Transition-Metal-Catalyzed C–C Bond Macrocyclization via Intramolecular C–H Bond Activation
Abstract
:1. Introduction
2. Polyketide Macrocycles
3. Polypeptide Macrocycles
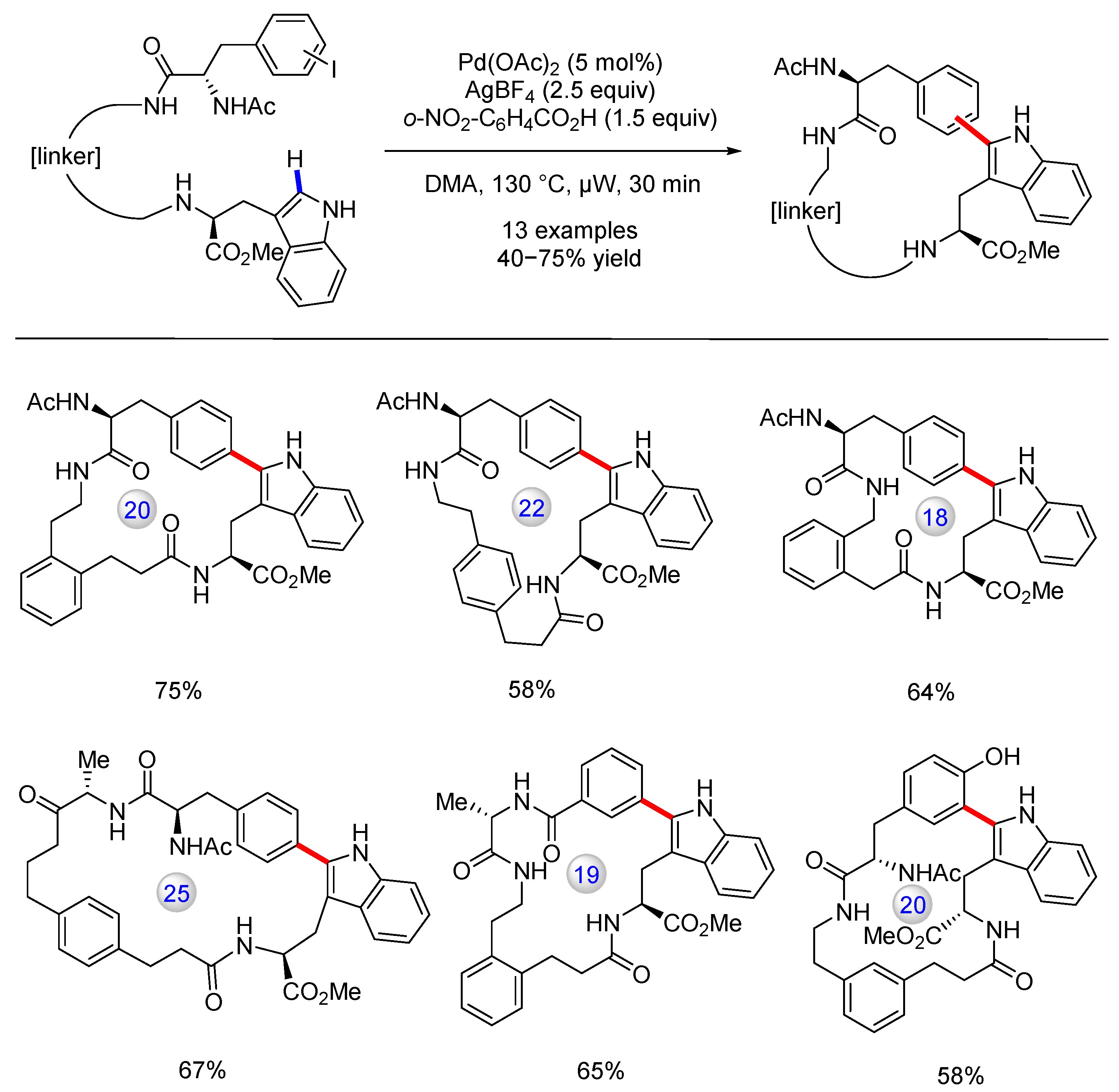
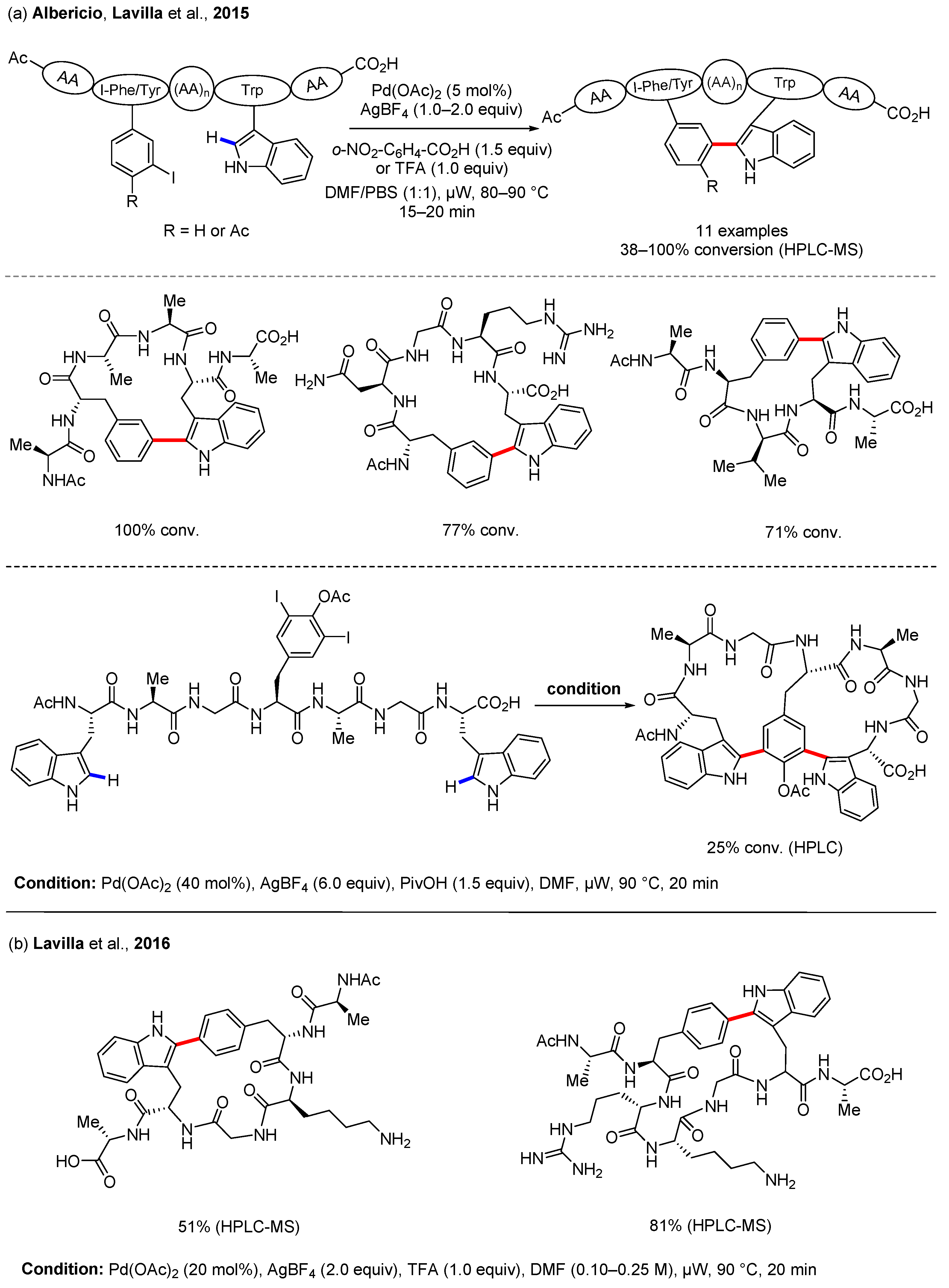
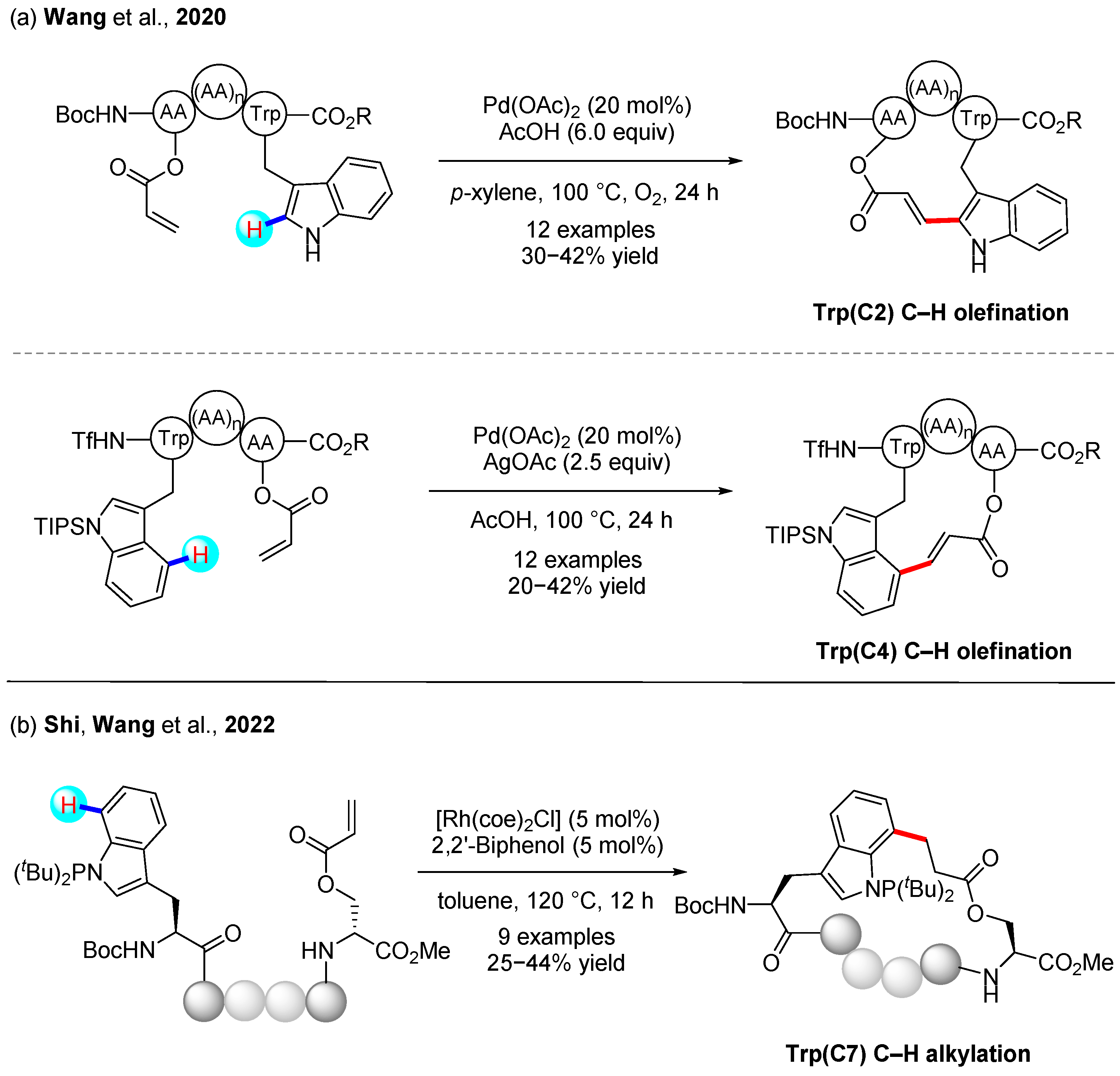
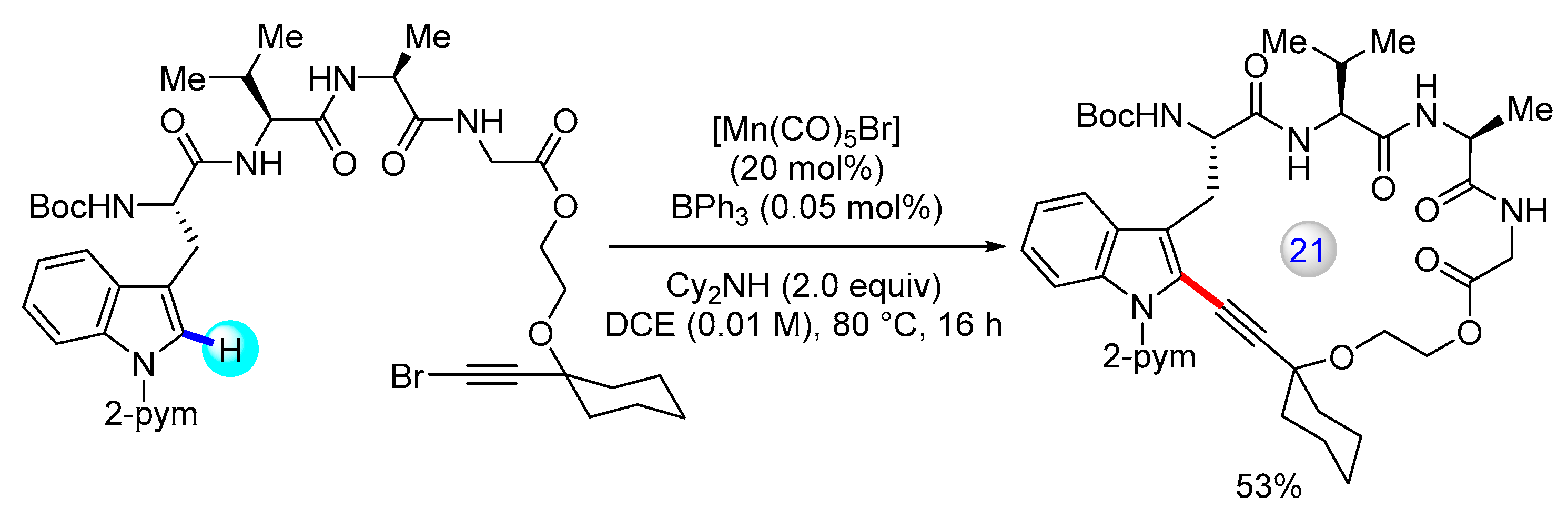
3.1. C(sp2)–H Bond Functionalization of Indoles
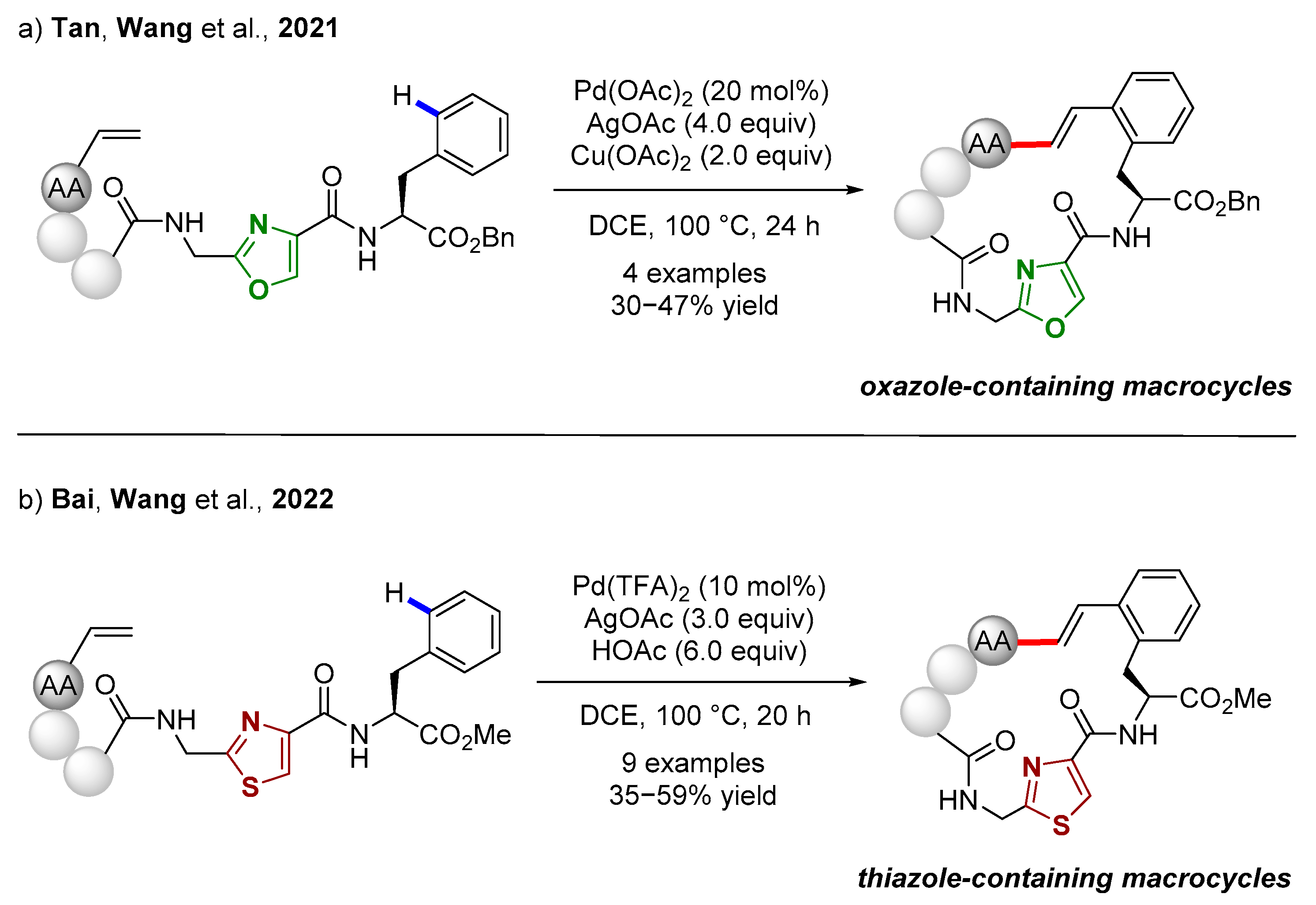
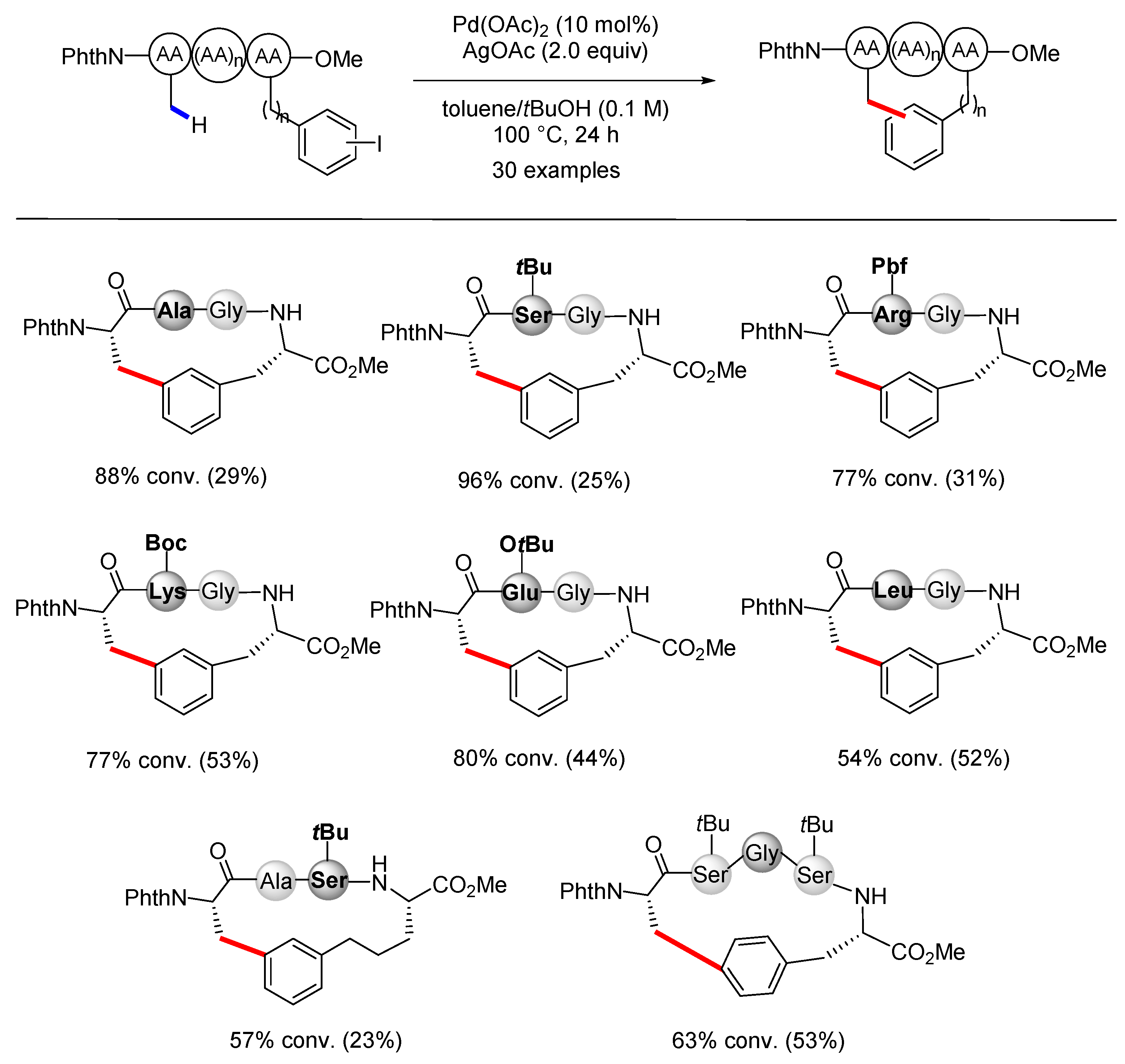
3.2. C(sp2)–H Bond Functionalization of Arenes
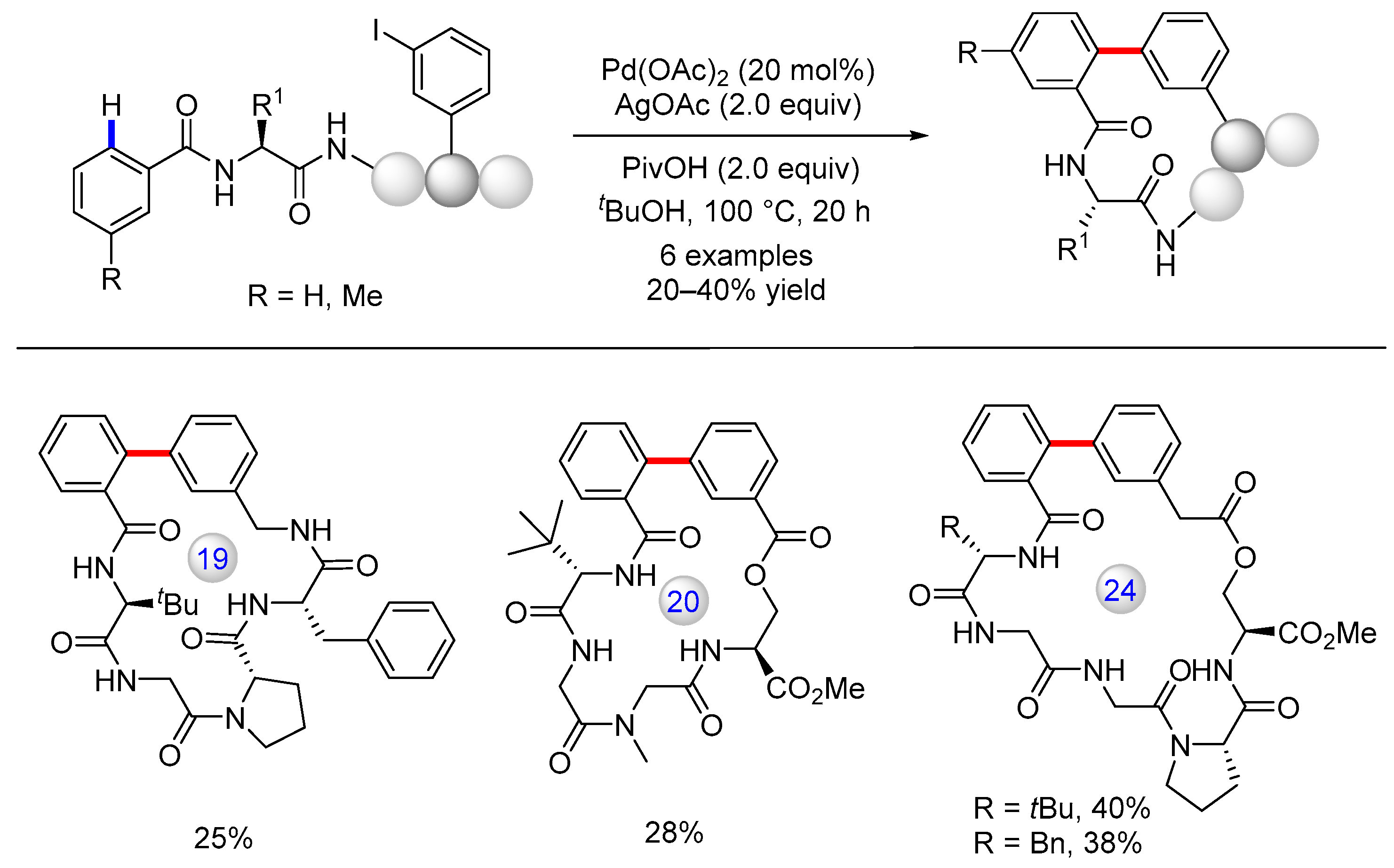
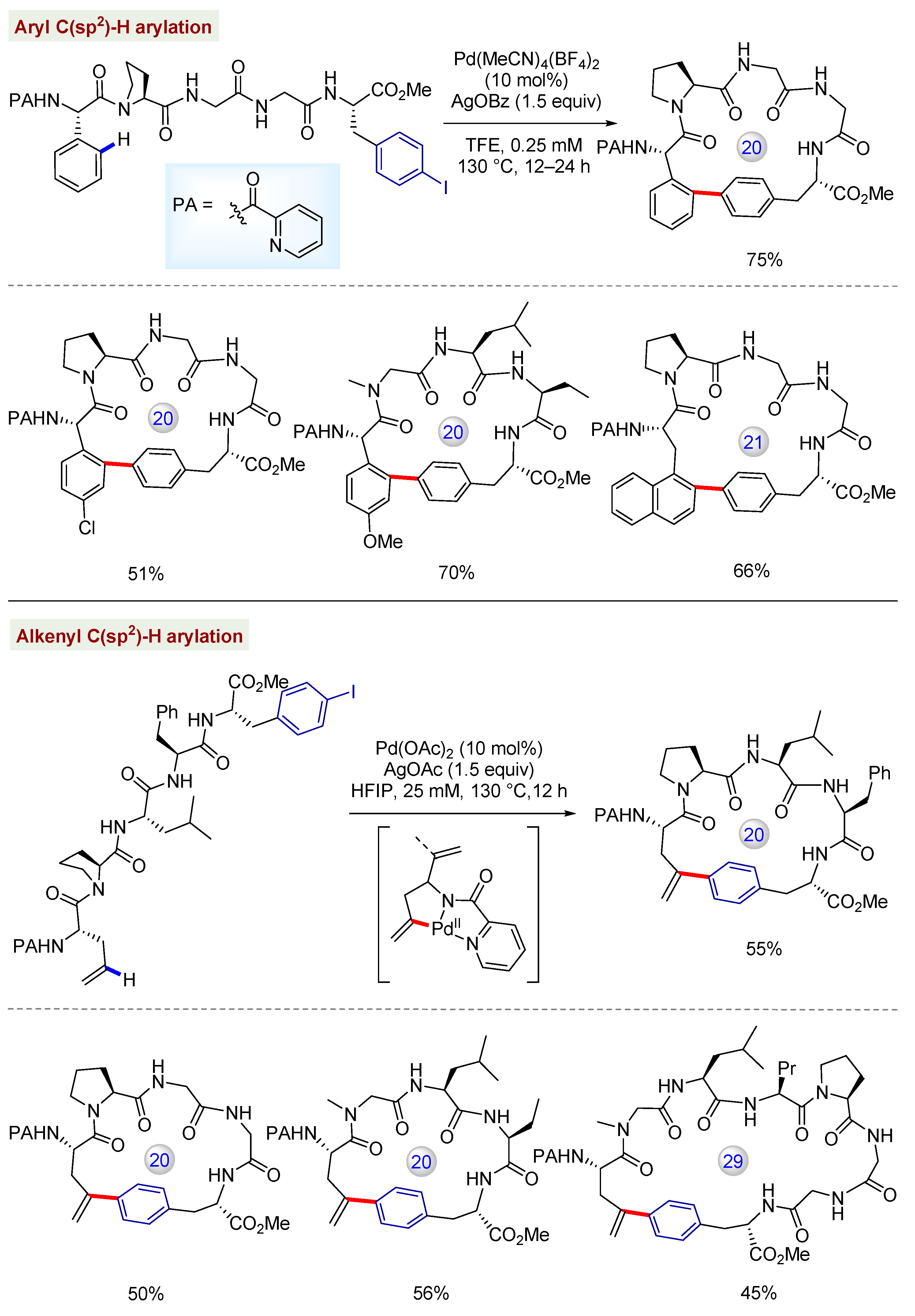
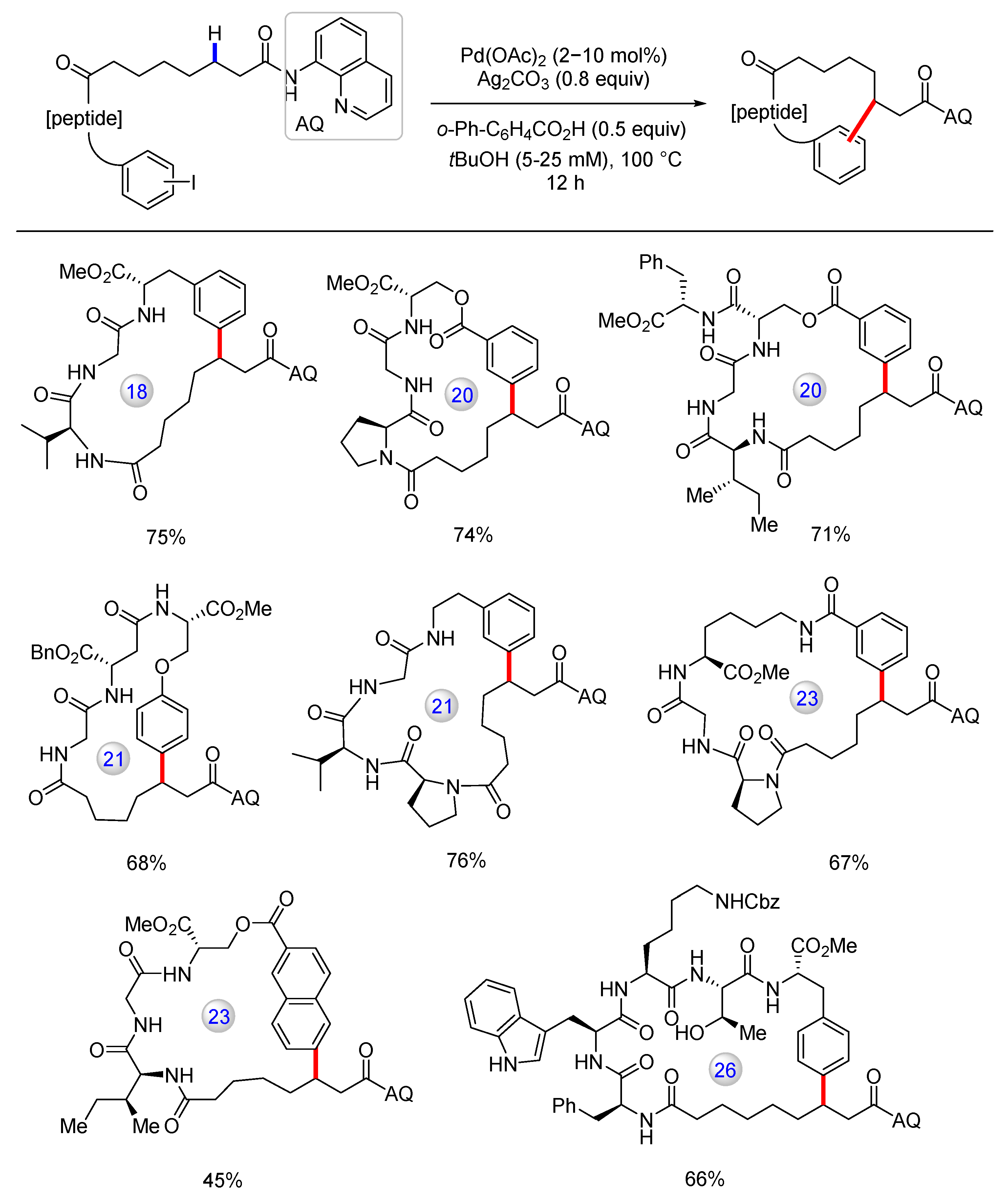
3.3. C(sp3)–H Bond Functionalization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gibson, S.E.; Lecci, C. Amino acid derived macrocycles—An area driven by synthesis or application? Angew. Chem. Int. Ed. 2006, 45, 1364–1377. [Google Scholar] [CrossRef]
- Yudin, A.K. Macrocycles: Lessons from the distant past, recent developments, and future directions. Chem. Sci. 2015, 6, 30–49. [Google Scholar] [CrossRef] [Green Version]
- Wessjohann, L.; Rivera, D.G.; Vercillo, O.E. Multiple multicomponent macrocyclizations (MiBs): A strategic development toward macrocycle diversity. Chem. Rev. 2009, 109, 796–814. [Google Scholar] [CrossRef]
- White, C.J.; Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011, 3, 509–524. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Pandey, M.D.; Burguete, M.I.; Luis, S.V. Macrocyclization reactions: The importance of conformational, configurational, and template-induced preorganization. Chem. Rev. 2015, 115, 8736–8834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Tang, J.; Chen, H.; He, Y.; Wang, H.; Yao, H. Recent developments in peptide macrocyclization. Tetrahedron Lett. 2018, 59, 325–333. [Google Scholar] [CrossRef]
- Mortensen, K.T.; Osberger, T.J.; King, T.A.; Sore, H.F.; Spring, D.R. Strategies for the diversity-oriented synthesis of macrocycles. Chem. Rev. 2019, 119, 10288–10317. [Google Scholar] [CrossRef]
- Reguera, L.; Rivera, D.G. Multicomponent reaction toolbox for peptide macrocyclization and stapling. Chem. Rev. 2019, 119, 9836–9860. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Hong, R. Stereoconfining macrocyclizations in the total synthesis of natural products. Nat. Prod. Rep. 2019, 36, 1546–1575. [Google Scholar] [CrossRef]
- Rivera, D.G.; Ojeda-Carralero, G.M.; Reguera, L.; Van der Eycken, E.V. Peptide macrocyclization by transition metal catalysis. Chem. Soc. Rev. 2020, 49, 2039–2059. [Google Scholar] [CrossRef]
