Recent Advances in the Efficient Synthesis of Useful Amines from Biomass-Based Furan Compounds and Their Derivatives over Heterogeneous Catalysts
Abstract
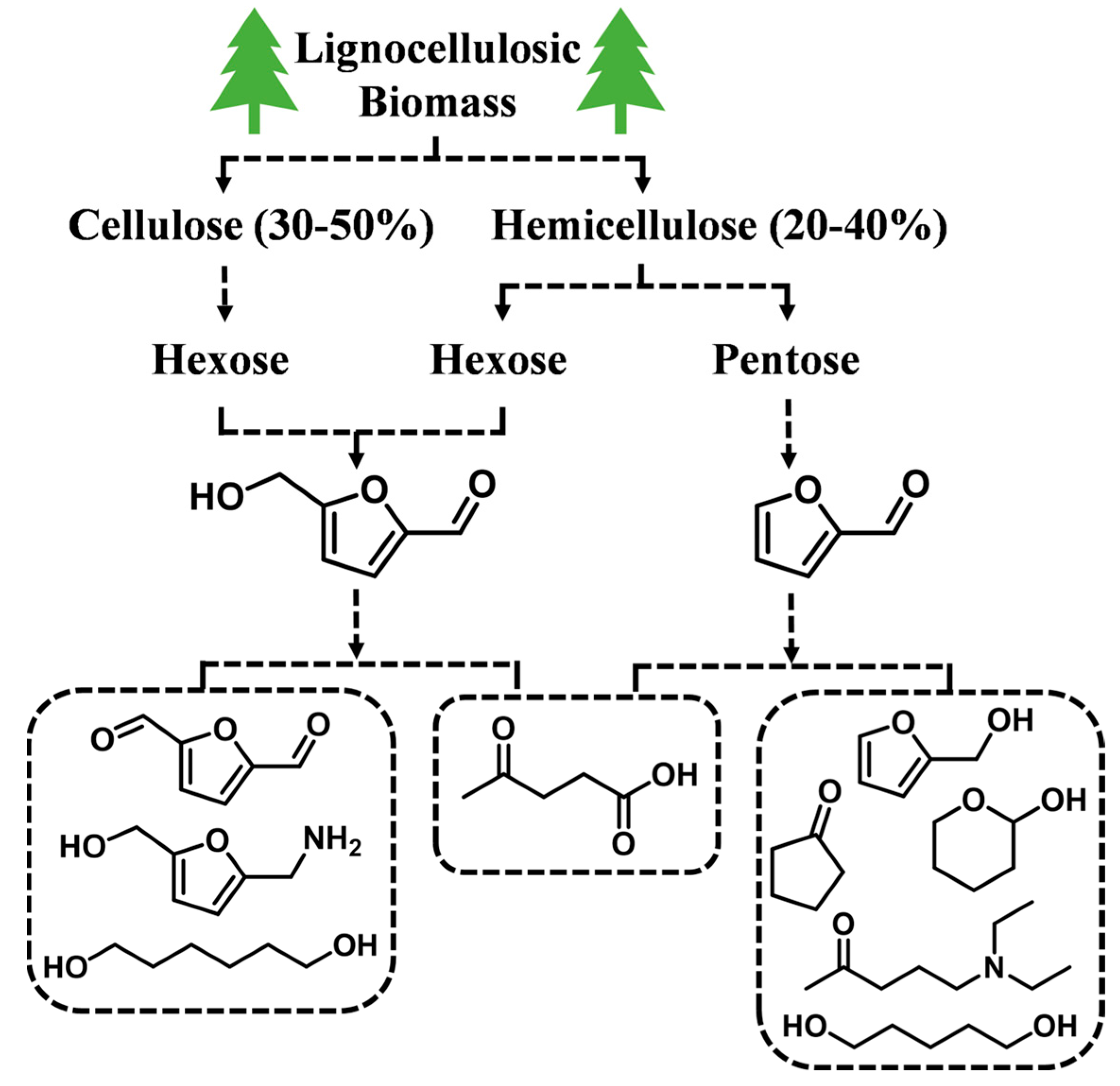
:1. Introduction
2. Reductive Amination of Bio-Based Furanic Aldehydes and Ketones
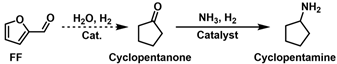
2.1. Reductive Amination of FF
2.1.1. Reductive Amination of FF with NH3
2.1.2. Reductive Amination of FF with Aniline
2.1.3. Reductive Amination of FF with HCOONH4
2.2. Reductive Amination of HMF
2.2.1. Reductive Amination of HMF with NH3
2.2.2. Reductive Amination of HMF with Aniline
2.3. Reductive Amination of Other Bio-Based Furanic Aldehydes and Ketones
2.3.1. Reductive Amination of 2-Hydroxytetrahydropyran (2-HTHP)
2.3.2. Reductive Amination of Cyclopentanone and 5-Diethylamino-2-Pentanone
2.3.3. Reductive Amination of 2,5-Diformylfuran (DFF)
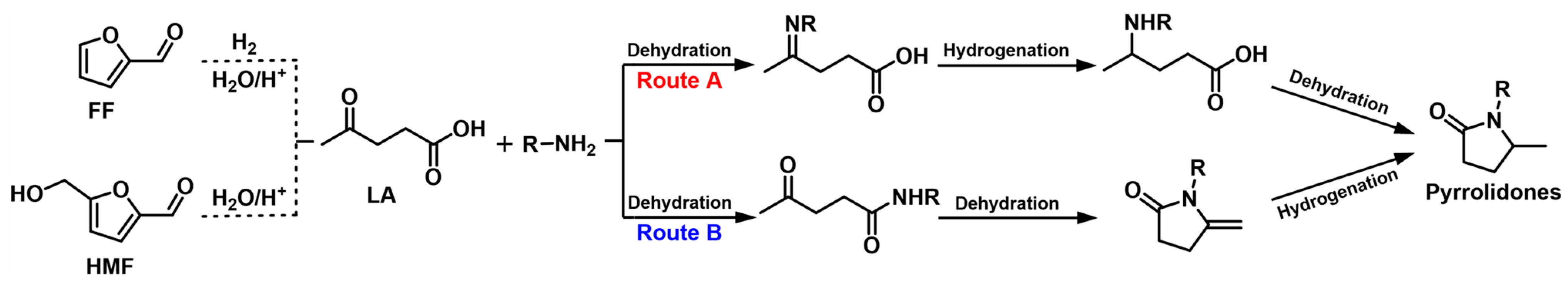
3. Reductive Amination of Levulinic Acid (LA)
3.1. Reductive Amination of LA with NH3 or Primary Amines
3.2. Co-Reductive Amination of LA with Nitrile or Aldehyde
4. Hydrogen-Borrowing Amination of Bio-Based Furanic Alcohols
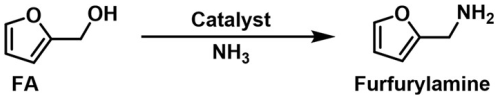
4.1. Hydrogen-Borrowing Amination of Furfuryl Alcohol (FA)
4.2. Hydrogen-Borrowing Amination of Bio-Based Furanic Alkanediols
4.2.1. Hydrogen-Borrowing Amination of 1,5-Pentanediol (PDO)
4.2.2. Hydrogen-Borrowing Amination of 1,6-Hexanediol (HDO)
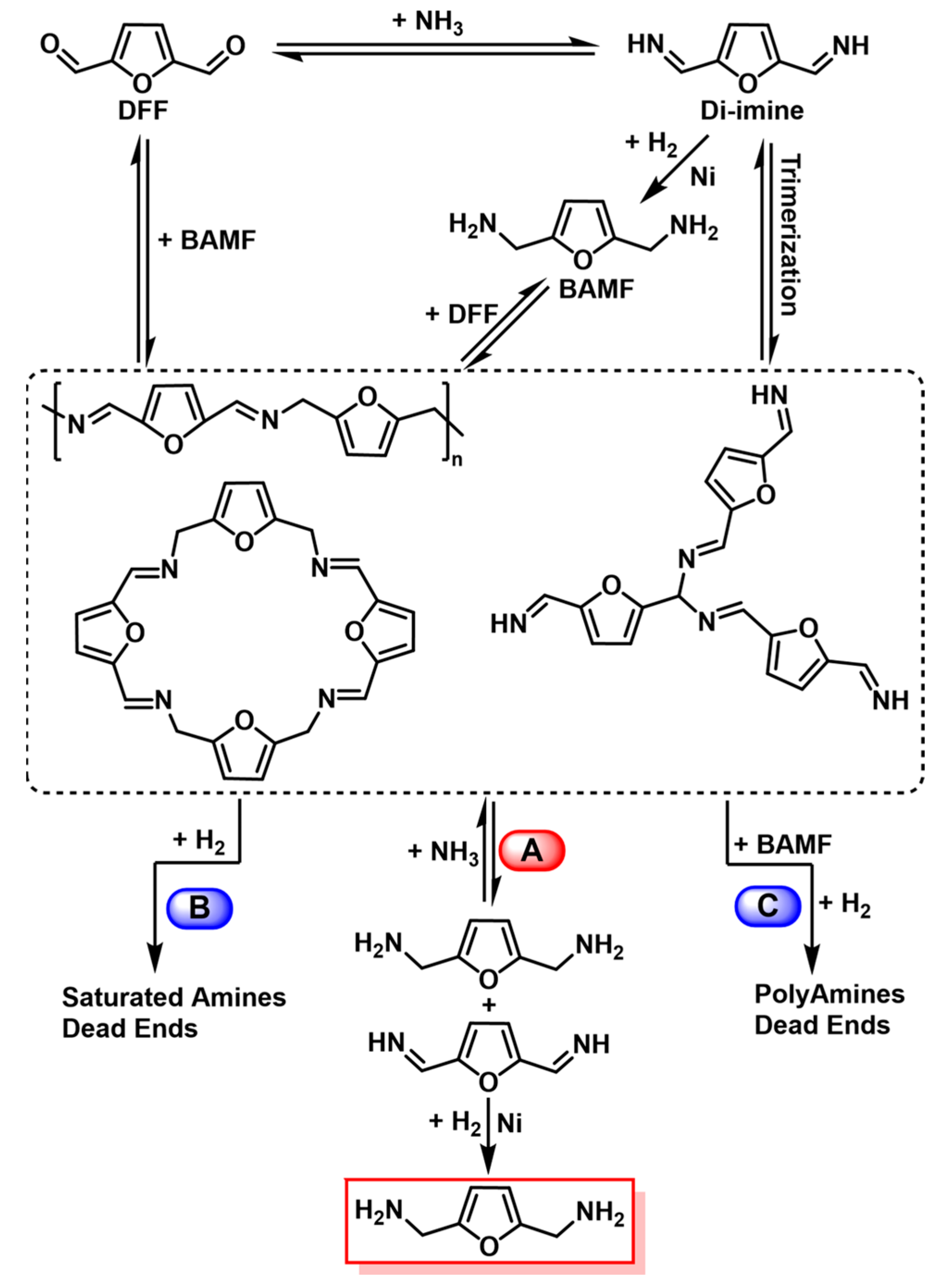
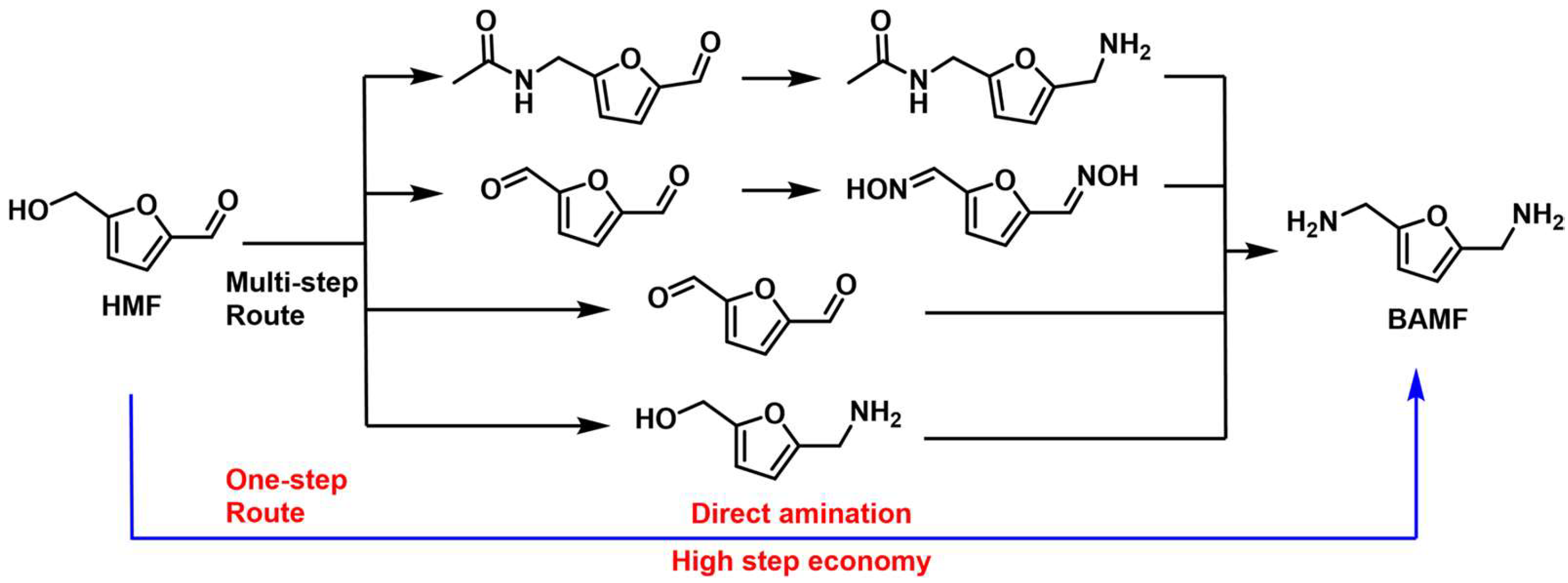
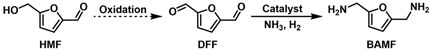
4.3. Reductive Amination and Hydrogen-Borrowing Amination of HMF to Synthesize BAMF
5. Conclusions and Prospects
5.1. Development of the Catalyst
5.2. Optimization of Reaction Conditions and Process
5.3. Studies on Reaction Mechanism and Kinetics
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FF | furfural |
| HMF | 5-hydroxymethylfurfural |
| FAM | furfurylamine |
| SACs | single-atom catalysts |
| NPs | nanoparticles |
| HMFA | 5-(hydroxymethyl)-2-furfurylamine |
| 2-HTHP | 2-hydroxytetrahydropyran |
| 5-AP | 5-amino-1-pentano |
| HAP | hydroxyapatite |
| ATP | attapulgite |
| IM | impregnation |
| DP | deposition–precipitation |
| CP | coprecipitation |
| DFF | 2,5-diformylfuran |
| BAMF | 2,5-bis(aminomethyl)furan |
| LA | levulinic acid |
| 5-MeP | 5-methyl-2-pyrrolidone |
| ALD | atomic layer deposition |
| FA | furfuryl alcohol |
| PDO | 1,5-pentanediol |
| HDO | 1,6-hexanediol |
| HAD | 1,6-Hexanediamine |
| TMHDA | N,N,N′,N′-tetramethyl-1,6-hexanediamine |
References
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [PubMed]
- Feng, Y.; Long, S.; Tang, X.; Sun, Y.; Luque, R.; Zeng, X.; Lin, L. Earth-abundant 3d-transition-metal catalysts for lignocellulosic biomass conversion. Chem. Soc. Rev. 2021, 50, 6042–6093. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Gerardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.M. Continuous flow upgrading of selected C2-C6 platform chemicals derived from biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Mura, M.G.; Luca, L.D.; Giacomelli, G.; Porcheddu, A. Formic acid: A promising bio-renewable feedstock for fine chemicals. Adv. Synth. Catal. 2012, 354, 3180–3186. [Google Scholar] [CrossRef]
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar]
- Bhaumik, P.; Dhepe, P.L. Solid acid catalyzed synthesis of furans from carbohydrates. Catal. Rev. 2016, 58, 36–112. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Jiang, S.; Ramdani, W.; Muller, E.; Ma, C.; Pera-Titus, M.; Jerome, F.; De Oliveira Vigiera, K. Direct catalytic conversion of furfural to furan-derived amines in the presence of Ru-based catalyst. ChemSusChem 2020, 13, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Senthamarai, T.; Chandrashekhar, V.G.; Natte, K.; Kamer, P.C.J.; Beller, M.; Jagadeesh, R.V. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines. Chem. Soc. Rev. 2020, 49, 6273–6328. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Liu, S.; Song, K.; Yang, S.; Riisager, A. Sustainable access to renewable N-containing chemicals from reductive amination of biomass-derived platform compounds. Green Chem. 2020, 22, 6714–6747. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Wang, J. Lignocellulosic biomass upgrading into valuable nitrogen-containing compounds by heterogeneous catalysts. Ind. Eng. Chem. Res. 2020, 59, 17008–17025. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrashekhar, V.G.; Baumann, W.; Beller, M.; Jagadeesh, R.V. Nickel-catalyzed hydrogenative coupling of nitriles and amines for general amine synthesis. Science 2022, 376, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.P.; Boutevin, B. Biobased amines: From synthesis to polymers; present and future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, S.; Li, H.; Gözaydın, G.; Yan, N. Expanding the boundary of biorefinery: Organonitrogen chemicals from biomass. Acc. Chem. Res. 2021, 54, 1711–1722. [Google Scholar] [CrossRef]
