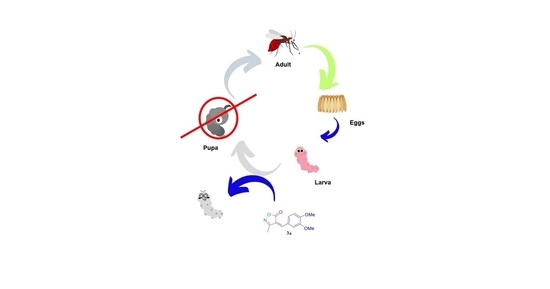
Continuous Flow Photochemical Synthesis of 3-Methyl-4-arylmethylene Isoxazole-5(4H)-ones through Organic Photoredox Catalysis and Investigation of Their Larvicidal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solubility Evaluation under Microwave Heating
2.2. Screening of Light Source in the MCR Photoinduced Batch Procedure
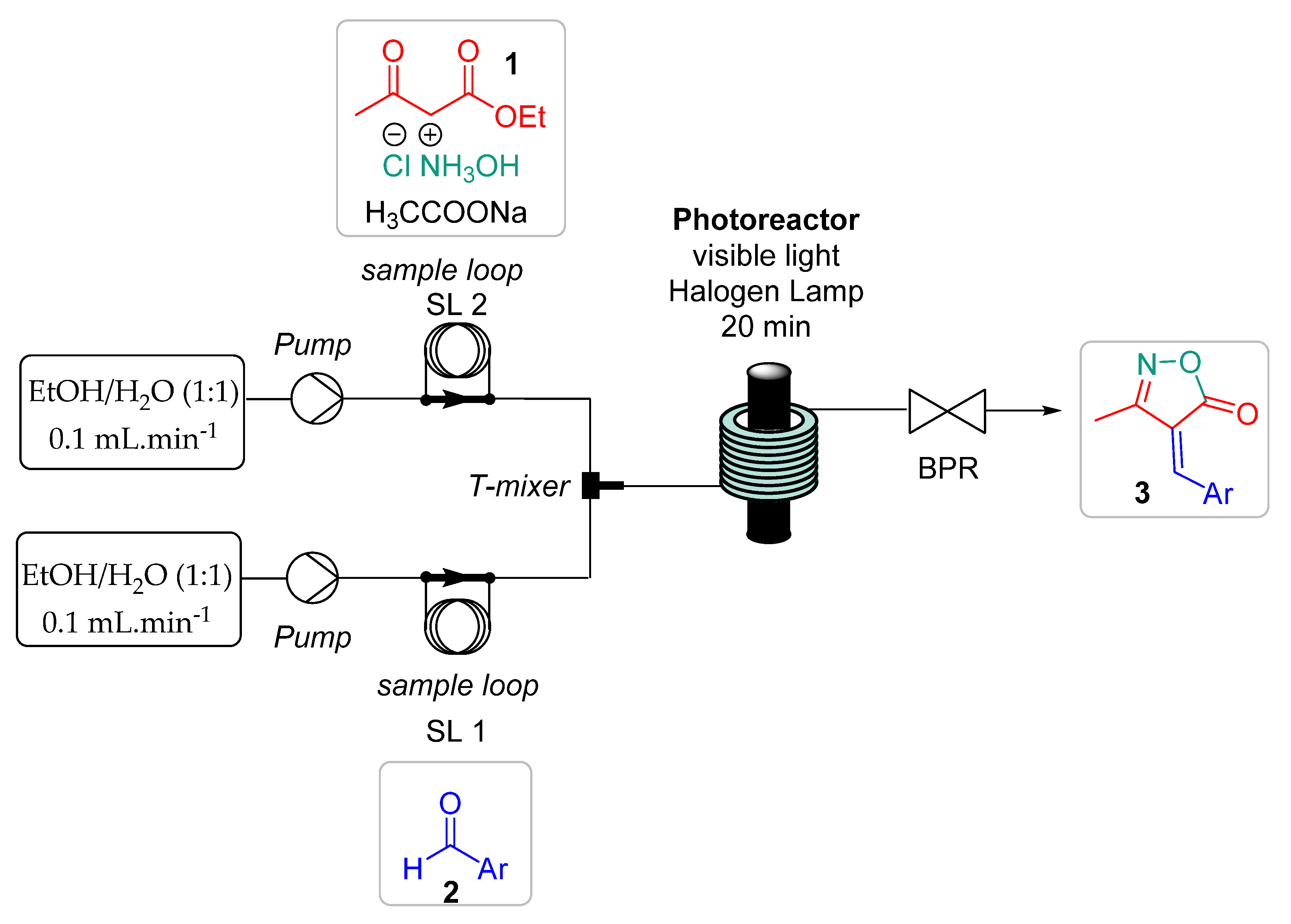
2.3. Continuous Flow Photochemistry Protocol for the Synthesis of Isoxazole-5(4H)-one Derivatives
2.4. Larvicidal Tests against Ae. aegypti
2.5. Mechanistic Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabir, S.; Alhazza, M.I.; Ibrahim, A.A. A review on heterocyclic moieties and their applications. Catal. Sustain. Energy 2016, 2, 99–115. [Google Scholar] [CrossRef]
- Prandi, C.; Occhiato, E.G. From synthetic control to natural products: A focus on N-heterocycles. Pest Manag. Sci. 2019, 75, 2385–2402. [Google Scholar] [CrossRef] [PubMed]
- Qadir, T.; Amin, A.; Sharma, P.K.; Jeelani, I.; Abe, H. A review on medicinally important heterocyclic compounds. Open Med. Chem. J. 2022, 16, e187410452202280. [Google Scholar] [CrossRef]
- Lamberth, C. Pyridazine chemistry in crop protection. J. Heterocycl. Chem. 2017, 54, 2974–2984. [Google Scholar] [CrossRef]
- Pozharskii, A.F.; Katritzky, A.R.; Soldatenkov, A. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications, 2nd ed.; John Wiley & Sons Ltd.: London, UK, 2011. [Google Scholar]
- Pathan, S.I.; Chundawat, N.S.; Chauhan, N.P.S.; Singh, G.P. A review on synthetic approaches of heterocycles via insertion-cyclization reaction. Synth. Commun. 2020, 50, 1251–1285. [Google Scholar] [CrossRef]
- Lv, Y.; Meng, J.; Li, C.; Wang, X.; Ye, Y.; Sun, K. Update on the synthesis of N-heterocycles via cyclization of hydrazones (2017–2021). Adv. Synth. Catal. 2021, 363, 5235–5265. [Google Scholar] [CrossRef]
- Jiang, B.; Rajale, T.; Wever, W.; Tu, S.J.; Li, G. Multicomponent reactions for the synthesis of heterocycles. Chem. Asian J. 2010, 5, 2318–2335. [Google Scholar] [CrossRef]
- Nishanth Rao, R.; Jena, S.; Mukherjee, M.; Maiti, B.; Chanda, K. Green synthesis of biologically active heterocycles of medicinal importance: A review. Environ. Chem. Lett. 2021, 19, 3315–3358. [Google Scholar] [CrossRef]
