Vertical Growth of WO3 Nanosheets on TiO2 Nanoribbons as 2D/1D Heterojunction Photocatalysts with Improved Photocatalytic Performance under Visible Light
Abstract
1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. FTIR Analysis
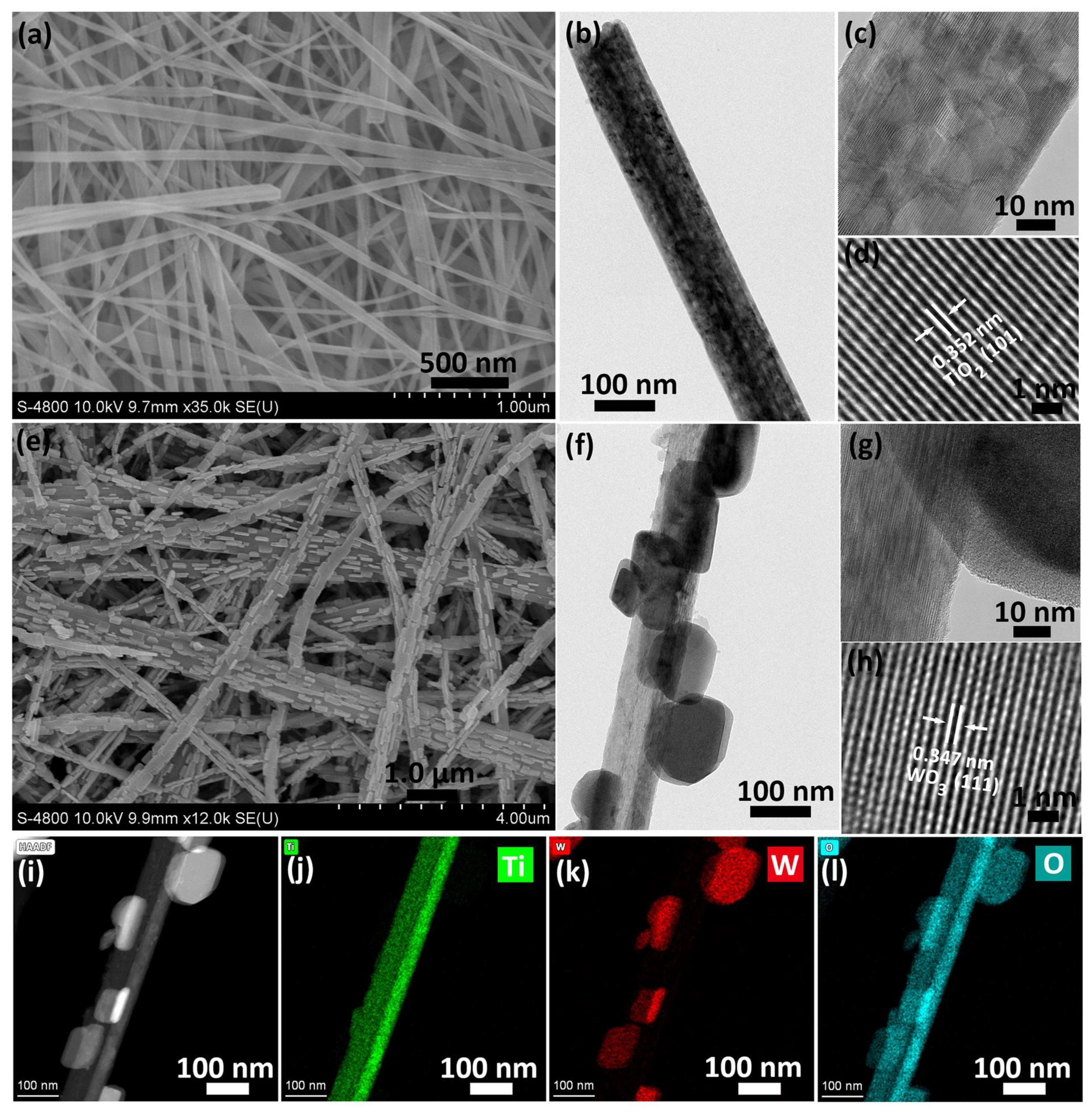
2.3. SEM and TEM Analysis
