Dimethyl Ether to Olefins on Hybrid Intergrowth Structure Zeolites
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
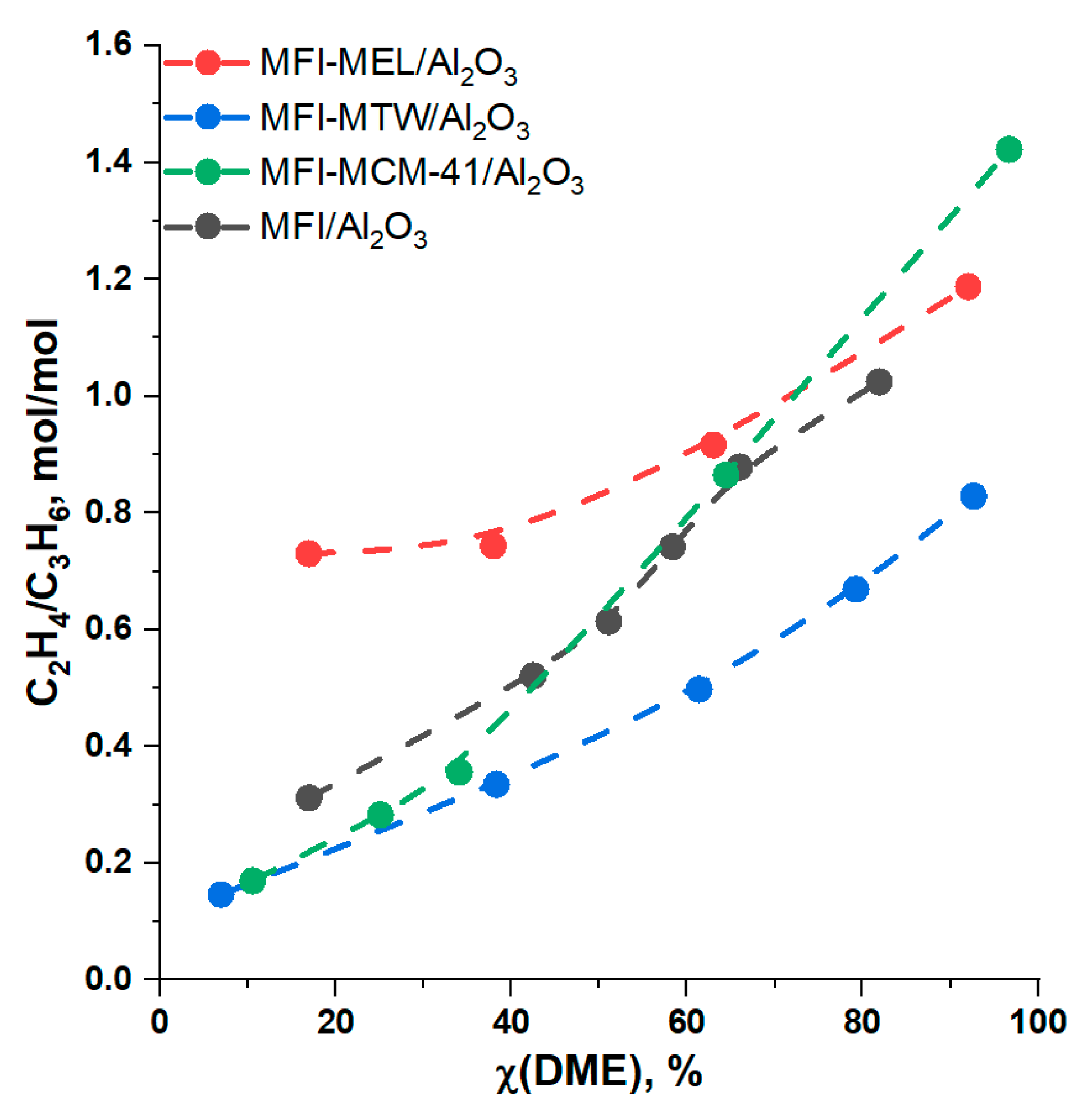
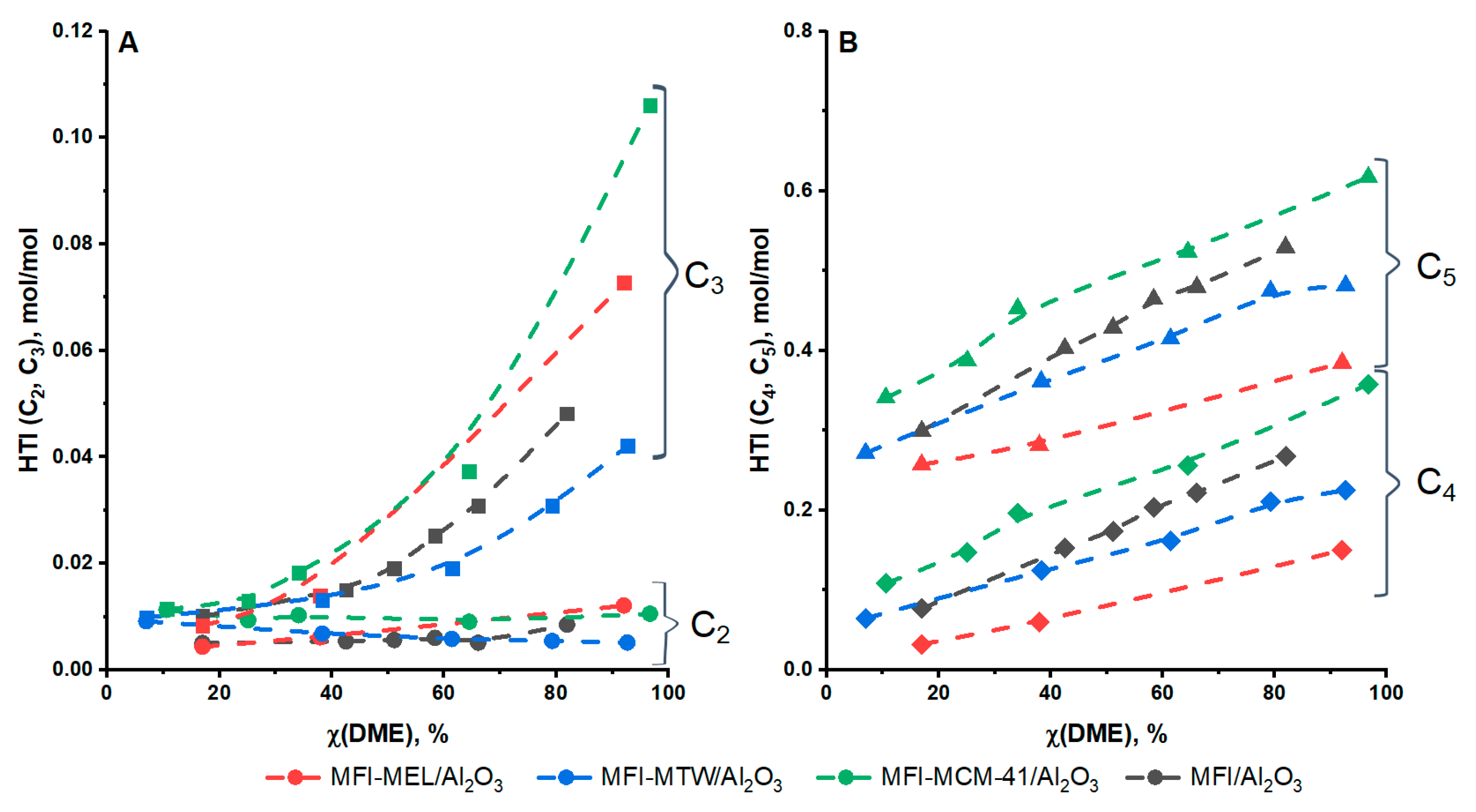
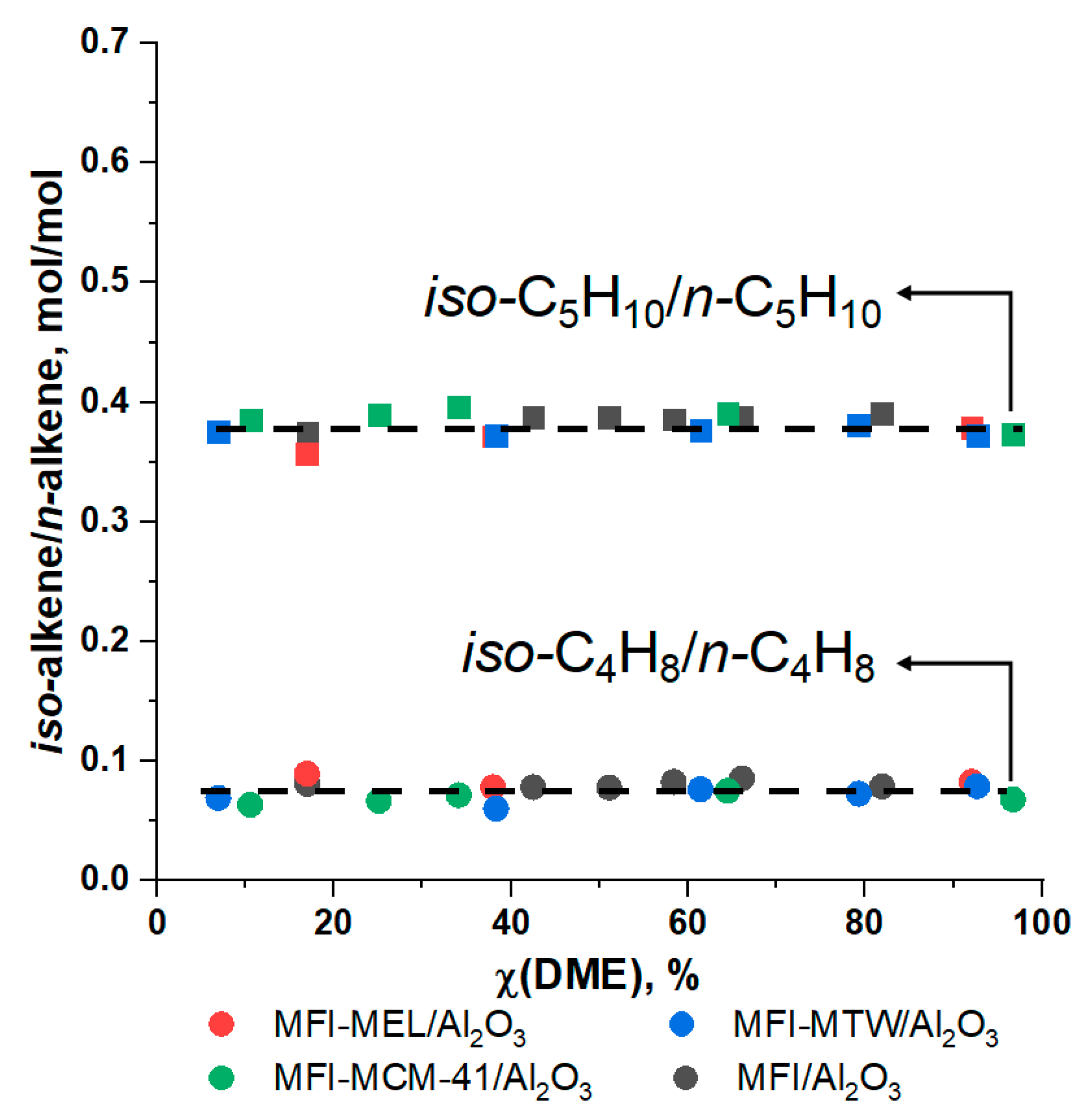
2.2. DME Conversion to Olefin
3. Materials and Methods
3.1. Catalyst Synthesis
3.1.1. MFI-MEL Synthesis
3.1.2. MFI-MTW Synthesis
3.1.3. MFI-MCM-41 Synthesis
3.1.4. Catalyst Preparation
3.1.5. Hydrothermal Treatment
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Yang, M.; Fan, D.; Wei, Y.; Tian, P.; Liu, Z. Recent Progress in Methanol-to-Olefins (MTO) Catalysts. Adv. Mater. 2019, 31, 1902181. [Google Scholar] [CrossRef]
- Chang, C.D.; Silvestri, A.J.; Smith, R.L. (MTG) Production of Gasoline Hydrocarbons. U.S. Patent 3,928,483, 23 December 1975. [Google Scholar]
- Chang, C.D.; Lang, W.H. Process for Manufacturing Olefins. U.S. Patent 566,166, 24 May 1977. [Google Scholar]
- Li, J.; Wei, Z.; Chen, Y.; Jing, B.; He, Y.; Dong, M.; Jiao, H.; Li, X.; Qin, Z.; Wang, J.; et al. A route to form initial hydrocarbon pool species in methanol conversion to olefins over zeolites. J. Catal. 2014, 317, 277–283. [Google Scholar] [CrossRef]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Liu, Y.; Müller, S.; Berger, D.; Jelic, J.; Reuter, K.; Tonigold, M.; Sanchez-Sanchez, M.; Lercher, J.A. Formation mechanism of the first carbon–carbon bond and the first olefin in the methanol conversion into hydrocarbons. Angew. Chem. Int. Ed. 2016, 55, 5723–5726. [Google Scholar] [CrossRef]
