Abstract
The catalytic transformation of sugars into lactic acid has shown great potential for the scalable utilization of renewable biomass. Herein, RuOx/MoS2 catalysts were synthesized with the assistance of CaO for the one-pot conversion of glucose to lactic acid. Under the reaction conditions of 120 °C and 1 MPa O2, a 96.6% glucose conversion and a 54.3% lactic acid selectivity were realized in the one-pot catalytic reaction, with relatively high stability after four successive cycles. This catalytic system was also effective for the conversion of many other carbohydrate substrates, such as fructose, xylose and cellulose (selectivity 68.9%, 78.2% and 50.6%, respectively). According to catalyst characterizations and conditional experiments, the highly dispersed RuOx species on the surface of MoS2, together with OH−, promoted isomerization, retro-aldol condensation, dehydration and hydration reactions, resulting in a relatively high lactic acid yield for sugar conversions.
1. Introduction
As the only abundant renewable carbon resource, the efficient conversion of biomass into fuel and chemicals has attracted significant attention [1,2]. Due to their abundance and wide availability, cellulose and sugars have been used in central research on biomass conversion and converted into a range of oxygen-containing compounds [3,4,5,6]. Among these attractive chemicals, lactic acid (LA) has emerged as one of the most promising due to its fast-growing market [7,8,9]. Currently, LA is widely used in the food and beverage sector as well as the cosmetics and pharmaceutics industries. Additionally, LA is also an important raw material for the production of bioplastics, polylactide in particular, which is regarded as one of the top ten industrially important chemicals by the United States Department of Energy [10,11].
At present, the fermentation of starch is the most common method for LA production, providing more than 90% of LA in market [12,13]. However, the bacterial fermentation method has the disadvantages of multiple treatment steps and low productivity. Alternatively, the catalytic conversion method of LA production from biomass has attracted increasing research interest during recent years thanks to its versatile feedstocks and high reaction efficiency [14]. To alleviate the condensation or decomposition reactions, the catalytic reaction is usually conducted under alcohol medium to produce esters or in water solution with metal salts to stabilize the final LA. Typically, Sn(IV)-beta or Sn(IV) grafted onto mesoporous silica support catalyzed the conversion of carbohydrates in an alcohol medium into a ca. 60–75% yield of alkyl lactate [15,16,17,18,19]. Alternatively, homogeneous metal salts were applied to crack sugars and cellulosic biomass into LA under hydrothermal conditions and yielded > 60% LA from the lignocellulose-based biomass [20,21,22,23,24]. The carbohydrates, including glucose, cellulose and starch, are feasibly transformed into LA in the presence of NaOH and/or Ca(OH)2 but suffer from low LA selectivity and fierce reaction conditions [25]. Therefore, a number of works have investigated heterogeneous catalysts to promote the conversion of sugars into LA. For instance, Murzin et al. developed a Sn(salen)/octylmethyl imidazolium bromide catalyst and achieved a 58% yield of LA with 68% glucose conversion at 473 K [26]. Zhang et al. reported that the introduction of indium and tin into Beta zeolites is able to promote the transformation of glucose to LA, affording a LA yield of 53% at 443 K [27]. Nakajima et al. synthesized a new type of solid acid-base bifunctional catalyst YNbO4 through a simple co-precipitation method, which obtained a 36% LA yield at 413 K for 5 h [28]. As for the catalysts with a base assistance, Choudhary et al. prepared a CTAB-capped Cu/MgO catalyst for the conversion of biomass-derived sugars in the presence of NaOH and obtained a 70% yield of LA at a mild temperature of 393 K [6]. Wang et al. employed the polymerizates of imidazole and epichlorohydrin ([IMEP]Cl) as catalysts to convert glucose to LA in water in the presence of 50 mM NaOH and yielded 63% LA with 99% glucose conversion at 373 K [29]. In spite of these impressive results, developing novel catalysts for the conversion of sugars to LA is still a hotly researched topic.
Ru-based catalysts have been widely used in the conversion of biomass-derived chemicals to organic acids due to the unique chemical properties of Ru/RuOx [30,31]. Additionally, MoS2 is a promising catalyst or stable support used in hydrothermal conditions [32,33]. Herein, a series of RuOx/MoS2 catalysts were synthesized for the conversion of sugars into LA with the assistance of CaO in aqueous solutions. Different catalysts and supports were first screened to yield the best RuOx/MoS2 catalyst. The influence of pivotal reaction parameters on the LA yield was investigated. Then, the optimized catalysts were systematically characterized to unveil the role of Ru species in the reaction and the reaction networks was proposed according to conditional experiments.
2. Results and Discussion
2.1. Catalytic Conversion of Glucose via Different Catalysts
In view of the versatile acidic and metallic properties of Ru-based catalysts in biomass conversions [30,34], a variety of 1 wt% Ru catalysts supported on different carriers were screened in glucose conversion for LA production. As shown in Table 1, under a mild condition of 2 h reaction at 120 °C under the pressure of 1.0 MPa O2, the glucose conversion ranged from 30% to 98%, and the LA selectivity varied greatly with the carbon balance changed within a large range of 40–85%, according to the HPLC, GC-MS and HPLC-MS analysis of the products. Via the activated carbon (AC)- and TS-1-supported RuOx catalysts (Table 1, Entries 1–2), fructose was the major product, with 30–50% selectivity and high conversions (>72%) of glucose, but LA selectivity was merely ca. 10%. This indicates that these two catalysts have notable activity in glucose isomerization but are much less active for retro-aldol reaction and C-C bond breakage [35]. On the other hand, besides the low yields of LA, the 1RuOx/MWW and 1RuOx/HPA catalysts (Table 1, Entries 3–4) exhibited distinguished selectivity (ca. 25%) for gluconic acid formation, reflecting the typical activity for catalytic oxidation usually observed via a noble metal catalyst [36,37]. With respect to the LA formation, the most remarkable performance was observed in the catalysts of 1RuOx/TiO2, 1RuOx/BN and 1RuOx/MoS2 (Table 1, Entries 5–7), which provided 30–46% LA selectivity and 65–95% glucose conversion. The carbon balance in the reaction via these RuOx catalysts changed within the large range of 40–85%, suggesting that certain side reactions often took place. The reactant solution was clear and colorless after the reaction. The HPLC analysis showed that no 5-hydroxylmethylfurfural (a product of fructose dehydration, often serving as a precursor for polymerization to form humins) was detected in the products, indicating the formation of heavier products was not the main cause of the carbon loss. The analysis of the gas phase product showed that CO2 was generated in the reaction. We speculated that the CO2 production largely accounted for the loss of carbon balance in the reaction. According to the highest LA selectivity and the high thermal stability of the MoS2-supported metal catalysts previously observed [38], the RuOx/MoS2 system was chosen for further investigation.

Table 1.
Product distribution for glucose conversion via different Ru-based catalysts.
Alkaline environments greatly contribute to the catalytic conversion of bio-based compounds to LA [39]. Therefore, two types of frequently used base, i.e., NaOH and CaO, with different alkalinities, were added into the reaction system for glucose conversion. As shown in Table 2, Entries 1 and 2, the addition of the base obviously improved the conversion of glucose, while the selectivity to LA was only increased under the assistance of CaO. According to the reaction results and previous studies [40,41,42], we speculated that CaO provided alkalinity and Ca2+ cations for the reaction. The glucose isomerization and the followed retro-aldol reaction were catalyzed by the suitable base, forming C3 intermediates as the precursor of LA. At the same time, the soluble Ca2+ combined with part of the generated LA to form more stable calcium lactate to a certain extent, which promoted the forward reaction. Via the 1RuOx/MoS2+CaO catalyst, the LA selectivity was improved to 50.5% with a 98.9% glucose conversion. To investigate the role of different sectors in the catalytic conversion, CaO, MoS2 and MoS2+CaO were evaluated in the catalytic system, respectively. As shown in Table 2, Entries 3–5, the presence of MoS2 merely showed a remarkable selectivity to gluconic acid but not to the LA product (38.8% vs. 9.2%). On the other hand, CaO afforded 29.5% yield of LA along with 15.2% gluconic acid formation. Combining the MoS2+CaO in the reaction simply obtained a compromised performance but without the synergistic effect on LA or gluconic acid production. In another case, Ru was loaded on CaO to detect the collaboration between them. Over the 1RuOx/CaO catalyst (Table 2, Entry 6), LA selectivity was very similar to that of CaO, but the fructose selectivity was increased by more than 25%, similar to that via 1RuOx/TiO2 (Table 1, entry 5). Thus, from these conditional experiments, it can be learned that merely through the RuOx/MoS2+CaO catalyst, synergistic effects were observed, which realized a higher yield of LA and glucose conversion simultaneously. Note that the carrier CaO was partially hydrolyzed during the reaction process, which may induce the supported RuOx species to being dissolved in aqueous solution. In contrast, the MoS2 is very stable under hydrothermal conditions, providing a potential stable support to load Ru species [43]. Thus, the RuOx/MoS2 catalyst was optimized for conversions under different conditions.