2.4. XPS Analysis
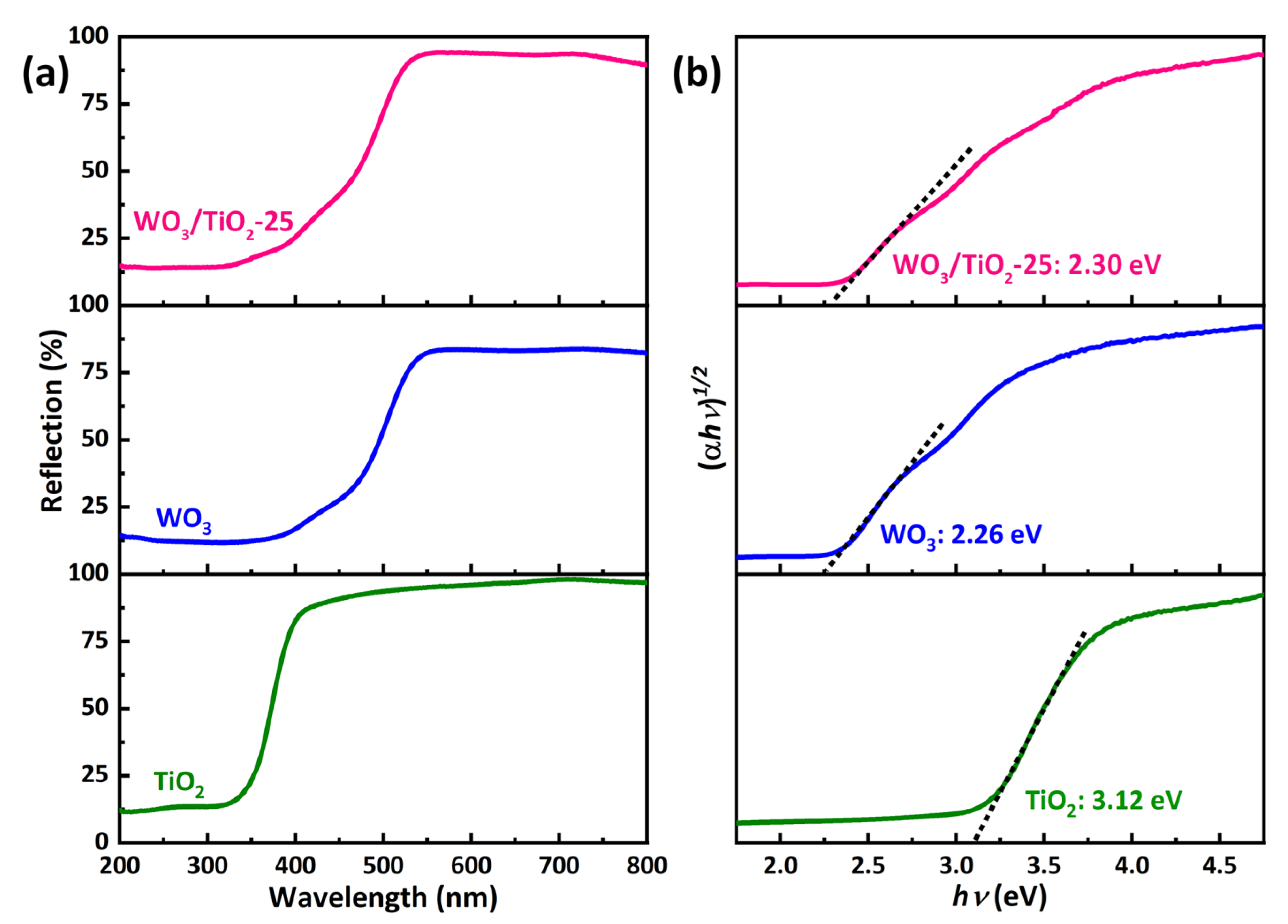
2.5. Optical Analysis
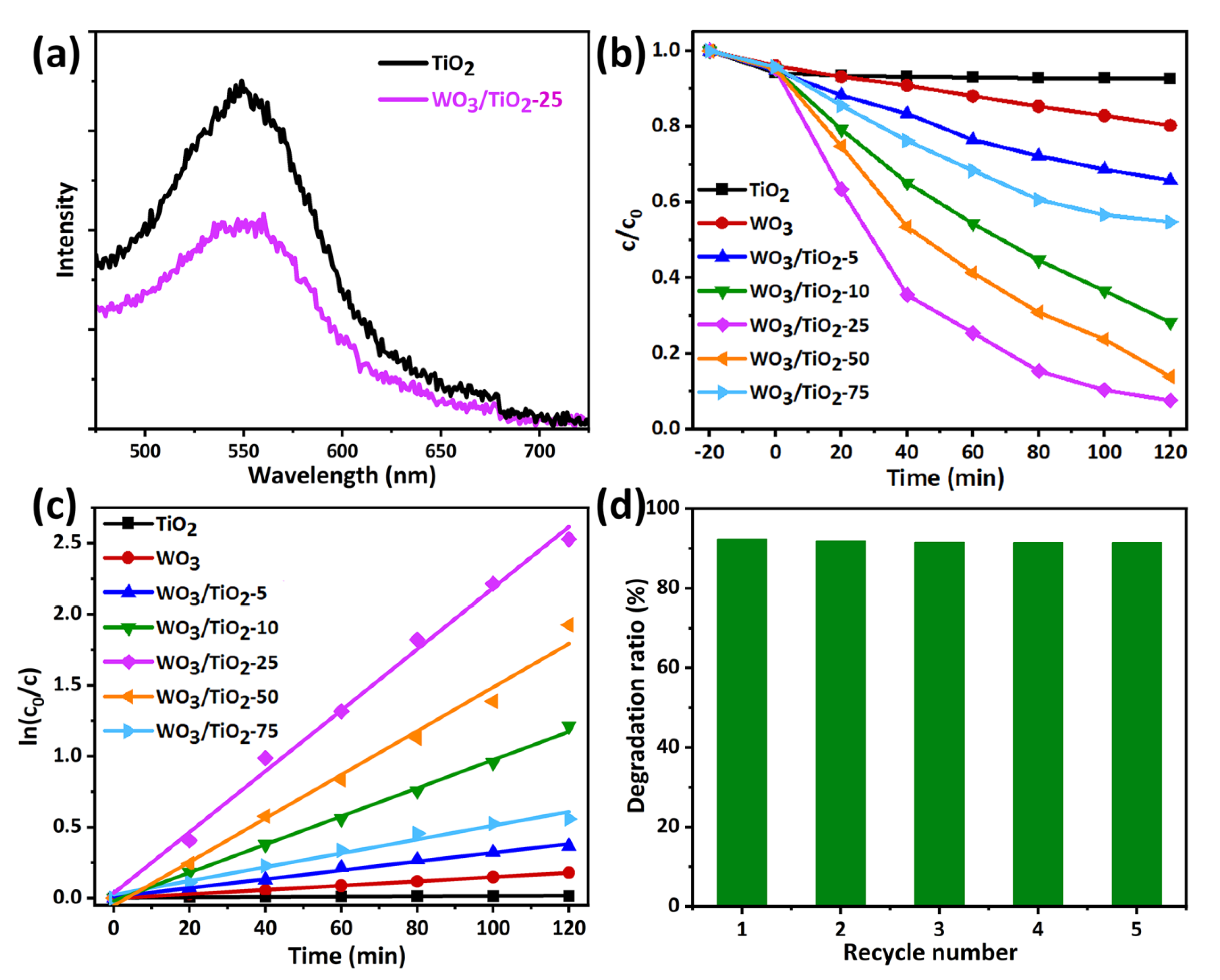
2.6. Photoluminescence Analysis
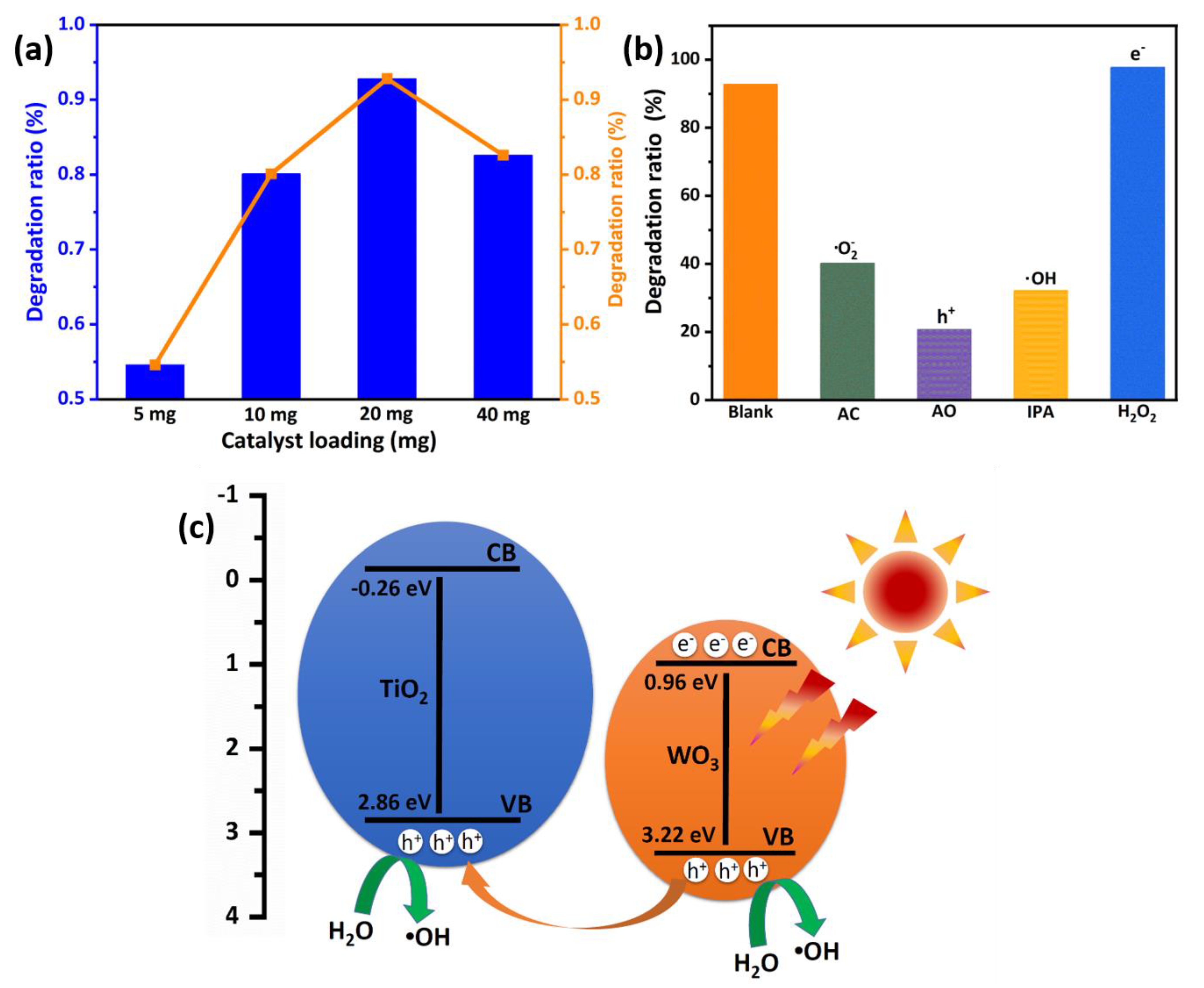
2.7. Photocatalytic Performance
3. Materials and Methods
3.1. Preparation of WO3/TiO2
3.2. Material Characterization
3.3. Photodegradation and Photocurrent Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McDonald, R.I.; Green, P.; Balk, D.; Montgomery, M. Urban growth, climate change, and freshwater availability. Proc. Natl. Acad. Sci. USA 2011, 108, 6312–6317. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Gadgil, A. Drinking Water in Developing Countries. Annu. Rev. Energy Environ. 1998, 23, 253–286. [Google Scholar] [CrossRef]
- Water Scarcity Threats. Available online: https://www.worldwildlife.org/threats/water-scarcity (accessed on 12 January 2023).