- Parenty, A.; Moreau, X.; Campagne, J.-M. Macrolactonizations in the total synthesis of natural products. Chem. Rev. 2006, 106, 911–939. [Google Scholar] [CrossRef]
- Li, Y.; Yin, X.; Dai, M. Catalytic macrolactonizations for natural product synthesis. Nat. Prod. Rep. 2017, 34, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, X.; Zhao, J. Ynamide-mediated macrolactonization. ACS Catal. 2020, 10, 5230–5235. [Google Scholar] [CrossRef]
- Ishihara, K.; Kuroki, Y.; Hanaki, N.; Ohara, S.; Yamamoto, H. Antimony-templated macrolactamization of tetraamino esters. facile synthesis of macrocyclic spermine alkaloids, (±)-buchnerine, (±)-verbacine, (±)-verbaskine, and (±)-verbascenine. J. Am. Chem. Soc. 1996, 118, 1569–1570. [Google Scholar] [CrossRef]
- Doi, T.; Kamioka, S.; Shimazu, S.; Takahashi, T. A synthesis of RGD model cyclic peptide by palladium-catalyzed carbonylative macrolactamization. Org. Lett. 2008, 10, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.-K.; Linghu, X.; Wong, N.; Zhang, H.; Sowell, C.G.; Gosselin, F. Macrolactamization approaches to arylomycin antibiotics core. Org. Lett. 2019, 21, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.M.; Yohannes, D.; Danishefsky, S.M. Total synthesis of rapamycin via a novel titanium-mediated aldol macrocyclization reaction. J. Am. Chem. Soc. 1993, 115, 9345–9346. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Scheid, G.O.; Eichelberger, U.; Umbreen, S. Total synthesis of epothilone D: The nerol/macroaldolization approach. J. Org. Chem. 2013, 78, 10588–10595. [Google Scholar] [CrossRef]
- Abramite, J.A.; Sammakia, T. Application of the intramolecular Yamamoto vinylogous aldol reaction to the synthesis of macrolides. Org. Lett. 2007, 9, 2103–2106. [Google Scholar] [CrossRef]
- Gradillas, A.; Pérez-Castells, J. Macrocyclization by ring-closing metathesis in the total synthesis of natural products: Reaction conditions and limitations. Angew. Chem. Int. Ed. 2006, 45, 6086–6101. [Google Scholar] [CrossRef]
- Denmark, S.E.; Muhuhi, J.M. Development of a general, sequential, ring-closing metathesis/intramolecular cross-coupling reaction for the synthesis of polyunsaturated macrolactones. J. Am. Chem. Soc. 2010, 132, 11768–11778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Wang, C.; Kyle, A.F.; Jakubec, P.; Dixon, D.; Schrock, R.R.; Hoveyda, A.H. Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Nature 2011, 479, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecourt, C.; Dhambri, S.; Allievi, L.; Sanogo, Y.; Zeghbib, N.; Othman, R.B.; Lannou, M.-I.; Sorin, G.; Ardisson, J. Natural products and ring-closing metathesis: Synthesis of sterically congested olefins. Nat. Prod. Rep. 2018, 35, 105–124. [Google Scholar] [CrossRef]