Table 2.
Effects of different components via catalysts on the glucose conversions.
The effect of Ru loading on catalytic performance was finally investigated. As shown in Table 2, Entries 7–9, the LA yield was maximized to 52.4% via the 2RuOx/MoS2 catalyst. At lower Ru loadings (0.5% and 1%), the main by-product was C6 acids, indicating the weak activity of catalysts in retro-aldol condensation reactions. In contrast, at a high Ru loading of 5%, glyceric acid and fructose were formed largely due to the hydration of dihydroxyacetone and/or glyceraldehyde and depressed retro-aldol condensation. Thus, a reasonable Ru content, i.e., 2%, should be loaded on MoS2 to balance the retro-aldol condensation and hydration reactions for high LA yields.
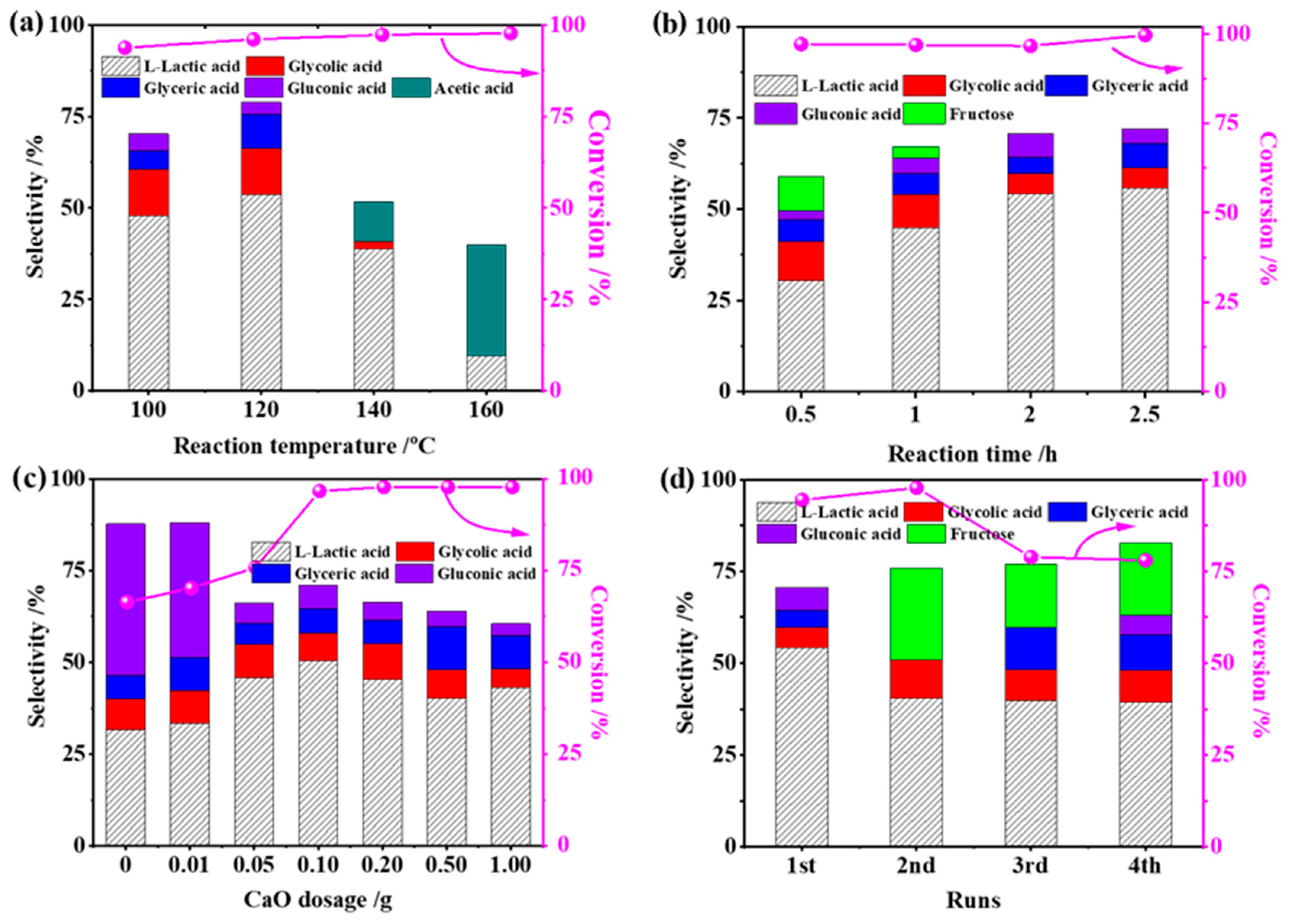
For the optimized 2RuOx/CaO catalyst, the reaction parameters, including reaction temperature, time, CaO dosage and catalyst stability, were investigated for the conversion of glucose to LA. As shown in Figure 1a, the selectivity to LA displayed a volcano curve as a function of temperature and was maximized to 54.3% at 120 °C with the conversion of glucose maintained at 95% at temperatures between 100 °C and 160 °C. When the reaction temperature was higher than the optimal point of 120 °C, the yield of LA decreased dramatically with a concomitantly increased yield of acetic acid. The reason for this should be attributed to the random decomposition of glucose and the oxidative decarbonylation of LA due to the metastable of glucose and LA at high temperatures. The influence of reaction time is shown in Figure 1b. At the 0.5 h reaction, the glucose conversion reached 93.1% with ca. 30% LA selectivity. Prolonging the reaction time to 2 h led to the increased LA yield, mainly due to the cascade conversion of the key intermediate of fructose to the downstream product. It is worth noting that even further extending the reaction time, the product compositions remained almost unchanged, confirming the good stability of LA in the present reaction system. The reaction atmosphere was also tested, as seen in Figure S1. Although the conversion of glucose to LA did not need the participation of O2, the presence of O2 herein encouraged the synthesis of LA in comparison to that in the N2 atmosphere. The enhanced performance should be related to the catalyst state under the oxidation conditions, e.g., RuOx species.
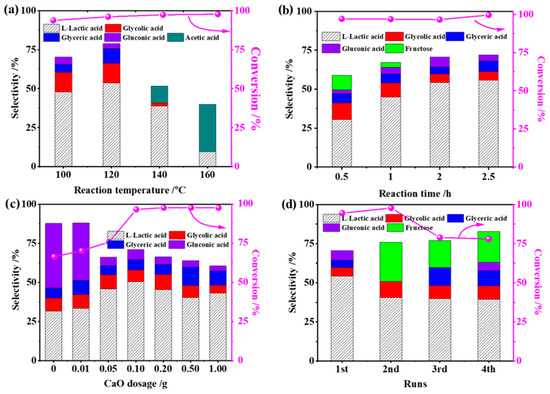
Figure 1.