- Eycken, E.V.; Sharma, U.K. Green synthesis of heterocycles via MCRs. In Multicomponent Reactions towards Heterocycles: Concepts and Applications; Wiley-VCH GmbH: Weinheim, Germany, 2021; pp. 163–209. [Google Scholar]
- Eycken, E.V.; Sharma, U.K. The use of flow chemistry in the multicomponent synthesis of heterocycles. In Multicomponent Reactions towards Heterocycles: Concepts and Applications; Wiley-VCH GmbH: Weinheim, Germany, 2021; pp. 211–235. [Google Scholar]
- Rehm, T.H. Flow photochemistry as a tool in organic synthesis. Chem. Eur. J. 2020, 26, 16952–16974. [Google Scholar] [CrossRef]
- Reihani, N.; Kiyani, H. Three-component synthesis of 4-arylidene-3-alkylisoxazol-5(4H)-ones in the presence of potassium 2,5-dioxoimidazolidin-1-ide. Curr. Org. Chem. 2021, 25, 950–962. [Google Scholar] [CrossRef]
- Atharifar, H.; Keivanloo, A.; Maleki, B. Greener synthesis of 3,4-disubstituted isoxazole-5(4H)-ones in a deep eutectic solvent. Org. Prep. Proc. Int. 2020, 52, 517–523. [Google Scholar] [CrossRef]
- Agrawal, N.; Mishra, P. The synthetic and therapeutic expedition of isoxazole and its analogs. Med. Chem. Res. 2018, 27, 1309–1344. [Google Scholar] [CrossRef] [Green Version]
- Vergelli, C.; Schepetkin, I.A.; Crocetti, L.; Iacovone, A.; Giovannoni, M.P.; Guerrini, G.; Khlebnikov, A.I.; Ciattini, S.; Ciciani, G.; Quinn, M.T. Isoxazol-5(2H)-one: A new scaffold for potent human neutrophil elastase (HNE) inhibitors. J. Enz. Inhib. Med. Chem. 2017, 32, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Da Silva-Alves, D.C.; dos Anjos, J.V.; Cavalcante, N.N.; Santos, G.K.; Navarro, D.M.; Srivastava, R.M. Larvicidal isoxazoles: Synthesis and their effective susceptibility towards Aedes aegypti larvae. Bioorg. Med. Chem. 2013, 21, 940–947. [Google Scholar] [CrossRef]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Liu-Helmersson, J.; Brännström, Å.; Sewe, M.O.; Semenza, J.C.; Rocklöv, J. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front. Public Health 2019, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Bortel, W.V.