- Gensch, T.; Hopkinson, M.N.; Glorius, F.; Wencel-Delord, J. Mild metal-catalyzed C–H activation: Examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, Q.; Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef]
- Loup, J.; Dhawa, U.; Pesciaioli, F.; Wencel-Delord, J.; Ackermann, L. Enantioselective C–H activation with earth-abundant 3d transition metals. Angew. Chem. Int. Ed. 2019, 58, 12803–12818. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, L.; Tan, J.-F.; Nguyen, Q.-H.; Madron du Vigne, A.; Smal, V.; Cao, Y.-X.; Cramer, N. Catalytic enantioselective functionalizations of C–H bonds by chiral iridium complexes. Chem. Rev. 2020, 120, 10516–10543. [Google Scholar] [CrossRef]
- Liu, B.; Romine, A.M.; Rubel, C.Z.; Engle, K.M.; Shi, B.-F. Transition-metal-catalyzed, coordination-assisted functionalization of nonactivated C(sp3)–H bonds. Chem. Rev. 2021, 121, 14957–15074. [Google Scholar] [CrossRef]
- Murali, K.; Machado, L.A.; Carvalho, R.L.; Pedrosa, L.F.; Mukherjee, R.; da Silva Júnior, E.N.; Maiti, D. Decoding directing groups and their pivotal role in C–H activation. Chem. Eur. J. 2021, 27, 12453–12508. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Almeida, R.G.; Murali, K.; Machado, L.A.; Pedrosa, L.F.; Dolui, P.; Maiti, D.; da Silva Júnior, E.N. Removal and modification of directing groups used in metal-catalyzed C–H functionalization: The magical step of conversion into ‘conventional’ functional groups. Org. Biomol. Chem. 2021, 19, 525–547. [Google Scholar] [CrossRef]
- Sinha, S.K.; Guin, S.; Maiti, S.; Biswas, J.P.; Porey, S.; Maiti, D. Toolbox for distal C–H bond functionalizations in organic molecules. Chem. Rev. 2022, 122, 5682–5841. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauermann, N.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Electrocatalytic C–H activation. ACS Catal. 2018, 8, 7086–7103. [Google Scholar] [CrossRef]
- Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition metals for C–H activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef] [PubMed]
- Stepek, I.A.; Itami, K. Recent advances in C–H activation for the synthesis of π-extended materials. ACS Mater. Lett. 2020, 2, 951–974. [Google Scholar] [CrossRef]