Catalytic conversion of glucose at different (a) reaction temperatures, (b) reaction times, (c) amounts of CaO and (d) stabilities of catalysts (typical reaction conditions: 20 mL 1% glucose, 0.10 g catalyst, 120 °C, 1.0 MPa O2, 2 h. One condition was altered while other factors were kept the same in each case).
As the key catalytic component in this catalytic system, the effect of CaO dosage was investigated and is shown in Figure 1c. As expected, LA formation is very dependent on the amount of CaO, reaching a maximum with a 0.10 g addition of CaO. Concurrently, the undesired glucose oxidation route was greatly depressed by the competition of base-catalyzed glucose isomerization and retro-aldol condensation reaction. Excessive CaO dosage played negative roles in LA production, which should be attributed to the low saturated solubility of Ca(OH)2 (~0.025 M at room temperature) and uncontrollable basicity. The reusability of RuOx/MoS2 catalyst was evaluated, and results are shown in Figure 1d. After four cycles, the glucose conversion slightly decreased from 96% to 76% with a relatively stable LA selectivity of ca. 45%, indicating the relatively high stability of the catalyst. After the second cycle, the key intermediate of fructose emerged, which can be attributed to the coverage of the Ru species with a strongly interacting Ca species.
2.2. Active Sites for Glucose Conversions
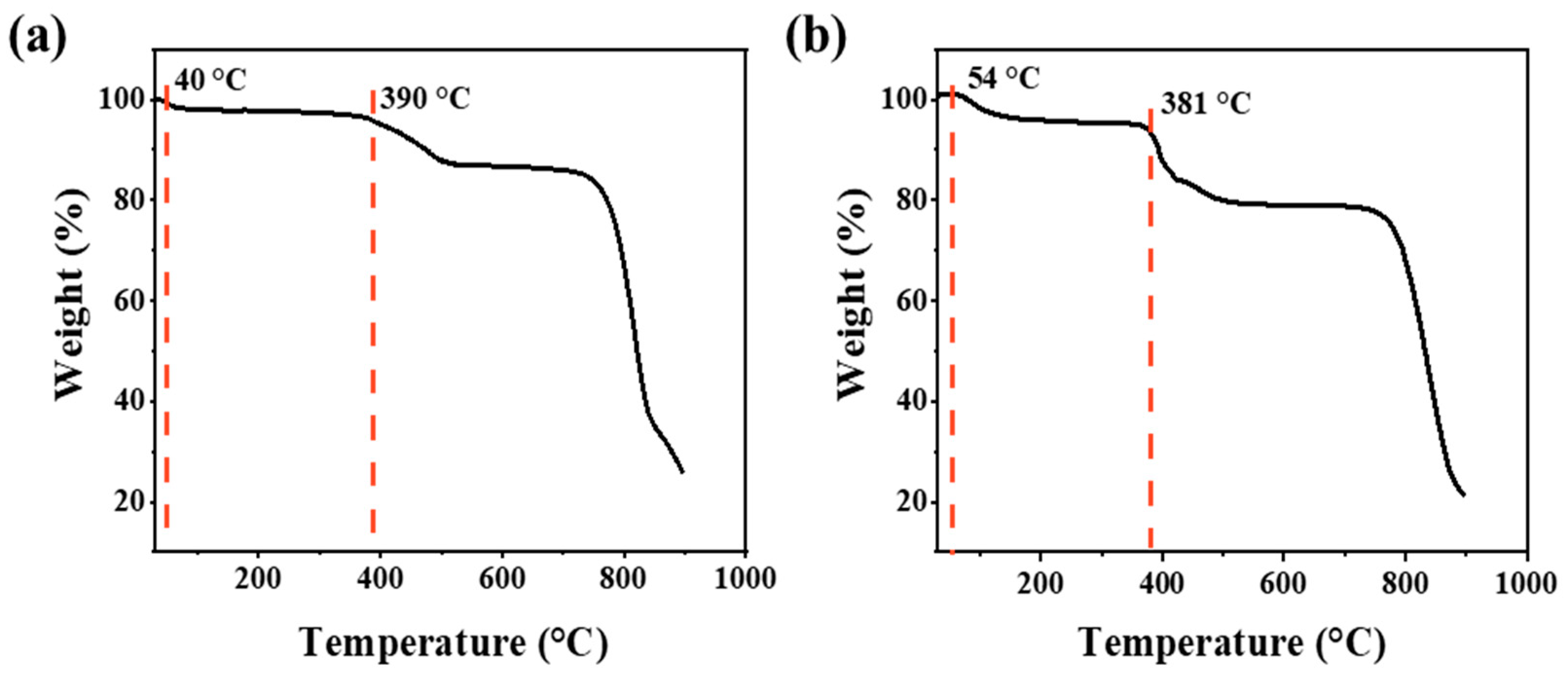
To verify the stability of support and the optimized catalyst under oxygen-containing hydrothermal reaction conditions, TGA was carried out to evaluate the decomposition temperature of the MoS2 and the supported 2RuOx/MoS2 in air. As shown in Figure 2a, the first weight loss stage from room temperature to 54 °C corresponds to the desorption of physically adsorbed water. The second and third weight losses distributed at around 390 °C and 720 °C could be attributed to the decomposition of the surficial and complete oxidation of MoS2, respectively [44]. Figure 2b illustrates that the introduction of the Ru species has no discernible impact on the thermal stability of the sample. Thus, at low reaction temperatures between 100 °C and 160 °C, the catalyst structure will not suffer from oxidative deterioration, which provides solid guarantee for the high stability of catalysts in the reaction.
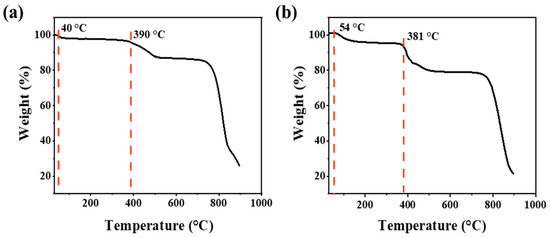
Figure 2.
TGA curves of (a) MoS2 and (b) 2RuOx/MoS2 catalyst.