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant natural products for the control of Aedes aegypti: The main vector of important arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wang, C.; Zhang, M.Y.; Cui, P.L.; Li, Y.M.; Zhou, X.; Li, J.C. Condensation reactions of aromatic aldehydes with 3-methyl-4,5-dihydroisoxazol-5-one without solvent and catalyst. Chin. J. Org. Chem. 2008, 28, 914–917. [Google Scholar]
- Zhang, Y.Q.; Ma, J.J.; Wang, C.; Li, J.C.; Zhang, D.N.; Zang, X.H.; Li, J. One-pot synthesis of 3-methyl-4-arylmethylene-isoxazol-5(4H)-ones. Chin. J. Org. Chem. 2008, 28, 141–144. [Google Scholar]
- Liu, Q.; Zhang, Y.N. One-pot synthesis of 3-Methyl-4-arylmethylene-isoxazol-5(4H)-ones catalyzed by sodium benzoate in aqueous media: A green chemistry strategy. Bull. Kor. Chem. Soc. 2011, 32, 3559–3560. [Google Scholar] [CrossRef] [Green Version]
- Ghogare, R.S.; Patankar-Jain, K.; Momin, S.A.H. A simple and efficient protocol for the synthesis of 3,4-disubstituted isoxazol-5(4H)-ones catalyzed by succinic acid using water as green reaction medium. Lett. Org. Chem. 2021, 18, 83–87. [Google Scholar] [CrossRef]
- Maleki, B.; Chahkandi, M.; Tayebee, R.; Kahrobaei, S.; Alinezhad, H.; Hemmati, S. Synthesis and characterization of nanocrystalline hydroxyapatite and its catalytic behavior towards synthesis of 3,4-disubstituted isoxazole-5(4H)-ones in water. Appl. Organometal. Chem. 2019, 33, e5118. [Google Scholar] [CrossRef]
- Oliveira, G.H.C.; Ramos, L.M.; Paiva, R.K.C.; Passos, S.T.A.; Simões, M.M.; Machado, F.; Correa, J.R.; Neto, B.A.D. Synthetic enzyme-catalyzed multicomponent reaction for isoxazol-5(4H)-one syntheses, their properties and biological application. Why to study mechanisms? Org. Biomol. Chem. 2020, 19, 1514–1531. [Google Scholar] [CrossRef]
- Saikh, F.; Das, J.; Ghosh, S. Synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones by visible light in aqueous ethanol. Tetrahedron Lett. 2013, 54, 4679–4682. [Google Scholar] [CrossRef]
- Gadkari, Y.U.; Jadhav, N.L.; Hatvate, N.T.; Telvekar, V.N. Concentrated solar radiation aided green approach for preparative scale and solvent-free synthesis of 3-methyl-4-(hetero)arylmethylene isoxazole-5(4H)-ones. ChemistrySelect 2020, 5, 12320–12323. [Google Scholar] [CrossRef]