- Achar, T.K.; Maiti, S.; Jana, S.; Maiti, D. Transition metal catalyzed enantioselective C(sp2)–H bond functionalization. ACS Catal. 2020, 10, 13748–13793. [Google Scholar] [CrossRef]
- Meng, G.; Lam NY, S.; Lucas, E.L.; Saint-Denis, T.G.; Verma, P.; Chekshin, N.; Yu, J.-Q. Achieving site-selectivity for C–H activation processes based on distance and geometry: A carpenter’s approach. J. Am. Chem. Soc. 2020, 142, 10571–10591. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, B.-F. 2-(Pyridin-2-yl)isopropyl (PIP) amine: An enabling directing group for divergent and asymmetric functionalization of unactivated methylene C(sp3)–H bonds. Acc. Chem. Res. 2021, 54, 2750–2763. [Google Scholar] [CrossRef]
- Lam NY, S.; Wu, K.; Yu, J.-Q. Advancing the logic of chemical synthesis: C–H activation as strategic and tactical disconnections for C–C bond construction. Angew. Chem. Int. Ed. 2021, 60, 15767–15790. [Google Scholar] [CrossRef]
- Kharitonov, V.B.; Muratov, D.V.; Loginov, D.A. Cyclopentadienyl complexes of group 9 metals in the total synthesis of natural products. Coord. Chem. Rev. 2022, 471, 214744. [Google Scholar] [CrossRef]
- Mandal, D.; Roychowdhury, S.; Biswas, J.P.; Maiti, S.; Maiti, D. Transition-metal-catalyzed C–H bond alkylation using olefins: Recent advances and mechanistic aspects. Chem. Soc. Rev. 2022, 51, 7358–7426. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.L.; Lam NY, S.; Zhuang, Z.; Chan HS, S.; Strassfeld, D.A.; Yu, J.-Q. Palladium-catalyzed enantioselective β-C(sp3)–H activation reactions of aliphatic acids: A retrosynthetic surrogate for enolate alkylation and conjugate addition. Acc. Chem. Res. 2022, 55, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-Z.; Goh, J.; Maraswami, M.; Jia, Z.; Tian, J.-S.; Loh, T.-P. Recent advances in alkenyl sp2 C–H and C–F bond functionalizations: Scope, mechanism, and applications. Chem. Rev. 2022, 122, 17479–17646. [Google Scholar] [CrossRef]
- Sengupta, S.; Mehta, G. Late stage modification of peptides via C–H activation reactions. Tetrahedron Lett. 2017, 58, 1357–1372. [Google Scholar] [CrossRef]
- Lu, X.; He, S.-J.; Cheng, W.-M.; Shi, J. Transition-metal-catalyzed C–H functionalization for late-stage modification of peptides and proteins. Chin Chem Lett. 2018, 29, 1001–1008. [Google Scholar] [CrossRef]
- Wang, W.; Lorion, M.M.; Shah, J.; Kapdi, A.R.; Ackermann, L. Late-stage peptide diversification by position-selective C–H activation. Angew. Chem. Int. Ed. 2018, 57, 14700–14717. [Google Scholar] [CrossRef]