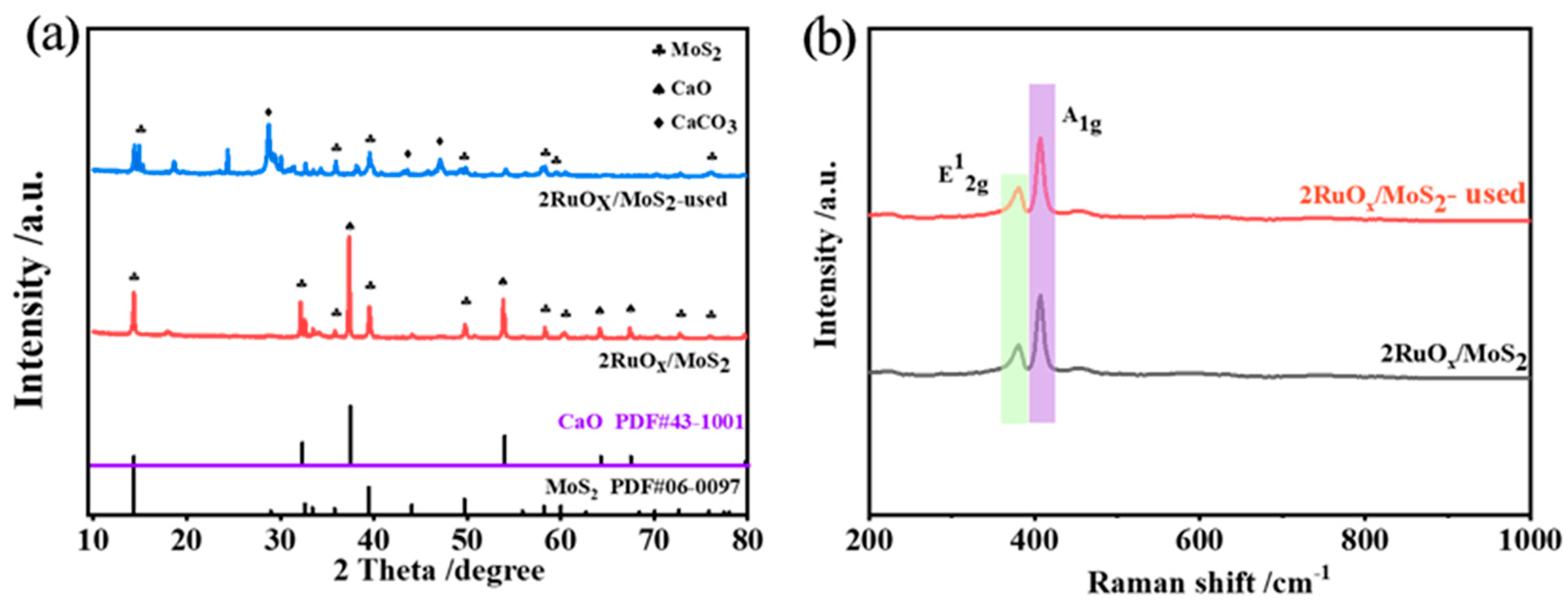
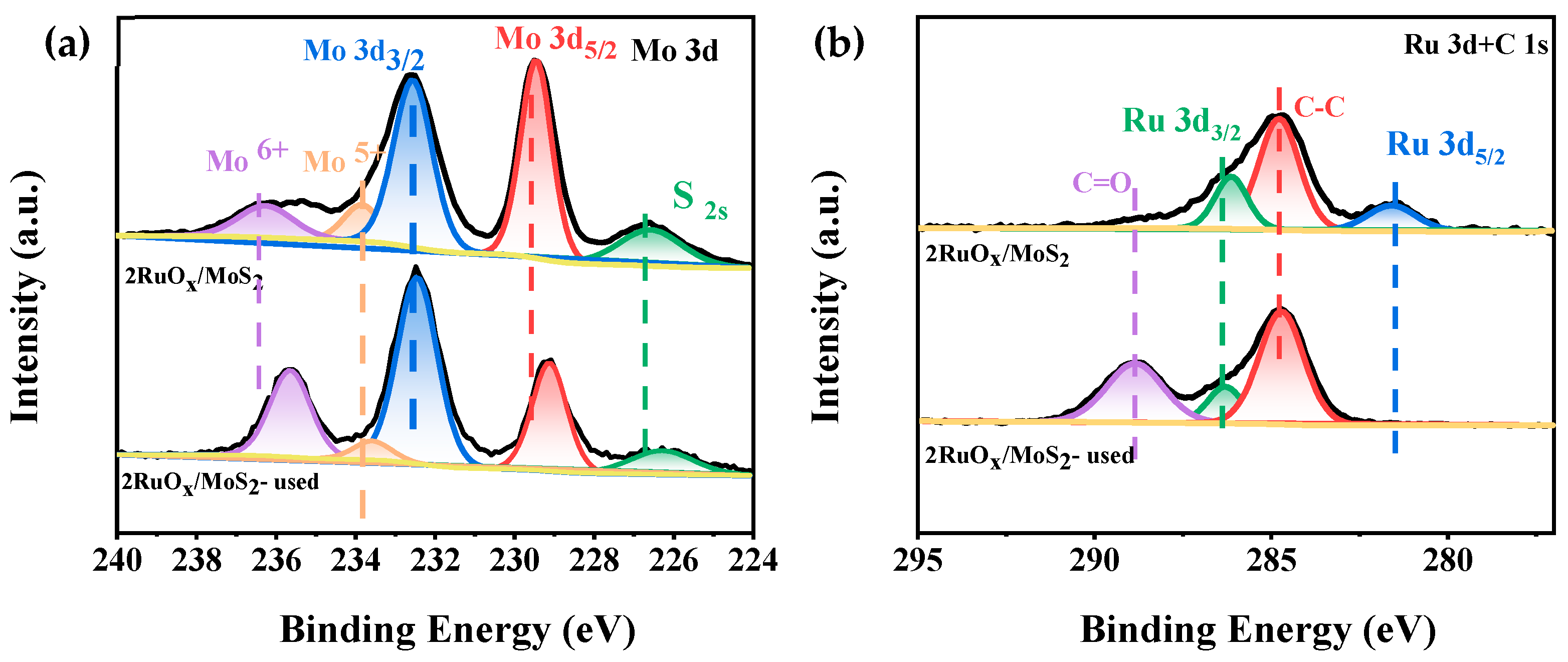
The XRD patterns of the fresh and used catalysts are displayed in Figure 3a. For the fresh 2RuOx/MoS2 catalyst, the diffraction peaks at 14.4°, 32.2°, 39.6°, 49.8°, 58.8° (labeled with clubs), and 37.4° and 53.8° (labeled with spades) were attributed to MoS2 and CaO, respectively. No characteristic peaks corresponding to RuOx were detected, indicating the high dispersion of the Ru species. For the used catalyst, new peaks attributed to the CaCO3 phase appeared, which may be caused by the reaction of CaO with CO2 generated in the reaction. Raman spectra in Figure 3b further reveal the structure of catalysts. Both MoS2 and 2RuOx/MoS2 showed two characteristic peaks at 409 cm−1 and 383 cm−1, corresponding to the E12g and A1g characteristic vibration modes of MoS2-2H phase, respectively [45]. Similar to the XRD patterns, the RuOx species were not detected, confirming the high dispersion of the Ru species.
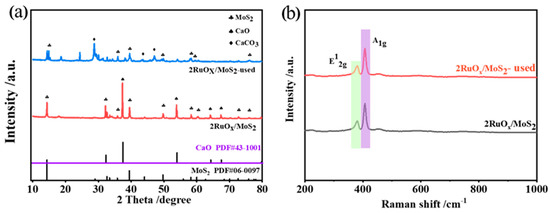
Figure 3.
(a) XRD patterns and (b) Raman spectra of the fresh and used 2RuOx/MoS2+CaO catalyst.