- Wu, X.; Xu, S.; Zhang, W.; Huang, J.; Li, J.; Yu, B.; Wei, Y.; Liu, Z. Direct mechanism of the first carbon–carbon bond formation in the methanol-to-hydrocarbons process. Angew. Chem. Int. Ed. 2017, 56, 9039–9043. [Google Scholar] [CrossRef]
- Salehirad, F.; Anderson, M.W. Solid-state 13C MAS NMR study of methanol-to-hydrocarbon chemistry over H-SAPO-34. J. Catal. 1996, 164, 301–314. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Pomakhina, E.B.; Rebrov, A.I.; Hunger, M.; Kolyagin, Y.G.; Weitkamp, J. Surface species formed during aniline methylation on zeolite H-Y investigated by in situ MAS NMR spectroscopy. J. Catal. 2001, 203, 375–381. [Google Scholar] [CrossRef]
- Van Der Mynsbrugge, J.; Moors, S.L.C.; De Wispelaere, K.; Van Speybroeck, V. Insight into the formation and reactivity of framework-bound methoxide species in h-zsm-5 from static and dynamic molecular simulations. ChemCatChem 2014, 6, 1906–1918. [Google Scholar] [CrossRef]
- Svelle, S.; Joensen, F.; Nerlov, J.; Olsbye, U.; Lillerud, K.P.; Kolboe, S.; Bjørgen, M. Conversion of methanol into hydrocarbons over zeolite H-ZSM-5: Ethene formation is mechanistically separated from the formation of higher alkenes. J. Am. Chem. Soc. 2006, 128, 14770–14771. [Google Scholar] [CrossRef] [PubMed]
- Bjørgen, M.; Svelle, S.; Joensen, F.; Nerlov, J.; Kolboe, S.; Bonino, F.; Palumbo, L.; Bordiga, S.; Olsbye, U. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5: On the origin of the olefinic species. J. Catal. 2007, 249, 195–207. [Google Scholar] [CrossRef]
