa Smirnova. 2023. "Photocatalytic Decomposition of Azo Dyes and Phenols Using Polymer Composites Containing Nanostructured Poly(Titanium Oxide) Doped with Gold or Silver Nanoparticles under Light Irradiation in a Wide Wavelength Range" Catalysts 13, no. 2: 423. https://doi.org/10.3390/catal13020423
APA StyleSalomatina, E., Shelud’ko, P., Kuz’michev, V., & Smirnova, L. (2023). Photocatalytic Decomposition of Azo Dyes and Phenols Using Polymer Composites Containing Nanostructured Poly(Titanium Oxide) Doped with Gold or Silver Nanoparticles under Light Irradiation in a Wide Wavelength Range. Catalysts, 13(2), 423. https://doi.org/10.3390/catal13020423