- Gupta, N.K.; Reif, P.; Palenicek, P.; Rose, M. Toward renewable amines: Recent advances in the catalytic amination of biomass-derived oxygenates. ACS Catal. 2022, 12, 10400–10440. [Google Scholar] [CrossRef]
- Pelckmans, M.; Renders, T.; Van de Vyver, S.; Sels, B.F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 2017, 19, 5303–5331. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Shi, F. Catalytic amination of biomass-based alcohols. ChemSusChem 2014, 7, 720–722. [Google Scholar] [CrossRef]
- Saini, M.K.; Kumar, S.; Li, H.; Babu, S.A.; Saravanamurugan, S. Advances in the catalytic reductive amination of furfural to furfural amine: The momentous role of active metal sites. ChemSusChem 2022, 15, e202200107. [Google Scholar] [CrossRef] [PubMed]
- Bukhtiyarova, M.V.; Bukhtiyarova, G.A. Reductive amination of levulinic acid or its derivatives to pyrrolidones over heterogeneous catalysts in the batch and continuous flow reactors: A review. Renew. Sustain. Energy Rev. 2021, 143, 110876. [Google Scholar] [CrossRef]
- Truong, C.C.; Mishra, D.K.; Suh, Y.W. Recent catalytic advances on the sustainable production of primary furanic amines from the one-pot reductive amination of 5-hydroxymethylfurfural. ChemSusChem 2023, 16, e202201846. [Google Scholar] [CrossRef]
- Xie, C.; Song, J.L.; Wu, H.R.; Hu, Y.; Liu, H.Z.; Zhang, Z.R.; Zhang, P.; Chen, B.F.; Han, B.X. Ambient reductive amination of levulinic acid to pyrrolidones over Pt nanocatalysts on porous TiO2 nanosheets. J. Am. Chem. Soc. 2019, 141, 4002–4009. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, F.; Zhang, L.; Li, L.; Su, Y.; Yang, J.; Hao, R.; Wang, A.; Zhang, T. Modulating trans-imination and hydrogenation towards the highly selective production of primary diamines from dialdehydes. Green Chem. 2020, 22, 6897–6901. [Google Scholar] [CrossRef]
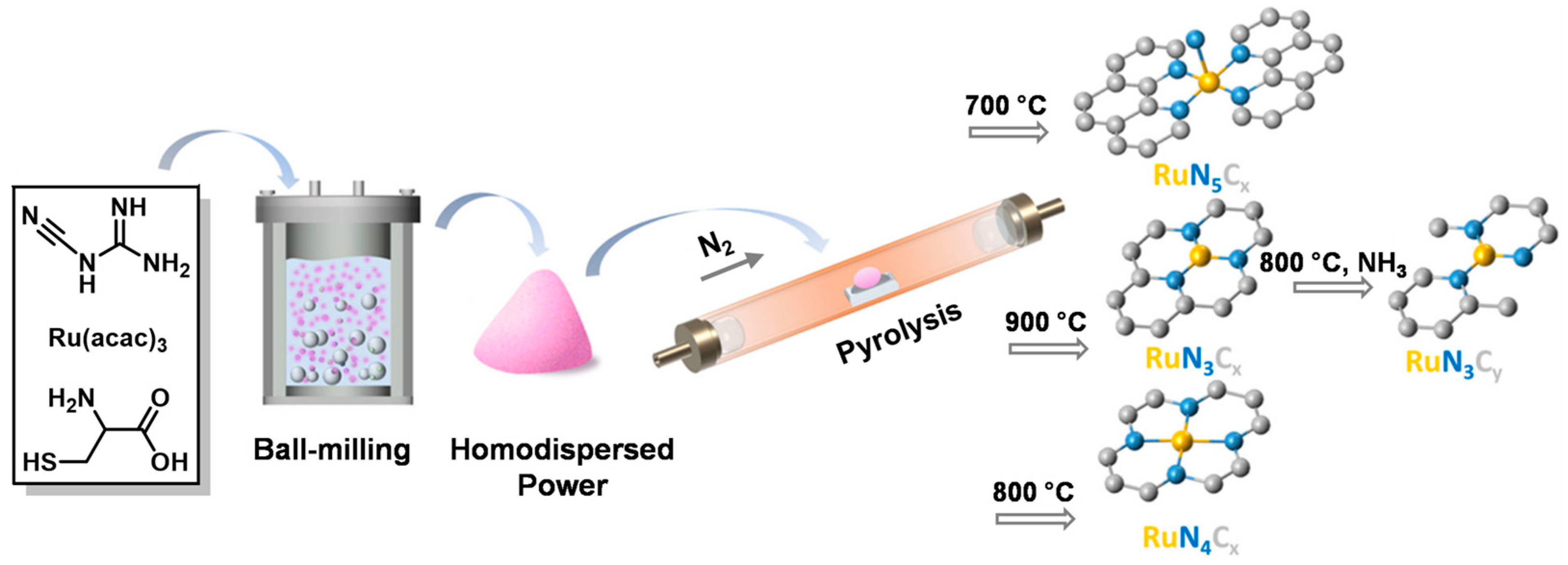
- Qi, H.; Yang, J.; Liu, F.; Zhang, L.; Yang, J.; Liu, X.; Li, L.; Su, Y.; Liu, Y.; Hao, R.; et al. Highly selective and robust single-atom catalyst Ru1/NC for reductive amination of aldehydes/ketones. Nat. Commun. 2021, 12, 3295. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. Transition-metal-catalyzed reductive amination employing hydrogen. Chem. Rev. 2020, 120, 9583–9674. [Google Scholar] [CrossRef]
- Wang, Y.; Furukawa, S.; Fu, X.; Yan, N. Organonitrogen chemicals from oxygen-containing feedstock over heterogeneous catalysts. ACS Catal. 2019, 10, 311–335. [Google Scholar] [CrossRef]
- Luo, D.; He, Y.; Yu, X.; Wang, F.; Zhao, J.; Zheng, W.; Jiao, H.; Yang, Y.; Li, Y.; Wen, X. Intrinsic mechanism of active metal dependent primary amine selectivity in the reductive amination of carbonyl compounds. J. Catal. 2021, 395, 293–301. [Google Scholar] [CrossRef]
- Liang, G.; Wang, A.; Li, L.; Xu, G.; Yan, N.; Zhang, T. Production of primary amines by reductive amination of biomass-derived aldehydes/ketones. Angew. Chem. Int. Ed. 2017, 56, 3050–3054. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Song, J.; Hua, M.; Hu, Y.; Huang, X.; Wu, H.; Yang, G.; Han, B. Ambient-temperature synthesis of primary amines via reductive amination of carbonyl compounds. ACS Catal. 2020, 10, 7763–7772. [Google Scholar] [CrossRef]
- Ho, C.R.; Defalque, V.; Zheng, S.; Bell, A.T. Propanol amination over supported nickel catalysts: Reaction mechanism and role of the support. ACS Catal. 2019, 9, 2931–2939. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, G.; Hao, W.; Tang, X.; Sun, Y.; Lin, L.; Liu, S. Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2012, 2, 11184–11206. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Saha, B.; Alam, M.I. Advances in conversion of hemicellulosic biomass to furfural and upgrading to biofuels. Catal. Sci. Technol. 2012, 2, 2025–2036. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Le, G.; Xie, L.; Wang, Y.; Dai, L. Efficient conversion of furfural to furfural amine over 4Ru1Co/AC catalyst. Appl. Catal. A Gen. 2022, 647, 118902. [Google Scholar]