- Bjørgen, M.; Lillerud, K.P.; Olsbye, U.; Svelle, S. Conversion of methanol to hydrocarbons: Hints to rational catalyst design from fundamental mechanistic studies on H-ZSM-5. Stud. Surf. Sci. Catal. 2007, 167, 463–468. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjrgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef]
- Lu, C.; Yang, H.; Wang, J.; Tan, Q.; Fu, L. Utilization of iron tailings to prepare high-surface area mesoporous silica materials. Sci. Total Environ. 2020, 736, 139483. [Google Scholar] [CrossRef]
- Hunger, M.; Horvath, T. Adsorption of methanol on Bronsted acid sites in zeolite H-ZSM-5 investigated by multinuclear solid-state NMR spectroscopy. J. Am. Chem. Soc. 1996, 118, 12302–12308. [Google Scholar] [CrossRef]
- Seiler, M.; Schenk, U.; Hunger, M. Conversion of methanol to hydrocarbons on zeolite HZSM-5 investigated by in situ MAS NMR spectroscopy under flow conditions and on-line gas chromatography. Catal. Lett. 1999, 62, 139–145. [Google Scholar] [CrossRef]
- Seiler, M.; Wang, W.; Hunger, M. Local structure of framework aluminum in zeolite H-ZSM-5 during conversion of methanol investigated by in situ NMR spectroscopy. J. Phys. Chem. B 2001, 105, 8143–8148. [Google Scholar] [CrossRef]
- Hunger, M.; Seiler, M.; Buchholz, A. In situ MAS NMR spectroscopic investigation of the conversion of methanol to olefins on silicoaluminophosphates SAPO-34 and SAPO-18 under continuous flow conditions. Catal. Lett. 2001, 74, 61–68. [Google Scholar] [CrossRef]