- Tong, H.-R.; Li, B.; Li, G.; He, G.; Chen, G. Postassembly modifications of peptides via metal-catalyzed C–H functionalization. CCS Chem. 2020, 2, 1797–1820. [Google Scholar] [CrossRef]
- Sengupta, S.; Mehta, G. Macrocyclization via C–H functionalization: A new paradigm in macrocycle synthesis. Org. Biomol. Chem. 2020, 18, 1851–1876. [Google Scholar] [CrossRef]
- Minami, A.; Eguchi, T. Substrate flexibility of vicenisaminyltransferase vinC involved in the biosynthesis of vicenistatin. J. Am. Chem. Soc. 2007, 129, 5102–5107. [Google Scholar] [CrossRef]
- Shinohara, Y.; Kudo, F.; Eguchi, T. A natural protecting group strategy to carry an amino acid starter unit in the biosynthesis of macrolactam polyketide antibiotics. J. Am. Chem. Soc. 2011, 133, 18134–18137. [Google Scholar] [CrossRef]
- Miyanaga, A.; Iwasawa, S.; Shinohara, Y.; Kudo, F.; Eguchi, T. Structure-based analysis of the molecular interactions between acyltransferase and acyl carrier protein in vicenistatin biosynthesis. PNAS 2016, 113, 1802–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, H.-L.; Panek, J.S. Total synthesis of the Hsp90 inhibitor geldanamycin. Org. Lett. 2008, 10, 2477–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilton, M.J.; Brackett, C.M.; Mercado, B.Q.; Blagg BS, J.; Miller, S.J. Catalysis-enabled access to cryptic geldanamycin oxides. ACS Cent. Sci. 2020, 6, 426–435. [Google Scholar] [CrossRef]
- Nazare, M.; Waldmann, H. Synthesis of the (9S,18R) diastereomer of cyclamenol A. Angew. Chem. Int. Ed. 2000, 39, 1125–1128. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, T.-P. Ruthenium- and rhodium-catalyzed cross-coupling reaction of acrylamides with alkenes: Efficient access to (Z,E)-dienamides. Chem. Commun. 2012, 48, 11232–11234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhao, M.; Li, S.-S.; Xu, Y.-H.; Loh, T.-P. Macrolide synthesis through intramolecular oxidative cross-coupling of alkenes. Angew. Chem. Int. Ed. 2018, 57, 555–559. [Google Scholar] [CrossRef]
- Maraswami, M.; Goh, J.; Loh, T.-P. Macrolactam synthesis via ring-closing alkene-alkene cross-coupling reactions. Org. Lett. 2020, 22, 9724–9728. [Google Scholar] [CrossRef]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discovery 2008, 7, 608–624. [Google Scholar] [CrossRef]
- Shchelik, I.S.; Gademann, K. Thiol- and disulfide-containing vancomycin derivatives against bacterial resistance and biofilm formation. ACS Med. Chem. Lett. 2021, 12, 1898–1904. [Google Scholar] [CrossRef]