- Zhang, X.; Xu, S.; Li, Q.; Zhou, G.; Xia, H. Recent advances in the conversion of furfural into bio-chemicals through chemo- and bio-catalysis. RSC Adv. 2021, 11, 27042–27058. [Google Scholar] [CrossRef] [PubMed]
- Caetano, J.A.T.; Fernandes, A.C. One-pot synthesis of amines from biomass resources catalyzed by HReO4. Green Chem. 2018, 20, 2494–2498. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Ma, L. Earth-abundant metal-catalyzed reductive amination: Recent advances and prospect for future catalysis. Chem. Asian J. 2021, 16, 2371–2391. [Google Scholar] [CrossRef]
- Komanoya, T.; Kinemura, T.; Kita, Y.; Kamata, K.; Hara, M. Electronic effect of ruthenium nanoparticles on efficient reductive amination of carbonyl compounds. J. Am. Chem. Soc. 2017, 139, 11493–11499. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Kita, Y.; Kamata, K.; Hara, M. Low-temperature reductive amination of carbonyl compounds over Ru deposited on Nb2O5·nH2O. ACS Sustain. Chem. Eng. 2018, 7, 4692–4698. [Google Scholar] [CrossRef]
- Chandra, D.; Inoue, Y.; Sasase, M.; Kitano, M.; Bhaumik, A.; Kamata, K.; Hosono, H.; Hara, M. A high performance catalyst of shape-specific ruthenium nanoparticles for production of primary amines by reductive amination of carbonyl compounds. Chem. Sci. 2018, 9, 5949–5956. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Jia, X.; Ma, J.; Fan, X.; Gao, J.; Xu, J. Self-regulated catalysis for the selective synthesis of primary amines from carbonyl compounds. Green Chem. 2021, 23, 7115–7121. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms. Chem. Rev. 2020, 120, 683–733. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, B.; Zhou, X.; Kang, S.; Xu, Y.; Wei, J. Selective synthesis of furfurylamine by reductive amination of furfural over Raney cobalt. ChemCatChem 2019, 11, 5562–5569. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Zhong, S.; Ma, L. Selective catalysis for the reductive amination of furfural toward furfurylamine by graphene-co-shelled cobalt nanoparticles. Green Chem. 2022, 24, 271–284. [Google Scholar] [CrossRef]
- Yogita; Rao, K.T.V.; Kumar, P.M.; Lingaiah, N. Cobalt nanoparticles embedded in a nitrogen-doped carbon matrix for reductive amination of biomass-derived furfural to furfurylamine. Sustain. Energy Fuels 2022, 6, 4692–4705. [Google Scholar] [CrossRef]
- Dong, C.; Wu, Y.; Wang, H.; Peng, J.; Li, Y.; Samart, C.; Ding, M. Facile and efficient synthesis of primary amines via reductive amination over a Ni/Al2O3 catalyst. ACS Sustain. Chem. Eng. 2021, 9, 7318–7327. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Li, X.; Liu, H.; Wang, A.; Xia, C.; Chen, J.; Huang, Z. Efficient synthesis of pharmaceutical intermediates from biomass-derived aldehydes and ketones over robust NixAl nanocatalysts. ACS Sustain. Chem. Eng. 2022, 10, 5526–5537. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Zhou, L.L.; Wang, X.C.; Zhang, L.Y.; Cheng, H.Y.; Zhao, F.Y. Catalytic reductive amination of furfural to furfurylamine on robust ultra-small Ni nanoparticles. Nano Res. 2022. [Google Scholar] [CrossRef]
- Song, W.; Wan, Y.; Li, Y.; Luo, X.; Fang, W.; Zheng, Q.; Ma, P.; Zhang, J.; Lai, W. Electronic Ni–N interaction enhanced reductive amination on an N-doped porous carbon supported Ni catalyst. Catal. Sci. Technol. 2022, 12, 7208–7218. [Google Scholar] [CrossRef]
- Bhunia, M.K.; Chandra, D.; Abe, H.; Niwa, Y.; Hara, M. Synergistic effects of earth-abundant metal-metal oxide enable reductive amination of carbonyls at 50 °C. ACS Appl. Mater. Interfaces 2022, 14, 4144–4154. [Google Scholar] [CrossRef]
- Martínez, J.J.; Nope, E.; Rojas, H.; Brijaldo, M.H.; Passos, F.; Romanelli, G. Reductive amination of furfural over Me/SiO2–SO3H (Me: Pt, Ir, Au) catalysts. J. Mol. Catal. A Chem. 2014, 392, 235–240. [Google Scholar] [CrossRef]
- Lin, C.; Zhou, J.; Zheng, Z.; Chen, J. An efficient approach to biomass-based tertiary amines by direct and consecutive reductive amination of furfural. J. Catal. 2022, 410, 164–179. [Google Scholar] [CrossRef]
