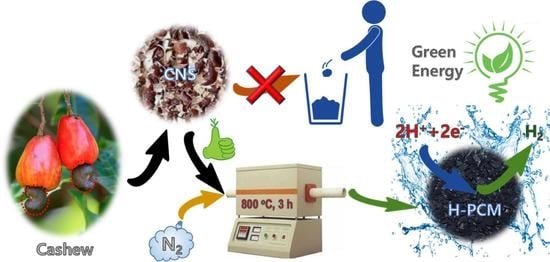
Biowaste-Derived Heteroatom-Doped Porous Carbon as a Sustainable Electrocatalyst for Hydrogen Evolution Reaction
Abstract
1. Introduction
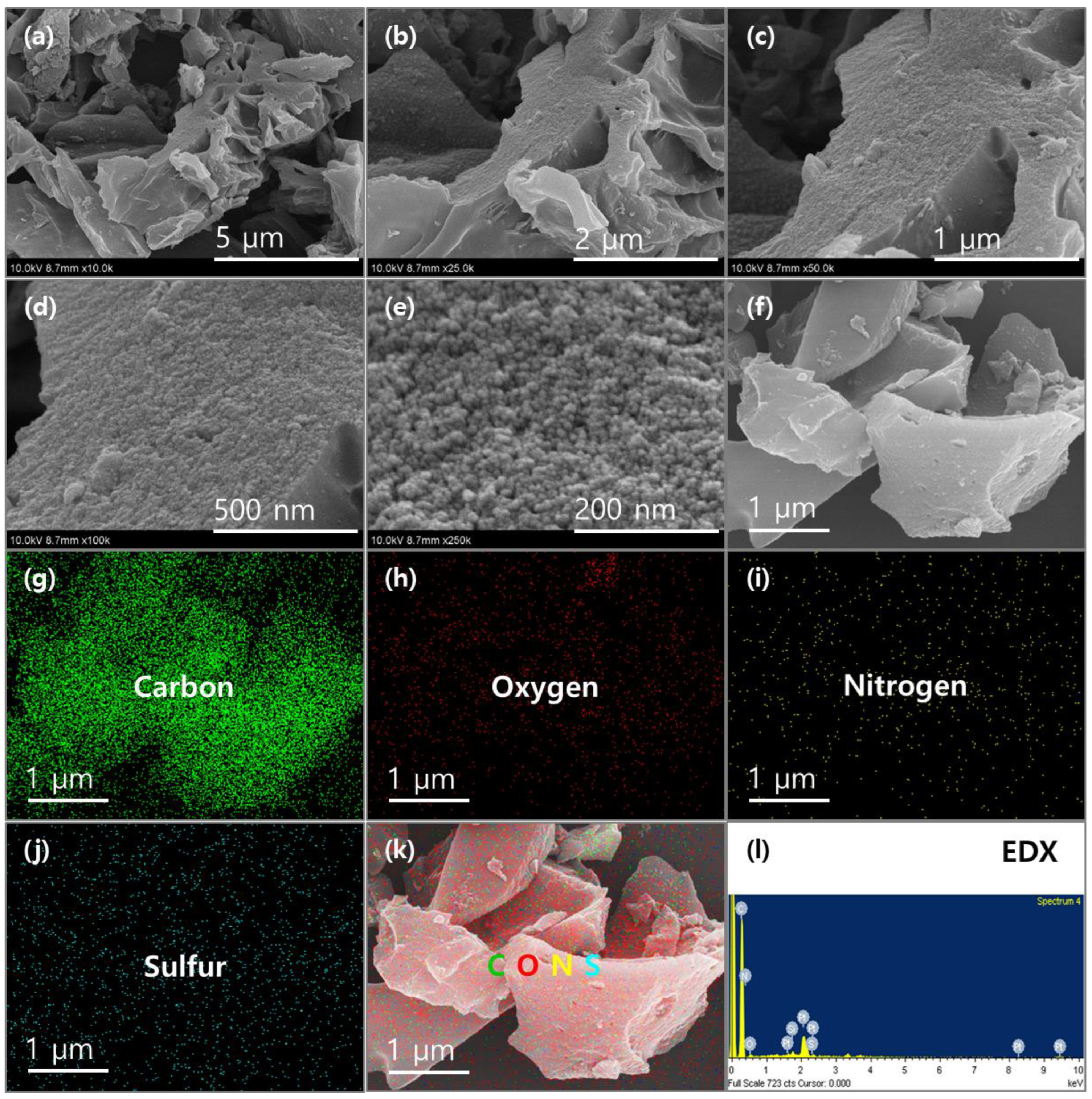
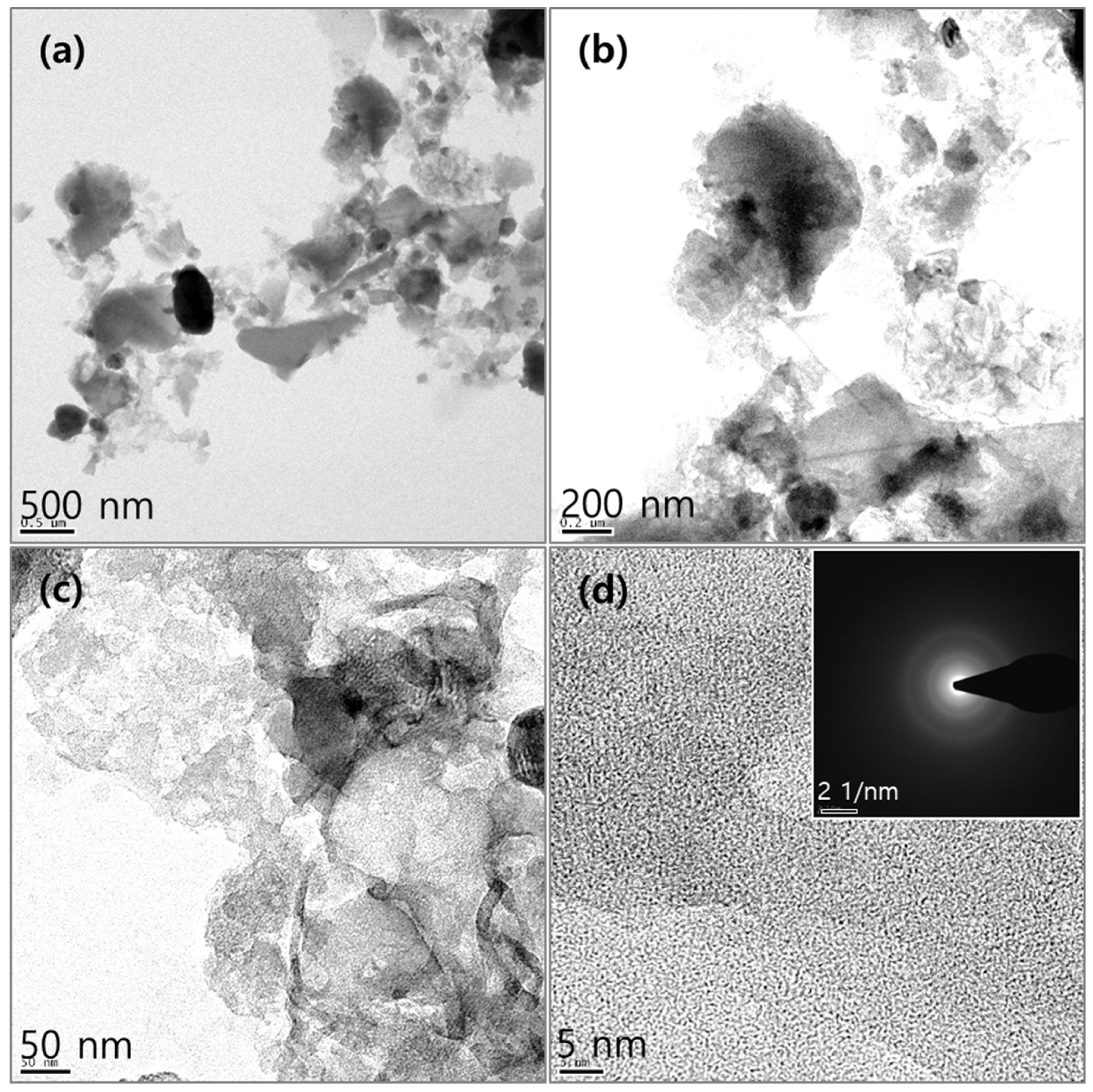
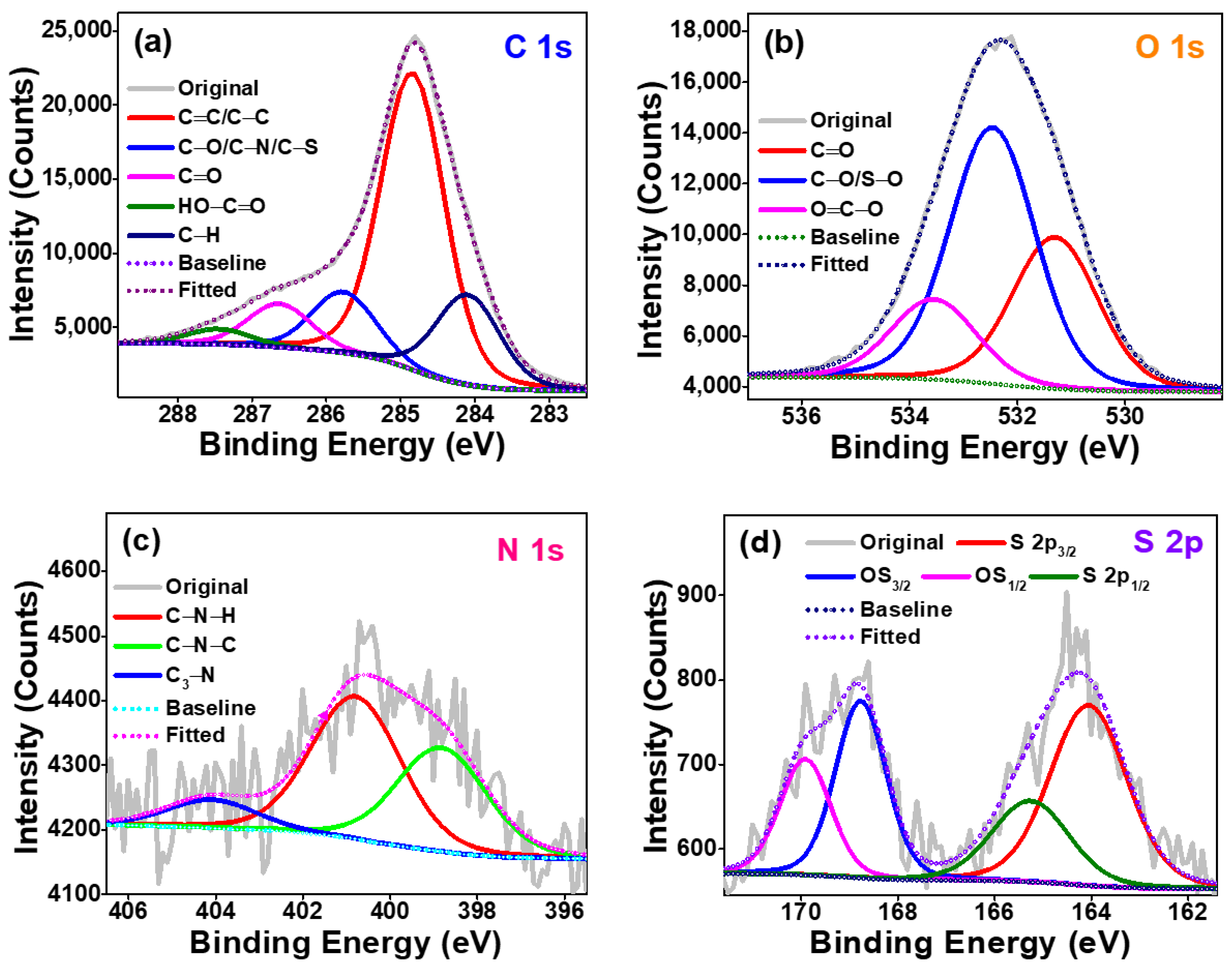
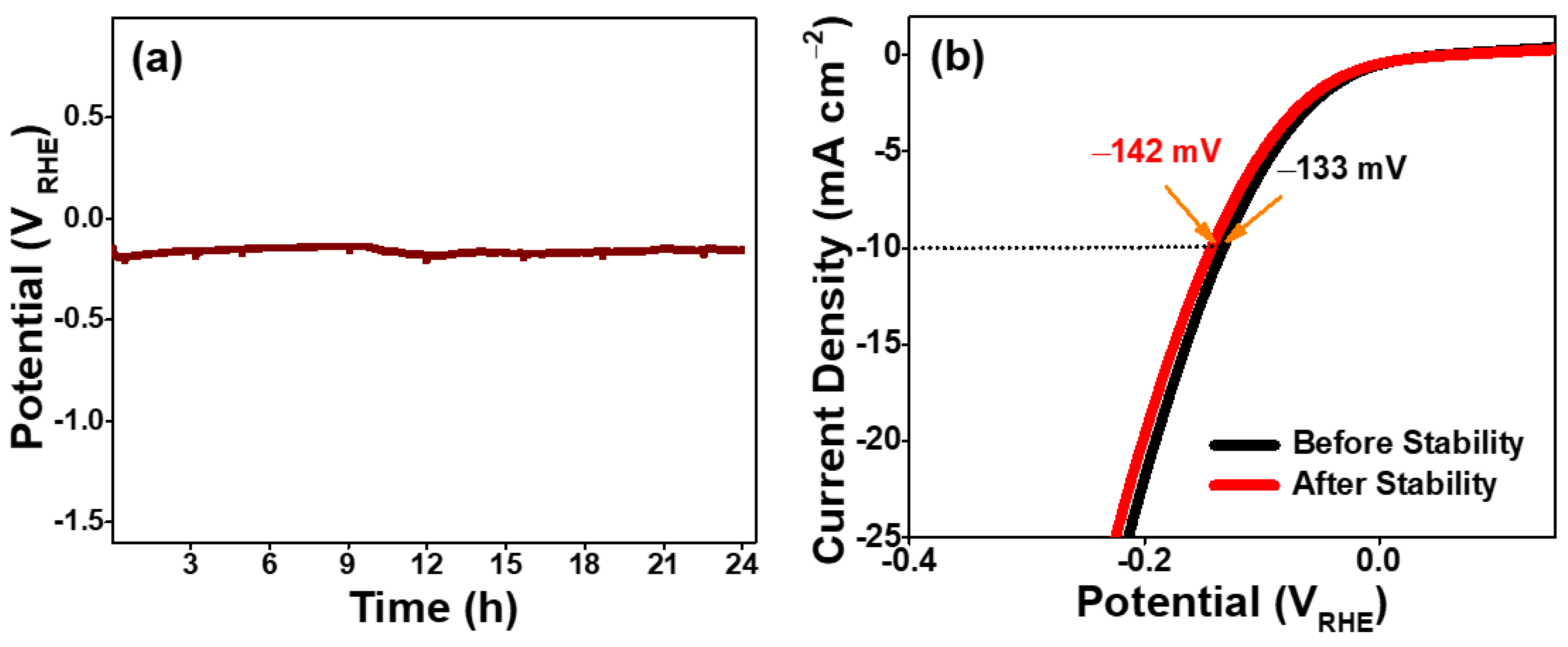
2. Results and Discussion
| Electrocatalysts | Preparation Method | Overpotential at 10 mA cm−2 (mV) | Tafel Slope (mV dec–1) | Reference |
|---|---|---|---|---|
| SrTiO3@MoS2 | Calcination | 165 | 81.41 | [66] |
| NA9 | Carbonization | 184 | 164 | [67] |
| NPCF | Annealing | 248 | 135 | [64] |
| S–MoS2/rGO/CNTs | Carbonization | 159 | 85 | [68] |
| Mo2C/C | Pyrolysis | 133 | 71 | [69] |
| LS-PC | Pyrolysis | 135 | 85 | [45] |
| PS/MoS2 | Pyrolysis | 154 | 71 | [70] |
| Porous Mo2C/C | Sintering | 556 | 123.9 | [59] |
| Ni/P-doped carbon | Pyrolysis | 297 | 134.9 | [71] |
| BS-800 | Calcination | 413 | 98 | [72] |
| H-PCM | Pyrolysis | 133 | 75 | This work |
3. Materials and Methods
Synthesis of Heteroatom-Doped Porous Carbon Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Wang, H.; Priest, C.; Li, S.; Xu, P.; Wu, G. Advanced Electrocatalysis for Energy and Environmental Sustainability via Water and Nitrogen Reactions. Adv. Mater. 2021, 33, 2000381. [Google Scholar] [CrossRef] [PubMed]
- Kaeffer, N.; Leitner, W. Electrocatalysis with Molecular Transition-Metal Complexes for Reductive Organic Synthesis. JACS Au 2022, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, J.; Kanokkanchana, K.; Tschulik, K. Design Strategies for Electrocatalysts from an Electrochemist’s Perspective. ACS Catal. 2021, 11, 5318–5346. [Google Scholar] [CrossRef]
- Chen, G.; Wei, C.; Zhu, Y.; Huang, H. Emerging chemical driving force in electro-catalytic water splitting. EcoMat 2023, 5, e12294. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Chandrasekaran, S.; Perumal, S.; Raja, P.B.; Perumal, V.; Lee, Y.R. Deep eutectic solvent assisted electrosynthesis of ruthenium nanoparticles on stainless steel mesh for electro-catalytic hydrogen evolution reaction. Fuel 2021, 297, 120786. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Jebakumar Immanuel Edison, T.N.; Aldawood, S.; Vinodh, R.; Sundramoorthy, A.K.; Ghodake, G.; Lee, Y.R. Facile synthesis of novel molybdenum disulfide decorated banana peel porous carbon electrode for hydrogen evolution reaction. Chemosphere 2022, 307, 135712. [Google Scholar] [CrossRef]