- Damn, M.; Glasnov, T.N.; Kappe, C.O. Translating high-temperature microwave chemistry to scalable continuous flow process. Org. Process Res. Dev. 2010, 14, 215–224. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Kappe, C.O. The microwave-to-flow paradigm: Translating high-temperature batch microwave chemistry to scalable continuous-flow processes. Chem. Eur. J. 2011, 17, 11956–11968. [Google Scholar] [CrossRef]
- Protti, S.; Ravelli, D.; Fagnoni, M. Wavelength dependence and wavelength selectivity in photochemical reactions. Photochem. Photobiol. Sci. 2019, 18, 2094–2101. [Google Scholar] [CrossRef]
- Kerzig, C.; Wenger, S. Reactivity control of a photocatalytic system by changing the light intensity. Chem. Sci. 2019, 10, 11023. [Google Scholar] [CrossRef] [Green Version]
- Kundu, B.K.; Das, M.; Ganguly, R.; Bhobe, P.A.; Mukhopadhyay, S. Role of zeolite encapsulated Cu(II) complexes in electron transfer as well as peroxy radical intermediates formation during oxidation of thioanisole. J. Catal. 2020, 289, 305–316. [Google Scholar] [CrossRef]
- Brandão, P.; Pinheiro, M.; Melo, T.M.V.D.P. Flow chemistry: Towards a more sustainable heterocyclic synthesis. Eur. J. Org. Chem. 2019, 43, 7187–7217. [Google Scholar] [CrossRef]
- Rossett, I. Flow chemistry: New concepts from batch to continuous organic chemistry. Ind. Chem. 2016, 2, 1000e102. [Google Scholar] [CrossRef] [Green Version]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. Guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Andrade, C.K.Z.; Dar, A.R. Applying green processes and techniques to simplify reaction work-ups. Tetrahedron 2016, 72, 7375–7391. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; WHO: Geneva, Switzerland, 2005; pp. 1–41. [Google Scholar]
- De Sousa, D.P.; Vieira, Y.W.; Uliana, M.P.; Melo, M.A.; Brocksom, T.J.; Cavalcanti, S.C.H. Larvicidal activity of para-Benzoquinones. Parasitol. Res. 2010, 107, 741–745. [Google Scholar] [CrossRef]
- Diretrizes Nacionais para a Prevenção e Controle de Epidemias de Dengue; Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica–Brasília: Ministério da Saúde, Brazil, 2009; ISBN 978-85-334-1602-4.