- Gould, N.S.; Landfield, H.; Dinkelacker, B.; Brady, C.; Yang, X.; Xu, B. Selectivity control in catalytic reductive amination of furfural to furfurylamine on supported catalysts. ChemCatChem 2020, 12, 2106–2115. [Google Scholar] [CrossRef]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US department of energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Chandrashekhar, V.G.; Natte, K.; Alenad, A.M.; Alshammari, A.S.; Kreyenschulte, C.; Jagadeesh, R.V. Reductive amination, hydrogenation and hydrodeoxygenation of 5-hydroxymethylfurfural using silica-supported cobalt-nanoparticles. ChemCatChem 2021, 14, e202101234. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, S.; Wu, Y.; Xu, H.; Li, G.; Zhou, Y.; Wang, J. Ambient-temperature reductive amination of 5-hydroxymethylfurfural over Al2O3-supported carbon-doped nickel catalyst. ChemSusChem 2022, 15, e202200192. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, Y.; Du, J.; Si, Z.; Tang, X.; Zeng, X.; Lin, L.; Liu, S.; Lei, T. Preparation of 5-(aminomethyl)-2-furanmethanol by direct reductive amination of 5-hydroxymethylfurfural with aqueous ammonia over the Ni/SBA-15 catalyst. J. Appl. Chem. Biotechnol. 2018, 93, 3028–3034. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.-P.; Su, F.; Yan, Z.; Kusema, B.T.; Streiff, S.; Huang, Y.; Pera-Titus, M.; Shi, F. Reductive amination of furanic aldehydes in aqueous solution over versatile NiyAlOx catalysts. ACS Omega 2019, 4, 2510–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ortiz, A.; Vidal, J.D.; Climent, M.J.; Concepción, P.; Corma, A.; Iborra, S. Chemicals from biomass: Selective synthesis of N-substituted furfuryl amines by the one-pot direct reductive amination of furanic aldehydes. ACS Sustain. Chem. Eng. 2019, 7, 6243–6250. [Google Scholar] [CrossRef]
- Karve, V.V.; Sun, D.T.; Trukhina, O.; Yang, S.; Oveisi, E.; Luterbacher, J.; Queen, W.L. Efficient reductive amination of HMF with well dispersed Pd nanoparticles immobilized in a porous MOF/polymer composite. Green Chem. 2020, 22, 368–378. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bukhtiyarov, V.I. Two-step one-pot reductive amination of furanic aldehydes using CuAlOx catalyst in a flow reactor. Molecules 2020, 25, 4771. [Google Scholar] [CrossRef]
- Li, B.; Jiang, X.; Chen, S. Preparation of 5-amino-1-pentanol. Fine Chem. Intermed. 2014, 44, 41–48. [Google Scholar]
- Li, X.; Tian, J.; Liu, H.; Tang, C.; Xia, C.; Chen, J.; Huang, Z. Effective synthesis of 5-amino-1-pentanol by reductive amination of biomass-derived 2-hydroxytetrahydropyran over supported Ni catalysts. Chin. J. Catal. 2020, 41, 631–641. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Tian, J.; Liu, H.; Li, X.; Fang, W.; Hu, X.; Xia, C.; Chen, J.; Huang, Z. Reductive amination of bio-based 2-hydroxytetrahydropyran to 5-amino-1-pentanol over nano-Ni–Al2O3 catalysts. New J. Chem. 2021, 45, 4236–4245. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Zhang, J.; Tian, J.; Yao, X.; Liu, H.; Xia, C.; Huang, Z. Reductive amination of biomass-derived 2-hydroxytetrahydropyran into 5-amino-1-pentanol over hydroxylapatite nanorod supported Ni catalysts. Catal. Lett. 2022. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Li, X.; Mu, B.; Liu, H.; Xia, C.; Wang, A.; Huang, Z. Natural attapulgite supported nano-Ni catalysts for the efficient reductive amination of biomass-derived aldehydes and ketones. Green Synth. Catal. 2023. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Liu, H.; Hu, X.; Zhang, J.; Xia, C.; Chen, J.; Liu, H.; Huang, Z. Efficient synthesis of 5-amino-1-pentanol from biomass-derived dihydropyran over hydrotalcite-based Ni–Mg3AlOx catalysts. ACS Sustain. Chem. Eng. 2020, 8, 6352–6362. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Zhang, J.; Liu, H.; Tian, J.; Xia, C.; Huang, Z. Biomass-derived 2-hydroxytetrahydropyran to 5-amino-1-pentanol over bimetallic Ni-Fe/Al2O3 catalysts. J. Mol. Catal. 2022, 36, 1–10. [Google Scholar]
- Yang, J.; Zhang, J.; Benassi, E.; Li, X.; Liu, H.; Fang, W.; Tian, J.; Xia, C.; Huang, Z. NiCo/Al2O3 nanocatalysts for the synthesis of 5-amino-1-pentanol and 1,5-pentanediol from biomass-derived 2-hydroxytetrahydropyran. Green Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, K.; Shu, H.; Liu, H.; Lou, J.; Guo, D.; Wei, Z.; Li, X. Switchable synthesis of furfurylamine and tetrahydrofurfurylamine from furfuryl alcohol over RANEY® nickel. Catal. Sci. Technol. 2017, 7, 4129–4135. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, H.; Shu, H.; Xiao, S.; Guo, D.; Liu, Y.; Wei, Z.; Li, X. A comprehensive study on the reductive amination of 5-hydroxymethylfurfural into 2,5-bisaminomethylfuran over Raney Ni through DFT calculations. ChemCatChem 2019, 11, 2649–2656. [Google Scholar] [CrossRef]