- Jebakumar Immanuel Edison, T.N.; Atchudan, R.; Karthik, N.; Lee, Y.R. Green synthesized N-doped graphitic carbon sheets coated carbon cloth as efficient metal free electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 14390–14399. [Google Scholar] [CrossRef]
- Li, Z.; Ge, R.; Su, J.; Chen, L. Recent Progress in Low Pt Content Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2020, 7, 2000396. [Google Scholar] [CrossRef]
- Li, D.Y.; Liao, L.L.; Zhou, H.Q.; Zhao, Y.; Cai, F.M.; Zeng, J.S.; Liu, F.; Wu, H.; Tang, D.S.; Yu, F. Highly active non-noble electrocatalyst from Co2P/Ni2P nanohybrids for pH-universal hydrogen evolution reaction. Mater. Today Phys. 2021, 16, 100314. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef]
- Long, G.-F.; Wan, K.; Liu, M.-Y.; Liang, Z.-X.; Piao, J.-H.; Tsiakaras, P. Active sites and mechanism on nitrogen-doped carbon catalyst for hydrogen evolution reaction. J. Catal. 2017, 348, 151–159. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Zhang, X.; Davey, K.; Dai, S.; Qiao, S.Z. Promotion of Electro-catalytic Hydrogen Evolution Reaction on Nitrogen-Doped Carbon Nanosheets with Secondary Heteroatoms. ACS Nano 2017, 11, 7293–7300. [Google Scholar] [CrossRef] [PubMed]
- Onyancha, R.B.; Ukhurebor, K.E.; Aigbe, U.O.; Osibote, O.A.; Kusuma, H.S.; Darmokoesoemo, H. A Methodical Review on Carbon-Based Nanomaterials in Energy-Related Applications. Adsorpt. Sci. Technol. 2022, 2022, 4438286. [Google Scholar] [CrossRef]
- Falcao, E.H.; Wudl, F. Carbon allotropes: Beyond graphite and diamond. J. Chem. Technol. Biotechnol. 2007, 82, 524–531. [Google Scholar] [CrossRef]
- Okwundu, O.S.; Aniekwe, E.U.; Nwanno, C.E. Unlimited potentials of carbon: Different structures and uses (a Review). Metall. Mater. Eng. 2018, 24, 145–171. [Google Scholar] [CrossRef]
- Arul, V.; Edison, T.N.J.I.; Lee, Y.R.; Sethuraman, M.G. Biological and catalytic applications of green synthesized fluorescent N-doped carbon dots using Hylocereus undatus. J. Photochem. Photobiol. B Biol. 2017, 168, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, J.; Pathak, T.S.; Pak, D. Heteroatom-Doped Metal-Free Carbon Nanomaterials as Potential Electrocatalysts. Molecules 2022, 27, 670. [Google Scholar] [CrossRef]