- Lelle, M.; Kaloyanova, S.; Freidel, C.; Theodoropoulou, M.; Musheev, M.; Niehrs, C.; Stalla, G.; Peneva, K. Octreotide-mediated tumor-targeted drug delivery via a cleavable doxorubicin–peptide conjugate. Mol. Pharm. 2015, 12, 4290–4300. [Google Scholar] [CrossRef]
- Peruzzi, M.T.; Gallou, F.; Lee, S.J.; Gagné, M.R. Site selective amide reduction of cyclosporine A enables diverse derivation of an important cyclic peptide. Org. Lett. 2019, 21, 3451–3455. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.K.; Adhikari, N.; Amin, S.A.; Biswas, S.; Jha, T.; Ghosh, B. Small molecule drug conjugates (SMDCs): An emerging strategy for anticancer drug design and discovery. N. J. Chem. 2021, 45, 5291–5321. [Google Scholar] [CrossRef]
- Dong, H.; Limberakis, C.; Liras, S.; Price, D.; James, K. Peptidic macrocyclization via palladium-catalyzed chemoselective indole C-2 arylation. Chem. Commun. 2012, 48, 11644–11646. [Google Scholar] [CrossRef]
- Mendive-Tapia, L.; Preciado, S.; García, J.; Ramón, R.; Kielland, N.; Albericio, F.; Lavilla, R. New peptide architectures through C-H activation stapling between tryptophan-phenylalanine/tyrosine residues. Nat. Commun. 2015, 6, 7160–7169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendive-Tapia, L.; Bertran, A.; García, J.; Acosta, G.; Albericio, F.; Lavilla, R. Constrained cyclopeptides: Biaryl formation through Pd-catalyzed C-H activation in peptides-structural control of the cyclization vs. cyclodimerization outcome. Chem.–Eur. J. 2016, 22, 13114–13119. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, C.; Khamrakulov, M.; Wang, J.; Liu, H. Rhodium(III)-catalyzed C–H alkenylation: Access to maleimide-decorated tryptophan and tryptophan-containing peptides. Org. Lett. 2020, 22, 1535–1541. [Google Scholar] [CrossRef]
- Bai, Z.; Cai, C.; Sheng, W.; Ren, Y.; Wang, H. Late-stage peptide macrocyclization by palladium-catalyzed site-selective C–H olefination of tryptophan. Angew. Chem. Int. Ed. 2020, 59, 14686–14692. [Google Scholar] [CrossRef]
- Liu, L.; Fan, X.; Wang, B.; Deng, H.; Wang, T.; Zheng, J.; Chen, J.; Shi, Z.; Wang, H. PIII-Directed late-stage ligation and macrocyclization of peptides with olefins by rhodium catalysis. Angew. Chem. Int. Ed. 2022, 61, e202206177. [Google Scholar]
- Ruan, Z.; Sauermann, N.; Manoni, E.; Ackermann, L. Manganese-catalyzed C–H alkynylation: Expedient peptide synthesis and modification. Angew. Chem. Int. Ed. 2017, 56, 3172–3176. [Google Scholar] [CrossRef]