- Hong, C.-B.; Li, G.; Liu, H. Selective hydrogenolysis of bio-renewable tetrahydrofurfurylamine to piperidine on ReOx-modified Rh catalysts. Green Chem. 2023. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Highly selective hydrogenation of furfural to cyclopentanone over a NiFe bimetallic catalyst in a methanol/water solution with a solvent effect. ACS Sustain. Chem. Eng. 2019, 7, 15221–15229. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Z.; Huang, Y.; Lu, F.; Wang, F.; Gao, J.; Xu, J. Conversion of furfural into cyclopentanone over Ni–Cu bimetallic catalysts. Green Chem. 2013, 15, 15–16. [Google Scholar] [CrossRef]
- Guo, W.; Tong, T.; Liu, X.; Guo, Y.; Wang, Y. Morphology-tuned activity of Ru/Nb2O5 catalysts for ketone reductive amination. ChemCatChem 2019, 11, 4130–4138. [Google Scholar] [CrossRef]
- Bonacci, S.; Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.; Oliverio, M.; Procopio, A. Montmorillonite K10-catalyzed solvent-free conversion of furfural into cyclopentenones. Catalysts 2019, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Duspara, P.A.; Batey, R.A. A short total synthesis of the marine sponge pyrrole-2-aminoimidazole alkaloid (+/-)-agelastatin a. Angew. Chem. Int. Ed. 2013, 52, 10862–10866. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Cavaca, L.A.S.; Gonçalves, J.M.; Ramos, R.; Peixoto, A.F.; Arias-Serrano, B.I.; Afonso, C.A.M. Silica-supported copper for the preparation of trans-4,5-diamino-cyclopent-2-enones under continuous flow conditions. ACS Sustain. Chem. Eng. 2021, 9, 16038–16043. [Google Scholar] [CrossRef]
- Cavaca, L.A.S.; Coelho, J.A.S.; Lucas, S.D.; Loureiro, R.M.S.; Gomes, R.F.A.; Afonso, C.A.M. Upgrading furanic platforms to α-enaminones: Tunable continuous flow hydrogenation of bio-based cyclopentenones. React. Chem. Eng. 2023, 8, 482–489. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, M.; Yao, X.; Tu, S.; Hou, Z.; Jie En, V.S.; Xiang, X.; Lin, J.; Cai, T.; Shen, N.; et al. Dose selection of chloroquine phosphate for treatment of COVID-19 based on a physiologically based pharmacokinetic model. Acta Pharm. Sin. B 2020, 10, 1216–1227. [Google Scholar] [CrossRef]
- Huang, M.; Li, M.; Xiao, F.; Pang, P.; Liang, J.; Tang, T.; Liu, S.; Chen, B.; Shu, J.; You, Y.; et al. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. Natl. Sci. Rev. 2020, 7, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, F.W. Unsaturated O- and N-heterocycles from carbohydrate feedstocks. Acc. Chem. Res. 2002, 35, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Kreye, O.; Mutlu, H.; Meier, M.A.R. Sustainable routes to polyurethane precursors. Green Chem. 2013, 15, 1431–1455. [Google Scholar] [CrossRef]
- Le, N.-T.; Byun, A.; Han, Y.; Lee, K.-I.; Kim, H. Preparation of 2,5-bis(aminomethyl)furan by direct reductive amination of 2,5-diformylfuran over nickel-Raney catalysts. Green Sustain. Chem. 2015, 5, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Mellmer, M.A.; Gallo, J.M.R.; Martin Alonso, D.; Dumesic, J.A. Selective production of levulinic acid from furfuryl alcohol in THF solvent systems over H-ZSM-5. ACS Catal. 2015, 5, 3354–3359. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Wu, P.d.; Li, H.; Fang, Z. Synergistic catalysis of Co-Zr/CNx bimetallic nanoparticles enables reductive amination of biobased levulinic acid. Adv. Sustain. Syst. 2022, 6, 2100321. [Google Scholar] [CrossRef]
- Touchy, A.S.; Hakim Siddiki, S.M.A.; Kon, K.; Shimizu, K.-i. Heterogeneous Pt catalysts for reductive amination of levulinic acid to pyrrolidones. ACS Catal. 2014, 4, 3045–3050. [Google Scholar] [CrossRef]
- Vidal, J.D.; Climent, M.J.; Concepcion, P.; Corma, A.; Iborra, S.; Sabater, M.J. Chemicals from biomass: Chemoselective reductive amination of ethyl levulinate with amines. ACS Catal. 2015, 5, 5812–5821. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, B.; Wang, L.; Yi, X.; Wang, C.; Wang, G.; Dai, Z.; Zheng, A.; Xiao, F.-S. Zirconium oxide supported palladium nanoparticles as a highly efficient catalyst in the hydrogenation-amination of levulinic acid to pyrrolidones. ChemCatChem 2017, 9, 2661–2667. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Wang, H.; Zhang, F.; Li, R.; Xiang, J.; Wang, Z.; Han, B.; Liu, Z. Ambient reductive synthesis of N-heterocyclic compounds over cellulose-derived carbon supported Pt nanocatalyst under H2 atmosphere. Green Chem. 2020, 22, 3820–3826. [Google Scholar] [CrossRef]