- Nakate, Y.T.; Sangale, S.S.; Shaikh, S.F.; Shinde, N.M.; Shirale, D.J.; Mane, R.S. Human urine-derived naturally heteroatom doped highly porous carbonaceous material for gas sensing and supercapacitor applications. Ceram. Int. 2022, 48, 28942–28950. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis–A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C.-H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Z.; Cao, B.; Chen, X.; Zhang, C.; Shaymurat, T.; Duan, H.; Zhang, J.; Zhang, M. Gas sensing performance of biomass carbon materials promoted by nitrogen doping and p-n junction. Appl. Surf. Sci. 2022, 592, 153254. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Park, J.-S. Biomass-derived N-doped porous carbon nanosheets for energy technologies. Chem. Eng. J. 2021, 425, 129017. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Yang, L.; Li, L.; Zhang, Z.; Ke, Y.; Chen, S. Nitrogen and sulfur co-doped porous carbon derived from human hair as highly efficient metal-free electrocatalysts for hydrogen evolution reactions. J. Mater. Chem. A 2015, 3, 8840–8846. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High Catalytic Activity of Nitrogen and Sulfur Co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Murugesan, C.; Senthilkumar, B.; Barpanda, P. Biowaste-Derived Highly Porous N-Doped Carbon as a Low-Cost Bifunctional Electrocatalyst for Hybrid Sodium–Air Batteries. ACS Sustain. Chem. Eng. 2022, 10, 9077–9086. [Google Scholar] [CrossRef]
- Li, Z.; Bai, Z.; Mi, H.; Ji, C.; Gao, S.; Pang, H. Biowaste-Derived Porous Carbon with Tuned Microstructure for High-Energy Quasi-Solid-State Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 13127–13135. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-doped Porous Carbon Derived from KOH-Activated Waste Wool: A Promising Material for Selective Adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Lv, H.; Chen, A. Silicate-assisted activation of biomass towards N-doped porous carbon sheets for supercapacitors. J. Alloys Compd. 2021, 853, 157091. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, S.; Huang, C.; Wang, X.; Cai, S.; Xiang, K.; Zhang, Y.; Xue, J. Honeycomb-like Nitrogen and Sulfur Dual-Doped Hierarchical Porous Biomass-Derived Carbon for Lithium–Sulfur Batteries. ChemSusChem 2017, 10, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Guan, Z.; Li, Z.; Liu, J.; Yu, K. Characterization and Preparation of Nano-porous Carbon Derived from Hemp Stems as Anode for Lithium-Ion Batteries. Nanoscale Res. Lett. 2019, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Lu, Y.; Zhao, F.; Chao, C.; Zhang, B.; Zhang, H. Preparing high surface area porous carbon from biomass by carbonization in a molten salt medium. RSC Adv. 2015, 5, 75728–75734. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Sangaraju, S.; Babu, R.S.; Lee, Y.R. Sustainable Synthesis of Bright Fluorescent Nitrogen-Doped Carbon Dots from Terminalia chebula for In Vitro Imaging. Molecules 2022, 27, 8085. [Google Scholar] [CrossRef]
- Lei, W.; Liu, H.; Xiao, J.; Wang, Y.; Lin, L. Moss-Derived Mesoporous Carbon as Bi-Functional Electrode Materials for Lithium–Sulfur Batteries and Supercapacitors. Nanomaterials 2019, 9, 84. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K. Converting Corncob to Activated Porous Carbon for Supercapacitor Application. Nanomaterials 2018, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yu, J.; Yang, X.; Zhang, G. Activated Carbon Fibers with Hierarchical Nanostructure Derived from Waste Cotton Gloves as High-Performance Electrodes for Supercapacitors. Nanoscale Res. Lett. 2017, 12, 379. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Ramalingam, S.; Aldawood, S.; Devarajan, N.; Lee, W.; Lee, Y.R. Silver nanoparticles loaded graphene-poly-vinylpyrrolidone composites as an effective recyclable antimicrobial agent. Environ. Res. 2023, 216, 114706. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, Y.R. Synthesis of Water-Dispersed Sulfobetaine Methacrylate–Iron Oxide Nanoparticle-Coated Graphene Composite by Free Radical Polymerization. Polymers 2022, 14, 3885. [Google Scholar]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Huang, C. Development of nitrogen and sulfur-doped carbon dots for cellular imaging. J. Pharm. Anal. 2019, 9, 127–132. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Y.; Li, Z.; He, W.; Li, Z. Selective Detection of Fe3+ by Nitrogen–Sulfur-Doped Carbon Dots Using Thiourea and Citric Acid. Coatings 2022, 12, 1042. [Google Scholar] [CrossRef]