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E.; Gusev, A.A.; Bardow, A.; Semiatf, R.; Kuriharag, M. Efficient technologies for worldwide clean water supply. Chem. Eng. Process. 2012, 51, 2–17. [Google Scholar] [CrossRef]
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: A review. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef]
- Ouachtak, H.; Akhouairi, S.; Haounati, R.; Addi, A.A.; Jada, A.; Taha, M.L.; Douch, J. 3,4-Dihydroxybenzoic acid removal from water by goethite modified natural sand column fixed-bed: Experimental study and mathematical modeling. Desalin. Water Treat. 2020, 194, 439–449. [Google Scholar] [CrossRef]
- Pendergast, M.T.M.; Eric, M.V.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Andrew, M.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar]
- Parvulescu, V.I.; Epron, F.; Garcia, H.; Granger, P. Recent progress and prospects in catalytic water treatment. Chem. Rev. 2021, 122, 2981–3121. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.; Krishnan, V. Recent advances in plasmonic photocatalysis based on TiO2 and noble metal nanoparticles for energy conversion, environmental remediation, and organic synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Lu, K.Q.; Tang, Z.C.; Chen, H.M.; Xu, Y.J. Stabilizing ultrasmall Au clusters for enhanced photoredox catalysis. Nat. Commun. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, W.J.; Cai, H.; Li, H.; Sun, X.J.; Weng, B.; Yi, Z. A redox-active support for the synthesis of Au@SnO2 core-shell nanostructure and SnO2 quantum dots with efficient photoactivities. RSC Adv. 2020, 10, 33955–33961. [Google Scholar] [CrossRef] [PubMed]
- Haounati, R.; Alakhras, F.; Ouachtak, H.; Saleh, T.A.; Al-Mazaideh, G.; Alhajri, E.; Jada, A.; Hafid, N.; Addi, A.A. Synthesized of Zeolite@Ag2O Nanocomposite as Superb Stability Photocatalysis toward Hazardous Rhodamine B Dye from Water. Arab. J. Sci. Eng. 2023, 48, 169–179. [Google Scholar] [CrossRef]
- Nawaz, A.; Goudarzi, S.; Asghari, M.A.; Pichiah, S.; Selopal, G.S.; Rosei, F.; Wang, Z.M.; Zarrin, H. Review of Hybrid 1D/2D Photocatalysts for Light-Harvesting Applications. ACS Appl. Nano Mater. 2021, 4, 11323–11352. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X. Rational design of 1D/2D heterostructured photocatalyst for energy and environmental applications. Chem. Eng. J. 2020, 395, 125030. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, Y.; Feng, Y.; Xu, J. Compositing two-dimensional materials with TiO2 for photocatalysis. Catalysts 2018, 8, 590. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Feng, G.S.; Yan, L. One-dimensional nanostructured TiO2 for photocatalytic degradation of organic pollutants in wastewater. Int. J. Photoenergy 2014, 2014, 563879. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Fórizs, B.; Rosseler, O.; Szegedi, Á.; Németh, P.; Király, P.; Tárkányi, G.; Vajna, B.; Katalin, V.-J.; László, K. WO3 photocatalysts: Influence of structure and composition. J. Catal. 2012, 294, 119–127. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakurb, V.K.; Parvaz, K.A.A.; Sainie, V.; Asiri, A.M.; Singha, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Wang, F.; Valentin, C.D.; Pacchioni, G. Rational band gap engineering of WO3 photocatalyst for visible light water splitting. Mater. Sci. ChemCatChem. 2012, 4, 476–478. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Wang, J.; Peng, T.; Li, R. Direct Z-scheme 2D/2D photocatalyst based on ultrathin g-C3N4 and WO3 nanosheets for efficient visible-light-driven H2 generation. ACS Appl. Mater. Interfaces 2019, 11, 27913–27923. [Google Scholar] [CrossRef]