- Martínez, J.J.; Silva, L.; Rojas, H.A.; Romanelli, G.P.; Santos, L.A.; Ramalho, T.C.; Brijaldo, M.H.; Passos, F.B. Reductive amination of levulinic acid to different pyrrolidones on Ir/SiO2-SO3H: Elucidation of reaction mechanism. Catal. Today 2017, 296, 118–126. [Google Scholar] [CrossRef]
- Chaudhari, C.; Shiraishi, M.; Nishida, Y.; Sato, K.; Nagaoka, K. One-pot synthesis of pyrrolidones from levulinic acid and amines/nitroarenes/nitriles over the SPVP catalyst. Green Chem. 2020, 22, 7760–7764. [Google Scholar] [CrossRef]
- Gao, G.; Sun, P.; Li, Y.; Wang, F.; Zhao, Z.; Qin, Y.; Li, F. Highly stable porous-carbon-coated Ni catalysts for the reductive amination of levulinic acid via an unconventional pathway. ACS Catal. 2017, 7, 4927–4935. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, B.; Yan, F.; Xu, W.; Bai, G.; Li, Y.; Yan, X.; Chen, L. Boron modified Cu/Al2O3 catalysts for the selective reductive amination of levulinic acid to N-substituted pyrrolidinones. ChemCatChem 2022, 14, e202200311. [Google Scholar] [CrossRef]
- Cao, P.; Ma, T.; Zhang, H.-Y.; Yin, G.; Zhao, J.; Zhang, Y. Conversion of levulinic acid to N-substituted pyrrolidinones over a nonnoble bimetallic catalyst Cu15Pr3/Al2O3. Catal. Commun. 2018, 116, 85–90. [Google Scholar] [CrossRef]
- Chieffi, G.; Braun, M.; Esposito, D. Continuous reductive amination of biomass-derived molecules over carbonized filter paper-supported FeNi alloy. ChemSusChem 2015, 8, 3590–3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Romero, A.A.; Muñoz-Batista, M.J.; Luque, R. Benign-by-design orange peel-templated nanocatalysts for continuous flow conversion of levulinic acid to N-heterocycles. ACS Sustain. Chem. Eng. 2018, 6, 16637–16644. [Google Scholar] [CrossRef]
- Siddiki, S.M.A.H.; Touchy, A.S.; Bhosale, A.; Toyao, T.; Mahara, Y.; Ohyama, J.; Satsuma, A.; Shimizu, K.-i. Direct synthesis of lactams from keto acids, nitriles, and H2 by heterogeneous Pt catalysts. ChemCatChem 2018, 10, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Gokhale, T.A.; Raut, A.B.; Chawla, S.K.; Bhanage, B.M. Insights into cascade and sequential one-pot pathways for reductive amination of aldehydes paired with bio-derived levulinic acid to N-substituted pyrrolidones using molecular hydrogen. React. Chem. Eng. 2022, 7, 1005–1013. [Google Scholar] [CrossRef]
- Niu, F.; Bahri, M.; Ersen, O.; Yan, Z.; Kusema, B.T.; Khodakov, A.Y.; Ordomsky, V.V. A multifaceted role of a mobile bismuth promoter in alcohol amination over cobalt catalysts. Green Chem. 2020, 22, 4270–4278. [Google Scholar] [CrossRef]
- Niu, F.; Xie, S.; Yan, Z.; Kusema, B.T.; Ordomsky, V.V.; Khodakov, A.Y. Alcohol amination over titania-supported ruthenium nanoparticles. Catal. Sci. Technol. 2020, 10, 4396–4404. [Google Scholar] [CrossRef]
- Liang, G.; Zhou, Y.; Zhao, J.; Khodakov, A.Y.; Ordomsky, V.V. Structure-sensitive and insensitive reactions in alcohol amination over nonsupported Ru nanoparticles. ACS Catal. 2018, 8, 11226–11234. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Kita, Y.; Kuwabara, M.; Yamadera, S.; Kamata, K.; Hara, M. Effects of ruthenium hydride species on primary amine synthesis by direct amination of alcohols over a heterogeneous Ru catalyst. Chem. Sci. 2020, 11, 9884–9890. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, R.; Xiao, M.; Guo, D.; Cai, Z.; Kang, S.; Xu, Y.; Wei, J. Direct amination of biomass-based furfuryl alcohol and 5-(aminomethyl)-2-furanmethanol with NH3 over hydrotalcite-derived nickel catalysts via the hydrogen-borrowing strategy. ChemCatChem 2021, 13, 2074–2085. [Google Scholar] [CrossRef]
- Enjamuri, N.; Darbha, S. Solid catalysts for conversion of furfural and its derivatives to alkanediols. Catal. Rev. 2020, 62, 566–606. [Google Scholar] [CrossRef]
- He, J.; Huang, K.; Barnett, K.J.; Krishna, S.H.; Alonso, D.M.; Brentzel, Z.J.; Burt, S.P.; Walker, T.; Banholzer, W.F.; Maravelias, C.T.; et al. New catalytic strategies for alpha,omega-diols production from lignocellulosic biomass. Faraday Discuss. 2017, 202, 247–267. [Google Scholar] [CrossRef]
- Rani, V.R.; Srinivas, N.; Kulkarni, S.J.; Raghavan, K.V. Amino cyclization of terminal (alpha,omega)-diols over modified ZSM-5 catalysts. J. Mol. Catal. A Chem. 2002, 187, 237–246. [Google Scholar] [CrossRef]
- Niemeier, J.; Engel, R.V.; Rose, M. Is water a suitable solvent for the catalytic amination of alcohols? Green Chem. 2017, 19, 2839–2845. [Google Scholar] [CrossRef] [Green Version]