- Kundoo, S.; Saha, P.; Chattopadhyay, K.K. Electron field emission from nitrogen and sulfur-doped diamond-like carbon films deposited by simple electrochemical route. Mater. Lett. 2004, 58, 3920–3924. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, K.; Hu, J.; Liu, R.; Zhu, H. Nitrogen and sulphur co-doped carbon quantum dots and their optical power limiting properties. Mater. Adv. 2020, 1, 3176–3181. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Edison, T.N.J.I.; Albasher, G.; Sundramoorthy, A.K.; Vinodh, R.; Lee, Y.R. Lotus-biowaste derived sulfur/nitrogen-codoped porous carbon as an eco-friendly electrocatalyst for clean energy harvesting. Environ. Res. 2022, 214, 113910. [Google Scholar] [CrossRef]
- Ouyang, Z.; Lei, Y.; Chen, Y.; Zhang, Z.; Jiang, Z.; Hu, J.; Lin, Y. Preparation and Specific Capacitance Properties of Sulfur, Nitrogen Co-Doped Graphene Quantum Dots. Nanoscale Res. Lett. 2019, 14, 219. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Wang, P.; Yang, Y.; Wang, Y.; Xu, J.; Wang, Y.; Yu, W.W. Synthesis of Nitrogen and Sulfur Co-doped Carbon Dots from Garlic for Selective Detection of Fe3+. Nanoscale Res. Lett. 2016, 11, 110. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, L.; Zou, M.; Lan, C.; Zhan, Z.; Zhao, S. Nitrogen and sulfur co-doped carbon dots: A facile and green fluorescence probe for free chlorine. Sens. Actuators B Chem. 2015, 219, 50–56. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, X.; Li, X.; Zhang, X.; Peng, X.; Song, H.; Fu, J.; Ding, K.; Huang, X.; Gao, B. Nitrogen Doped Carbon Nanosheets Encapsulated in situ Generated Sulfur Enable High Capacity and Superior Rate Cathode for Li-S Batteries. Front. Chem. 2018, 6, 429. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Tyc, M.; Kowalska, K.; Kamedulski, P.; Zielinski, W.; Lukaszewicz, J.P. Green algae and gelatine derived nitrogen rich carbon as an outstanding competitor to Pt loaded carbon catalysts. Sci. Rep. 2021, 11, 7084. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.-P.; Antonietti, M. One-pot synthesis of nitrogen–sulfur-co-doped carbons with tunable composition using a simple isothiocyanate ionic liquid. J. Mater. Chem. A 2013, 1, 14097–14102. [Google Scholar] [CrossRef]