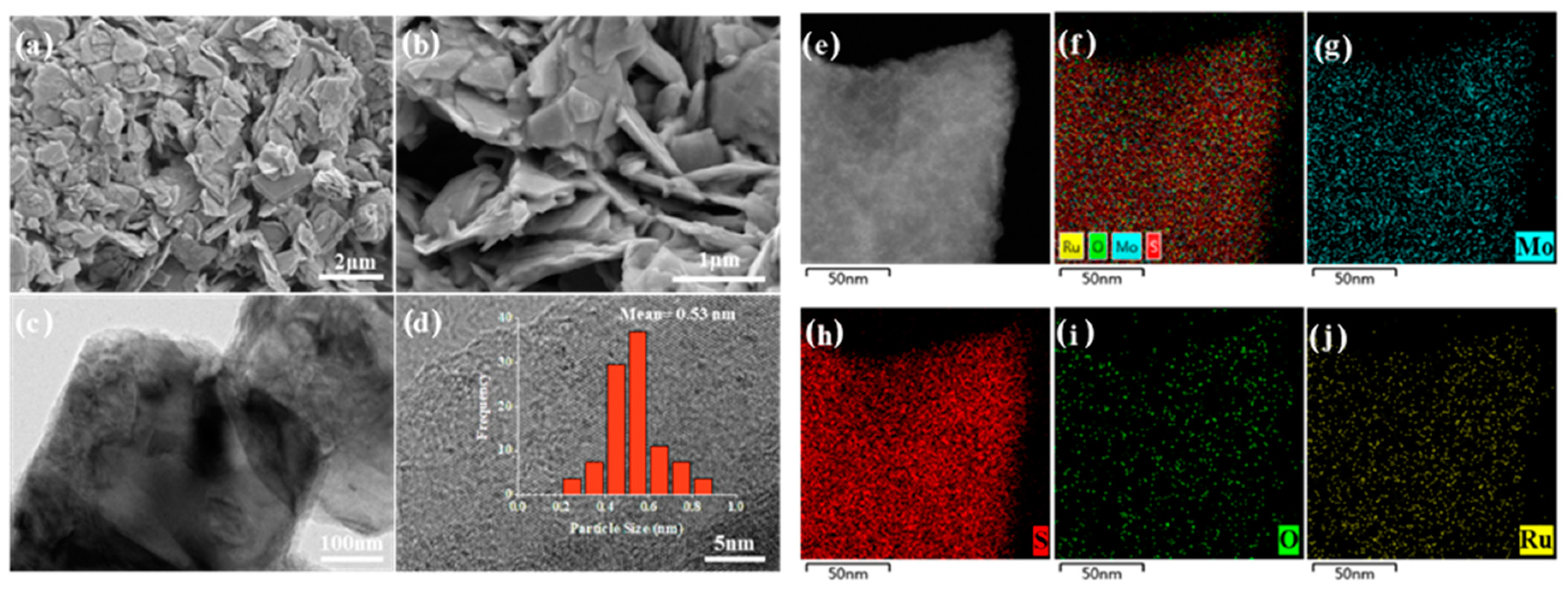
To disclose the metal dispersion and morphology of catalysts, the SEM and TEM images of the optimized catalyst are compiled in Figure 4. The carrier MoS2 showed a multilayer smooth sheet morphology with a thickness of about 100 nm. The highly dispersed of RuOx species are clearly shown in the high-resolution TEM images of Figure 4c,d. Even up to 2 wt% of the metal loading onto the MoS2 with a low BET surface area of < 10 m2/g (Figure S2), the average size of RuOx particles was centered at 0.53 nm. Meanwhile, the elemental mapping in Figure 4e–j reveals that the Ru particles were uniformly distributed on the catalyst surface. This is highly consistent with the results obtained via the Pt/MoS2 catalysts reported previously, owing to the uniquely strong interaction between MoS2 and noble metals [38]. The used catalyst was also characterized and is shown in Figure S3. According to the SEM image, a large number of amorphous particles attributed to Ca species were randomly distributed on the layered MoS2. In addition, the TEM and EDS analyses verified the remarkable structural stability of 2RuOx/MoS2. The intrinsic structures of MoS2 and RuOx were completely maintained through the catalytic reaction, similar to that of the fresh catalyst. The ICP-OES measurements in Table 3 further highlighted the fact that Ru leaching rarely occurred under hydrothermal reaction conditions. Above all, despite Ru sites being partially covered by Ca species, the 2RuOx/MoS2 catalyst was still able to produce a reasonable yield of LA in the subsequent cycling tests thanks to its excellent stability.
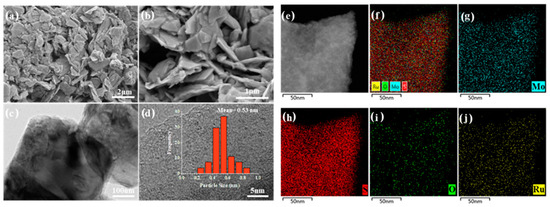
Figure 4.
(a,b) SEM images, (c,d) TEM images and (e–j) element mapping of the 2RuOx/MoS2 catalyst.

Table 3.
Elemental analysis of catalysts and product solution by ICP-OES.
The chemical states of Mo and Ru species on the catalysts were investigated by XPS (the whole spectrum is shown in Figure S4). As shown in Figure 5a, for the catalyst of 2RuOx/MoS2, three valence states of Mo species were identified, including the dominant Mo4+ (229.09 eV and 232.28 eV) and a small amount of Mo5+ (233.45 eV) and Mo6+ (235.56 eV) [46,47]. Since no MoO3 related signals were observed by Raman characterizations, it was speculated that the high-valence Mo species was distributed in the bulk phase of MoS2. Through the catalytic reaction, the proportion of Mo6+ increased by 8.95% after reaction, which may be caused by the partial oxidation of the carrier under the oxidizing atmosphere. For the Ru spectra in Figure 5b, the signal of Ru 3d overlaps with that of C ls. By means of deconvolution analysis, the Ru4+ species in the form of RuO2 (280.93 eV and 286.08 eV) were observed [48]. After the reaction, characteristic peaks assigned to RuO2 vanished, which should be attributed to the tendentiously deposition of CaCO3 onto the metal sites of the 2RuOx/MoS2 catalyst, agreeing with our hypothesis that the Ru species were covered to decrease the activity, rather than the deactivation of 2RuOx/MoS2 catalysts.
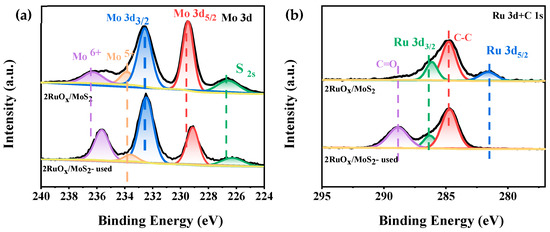
Figure 5.
XPS spectra of the fresh and used 2RuOx/MoS2 catalyst (a) Mo 3d, (b) Ru 3d and C1s.
2.3. Reaction Networks
Various C5 and C6 sugars, polyols and polyhydroxy acids were employed as feedstocks to evaluate the possible reaction networks via this catalytic system. As shown in Table 4, Entries 1–3, the RuOx/MoS2 catalyst with the assistance of CaO showed a good catalytic performance in the conversion of sugars, including glucose, fructose and xylose. As expected, because the obstacle of glucose isomerization was eliminated, the feedstock of fructose provided a higher selectivity to LA than glucose (68.9% vs. 54.3%). Interestingly, xylose exhibited the best performance for LA production, possibly due to the fewer side reactions occurring after the C5 reactant being degraded into C3 and C2 moieties. Moreover, it was difficult to effectively convert both polyols and gluconic acid into LA. It is noteworthy that the selectivity of LA reached 50.6% in the 8 h reaction at 160 °C when cellulose was used as the reactant (Table 4, Entry 7). This suggests that the RuOx/MoS2+ CaO catalyst has a potential to treat recalcitrant feedstock.

Table 4.
Catalytic performance of RuOx/MoS2 catalyst in the presence of CaO with different feedstocks a.
In addition, the activity of the catalyst was tested in glucose conversion at a high concentration (5 wt%). As shown in Table 4, Entry 8, the selectivity of LA was slightly decreased to 43.5%, providing LA productivity of 8.3 g/L/h, which is notably higher than that of the standard of LA production via industrial fermentation (5 g/L/h) [49].