- Dros, A.B.; Larue, O.; Reimond, A.; De Campo, F.; Pera-Titus, M. Hexamethylenediamine (HMDA) from fossil- vs. bio-based routes: An economic and life cycle assessment comparative study. Green Chem. 2015, 17, 4760–4772. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.Y.; Zhang, C.; Zhang, B.; Liu, T.; Wu, Q.F.; Su, X.L.N.; Lin, W.W.; Zhao, F.Y. Reductive amination of 1,6-hexanediol with Ru/Al2O3 catalyst in supercritical ammonia. Sci. China Chem. 2017, 60, 920–926. [Google Scholar] [CrossRef]
- Gupta, N.K.; Palenicek, P.; Nortmeyer, L.; Meyer, G.M.; Schäfer, T.; Hellmann, T.; Hofmann, J.P.; Rose, M. Modulating catalytic selectivity by base addition in aqueous reductive amination of 1,6-hexanediol using Ru/C. ACS Sustain. Chem. Eng. 2022, 10, 14560–14567. [Google Scholar] [CrossRef]
- Cai, X.; Ke, Y.; Wang, B.; Zeng, Y.; Chen, L.; Li, Y.; Bai, G.; Yan, X. Efficient catalytic amination of diols to diamines over Cu/ZnO/γ-Al2O3. Mol. Catal. 2021, 508, 111608. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, H.; Shi, F. Sustainable catalytic amination of diols: From cycloamination to monoamination. ACS Sustain. Chem. Eng. 2017, 6, 1061–1067. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N. Furans in polymer chemistry. Prog. Polym. Sci. 1997, 22, 1203–1379. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Li, Z.; Zeng, X.; Tang, X.; Sun, Y.; Lei, T.; Lin, L. Synthesis of bis(amino)furans from biomass based 5-hydroxymethylfurfural. J. Energy Chem. 2018, 27, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Jia, X.; Ma, J.; Gao, J.; Xia, F.; Li, X.; Xu, J. Selective synthesis of 2,5-bis(aminomethyl)furan via enhancing the catalytic dehydration–hydrogenation of 2,5-diformylfuran dioxime. Green Chem. 2018, 20, 2697–2701. [Google Scholar] [CrossRef]
- Yuan, H.; Kusema, B.T.; Yan, Z.; Streiff, S.; Shi, F. Highly selective synthesis of 2,5-bis(aminomethyl)furan via catalytic amination of 5-(hydroxymethyl)furfural with NH3 over a bifunctional catalyst. RSC Adv. 2019, 9, 38877–38881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Cheng, Y.; Huang, H.; Ma, Z.; Zhou, K.; Liu, Y. Reductive amination of 5-hydroxymethylfurfural to 2,5-bis(aminomethyl)furan over alumina-supported Ni-based catalytic systems. ChemSusChem 2022, 15, e202200233. [Google Scholar] [CrossRef]







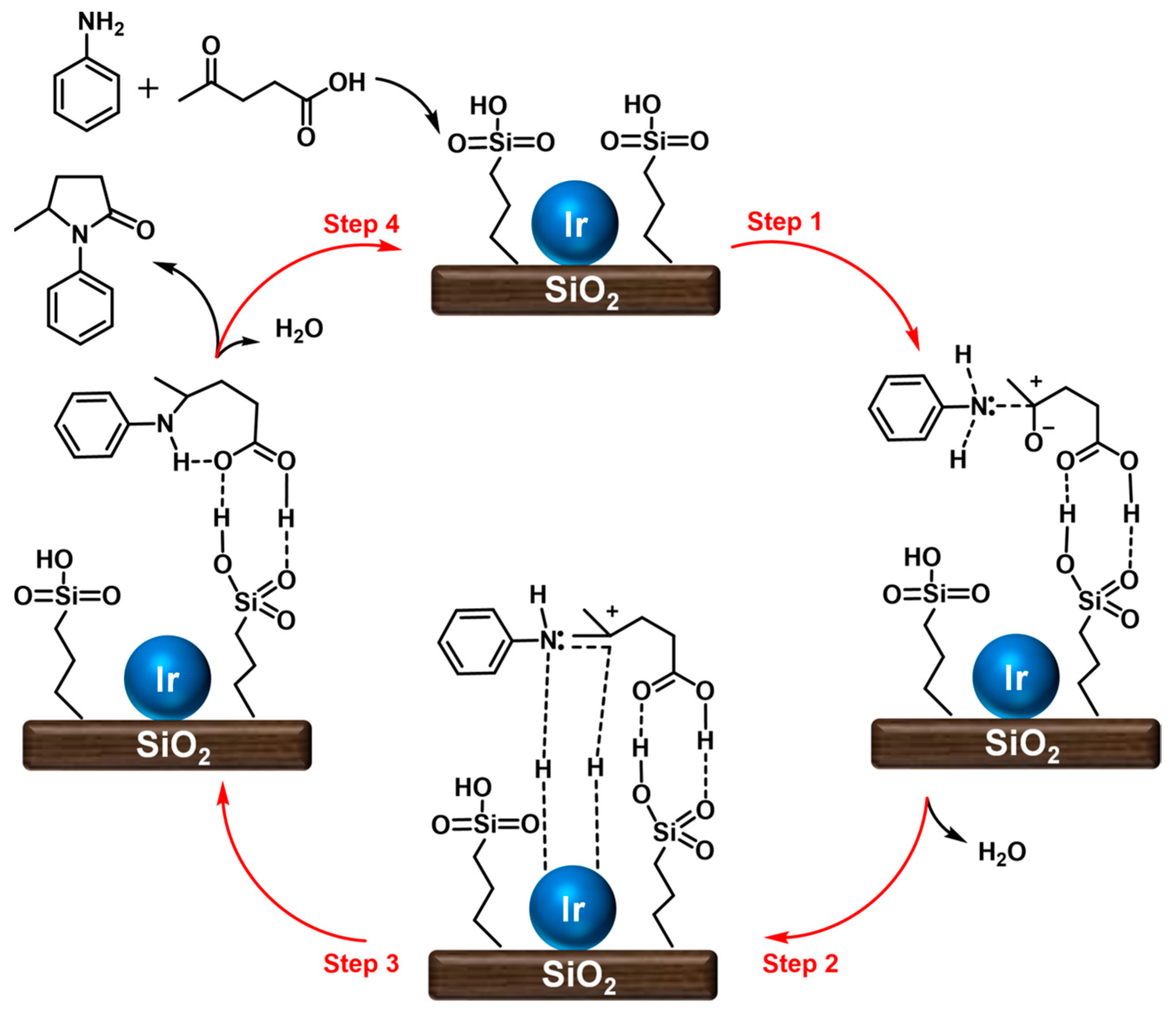

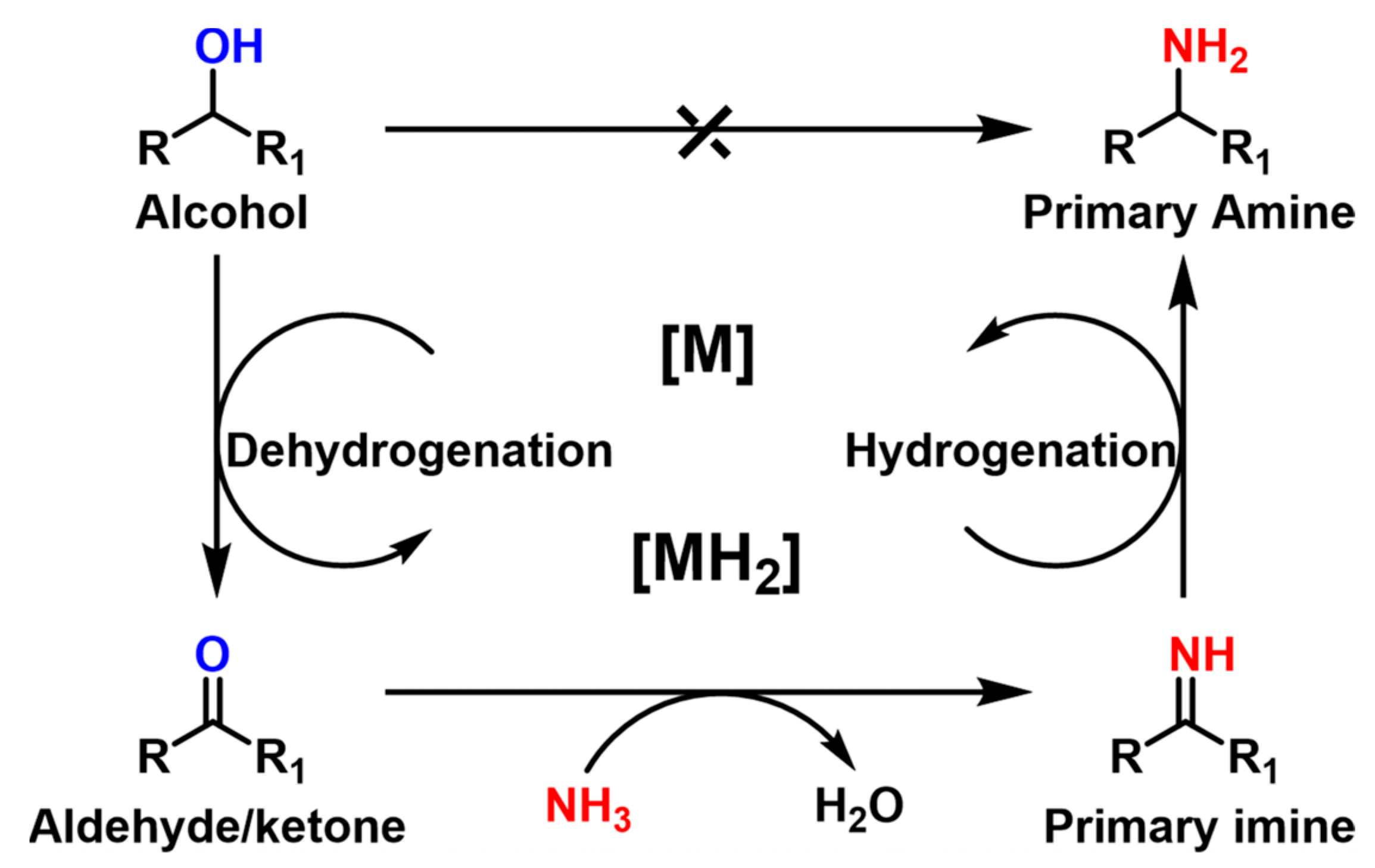

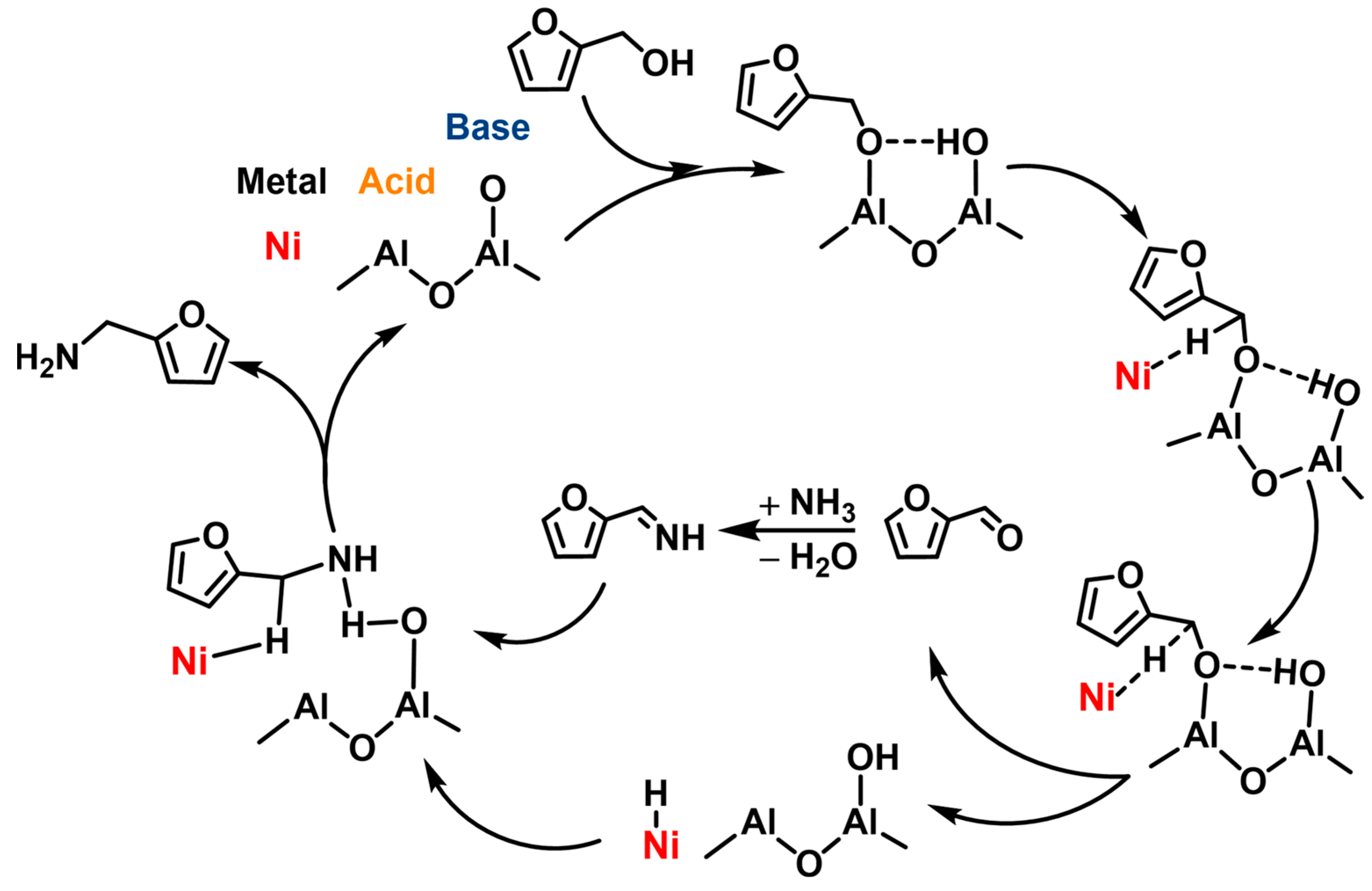

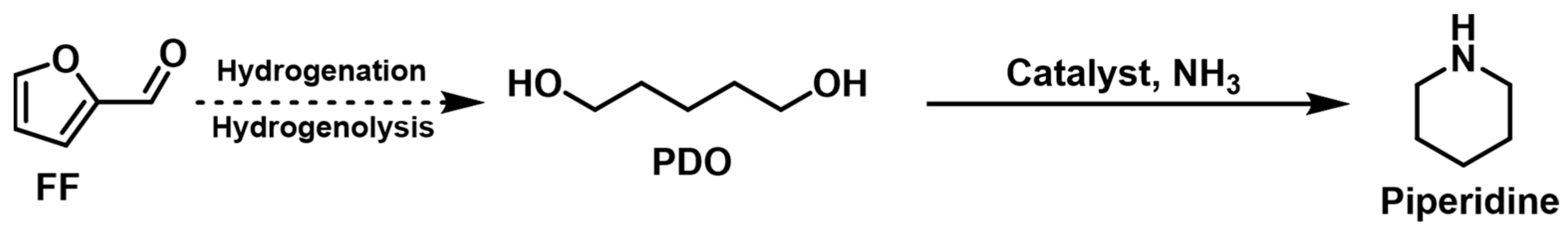
 | ||||||||
| Entry | Catalyst | Nitrogen Source | PH2 (MPa) | Temp. (°C) | Time (h) | Conv. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Ru/Nb2O5 | NH3 gas | 4 | 90 | 4 | 100 | 99 | [43] |
| 2 | Ru-NPs | NH3 in MeOH | 2 | 90 | 2 | 100 | 99 | [45] |
| 3 | Ru/BN-e | NH3 aqueous | 1 | 90 | 5 | 100 | 99 | [46] |
| 4 | 4Ru1Co/AC | NH3 aqueous | 2 | 80 | 1 | 100 | 92 | [39] |
| 5 | Ru1/NC-900-800NH3 | NH3 gas | 2 | 100 | 10 | 100 | 97 | [28] |
| 6 | Ru/TiP-100 | NH3 gas | 1.7 | 30 | 24 | - | 91 | [33] |
| 7 | Raney Co | NH3 gas | 1 | 120 | 2 | 100 | 99 | [51] |
| 8 | Co@C-600-EtOH | NH3 in MeOH | 2 | 90 | 2 | 100 | 87 | [52] |
| 9 | Co/NC-700 | NH3 in MeOH | 2 | 120 | 1 | 100 | 99 | [53] |
| 10 | 10Ni/Al2O3 | NH3 in MeOH | 2 | 100 | 2 | 100 | 92 | [54] |
| 11 | Ni1Al | NH3 aqueous | 2 | 80 | 1 | 100 | 91 | [55] |
| 12 | Ni/SiO2 | NH3 gas | 2 | 90 | 1.5 | 100 | 98 | [56] |
| 13 | Ni/pNC | NH3 gas | 4 | 60 | 6 | 100 | 99 | [57] |
| 14 | Ni@DS | NH3 gas | 0.9 | 50 | 6 | - | 89 | [58] |
| 15 | Ir/SiO2-SO3H | Aniline | 5 | 30 | 8 | 72 | 21 | [59] |
| 16 | Rh2P/NC | HCOONH4 | 3 | 60 | 24 | - | 92 | [60] |
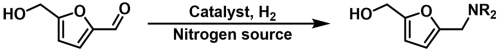 | ||||||||
| Entry | Catalyst | Nitrogen Source | PH2 (MPa) | Temp. (°C) | Time (h) | Conv. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Ni/SBA-15 | NH3 in MeOH | 1.5 | 100 | 4 | - | 90 | [66] |
| 2 | Ni6AlOx | NH3 gas | 0.1 | 100 | 6 | 100 | 99 | [67] |
| 3 | Ni1Al | NH3 aqueous | 2 | 80 | 1 | 100 | 99 | [55] |
| 4 | Ni@C/Al2O3-400 | NH3 in MeOH | 2 | 30 | 16 | 100 | 96 | [65] |
| 5 | Co-Co3O4@SiO2 | NH3 gas | 1 | 50 | 16 | 100 | 94 | [64] |
| 6 | Pd/C | Aniline | 0.3 | 100 | 1 | 100 | 100 | [68] |
| 7 | Pd/Al2O3 | Aniline | 0.3 | 100 | 1 | 100 | 95 | [68] |
| 8 | UiO-67/PpPDA/Pd | Aniline | 0.5 | 50 | 2 | - | 95 | [69] |
| 9 | CuAlOx | Aniline | 1 | 100 | 3 | - | 97 | [70] |
 | ||||||||