- Atchudan, R.; Chandra Kishore, S.; Gangadaran, P.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Rajendran, R.L.; Alagan, M.; Al-Rashed, S.; Ahn, B.-C.; Lee, Y.R. Tunable fluorescent carbon dots from biowaste as fluorescence ink and imaging human normal and cancer cells. Environ. Res. 2022, 204, 112365. [Google Scholar] [CrossRef] [PubMed]
- Karikalan, N.; Velmurugan, M.; Chen, S.-M.; Karuppiah, C. Modern Approach to the Synthesis of Ni(OH)2 Decorated Sulfur Doped Carbon Nanoparticles for the Nonenzymatic Glucose Sensor. ACS Appl. Mater. Interfaces 2016, 8, 22545–22553. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, D.; Zhang, W.; Bao, W.; Wang, G. A nitrogen–sulfur co-doped porous graphene matrix as a sulfur immobilizer for high performance lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 17381–17393. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wu, Z.; Wang, S.; Wu, Y.; Liu, X. Heteroatom (Nitrogen/Sulfur)-Doped Graphene as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions. Catalysts 2018, 8, 475. [Google Scholar] [CrossRef]
- Chen, H.; Yu, F.; Wang, G.; Chen, L.; Dai, B.; Peng, S. Nitrogen and Sulfur Self-Doped Activated Carbon Directly Derived from Elm Flower for High-Performance Supercapacitors. ACS Omega 2018, 3, 4724–4732. [Google Scholar] [CrossRef]
- Ji, H.; Wang, T.; Liu, Y.; Lu, C.; Yang, G.; Ding, W.; Hou, W. A novel approach for sulfur-doped hierarchically porous carbon with excellent capacitance for electrochemical energy storage. Chem. Commun. 2016, 52, 12725–12728. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kan, J.; Wang, H.; Zhao, X.; Zheng, Y.; Zhang, H.; Tao, L.; Huang, M.; Liu, W.; Shi, J. Nitrogen and Sulfur Co-doped Mesoporous Carbon for Sodium Ion Batteries. ACS Appl. Nano Mater. 2019, 2, 5643–5654. [Google Scholar] [CrossRef]
- Ding, J.; He, W.; Yu, C.; Liu, Z.; Deng, C.; Zhu, H. Waste biomass derived carbon supported Mo2C/C composite materials for heavy metal adsorption and hydrogen evolution reaction. Mater. Today Commun. 2022, 31, 103646. [Google Scholar] [CrossRef]
