Preparation of High-Performance Zn-Based Catalysts Using Printing and Dyeing Wastewater and Petroleum Coke as a Carrier in Acetylene Acetoxylation
Abstract
:1. Introduction
2. Results and Discussion
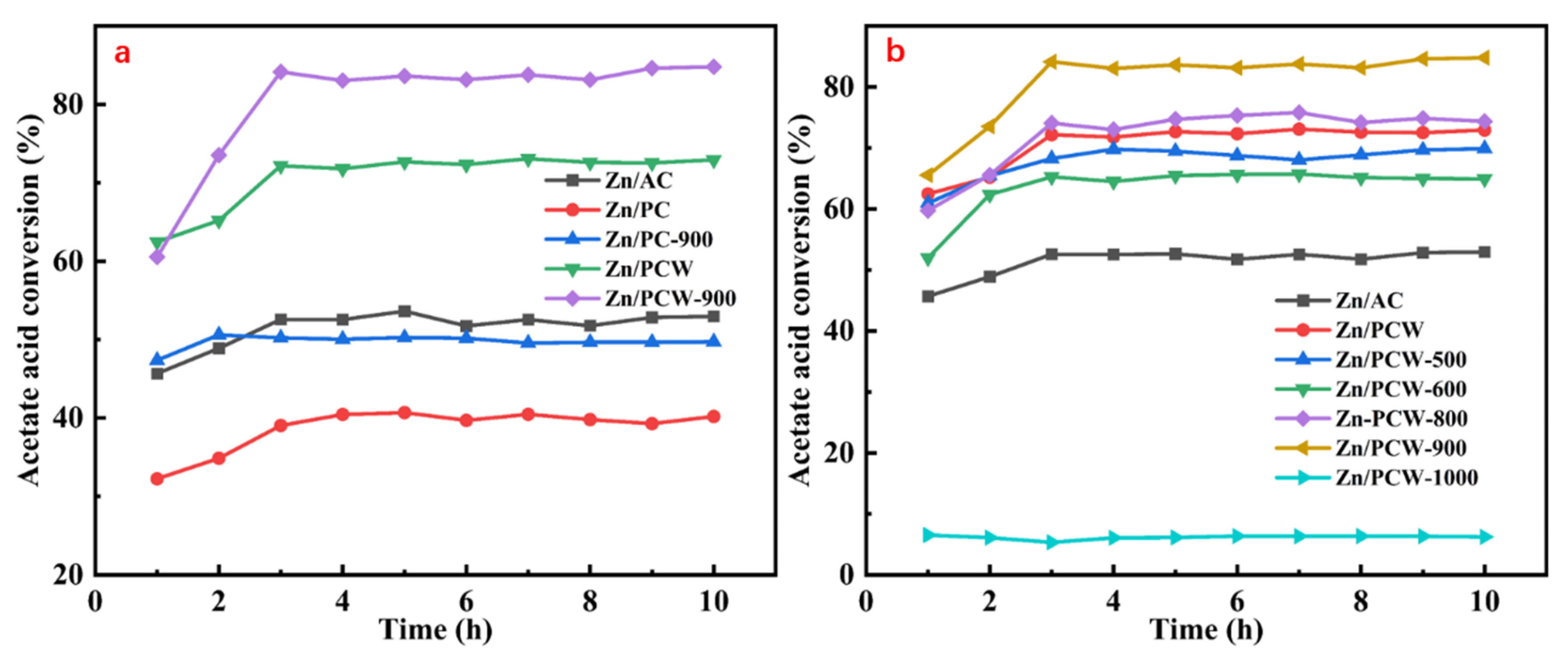
2.1. Activity Test
2.2. Catalyst Characterization
2.2.1. N2 Physical Adsorption and Desorption
2.2.2. ICP-MS
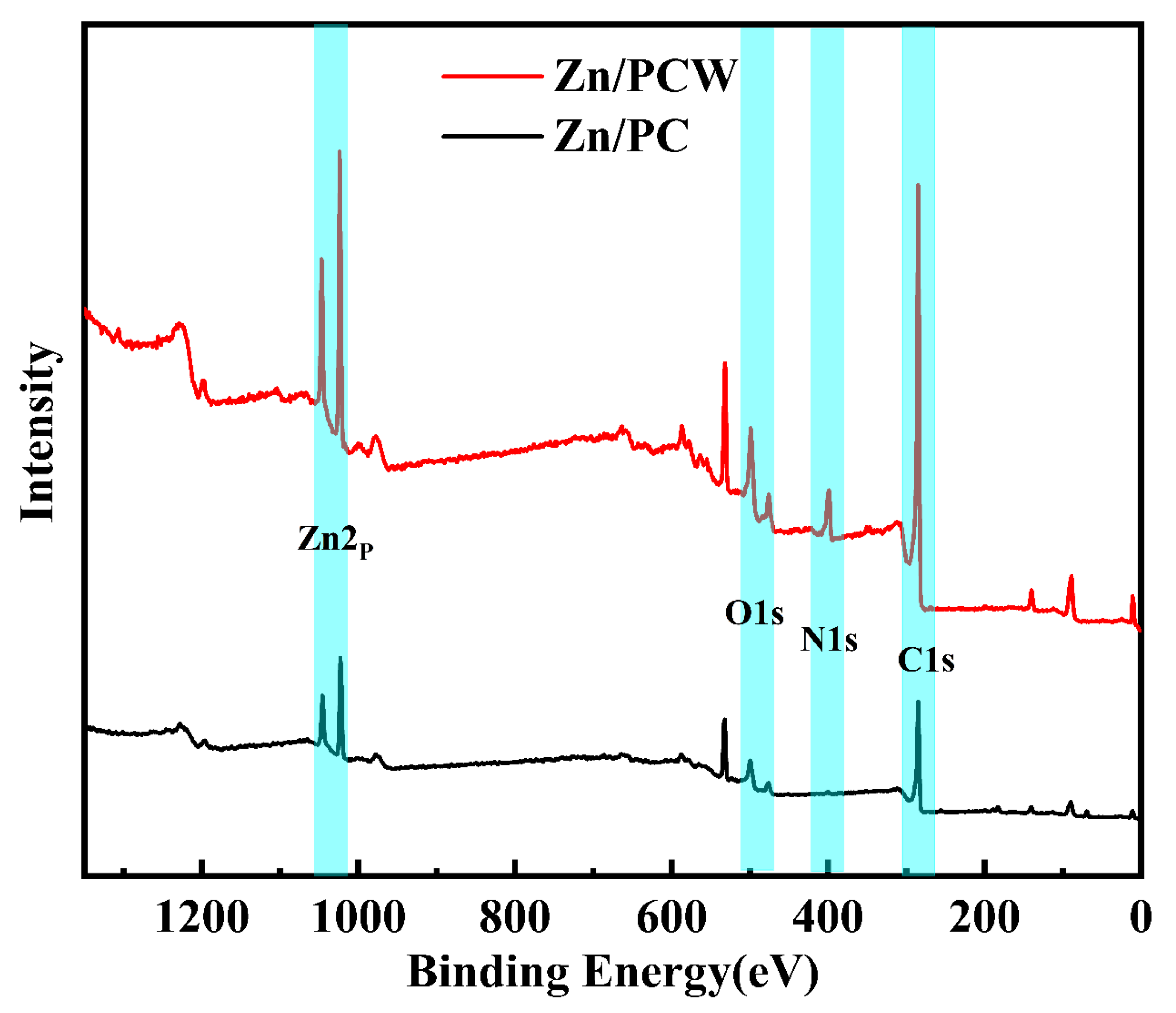
2.2.3. XPS (X-ray Photoelectron Spectroscopy)
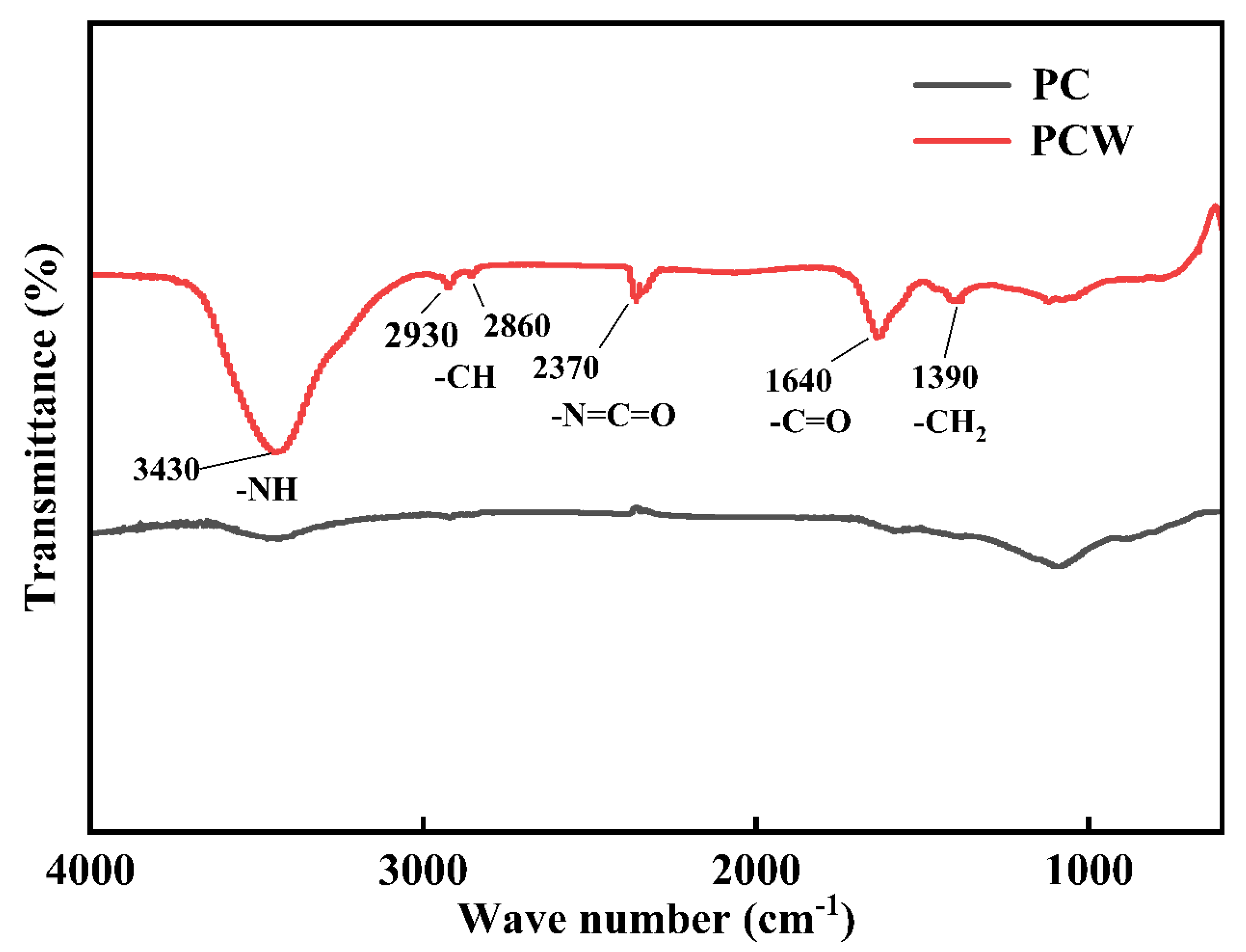
2.2.4. FTIR (Fourier Transform Infrared Spectrometer)
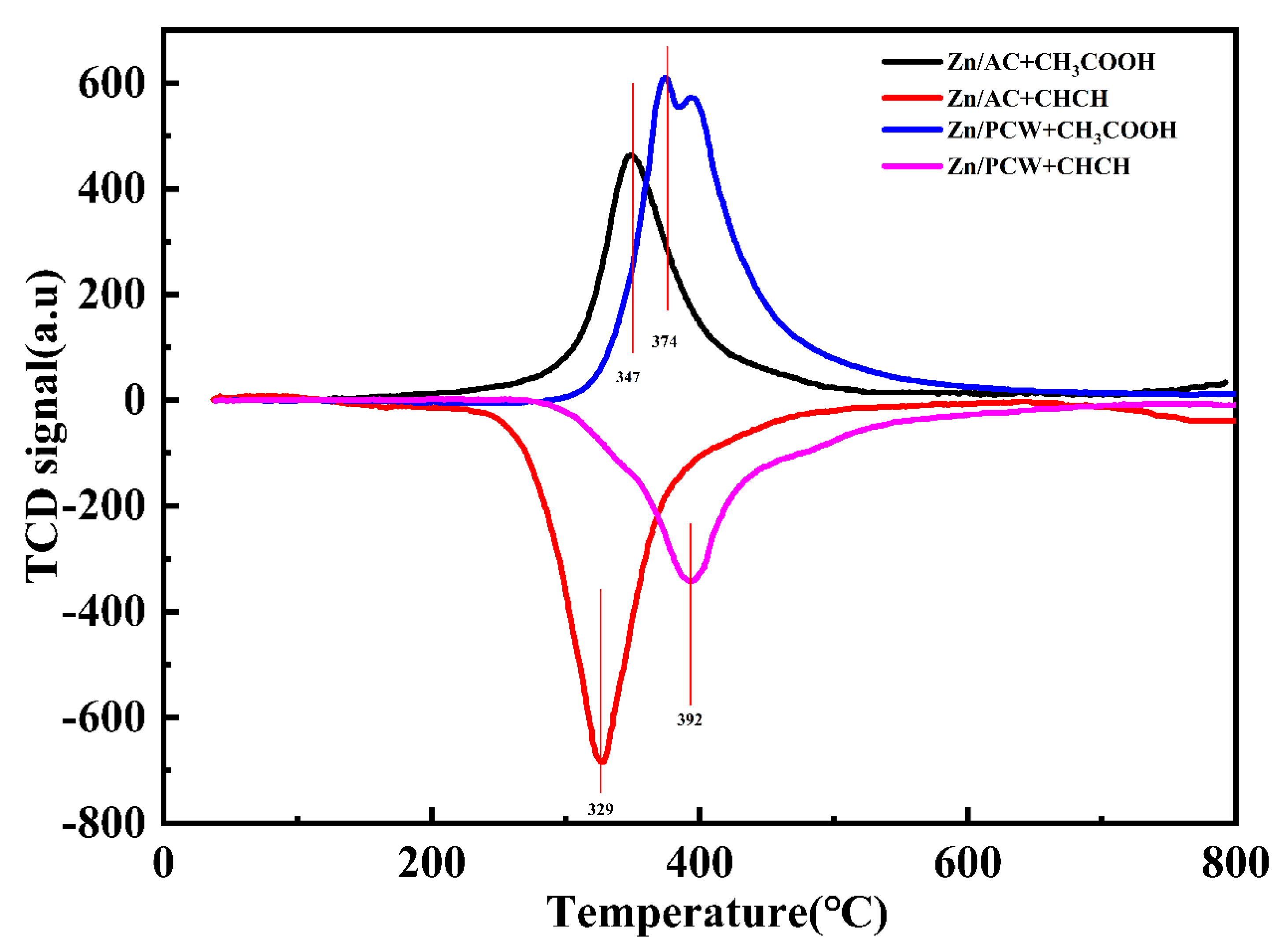
2.2.5. TPD (Temperature-Programmed Desorption)
3. Experiment
3.1. Raw Materials
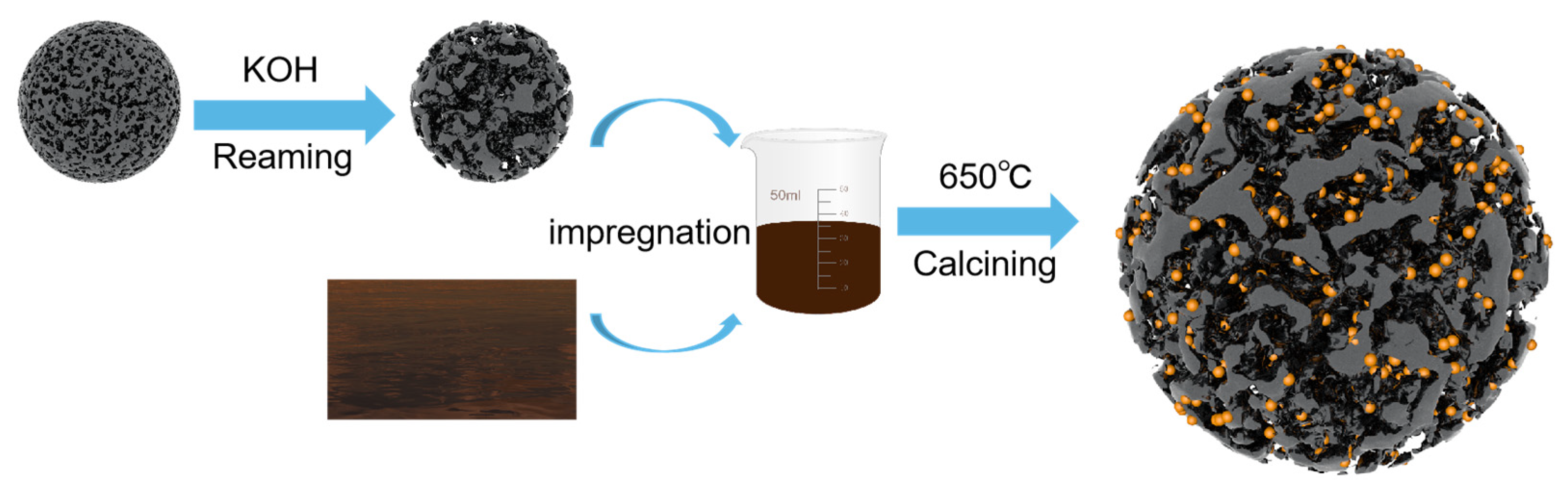
3.2. Carrier Preparation and Catalyst Synthesis
3.3. Characterization of Catalysts
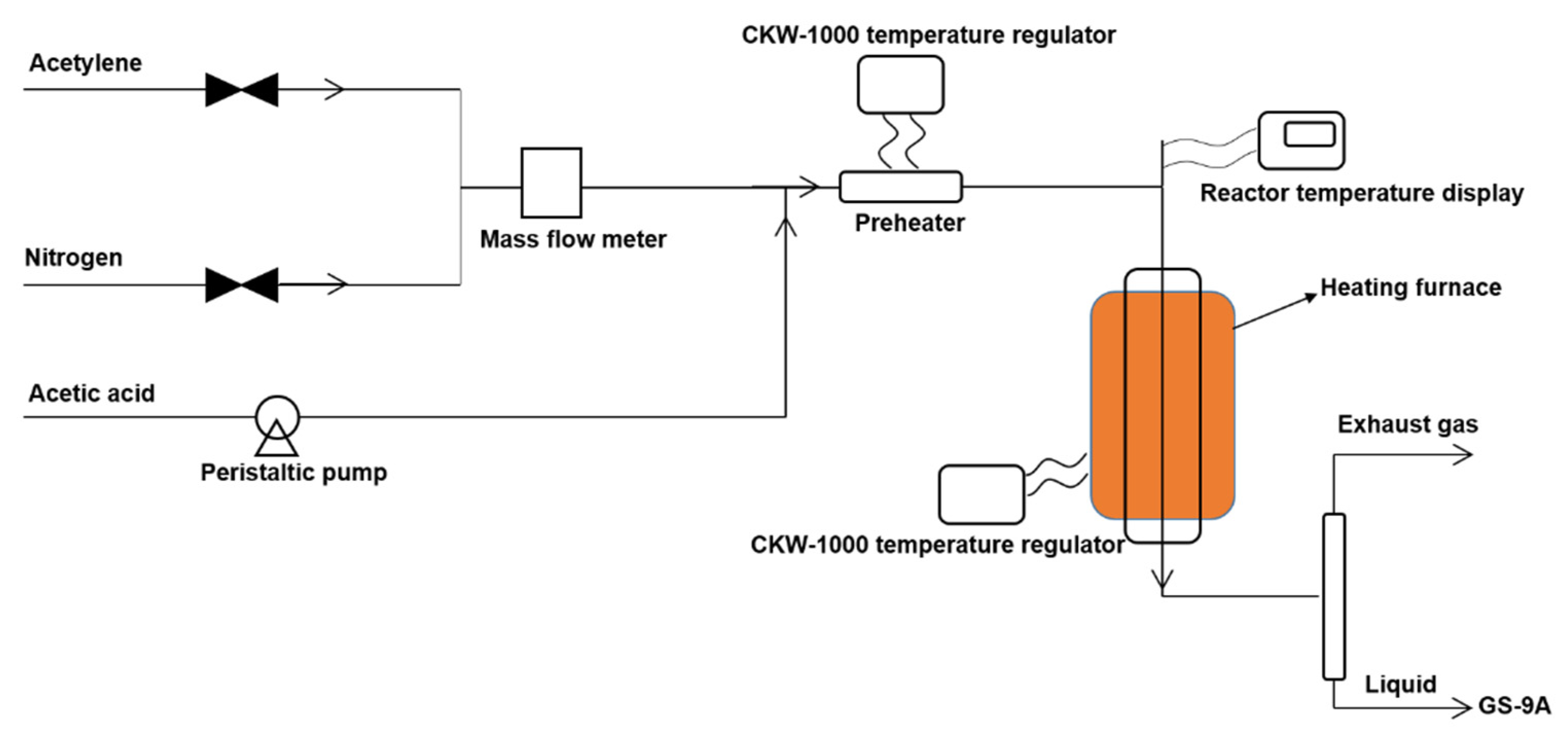
3.4. Catalyst Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manasrah, A.D.; Nassar, N.N.; Ortega, L.C. Conversion of petroleum coke into valuable products using oxy-cracking technique. Fuel 2018, 215, 865–878. [Google Scholar] [CrossRef]