| Entry | Catalyst | PH2 (MPa) | Temp. (°C) | Time (h) | Conv. (%) | Yield (%) a | Lifetime (h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 15Ni/ZrO2-IM | 2 | 80 | 1 | 100 | 85 (91) | <30 | [72] |
| 2 | 15Ni/Al2O3-IM | 2 | 80 | 1 | 100 | 81 | - | [72] |
| 3 | 15Ni/TiO2-IM | 2 | 80 | 1 | 100 | 43 | - | [72] |
| 4 | 15Ni/SiO2-IM | 2 | 80 | 1 | 99 | 21 | - | [72] |
| 5 | 15Ni/MgO-IM | 2 | 80 | 1 | 98 | 7 | - | [72] |
| 6 | 10Ni/HAP-DP | 2 | 80 | 1 | 100 | 92 | <60 | [74] |
| 7 | 10Ni/ATP-DP | 2 | 80 | 1 | 100 | 94 | 72 | [75] |
| 8 | 30Ni-MgAlOx-CP | 2 | 60 | 1 | 100 | 83 (90) | <90 | [76] |
| 9 | 40Ni-MgAlOx-CP | 2 | 60 | 2 | 100 | 93 | - | [76] |
| 10 | 50Ni-Al2O3-CP | 2 | 60 | 1 | 100 | 85 (91) | >150 | [73] |
| 11 | NiFe0.25/Al2O3-CP | 2 | 80 | 1 | 100 | 90 | >120 | [77] |
| 12 | Ni5Co1-Al2O3-CP | 2 | 60 | 1 | 100 | 87 | >180 | [78] |
 | |||||||
| Entry | Catalyst | PH2 (MPa) | Temp. (°C) | Time (h) | Conv. (%) | Yield (%) a | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Ru/Nb2O5-L | 2 | 90 | 4 | 100 | 84 | [84] |
| 2 | Ru/ZrO2 | 1.2 | 90 | 12 | 100 | 93 | [32] |
| 3 | Ni/Al2O3 | 4 | 80 | 2 | 100 | 97 | [73] |
 | ||||||||
| Entry | Catalyst | Nitrogen Source | PH2 (MPa) | Temp. (°C) | Time (h) | Conv. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | AT-Ni-Raney | NH3 gas | 1 | 120 | 6 | 100 | 43 | [93] |
| 2 | Co/ZrO2 | butylamine and NH3 gas | 2 | 100 | 10 | 100 | 95 | [27] |
 | ||||||||
| Entry | Catalyst | Nitrogen Source | PH2 (MPa) | Temp. (°C) | Time (h) | Conv. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Pt-MoOx/TiO2 | n-Octylamine | 0.3 | 100 | 20 | - | 99 | [97] |
| 2 | Pt/TiO2D | Aniline | 0.5 | 150 | 18 | 100 | 100 | [98] |
| 3 | Pd/ZrO2 | n-Octylamine | 0.5 | 90 | 12 | 99 | 98 | [99] |
| 4 | Pt/P-TiO2 | n-Octylamine | 0.1 | 30 | 3 | - | 97 | [26] |
| 5 | Pt/c-C | Aniline | 0.1 | 30 | 3 | - | 96 | [100] |
| 6 | Ir/SiO2-SO3H | Aniline | 3.5 | 100 | 8 | 63 | 63 | [101] |
| 7 | Ir-PVP | Aniline | 0.5 | 30 | 24 | 99 | 95 | [102] |
| 8 | CNF30@Ni@CNTs | Benzylamine | 3 | 130 | 6 | - | 99 | [103] |
| 9 | Cu10/AlB3O | n-Butylamine | 3 | 200 | - | 99 | 94 | [104] |
| 10 | Cu15Pr3/Al2O3 | n-Butylamine | 5 | 175 | 20 | 100 | 94 | [105] |
| 11 | FeNi/C | Phenethylamine | 8.5 | 150 | - | 93 | 93 | [106] |
| 12 | Co-Zr@Chitosan-20 | NH3 aqueous solution | 3 | 130 | 24 | 94 | 93 | [96] |
| 13 | Ru/TiO2 | Acetonitrile | 5 | 90 | - | 79 | 67 | [107] |
| 14 | Pt-MoOx/TiO2 | n-Octanenitrile | 0.7 | 110 | 24 | 100 | 92 | [108] |
| 15 | Ni-MMT | Benzaldehyde | 1.5 | 140 | 16 | - | 86 | [109] |
 | ||||||||
| Entry | Catalyst | Nitrogen Source | PH2 (MPa) | Temp. (°C) | Time (h) | Conv. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Ru-20MgO/TiO2 | NH3 gas | 0 | 110 | 20 | 100 | 94 | [114] |
| 2 | Raney Ni | NH3 gas | 0 | 180 | 24 | 79 | 77 | [79] |
| 3 | Ni2Al-600 | NH3 gas | 0 | 180 | 36 | - | 84 | [115] |
 | ||||||||
| Entry | Catalyst | Nitrogen Source | PH2 (MPa) | Temp. (°C) | Time (h) | Conv. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Ru/Al2O3 | Supercritical NH3 | 2 | 220 | 6 | 100 | 38 | [121] |
| 2 | Ru/C | NH3 aqueous solution | 2.5 | 190 | 2 | - | 34 | [122] |
| 3 | Cu/ZnO/γ-Al2O3 | Dimethylamine | 3 | 180 | - | 100 | 93 | [123] |
| 4 | CuNiAlOx | Aniline | 0 | 180 | 24 | 100 | 89 | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, J.; Li, X.; Liu, H.; Yao, X.; Xia, C.; Huang, Z. Recent Advances in the Efficient Synthesis of Useful Amines from Biomass-Based Furan Compounds and Their Derivatives over Heterogeneous Catalysts. Catalysts 2023, 13, 528. https://doi.org/10.3390/catal13030528
Zhang J, Yang J, Li X, Liu H, Yao X, Xia C, Huang Z. Recent Advances in the Efficient Synthesis of Useful Amines from Biomass-Based Furan Compounds and Their Derivatives over Heterogeneous Catalysts. Catalysts. 2023; 13(3):528. https://doi.org/10.3390/catal13030528
Chicago/Turabian StyleZhang, Jia, Jian Yang, Xuemei Li, Hailong Liu, Xiaolan Yao, Chungu Xia, and Zhiwei Huang. 2023. "Recent Advances in the Efficient Synthesis of Useful Amines from Biomass-Based Furan Compounds and Their Derivatives over Heterogeneous Catalysts" Catalysts 13, no. 3: 528. https://doi.org/10.3390/catal13030528
APA StyleZhang, J., Yang, J., Li, X., Liu, H., Yao, X., Xia, C., & Huang, Z. (2023). Recent Advances in the Efficient Synthesis of Useful Amines from Biomass-Based Furan Compounds and Their Derivatives over Heterogeneous Catalysts. Catalysts, 13(3), 528. https://doi.org/10.3390/catal13030528