- Kundu, B.K.; Han, G.; Sun, Y. Derivatized Benzothiazoles as Two-Photon-Absorbing Organic Photosensitizers Active under Near Infrared Light Irradiation. J. Am. Chem. Soc. 2023, 145, 3535–3542. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, J.; Chattopadhyay, S. A novel light induced Knoevenagel condensation of Meldrum’s acid with aromatic aldehydes in aqueous ethanol. Tetrahedron Lett. 2011, 52, 2869–2872. [Google Scholar] [CrossRef]
- Silva, R.; Demarque, D.P.; Dusi, R.G.; Sousa, J.P.B.; Albernaz, L.C.; Espíndola, L.S. Residual larvicidal activity of quinones against Aedes aegypti. Molecules 2020, 25, 3978. [Google Scholar] [CrossRef]






| Entry | Solvent a | Product Yield (%) b |
|---|---|---|
| 1 | H2O | 91 |
| 2 | EtOH/H2O (1:1) | 77 |
| 3 | EtOH | 52 |
| 4 | EtOAc | 68 |
| 5 | DMF | Trace |

| Entry | Light Source | Time (h) | Yield (%) a |
|---|---|---|---|
| 1 | No light | 24 | 14 |
| 2 | Ambient light | 24 | 26 |
| 3 | 150 W halogen lamp b | 10 min | 90 |
| 4 | 30 W white LED bulb c | 24 | 18 |
| 5 | 15 W blue LED bulb d | 24 | 56 |
| 6 | 6 W ultraviolet lamp e | 6 | 77 |
| 7 | 150 W halogen lamp b with TEMPO | 10 min | 0 |
| Entry | Isoxazol-5(4H)-ones | Yield (%) |
|---|---|---|
| 1 |  | 96 |
| 2 |  | 88 |
| 3 |  | 52 |
| 4 |  | 34 |
| 5 |  | 69 |
| 6 |  | 50 |
| 7 |  | 43 |
| 8 |  | 30 |
| 9 |  | 0 |
| 10 |  | 0 |
| Entry | Compound | Final Volume (mL) | Number of Larvae | LC50 (µg/mL) (95% CI a) | ||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||||
| 1 | 3a | 10 | 25 | 8.064 | 7.346 | 7.128 |
| 2 | 3b | 10 | 25 | 18.73 | 16.76 | 16.51 |
| 3 | 3c | 3 | 10 | - b | - b | - b |
| 4 | 3d | 3 | 10 | - b | - b | - b |
| 5 | 3e | 10 | 25 | 8.639 | 4.633 | 4.546 |
| 6 | 3f | 3 | 10 | - c | - c | - c |
| 7 | 3g | 3 | 10 | - c | - c | - c |
| 8 | 3h | 3 | 10 | - b | - b | - b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio, A.B.S.; Mori, M.S.S.; Albernaz, L.C.; Espindola, L.S.; Salvador, C.E.M.; Andrade, C.K.Z. Continuous Flow Photochemical Synthesis of 3-Methyl-4-arylmethylene Isoxazole-5(4H)-ones through Organic Photoredox Catalysis and Investigation of Their Larvicidal Activity. Catalysts 2023, 13, 518. https://doi.org/10.3390/catal13030518
Sampaio ABS, Mori MSS, Albernaz LC, Espindola LS, Salvador CEM, Andrade CKZ. Continuous Flow Photochemical Synthesis of 3-Methyl-4-arylmethylene Isoxazole-5(4H)-ones through Organic Photoredox Catalysis and Investigation of Their Larvicidal Activity. Catalysts. 2023; 13(3):518. https://doi.org/10.3390/catal13030518
Chicago/Turabian StyleSampaio, Ana Beatriz S., Mônica Shigemi S. Mori, Lorena C. Albernaz, Laila S. Espindola, Carlos Eduardo M. Salvador, and Carlos Kleber Z. Andrade. 2023. "Continuous Flow Photochemical Synthesis of 3-Methyl-4-arylmethylene Isoxazole-5(4H)-ones through Organic Photoredox Catalysis and Investigation of Their Larvicidal Activity" Catalysts 13, no. 3: 518. https://doi.org/10.3390/catal13030518
APA StyleSampaio, A. B. S., Mori, M. S. S., Albernaz, L. C., Espindola, L. S., Salvador, C. E. M., & Andrade, C. K. Z. (2023). Continuous Flow Photochemical Synthesis of 3-Methyl-4-arylmethylene Isoxazole-5(4H)-ones through Organic Photoredox Catalysis and Investigation of Their Larvicidal Activity. Catalysts, 13(3), 518. https://doi.org/10.3390/catal13030518