- Chen, C.; Diao, Y.; Lu, Y.; Chen, S.; Tian, L. Complete Reaction Mechanisms of Mercury Binding on Petroleum Coke and Brominated Petroleum Coke. Energy Fuels 2019, 3, 5488–5497. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Z.; Ma, W.; Zhang, H. Vacuum-assisted and alkali roasting for desulfurization of petroleum coke. J. Clean. Prod. 2022, 332, 130052. [Google Scholar] [CrossRef]
- Hamadeh, H.; Toor, S.Y.; Douglas, P.L.; Sarathy, S.M.; Dibble, R.W.; Croiset, E. Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke. Energies 2020, 13, 3463. [Google Scholar] [CrossRef]
- Ksepko, E.; Klimontko, J.; Kwiecinska, A. Industrial wastewater treatment wastes used as oxygen carriers in energy generation processes. J. Therm. Anal. Calorim. 2019, 138, 4247–4260. [Google Scholar] [CrossRef] [Green Version]
- Shamaei, L.; Khorshidi, B.; Islam, M.A.; Sadrzadeh, M. Industrial waste lignin as an antifouling coating for the treatment of oily wastewater: Creating wealth from waste. J. Clean. Prod. 2020, 256, 120304. [Google Scholar] [CrossRef]
- Wei, G.; Shao, L.; Mo, J.; Li, Z.; Zhang, L. Preparation of a new Fenton-like catalyst from red mud using molasses wastewater as partial acidifying agent. Environ. Sci. Pollut. Res. Int. 2017, 24, 15067–15077. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Adeniran, J.A.; Lototskyy, M.; Asadi, A. Simultaneous brewery wastewater treatment and hydrogen generation via hydrolysis using Mg waste scraps. J. Clean. Prod. 2020, 276, 123198. [Google Scholar] [CrossRef]
- Choi, J.; Barnard, Z.G.; Zhang, S.; Hill, J.M. Ni catalysts supported on activated carbon from petcoke and their activity for toluene hydrogenation. Can. J. Chem. Eng. 2012, 90, 631–636. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Cheng, L.; Xu, L.; Bi, X.; Liu, Q. Valorization of fluid petroleum coke for efficient catalytic destruction of biomass gasification tar. J. Hazard. Mater. 2022, 424, 127297. [Google Scholar] [CrossRef]
- Arcibar-Orozco, J.A.; Zili-Tomita, H.E.; Suárez-Toriello, V.A.; Saucedo-Lucero, J.O. Petcoke Revalorization as Support for ZnO-based Photocatalyst. Waste Biomass Valorization 2022, 13, 1681–1694. [Google Scholar] [CrossRef]
- Mei, S.; Gu, J.; Ma, T.; Li, X.; Hu, Y.; Li, W.; Zhang, J.; Han, Y. N-doped activated carbon from used dyeing wastewater adsorbent as a metal-free catalyst for acetylene hydrochlorination. Chem. Eng. J. 2019, 371, 118–129. [Google Scholar] [CrossRef]