- Bai, Q.; Bai, Z.; Wang, H. Macrocyclization of biaryl-bridged peptides through late-stage palladium-catalyzed C(sp2)–H arylation. Org. Lett. 2019, 21, 8225–8228. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Li, B.; Qi, L.; Yang, P.; He, G.; Chen, G. Construction of cyclophane-braced peptide macrocycles via palladium-catalyzed picolinamide-directed intramolecular C(sp2)–H arylation. Org. Lett. 2020, 22, 6879–6883. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Cai, C.; Yu, Z.; Wang, H. Backbone-enabled directional peptide macrocyclization through late-stage palladium-catalyzed δ-C(sp2)–H olefination. Angew. Chem. Int. Ed. 2018, 57, 13912–13916. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, H.; He, Y.; Sheng, W.; Bai, Q.; Wang, H. Peptide-guided functionalization and macrocyclization of bioactive peptidosulfonamides by Pd(II)-catalyzed late-stage C–H activation. Nat. Commun. 2018, 9, 3383–3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Wu, J.; Liu, S.; Yao, H.; Wang, H. Macrocyclization of peptidoarylacetamides with self-assembly properties through late-stage palladium-catalyzed C(sp2) –H olefination. Sci. Adv. 2019, 5, eaaw0323. [Google Scholar]
- Bai, Z.; Wang, H. Backbone-enabled peptide macrocyclization through late-stage palladium-catalyzed C–H activation. Synlett 2020, 31, 199–204. [Google Scholar] [CrossRef]
- Liu, S.; Cai, C.; Bai, Z.; Sheng, W.; Tan, J.; Wang, H. Late-stage macrocyclization of bioactive peptides with internal oxazole motifs via palladium-catalyzed C–H olefination. Org. Lett. 2021, 23, 2933–2937. [Google Scholar] [CrossRef]
- Cai, C.; Wang, F.; Xiao, X.; Sheng, W.; Liu, S.; Chen, J.; Zheng, J.; Xie, R.; Bai, Z.; Wang, H. Macrocyclization of bioactive peptides with internal thiazole motifs via palladium-catalyzed C–H olefination. Chem. Commun. 2022, 58, 4861–4864. [Google Scholar] [CrossRef]
- Noisier, A.F.M.; Brimble, M.A. C–H functionalization in the synthesis of amino acids and peptides. Chem. Rev. 2014, 114, 8775–8806. [Google Scholar] [CrossRef]
- He, G.; Wang, B.; Nack, W.A.; Chen, G. Syntheses and transformations of α-amino acids via palladium-catalyzed auxiliary-directed sp3 C–H functionalization. Acc. Chem. Res. 2016, 49, 635–645. [Google Scholar] [CrossRef]