- Lim, B.A.-L.; Lim, S.; Pang, Y.L.; Shuit, S.H.; Wong, K.H.; Ooi, J.B. Investigation on the Potential of Various Biomass Waste for the Synthesis of Carbon Material for Energy Storage Application. Sustainability 2022, 14, 2919. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Liu, Q.; Li, X. Preparation of porous carbon materials from biomass pyrolysis vapors for hydrogen storage. Appl. Energy 2022, 306, 118131. [Google Scholar] [CrossRef]
- Min, S.; Duan, Y.; Li, Y.; Wang, F. Biomass-derived self-supported porous carbon membrane embedded with Co nanoparticles as an advanced electrocatalyst for efficient and robust hydrogen evolution reaction. Renew. Energy 2020, 155, 447–455. [Google Scholar] [CrossRef]
- Chen, X.; Sun, J.; Guo, T.; Zhao, R.; Liu, L.; Liu, B.; Wang, Y.; Li, J.; Du, J. Biomass-derived carbon nanosheets coupled with MoO2/Mo2C electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 30959–30969. [Google Scholar] [CrossRef]
- Han, G.; Hu, M.; Liu, Y.; Gao, J.; Han, L.; Lu, S.; Cao, H.; Wu, X.; Li, B. Efficient carbon-based catalyst derived from natural cattail fiber for hydrogen evolution reaction. J. Solid State Chem. 2019, 274, 207–214. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, Y.; Huang, S.; He, S.; Li, X.; Tong, S.; Wu, M. Nitrogen-, Oxygen- and Sulfur-Doped Carbon-Encapsulated Ni3S2 and NiS Core–Shell Architectures: Bifunctional Electrocatalysts for Hydrogen Evolution and Oxygen Reduction Reactions. ACS Sustain. Chem. Eng. 2018, 6, 15582–15590. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, J.; Wei, K.; Li, B.; Jiao, T.; Chen, Y.; Zhou, J.; Peng, Q. Fabrication of hierarchical SrTiO3@MoS2 heterostructure nanofibers as efficient and low-cost electrocatalysts for hydrogen-evolution reactions. Nanotechnology 2020, 31, 205604. [Google Scholar] [CrossRef]
- Chinnadurai, D.; Karuppiah, P.; Chen, S.-M.; Kim, H.-j.; Prabakar, K. Metal-free multiporous carbon for electrochemical energy storage and electrocatalysis applications. New J. Chem. 2019, 43, 11653–11659. [Google Scholar] [CrossRef]
- Tian, J.; Li, J.; Feng, A.; Han, X.; Lv, Y.; Ma, M.; Lin, C. Self-limited conversion of MoO2 into ultramicro MoS2 nanosheets on graphene/CNTs matrix for hydrogen evolution with excellent stability. Sustain. Energy Fuels 2020, 4, 2869–2874. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Yusuf, B.A.; Zhou, C.; Xu, Y.; Ji, Q.; Xie, J.; Ma, H. Simplistic two-step fabrication of porous carbon-based biomass-derived electrocatalyst for efficient hydrogen evolution reaction. Energy Convers. Manag. 2021, 227, 113628. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, H.; Cao, X.; Zhang, Y.; Zhang, X.; Qiao, S. Castoff derived Biomass—carbon supported MoS2 nanosheets for hydrogen evolution reaction. Mater. Chem. Phys. 2020, 252, 123244. [Google Scholar] [CrossRef]
- Hoang, V.C.; Gomes, V.G.; Dinh, K.N. Ni- and P-doped carbon from waste biomass: A sustainable multifunctional electrode for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Electrochim. Acta 2019, 314, 49–60. [Google Scholar] [CrossRef]
- Cao, X.; Li, Z.; Chen, H.; Zhang, C.; Zhang, Y.; Gu, C.; Xu, X.; Li, Q. Synthesis of biomass porous carbon materials from bean sprouts for hydrogen evolution reaction electrocatalysis and supercapacitor electrode. Int. J. Hydrogen Energy 2021, 46, 18887–18897. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atchudan, R.; Perumal, S.; Jebakumar Immanuel Edison, T.N.; Sundramoorthy, A.K.; Karthik, N.; Sangaraju, S.; Choi, S.T.; Lee, Y.R. Biowaste-Derived Heteroatom-Doped Porous Carbon as a Sustainable Electrocatalyst for Hydrogen Evolution Reaction. Catalysts 2023, 13, 542. https://doi.org/10.3390/catal13030542
Atchudan R, Perumal S, Jebakumar Immanuel Edison TN, Sundramoorthy AK, Karthik N, Sangaraju S, Choi ST, Lee YR. Biowaste-Derived Heteroatom-Doped Porous Carbon as a Sustainable Electrocatalyst for Hydrogen Evolution Reaction. Catalysts. 2023; 13(3):542. https://doi.org/10.3390/catal13030542
Chicago/Turabian StyleAtchudan, Raji, Suguna Perumal, Thomas Nesakumar Jebakumar Immanuel Edison, Ashok K. Sundramoorthy, Namachivayam Karthik, Sambasivam Sangaraju, Seung Tae Choi, and Yong Rok Lee. 2023. "Biowaste-Derived Heteroatom-Doped Porous Carbon as a Sustainable Electrocatalyst for Hydrogen Evolution Reaction" Catalysts 13, no. 3: 542. https://doi.org/10.3390/catal13030542
APA StyleAtchudan, R., Perumal, S., Jebakumar Immanuel Edison, T. N., Sundramoorthy, A. K., Karthik, N., Sangaraju, S., Choi, S. T., & Lee, Y. R. (2023). Biowaste-Derived Heteroatom-Doped Porous Carbon as a Sustainable Electrocatalyst for Hydrogen Evolution Reaction. Catalysts, 13(3), 542. https://doi.org/10.3390/catal13030542