- Wu, X.; He, P.; Wang, X.; Dai, B. Zinc acetate supported on N-doped activated carbon as catalysts for acetylene acetoxylation. Chem. Eng. J. 2017, 309, 172–177. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, X.; Wei., J. A facile synthesis for nitrogen-doped carbon catalyst with high activity of oxygen reduction reaction in acidic media. Int. J. Energy Res 2021, 13, 45. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Li, M.; Wang, X.; Zhu, M.; Dai, B. A Highly Active In Situ Zn(CH3COO)2-NC Catalyst for the Acetoxylation of Acetylene. Ind. Eng. Chem. Res. 2022, 61, 1313–1321. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, X.; Sheng, W.; Tu, J.; Zhao, Z.; Shi, Y.; Xu, C.; Ge, G.; Jia, X. Isolated single iron atoms anchored on a N, S-codoped hierarchically ordered porous carbon framework for highly efficient oxygen reduction. J. Mater. Chem. A 2021, 9, 10110–10119. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Shang, N.; Gao, S.; Gao, Y.; Wang, C. Ultra dispersed cobalt anchored on nitrogen-doping ordered porous carbon as an efficient transfer hydrogenation catalyst. Appl. Surf. Sci. 2019, 491, 544–552. [Google Scholar] [CrossRef]