- Lei, B.; Cui, W.; Chen, P.; Chen, L.; Li, J.; Dong, F. C-doping induced oxygen-vacancy in WO3 nanosheets for CO2 activation and photoreduction. ACS Catal. 2022, 12, 9670–9678. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Zou, C.; Xu, K.; Luo, X.; Luo, T.; Li, J.; Yang, Q.; Shi, P.; Yuan, C. 2D ultra-thin WO3 nanosheets with dominant {002} crystal facets for high-performance xylene sensing and methyl orange photocatalytic degradation. J. Alloy. Compd. 2019, 783, 848–854. [Google Scholar] [CrossRef]
- Lai, C.W. WO3-TiO2 Nanocomposite and its applications: A review. Nano Hybrids Compos. 2018, 20, 1–26. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, M.; Yu, W.; Zhang, Q.; Xie, H.; Sun, Z.; Shao, Q.; Guo, X.; Hao, L.; Zheng, Y.; et al. Heterostructured TiO2/WO3 nanocomposites for photocatalytic degradation of toluene under visible light. J. Electrochem. Soc. 2017, 164, H1086–H1090. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, C.; Li, J.; Zou, T.; Zeng, D. New insights into the relationship between photocatalytic activity and photocurrent of TiO2/WO3 nanocomposite. Appl. Catal. A 2012, 433–434, 81–87. [Google Scholar] [CrossRef]
- Prabhu, S.; Cindrella, L.; Kwon, O.J.; Mohanrajubet, K. Photoelectrochemical and photocatalytic activity of TiO2-WO3 heterostructures boosted by mutual interaction. Mater. Sci. Semicond. Process. 2018, 88, 10–19. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Liu, H.; Wang, C.; Liu, S.; Sun, P.; Wang, L.; Liu, Y. Heterostructured TiO2/WO3 porous microspheres: Preparation, characterization and photocatalytic properties. Catal. Today 2013, 201, 195–202. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Hu, X.; Xua, L.; Chen, G.; Li, X. Hollow spherical WO3/TiO2 heterojunction for enhancing photocatalytic performance in visible-light. J. Water Process. Eng. 2021, 40, 101943. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, H.; Ge, H.; Bian, Z. The enhanced photoreduction of Cr (VI) to Cr (III) using carbon dots coupled TiO2 mesocrystals. Appl. Catal. B 2018, 226, 213–219. [Google Scholar] [CrossRef]
- Song, Y.Y.; Gao, Z.D.; Wang, J.H.; Xia, X.H.; Lynch, R. Multistage coloring electrochromic device based on TiO2 nanotube arrays modified with WO3 nanoparticles. Adv. Funct. Mater. 2011, 21, 1941–1946. [Google Scholar] [CrossRef]
- Paramasivam, I.; Nah, Y.C.; Das, C.; Shrestha, N.K.; Schmuki, P. WO3/TiO2 nanotubes with strongly enhanced photocatalytic activity. Chem. Eur. J. 2010, 16, 8993–8997. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Zhou, X.; Zheng, X.; Xu, Q.; Chen, Z.; Ren, Y.; Yan, B. Fabrication and assembly of two-dimensional TiO2/WO3·H2O heterostructures with type II band alignment for enhanced photocatalytic performance. Appl. Surf. Sci. 2017, 403, 564–571. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, W.K. Heterojunction-based two-dimensional N-doped TiO2/WO3 composite architectures for photocatalytic treatment of hazardous organic vapor. J. Hazard. Mater. 2016, 314, 22–31. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Li, C.; Chen, Y.; Zhang, Z. Optimal monolayer WO3 nanosheets/TiO2 heterostructure and its photocatalytic performance under solar light. Chem. Phys. Lett. 2022, 804, 139861. [Google Scholar] [CrossRef]
- Mei, Y.; Su, Y.; Li, Z.; Bai, S.; Yuan, M.; Li, L.; Yan, Z.; Wu, J.; Zhu, L.W. BiOBr nanoplates@TiO2 nanowires/carbon fiber cloth as a functional water transport network for continuous flow water purification. Dalton Trans. 2017, 46, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Ko, W.Y.; Yen, Y.C.; Chen, P.H.; Lin, K.J. Hydrothermally processed TiO2 nanowire electrodes with antireflective and electrochromic properties. ACS Nano 2012, 6, 6633–6639. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, C.; Zhu, L.; Guo, W.; Shen, L.; Wen, S.; Ruan, S. Enhanced toluene sensing performance of gold-functionalized WO3· H2O nanosheets. Sens. Actuators B Chem. 2016, 223, 761–767. [Google Scholar] [CrossRef]