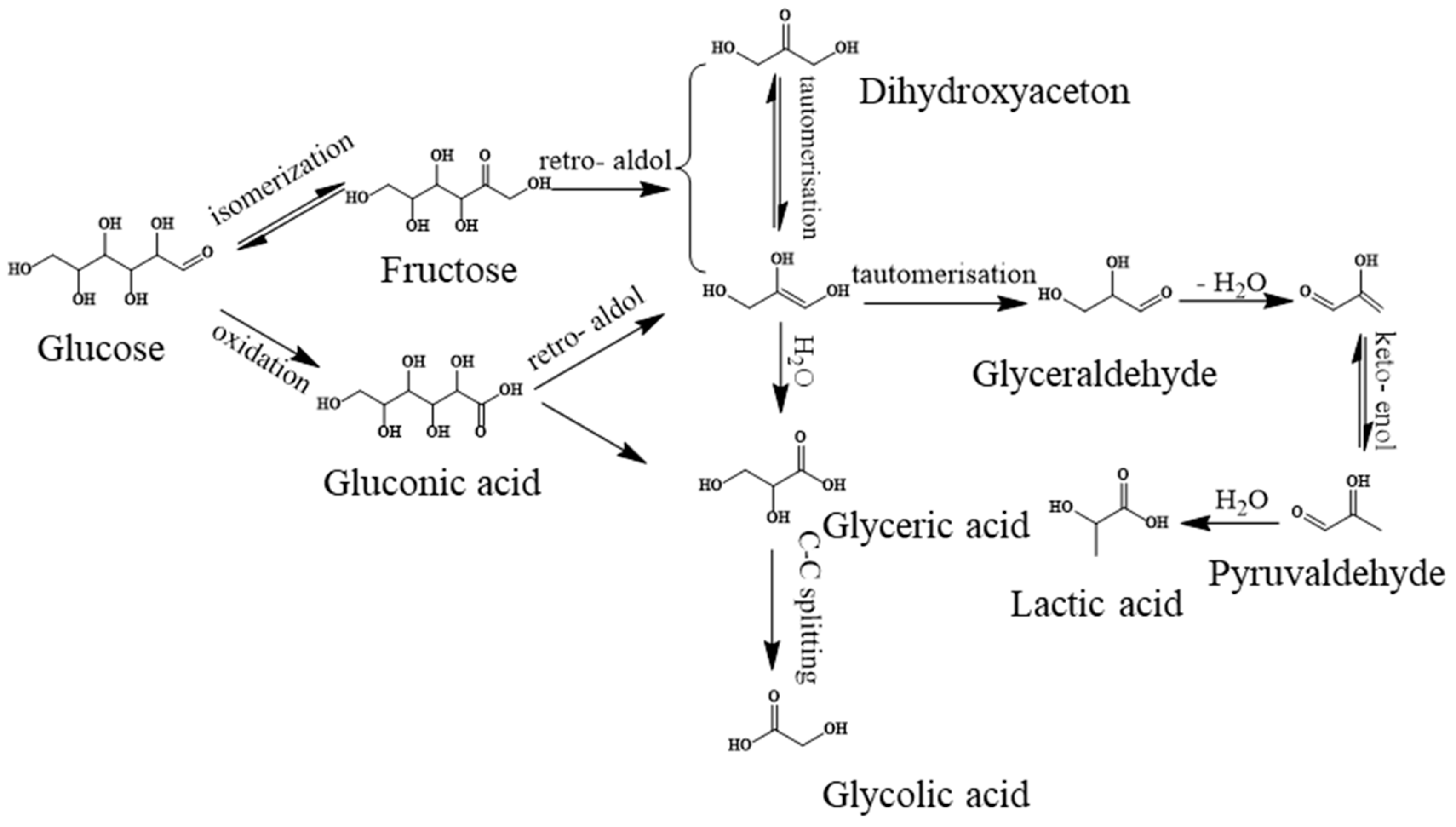
The above results demonstrate again that the base-catalyzed and RuOx/MoS2-catalyzed retro-aldol condensation route together contributed to the LA formation. CaO dissolved in water to release OH− and Ca2+. OH- catalyzed the isomerization of glucose to fructose, followed by degradation into dihydroxyacetone and glyceraldehyde through retro-aldol condensation. Afterward, glyceraldehyde was further catalytically dehydrated by the elimination reaction under the actions of OH− to generate the pyruvaldehyde intermediate [40,42]. Finally, obtained pyruvaldehyde can form metal chelates with Ca2+ and transform into LA and/or lactate via the 1,2-hydride shift [41]. On the other hand, the Ru species on the RuOx/MoS2 in an oxidation state played the crucial role in promoting retro-aldol condensation reactions.
Finally, the reaction pathway is briefly proposed in Scheme 1. Under the mild conditions of 120 °C and 1MPa O2, the CaO and Ru species catalyzed the isomerization of glucose into fructose. Afterward, the retro-aldol condensation occurred via the RuOx species with dihydroxyacetone and glyceraldehyde production. Finally, the obtained glyceraldehyde was transformed into LA via dehydration and hydration reactions via the Ru species. Simultaneously, side reactions, such as glucose direct oxidation and the direct conversion of dihydroxyacetone, occurred, resulting in the by-products of gluconic acid, glyceric acid, glycolic acid and acetic acid.
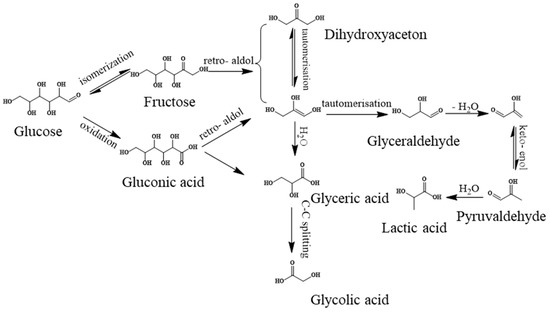
Scheme 1.
Proposed reaction pathways for the catalytic conversion of glucose to lactic acid via the RuOx/MoS2 catalyst in the presence of CaO [42,49].
3. Materials and Methods
3.1. Materials
All reagents were purchased commercially without further purification. Glucose (99%) and calcium oxide were purchased from Tianjin Damao Chemical Reagent Co., Ltd. (Tianjin, China). Sodium hydroxide was purchased from Tianjin Yongda Chemical Reagent Co. Ltd. Activated carbon (AC) was purchased from Norit Corporation. Calcium carbonate was purchased from Xilong Science Co., Ltd. (Shantou, China). Standard sample of lactic acid (LA) with 95% purity was purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Ruthenium chloride hydrate, molybdenum disulfide, hydroxyapatite (HPA), titanium dioxide, titanium silicalite-1 (TS-1), Boron nitride (BN) and all other chemicals were analytical reagents and purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
3.2. Catalysts Preparation
RuOx/support catalysts were prepared using the incipient wetness impregnation method with MoS2, AC, TiO2, TS-1, HPA and BN as supports. Typically, 2 g of support was mixed with 0.26–2.6mL aqueous solutions of RuCl3·3H2O (3.792% g/mL) and maintained at 25 °C for 12 h. Then, the sample was dried at 60 °C overnight and calcined at 350 °C for 4 h in air. The theoretical contents of Ru were controlled to remain at 0.5–5 wt%. The cost of preparing 1 g of 1RuOx/MoS2 catalyst was approximately CNY 1.1 (USD 0.16).
3.3. Catalysts Characterizations
X-ray powder diffraction (XRD, PANalytical B. V., Almelo, The Netherlands) patterns of catalysts were collected on a PANalytical X’pert Pro-1 equipped with Cu-Kα monochromatic radiation (0.1541 nm), operating at 40 kV and 40 mA. A continuous mode was used in the 2θ range from 10° to 80° at a scanning speed of 5°/min.
Thermogravimetric (TG, TA Instruments, Newcastle, DE, USA) analysis was conducted with SDT Q600 equipment under an air flow of 100 mL/min from 30 to 900 °C at a heating rate of 10 °C/min.
Scanning electron microscopy (SEM, HITACHI, Tokyo, Japan) images were obtained by using a Carl Zeiss Merlin Compact scanning electron microscope.
High-angle annular dark-field scanning-transmission electron microscopy (HAADF-STEM, JEOL, Tokyo, Japan) images and energy-dispersive X-ray spectroscopy (EDS, JEOL, Tokyo, Japan) elemental mapping were obtained by using a JEM-2100F microscope, operated at a voltage of 200 kV.
The contents of Ru and Mo in the synthesized catalysts and the leaching amount in the reaction solution were determined by an inductively coupled plasma optical emission spectrometer (ICP-OES, PerkinElmer, Waltham, MA, USA) on a Perkin Elmer ICP-OES 7300DV.
X-ray photoelectron spectra (XPS Thermo Fisher Scientific, Waltham, MA, USA) were recorded on a Thermo Scientific K-Alpha+ spectrometer with a monochromatic Al-Kα X-ray source as the excitation source. The binding energies were corrected by using the C1s peak at 284.4 eV as a reference.
3.4. Catalytic Conversion of Glucose
The catalytic conversion of glucose was carried out in a high-pressure batch reactor. Typically, 20 mL of 1% glucose aqueous solutions, 0.10 g of catalyst and 0.10 g of CaO were placed into the reactor. Then, the reactor was charged in O2 five times to remove the air. Finally, the oxygen pressure in the reactor was increased to 1.0 MPa and sealed for the reaction at 120 °C for 2 h.