- Guo, X.; Duan, J.; Li, C.; Zhang, Z.; Wang, W. Modified bamboo-based activated carbon as the catalyst carrier for the gas phase synthesis of vinyl acetate from acetylene and acetic acid. Int. J. Chem. React. Eng. 2021, 19, 331–340. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Wang, G.; Yu, B. Structure-activity relationship of zinc acetate/activated carbon catalysts. J. Catal. 1986, 7, 155–161. [Google Scholar]
- Hu, L.; Xu, Z.; He, P.; Wang, X.; Tian, Z.; Yuan, H.; Yu, F.; Dai, B. Zinc and Nitrogen-Doped Carbon In-Situ Wrapped ZnO Nanoparticles as a High-Activity Catalyst for Acetylene Acetoxylation. Catal. Lett. 2020, 150, 1155–1162. [Google Scholar] [CrossRef]
- Islam, M.T.; Dominguez, A.; Alvarado-Tenorio, B.; Bernal, R.A.; Montes, M.O.; Noveron, J.C. Sucrose-Mediated Fast Synthesis of Zinc Oxide Nanoparticles for the Photocatalytic Degradation of Organic Pollutants in Water. ACS Omega 2019, 4, 6560–6572. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.Y.; Cho, J.S. Porous Hybrid Nanofibers Comprising ZnSe/CoSe(2)/Carbon with Uniformly Distributed Pores as Anodes for High-Performance Sodium-Ion Batteries. Nanomaterials 2019, 9, 1362. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kang, L. Non-noble metal single atom catalysts with S, N co-doped defective graphene support: A theoretical study of highly efficient acetylene hydration. Mater. Today Commun. 2021, 27, 102216. [Google Scholar] [CrossRef]
- Zhou, D.; Xie, G.; Hu, X.; Cai, X.; Zhao, Y.; Hu, X.; Jin, Q.; Fu, X.; Tan, X.; Liang, C.; et al. Coupling of kenaf Biochar and Magnetic BiFeO3 onto Cross-linked Chitosan for Enhancing Separation Performance and Cr(VI) Ions Removal Efficiency. Int. J. Environ. Res. Public Health 2020, 17, 788. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xu, Z.; Chen, Y.; Shen, G.; Wang, X.; Dai, B. MOFs-Derived Zn-Based Catalysts in Acetylene Acetoxylation. Nanomaterials 2021, 12, 98. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Park, S.-K.; Kang, Y.C.; Cho, J.S. One-dimensional nanostructure comprising MoSe2 nanosheets and carbon with uniformly defined nanovoids as an anode for high-performance sodium-ion batteries. Chem. Eng. J. 2018, 351, 559–568. [Google Scholar] [CrossRef]
- Kent, P.R.C.; Annaberdiyev, A.; Benali, A.; Bennett, M.C.; Landinez Borda, E.J.; Doak, P.; Hao, H.; Jordan, K.D.; Krogel, J.T.; Kylanpaa, I.; et al. QMCPACK: Advances in the development, efficiency, and application of auxiliary field and real-space variational and diffusion quantum Monte Carlo. J. Chem. Phys. 2020, 152, 174105. [Google Scholar] [CrossRef]
- Rong, S.L.; Peng, F.G.; Hong, Z.Z.; Lin, L.Z.; Li, C.M.; Jian, W.; Yuan, F.L.; Feng, L.; Na, L.; Cheng, Z.H. Chiral nanoprobes for targeting and long-term imaging of the Golgi apparatus. Chem. Sci. 2017, 8, 6829–6835. [Google Scholar]
- Javan Bakht Dalir, S.; Djahaniani, H.; Nabati, F.; Hekmati, M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon 2020, 6, e03624. [Google Scholar] [CrossRef]
- He, P.; Wu, X.; Huang, L.; Zhu, M.; Dai, B. Acetoxylation of acetylene to vinyl acetate monomer over bimetallic Zn-Ni/AC catalysts. Catal. Commun. 2018, 112, 5–9. [Google Scholar] [CrossRef]
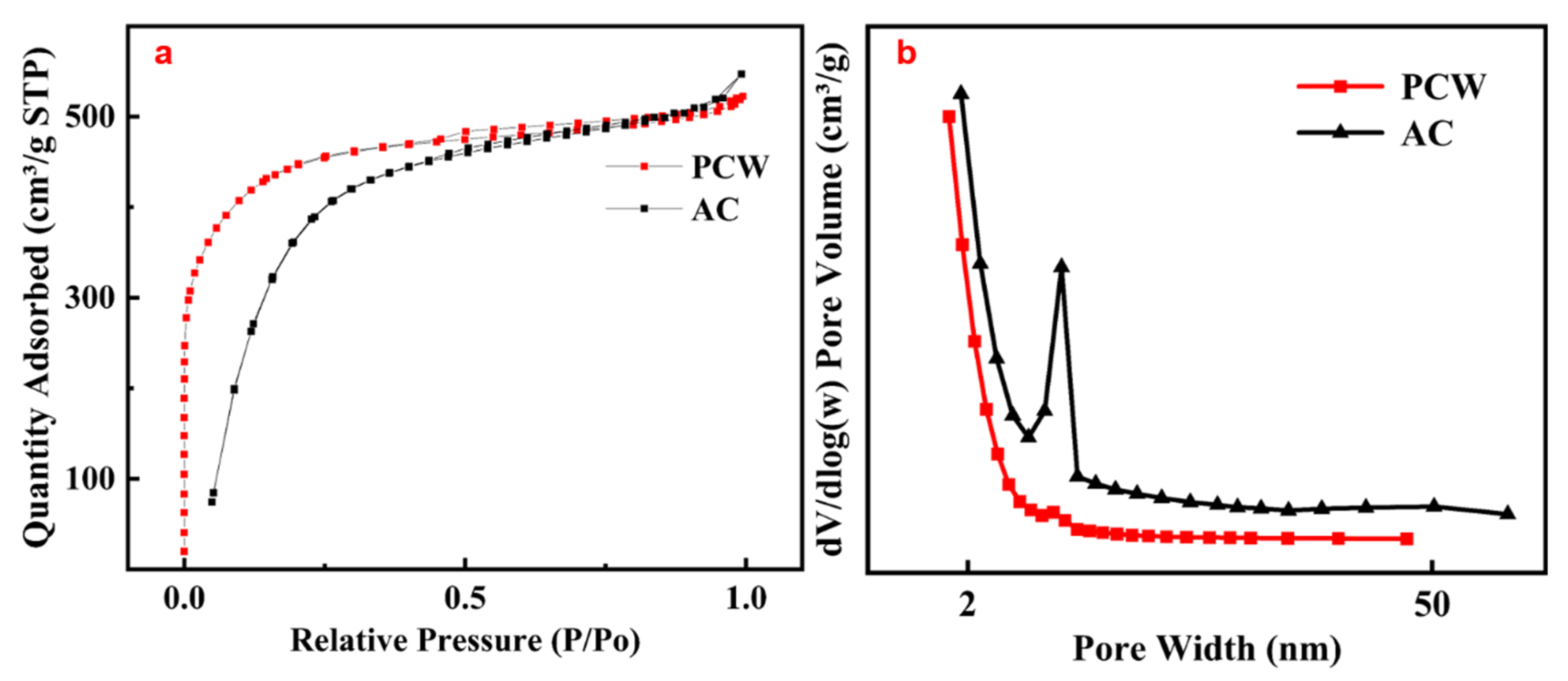

| Sample | AC | Zn/AC | PCW | Zn/PCW | Zn/PCW-600 | Zn/PCW-900 | Zn/PCW-1000 |
|---|---|---|---|---|---|---|---|
| SBET (m2/g) | 1476.9 | 629.9 | 1505.3 | 86.3 | 1105.8 | 1126.8 | 1412.3 |
| Pore volume (cm3/g) | 0.67 | 0.28 | 0.80 | 0.05 | 0.50 | 0.51 | 0.63 |