- Liu, W.; Du, T.; Ru, Q.; Zuo, S.; Cai, Y.; Yao, C. Preparation of graphene/WO3/TiO2 composite and its photocathodic protection performance for 304 stainless steel. Mater. Res. Bull. 2018, 102, 399–405. [Google Scholar] [CrossRef]
- Idriss, H.; Barteau, M.A. Characterization of TiO2 surfaces active for novel organic syntheses. Catal. Lett. 1994, 26, 123–139. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.; Gao, S.; Ca, G. The preparation of coupled WO3/TiO2 photocatalyst by ball milling. Powder Technol. 2005, 160, 198–202. [Google Scholar]
- Li, S.; Wang, P.; Wang, R.; Liu, Y.; Jing, R.; Zi, Z.; Meng, L.; Liu, Y.; Zhang, Q. One-step co-precipitation method to construct black phosphorus nanosheets/ZnO nanohybrid for enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2019, 497, 143682. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, K.; Feng, Z.; Bao, Y.; Dong, B. Hierarchical sheet-on-sheet ZnIn2S4/g-C3N4 heterostructure with highly efficient photocatalytic H2 production based on photoinduced interfacial charge transfer. Sci. Rep. 2016, 6, 19221. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Nehra, S.P.; Sharma, A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019, 9, 15381–15391. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Wang, Y.; Zheng, L.; He, G.; Deng, J.; Liu, M.; Li, Y.; Liu, Y.; Zhou, H. Fabrication of Z-Scheme TiO2/SnS2/MoS2 ternary heterojunction arrays for enhanced photocatalytic and photoelectrochemical performance under visible light. J. Solid State Chem. 2022, 307, 122737. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Li, Y.; Ho, W.; Yu, J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017, 391, 175–183. [Google Scholar] [CrossRef]
- Vembuli, T.; Thiripuranthagan, S.; Sureshkumar, T.; Erusappan, E.; Kumaravel, S.; Kasinathan, M.; Sivakumar, A. Degradation of Harmful Organics Using Visible Light Driven N-TiO2/rGO Nanocomposite. J. Nanosci. Nanotechnol. 2021, 21, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, L.; Zhou, Y.; Cao, S.; Cao, X. Direct production of a free-standing titanate and titania nanofiber membrane with selective permeability and cleaning performance. J. Mater. Chem. 2011, 21, 12503–12510. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, L.; Gu, L.; Cao, S.; Wang, L.; Cao, X. Guided growth and alignment of millimetre-long titanate nanofibers in solution. J. Mater. Chem. 2012, 22, 16890–16896. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xu, K.; Tang, H.; Zhu, L. Vertical Growth of WO3 Nanosheets on TiO2 Nanoribbons as 2D/1D Heterojunction Photocatalysts with Improved Photocatalytic Performance under Visible Light. Catalysts 2023, 13, 556. https://doi.org/10.3390/catal13030556
Wang L, Xu K, Tang H, Zhu L. Vertical Growth of WO3 Nanosheets on TiO2 Nanoribbons as 2D/1D Heterojunction Photocatalysts with Improved Photocatalytic Performance under Visible Light. Catalysts. 2023; 13(3):556. https://doi.org/10.3390/catal13030556
Chicago/Turabian StyleWang, Ling, Keyi Xu, Hongwang Tang, and Lianwen Zhu. 2023. "Vertical Growth of WO3 Nanosheets on TiO2 Nanoribbons as 2D/1D Heterojunction Photocatalysts with Improved Photocatalytic Performance under Visible Light" Catalysts 13, no. 3: 556. https://doi.org/10.3390/catal13030556
APA StyleWang, L., Xu, K., Tang, H., & Zhu, L. (2023). Vertical Growth of WO3 Nanosheets on TiO2 Nanoribbons as 2D/1D Heterojunction Photocatalysts with Improved Photocatalytic Performance under Visible Light. Catalysts, 13(3), 556. https://doi.org/10.3390/catal13030556