After reaction, the product was cooled to room temperature and analyzed with a high-performance liquid chromatograph HPLC, Agilent 1200, (Agilent Technologies, Palo Alto, CA, USA) equipped with a Rezex ROA-Orgenic Acid H+ column and a differential refractive index detector with 5 mM sulfuric acid aqueous as the mobile phase. In detail, the GC-MS (Agilent Technologies, Palo Alto, CA, USA) and HPLC-MS methods (Agilent Technologies, Palo Alto, CA, USA)were used to identify the products in reaction solutions or identify the products after methyl esterification treatment [31]. Then, the standard samples were used to further confirm and quantitatively analyze the products on the HPLC instrument.
Glucose conversion and product selectivity were calculated using following equations:
4. Conclusions
A series of RuOx/MoS2 catalysts were synthesized for the conversion of sugars into LA with the assistance of CaO. Unlike the conventional base-catalyst system, the 2RuOx/MoS2 catalyst exhibited enhanced catalytic activity and stability. Under the low reaction temperature of 120 °C with low alkalinity, the LA selectivity reached 54.3% at a 96.6% glucose conversion, even in the recycle runs. The RuOx species promoted the isomerization of glucose to fructose, and then the fructose was cracked into the C3 compound of glyceraldehyde via the RuOx species via the retro-aldol condensation reaction. Finally, the C3 intermediate was further converted into LA via dehydration and hydration reactions. Although the finally yield of LA still needs to be improved, this work still provides valuable information for the development of the novel heterogeneous catalyst for carbohydrates conversions. The future study may focus on how to further enhance the LA selectivity and the reaction efficiency, particularly in the cellulosic feedstock conversion, which involves the catalyst development as well as a kinetic reaction investigation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal13030545/s1: Figure S1. Results of catalytic conversion of glucose at different reaction atmospheres (reaction conditions: 20 mL 1% glucose, 0.10 g catalyst, 0.10 g CaO. 120 °C, 1.0 MPa O2, 2 h); Figure S2. Nitrogen adsorption–desorption isotherms BET surface area of 2RuOx/MoS2 catalyst; Figure S3. TEM and EDS images of the used 2RuOx/MoS2; Figure S4. The survey spectra of fresh and used 2RuOx/MoS2 catalysts.
Author Contributions
Data curation, Z.L.; formal analysis Z.L., X.L., P.W. and J.P.; supervision, S.Z. and M.Z.; writing—original draft, Z.L.; writing—review and editing, P.W., J.P. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (21721004, 21676039, 22108274), “Transformational Technologies for Clean Energy and Demonstration”, Strategic Priority Research Program of the Chinese Academy of Sciences (XDA 21060200).
Data Availability Statement
The data is included in the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nwosu, U.; Wang, A.; Palma, B.; Zhao, H.; Khan, M.A.; Kibria, M.; Hu, J. Selective biomass photoreforming for valuable chemicals and fuels: A critical review. Renew. Sustain. Energy Rev. 2021, 148, 111266. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.; Palkovits, R. Cellulose-based platform chemical: The path to application. Curr. Opin. Green Sustain. Chem. 2018, 14, 14–18. [Google Scholar] [CrossRef]
- Gao, X.; Zhong, H.; Yao, G.; Guo, W.; Jin, F. Hydrothermal conversion of glucose into organic acids with bentonite as a solid-base catalyst. Catal. Today 2016, 274, 49–54. [Google Scholar] [CrossRef]
- Wang, F.-F.; Liu, J.; Li, H.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Conversion of cellulose to lactic acid catalyzed by erbium-exchanged montmorillonite K10. Green. Chem. 2015, 17, 2455–2463. [Google Scholar] [CrossRef]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Synthesis of high-value organic acids from sugars promoted by hydrothermally loaded Cu oxide species on magnesia. Appl. Catal. B 2015, 162, 1–10. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Production of Lactic Acid/Lactates from Biomass and Their Catalytic Transformations to Commodities. Chem. Rev. 2014, 114, 1909–1971. [Google Scholar] [CrossRef] [PubMed]
- Chenebault, C.; Moscoviz, R.; Trably, E.; Escudié, R.; Percheron, B. Lactic acid production from food waste using a microbial consortium: Focus on key parameters for process upscaling and fermentation residues valorization. Bioresour. Technol. 2022, 354, 127230. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chen, C.-Y.; Ariyadasa, T.U.; Lee, D.-J.; Chang, J.-S. Macroalgal biomass as a potential resource for lactic acid fermentation. Chemosphere 2022, 309, 136694. [Google Scholar] [CrossRef]
- Becker, J.; Lange, A.; Fabarius, J.; Wittmann, C. Top value platform chemicals:Bio-based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, W.; Luo, J.; Qi, B.; Wan, Y. One step open fermentation for lactic acid production from inedible starchy biomass by thermophilic Bacillus coagulans IPE22. Bioresour. Technol. 2019, 272, 398–406. [Google Scholar] [CrossRef]
- Trakarnpaiboon, S.; Srisuk, N.; Piyachomkwan, K.; Yang, S.-T.; Kitpreechavanich, V. l-Lactic acid production from liquefied cassava starch by thermotolerant Rhizopus microsporus: Characterization and optimization. Process Biochem. 2017, 63, 26–34. [Google Scholar] [CrossRef]
- Li, S.; Deng, W.; Li, Y.; Zhang, Q.; Wang, Y. Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. J. Energy Chem. 2019, 32, 138–151. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of Sugars to Lactic Acid Derivatives Using Heterogeneous Zeotype Catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- de Clippel, F.; Dusselier, M.; Van Rompaey, R.; Vanelderen, P.; Dijkmans, J.; Makshina, E.; Giebeler, L.; Oswald, S.; Baron, G.V.; Denayer, J.F.M.; et al. Fast and Selective Sugar Conversion to Alkyl Lactate and Lactic Acid with Bifunctional Carbon–Silica Catalysts. J. Am. Chem. Soc. 2012, 134, 10089–10101. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fan, F.; Pidko, E.A.; van der Graaff, W.N.P.; Feng, Z.; Li, C.; Hensen, E.J.M. Highly Active and Recyclable Sn-MWW Zeolite Catalyst for Sugar Conversion to Methyl Lactate and Lactic Acid. ChemSusChem 2013, 6, 1352–1356. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Li, X.; Song, L.; Sun, R.; Sebastian, J.; Wang, A.; Wang, J.; Wang, X.; Zhang, T. Catalytic conversion of carbohydrates to methyl lactate using isolated tin sites in SBA-15. ChemistrySelect 2017, 2, 309–314. [Google Scholar] [CrossRef]
- Sun, P.; Liu, C.; Wang, H.; Liao, Y.; Li, X.; Liu, Q.; Sels, B.F.; Wang, C. Rational Positioning of Metal Ions to Stabilize Open Tin Sites in Beta Zeolite for Catalytic Conversion of Sugars. Angew. Chem. Int. Ed. 2023, 62, e202215737. [Google Scholar] [CrossRef]