| Average pore diameter (nm) | 1.81 | 1.8 | 2.1 | 2.3 | 1.80 | 1.81 | 1.79 |
| Sample | Zn/AC | Zn/PCW | Zn/PC |
|---|---|---|---|
| Zn | 12.54 | 6.15 | 12.11 |
| N | 3.36 | 7.84 | 1.75 |
| Sample | Zn/AC | Zn/PCW | Zn/PCW-900 | Zn/PCW-1000 | Zn/PC |
|---|---|---|---|---|---|
| Zn (wt%) | 14.01 | 13.75 | 11.98 | 0.52 | 12.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, M.; Xu, Z.; Shen, G.; Wang, X.; Dai, B. Preparation of High-Performance Zn-Based Catalysts Using Printing and Dyeing Wastewater and Petroleum Coke as a Carrier in Acetylene Acetoxylation. Catalysts 2023, 13, 539. https://doi.org/10.3390/catal13030539
Chen Y, Li M, Xu Z, Shen G, Wang X, Dai B. Preparation of High-Performance Zn-Based Catalysts Using Printing and Dyeing Wastewater and Petroleum Coke as a Carrier in Acetylene Acetoxylation. Catalysts. 2023; 13(3):539. https://doi.org/10.3390/catal13030539
Chicago/Turabian StyleChen, Yuhao, Mengli Li, Zhuang Xu, Guowang Shen, Xugeng Wang, and Bin Dai. 2023. "Preparation of High-Performance Zn-Based Catalysts Using Printing and Dyeing Wastewater and Petroleum Coke as a Carrier in Acetylene Acetoxylation" Catalysts 13, no. 3: 539. https://doi.org/10.3390/catal13030539