- Brandhofer, T.; Mancheño, O.G. Site-selective C–H bond activation/functionalization of alpha-amino acids and peptide-like derivatives. Eur. J. Org. Chem. 2018, 2018, 6050–6067. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, G.; Liu, T.; Giri, R.; Yu, J.-Q. Site-selective C(sp3)–H functionalization of di-, tri-, and tetrapeptides at the N-terminus. J. Am. Chem. Soc. 2014, 136, 16940–16946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noisier AF, M.; García, J.; Ionuţ, I.A.; Albericio, F. Stapled peptides by late-stage C(sp3)–H activation. Angew. Chem. Int. Ed. 2017, 56, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, Y.; Chen, H.; Sheng, W.; Wang, H. Synthesis of bioactive and stabilized cyclic peptides by macrocyclization using C(sp3)–H activation. Chem. Sci. 2017, 8, 4565–4570. [Google Scholar] [CrossRef] [Green Version]
- Daugulis, O.; Roane, J.; Tran, L.D. Bidentate, monoanionic auxiliary-directed functionalization of carbon–Hydrogen bonds. Acc. Chem. Res. 2015, 48, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, G.; Sun, M.; Mahankali, M.; Ma, Y.; Zhang, M.; Hua, W.; Hu, Y.; Wang, Q.; Chen, J.; et al. A general strategy for synthesis of cyclophane-braced peptide macrocycles via palladium-catalysed intramolecular sp3 C–H arylation. Nat. Chem. 2018, 10, 540–548. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Han, B.; Chen, Z.; Zhang, X.; He, G.; Chen, G. Construction of natural-product-like cyclophane-braced peptide macrocycles via sp3 C–H arylation. J. Am. Chem. Soc. 2019, 141, 9401–9407. [Google Scholar] [CrossRef]
- Li, X.; Qi, L.; Li, B.; Zhao, Z.; He, G.; Chen, G. Synthesis of cyclophane-braced peptide macrocycles via palladium-catalyzed intramolecular C(sp3)–H arylation of N-methyl alanine at C-termini. Org. Lett. 2020, 22, 6209–6213. [Google Scholar] [CrossRef]


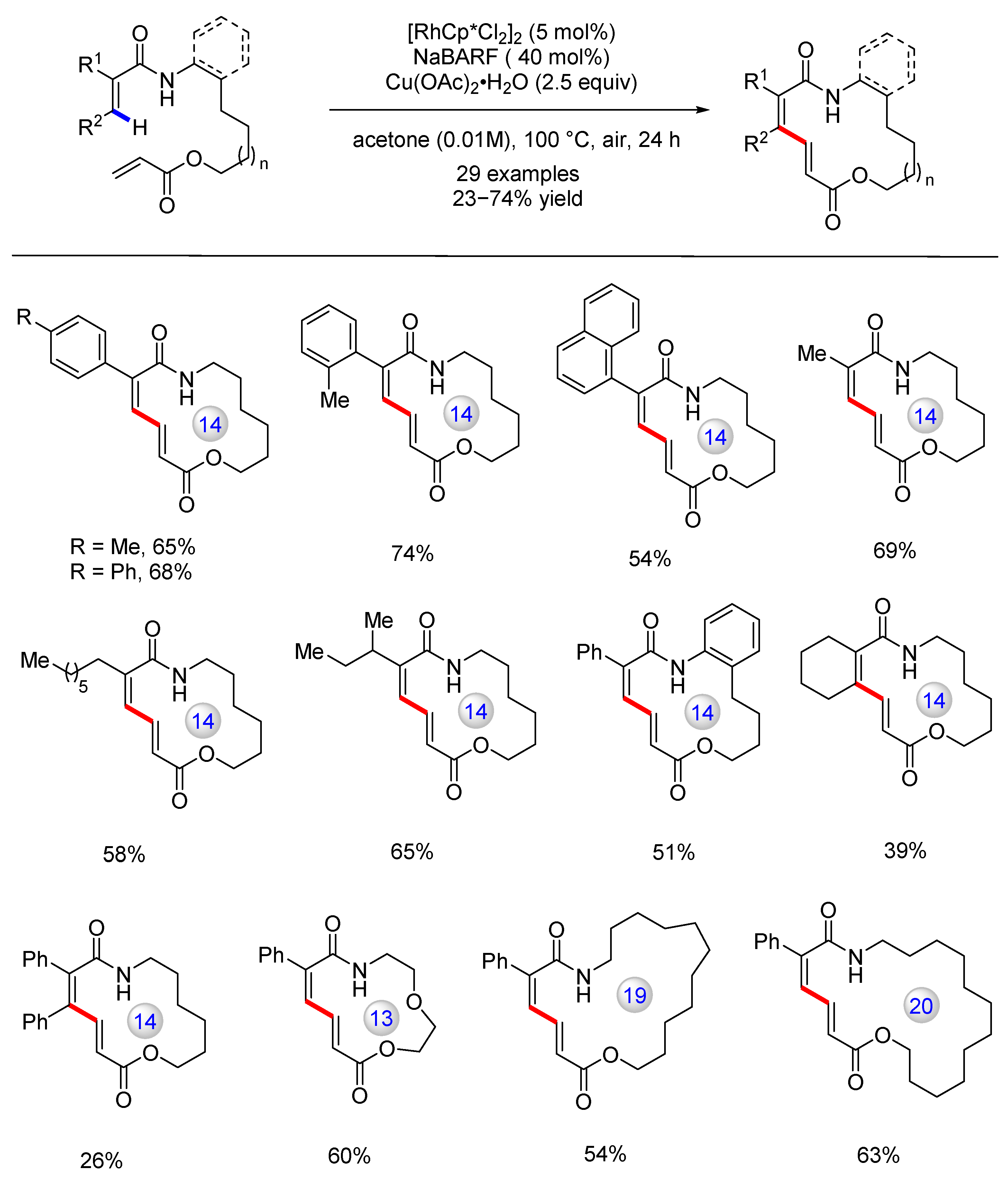
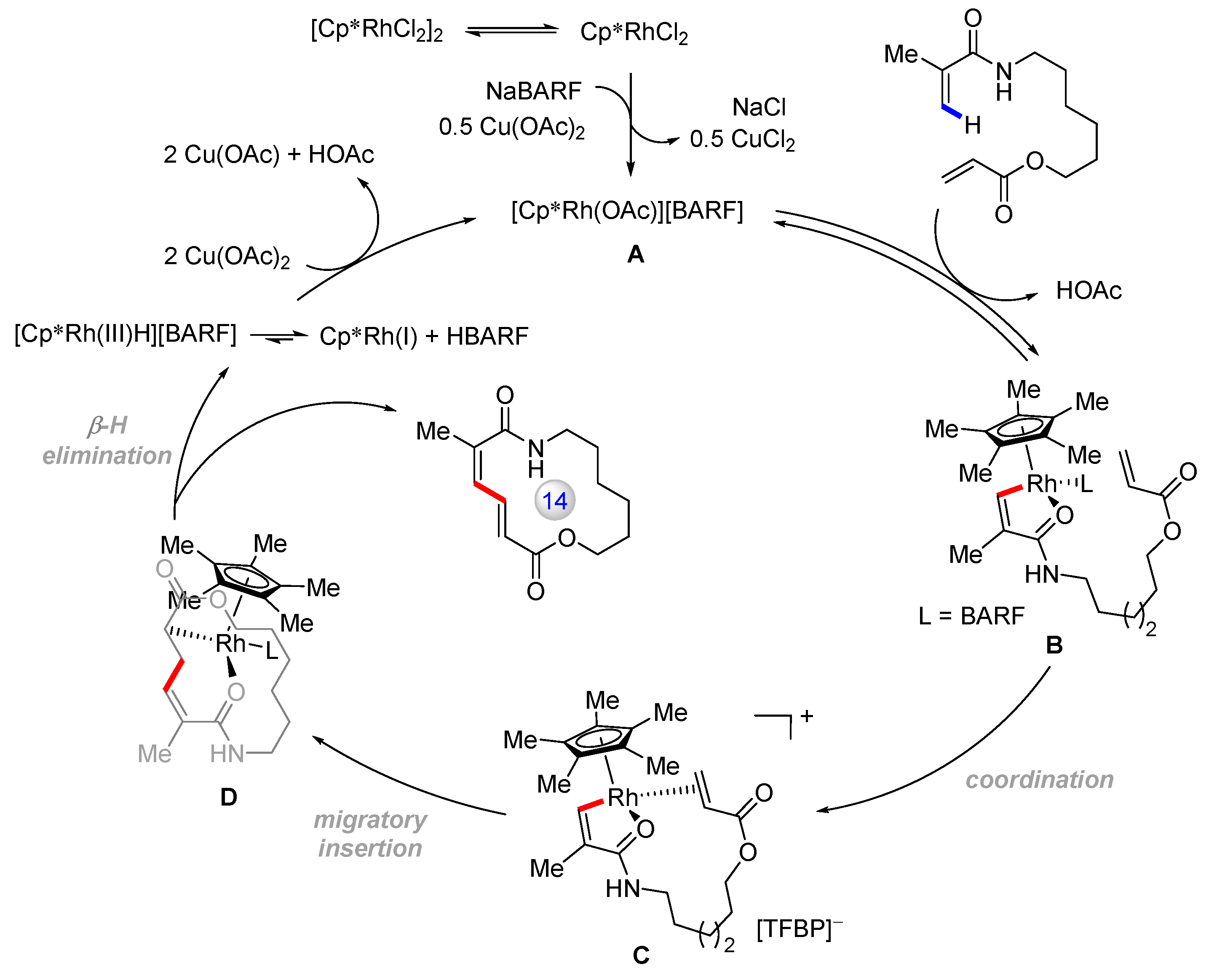
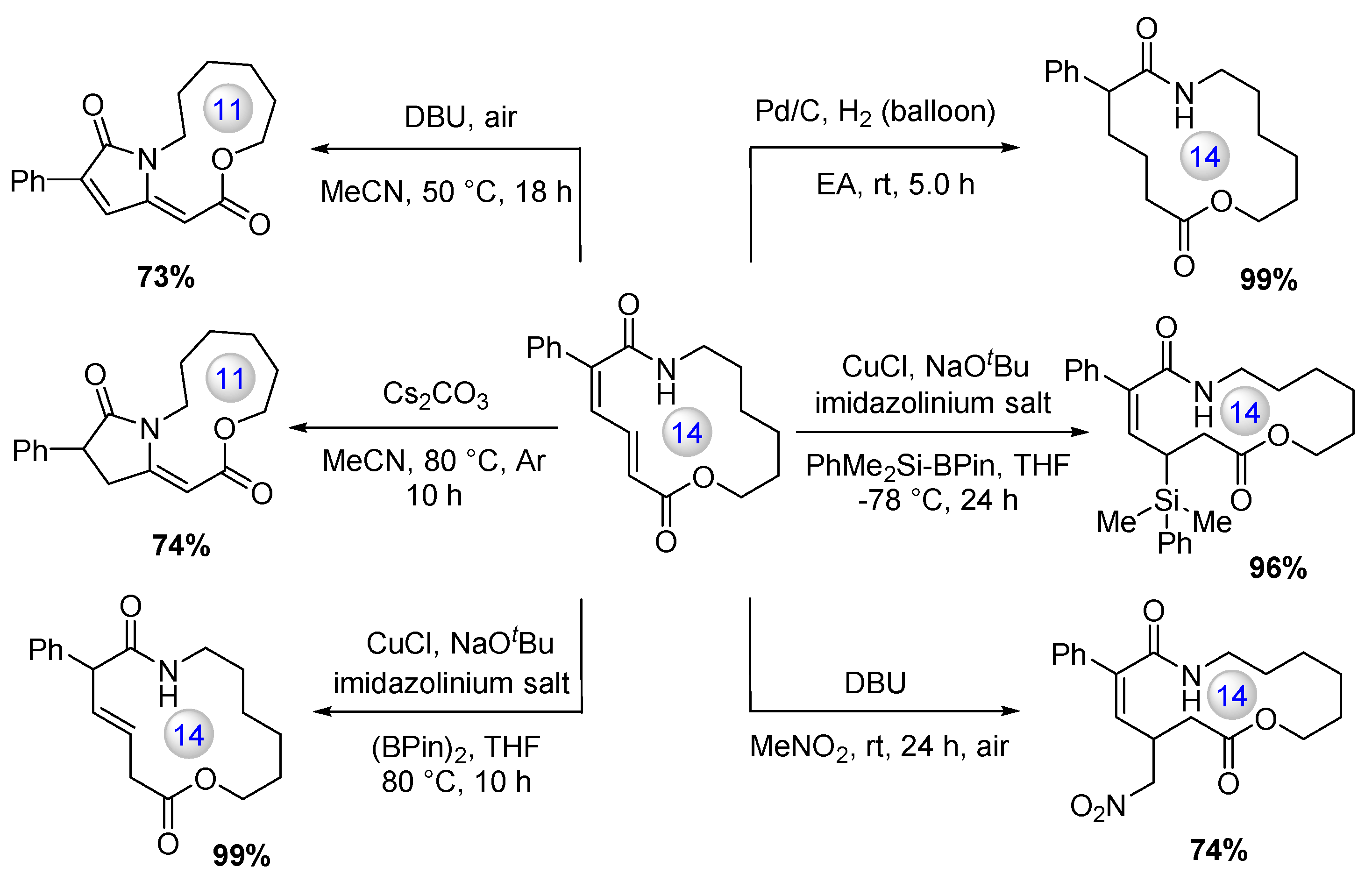
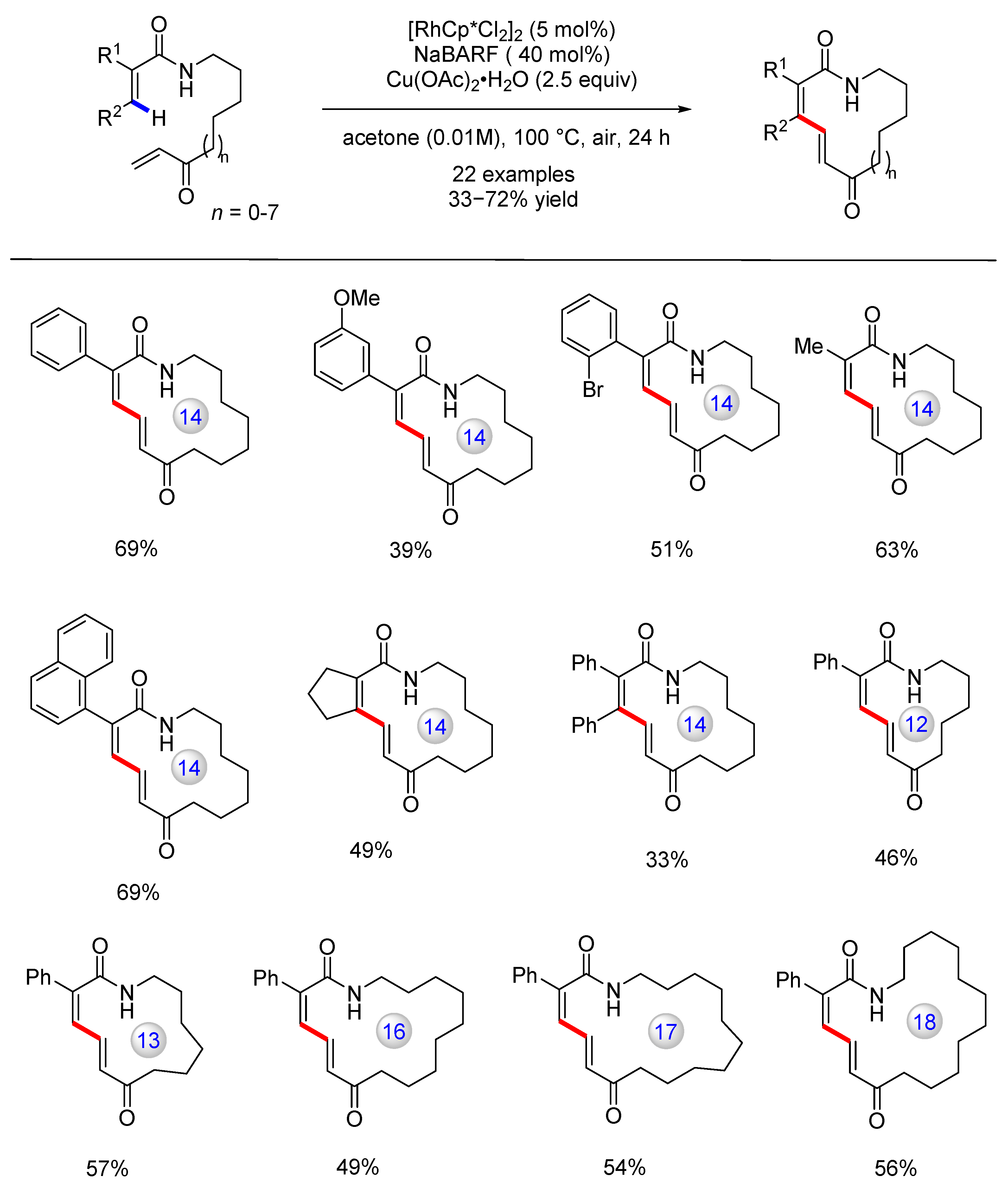





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lu, M.-Z.; Loh, T.-P. Transition-Metal-Catalyzed C–C Bond Macrocyclization via Intramolecular C–H Bond Activation. Catalysts 2023, 13, 438. https://doi.org/10.3390/catal13020438
Wang X, Lu M-Z, Loh T-P. Transition-Metal-Catalyzed C–C Bond Macrocyclization via Intramolecular C–H Bond Activation. Catalysts. 2023; 13(2):438. https://doi.org/10.3390/catal13020438
Chicago/Turabian StyleWang, Xiao, Ming-Zhu Lu, and Teck-Peng Loh. 2023. "Transition-Metal-Catalyzed C–C Bond Macrocyclization via Intramolecular C–H Bond Activation" Catalysts 13, no. 2: 438. https://doi.org/10.3390/catal13020438
APA StyleWang, X., Lu, M.-Z., & Loh, T.-P. (2023). Transition-Metal-Catalyzed C–C Bond Macrocyclization via Intramolecular C–H Bond Activation. Catalysts, 13(2), 438. https://doi.org/10.3390/catal13020438