- Kong, L.; Li, G.; Wang, H.; He, W.; Ling, F. Hydrothermal catalytic conversion of biomass for lactic acid production. J. Chem. Technol. Biot. 2008, 83, 383–388. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Wang, B.; Zhang, Q.; Wan, X.; Tang, Z.; Wang, Y.; Zhu, C.; Cao, Z.; Wang, G.; et al. Chemical synthesis of lactic acid from cellulose catalysed by lead(II) ions in water. Nat. Commun. 2013, 4, 2141. [Google Scholar] [CrossRef] [PubMed]
- Bayu, A.; Yoshida, A.; Karnjanakom, S.; Kusakabe, K.; Hao, X.; Prakoso, T.; Abudula, A.; Guan, G. Catalytic conversion of biomass derivatives to lactic acid with increased selectivity in an aqueous tin(ii) chloride/choline chloride system. Green Chem. 2018, 20, 4112–4119. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Y.; Li, J.; He, T.; Xiao, Y.; Zhou, C.; Hu, C. Directing the Simultaneous Conversion of Hemicellulose and Cellulose in Raw Biomass to Lactic Acid. ACS Sustain. Chem. Eng. 2020, 8, 4244–4255. [Google Scholar] [CrossRef]
- Kutrakul, N.; Liu, A.; Ratchahat, S.; Posoknistakul, P.; Laosiripojana, N.; Wu, K.C.W.; Sakdaronnarong, C. Highly selective catalytic conversion of raw sugar and sugarcane bagasse to lactic acid over YbCl3, ErCl3, and CeCl3 Lewis acid catalysts without alkaline in a hot-compressed water reaction system. Chem. Eng. Res. Des. 2022, 187, 549–569. [Google Scholar] [CrossRef]
- Yan, X.; Jin, F.; Tohji, K.; Kishita, A.; Enomoto, H. Hydrothermal conversion of carbohydrate biomass to lactic acid. AIChE J. 2010, 56, 2727–2733. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Aho, A.; Murzin, D.Y. Heterogeneous Catalytic Synthesis of Methyl Lactate and Lactic Acid from Sugars and Their Derivatives. ChemSusChem 2020, 13, 4833–4855. [Google Scholar] [CrossRef]
- Xia, M.; Dong, W.; Shen, Z.; Xiao, S.; Chen, W.; Gu, M.; Zhang, Y. Efficient production of lactic acid from biomass-derived carbohydrates under synergistic effects of indium and tin in In–Sn-Beta zeolites. Sustain. Energy Fuels 2020, 4, 5327–5338. [Google Scholar] [CrossRef]
- Kim, M.; Ronchetti, S.; Onida, B.; Ichikuni, N.; Fukuoka, A.; Kato, H.; Nakajima, K. Lewis Acid and Base Catalysis of YNbO4 toward Aqueous-Phase Conversion of Hexose and Triose Sugars to Lactic Acid in Water. ChemCatChem 2020, 12, 350–359. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Huang, C.; Liang, F.; Chen, B. Lactic acid production from glucose over polymer catalysts in aqueous alkaline solution under mild conditions. Green Chem. 2014, 16, 4234–4240. [Google Scholar] [CrossRef]
- Liang, G.; Wang, A.; Li, L.; Xu, G.; Yan, N.; Zhang, T. Production of Primary Amines by Reductive Amination of Biomass-Derived Aldehydes/Ketones. Angew. Chem. Int. Ed. 2017, 56, 3050–3054. [Google Scholar] [CrossRef]
- Song, L.; Wang, R.; Che, L.; Jiang, Y.; Zhou, M.; Zhao, Y.; Pang, J.; Jiang, M.; Zhou, G.; Zheng, M.; et al. Catalytic Aerobic Oxidation of Lignocellulose-Derived Levulinic Acid in Aqueous Solution: A Novel Route to Synthesize Dicarboxylic Acids for Bio-Based Polymers. ACS Catal. 2021, 11, 11588–11596. [Google Scholar] [CrossRef]
- Diao, X.; Ji, N.; Li, X.; Rong, Y.; Zhao, Y.; Lu, X.; Song, C.; Liu, C.; Chen, G.; Ma, L.; et al. Fabricating high temperature stable Mo-Co9S8/Al2O3 catalyst for selective hydrodeoxygenation of lignin to arenes. Appl. Catal. B 2022, 305, 121067. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Jeong, H.Y.; Lee, M.H.; Shin, H.S.; Lee, J.S. Efficient Hydrogen Evolution Reaction Catalysis in Alkaline Media by All-in-One MoS2 with Multifunctional Active Sites. Adv. Mater. 2018, 30, 1707105. [Google Scholar] [CrossRef]
- Kobayashi, H.; Matsuhashi, H.; Komanoya, T.; Hara, K.; Fukuoka, A. Transfer hydrogenation of cellulose to sugar alcohols over supported ruthenium catalysts. Chem. Commun. 2011, 47, 2366–2368. [Google Scholar] [CrossRef]
- Zheng, M.; Pang, J.; Sun, R.; Wang, A.; Zhang, T. Selectivity control for cellulose to diols:Dancing on eggs. ACS Catal. 2017, 7, 1939–1954. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, L.; Chen, Y.; Lin, J.; Hu, W.; Wang, S.; Lin, J.; Wan, S.; Wang, Y. One-pot synthesis of gluconic acid from biomass-derived levoglucosan using a Au/Cs2.5H0.5PW12O40 catalyst. Green Chem. 2019, 21, 6318–6325. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, Z.; Yu, I.K.M.; Tsang, D.C.W. Sustainable production of high-value gluconic acid and glucaric acid through oxidation of biomass-derived glucose: A critical review. J. Clean. Prod. 2021, 312, 127745. [Google Scholar] [CrossRef]
- Wang, J.; Fang, W.; Hu, Y.; Zhang, Y.; Dang, J.; Wu, Y.; Chen, B.; Zhao, H.; Li, Z. Single atom Ru doping 2H-MoS2 as highly efficient hydrogen evolution reaction electrocatalyst in a wide pH range. Appl. Catal. B Environ. 2021, 298, 120490. [Google Scholar] [CrossRef]
- Yan, X.; Jin, F.; Tohji, K.; Moriya, T.; Enomoto, H. Production of lactic acid from glucose by alkaline hydrothermal reaction. J. Mater. Sci. 2007, 42, 9995–9999. [Google Scholar] [CrossRef]
- Wang, Z.; Mo, C.; Xu, S.; Chen, S.; Deng, T.; Zhu, W.; Wang, H. Ca(OH)2 induced a controlled-release catalytic system for the efficient conversion of high-concentration glucose to lactic acid. Mol. Catal. 2021, 502, 111406. [Google Scholar] [CrossRef]
- Lux, S.; Siebenhofer, M. Synthesis of lactic acid from dihydroxyacetone:Use of alkaline-earth metal hydroxides. Catal. Sci. Technol. 2013, 3, 1380–1385. [Google Scholar] [CrossRef]
- Esposito, D.; Antonietti, M. Chemical Conversion of Sugars to Lactic Acid by Alkaline Hydrothermal Processes. ChemSusChem 2013, 6, 989–992. [Google Scholar] [CrossRef]
- Li, X.; Han, S.; Qiao, Z.; Zeng, X.; Cao, D.; Chen, J. Ru monolayer island doped MoS2 catalysts for efficient hydrogen evolution reaction. Chem. Eng. J. 2023, 453, 139803. [Google Scholar] [CrossRef]
- Li, X.; Pang, J.; Luo, W.; Zhao, Y.; Pan, X.; Zheng, M. Catalytic Conversion of Tetrahydrofurfuryl Alcohol over Stable Pt/MoS2 Catalysts. Catal. Lett. 2021, 151, 2734–2747. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Song, W.; Lai, W.; Lian, Y.; Jiang, X.; Yang, W. Sulfated ZrO2 supported CoMo sulfide catalyst by surface exsolution for enhanced hydrodeoxygenation of lignin-derived ethers to aromatics. Fuel 2020, 263, 116705. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, G.; Ke, X.; Chen, X.; Chen, X.; Wang, Y.; Huang, G.; Dong, J.; Chu, S.; Sui, M. Direct Synthesis of Stable 1T-MoS2 Doped with Ni Single Atoms for Water Splitting in Alkaline Media. Small 2022, 18, e2107238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xue, J.; Zeng, L.; Shang, J.; Lu, S.; Cao, X.; Abrahams, B.F.; Gu, H.; Lang, J. One-pot pyrolysis synthesis of highly active Ru/RuOX nanoclusters for water splitting. Nano Res. 2022, 15, 1020–1026. [Google Scholar] [CrossRef]
- Jin, F.; Zhou, Z.; Enomoto, H.; Moriya, T.; Higashijima, H. Conversion Mechanism of Cellulosic Biomass to Lactic Acid in Subcritical Water and Acid–base Catalytic Effect of Subcritical Water. Chem. Lett. 2004, 33, 126–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).