- Wang, W.; Seiler, M.; Hunger, M. Role of surface methoxy species in the conversion of methanol to dimethyl ether on acidic zeolites investigated by in situ stopped-flow MAS NMR spectroscopy. J. Phys. Chem. B 2001, 105, 12553–12558. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Xu, J.; Qi, G.; Wang, W.; Zhao, X.; Li, W.; Wang, Q.; Deng, F. Impact of temporal and spatial distribution of hydrocarbon pool on methanol conversion over H-ZSM-5. J. Catal. 2017, 354, 138–151. [Google Scholar] [CrossRef]
- Yarulina, I.; De Wispelaere, K.; Bailleul, S.; Goetze, J.; Radersma, M.; Abou-Hamad, E.; Vollmer, I.; Goesten, M.; Mezari, B.; Hensen, E.J.M.; et al. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nat. Chem. 2018, 10, 804–812. [Google Scholar] [CrossRef]
- Liu, C.; Uslamin, E.A.; Pidko, E.A.; Kapteijn, F. Direct discerning reaction pathways in methanol-to-hydrocarbons by transient operation–FASPA. Chem. Eng. J. 2023, 453, 139696. [Google Scholar] [CrossRef]
- Magomedova, M.; Starozhitskaya, A.; Davidov, I.; Maximov, A.; Kravtsov, M. Dual-cycle mechanism based kinetic model for dme-to-olefin synthesis on hzsm-5-type catalysts. Catalysts 2021, 11, 1459. [Google Scholar] [CrossRef]
- Dahl, I.M.; Kolboe, S. On the reaction mechanism for hydrocarbon formation from methanol over SAPO-34. I. Isotopic labeling studies of the co-reaction of ethene and methanol. J. Catal. 1994, 149, 458–464. [Google Scholar] [CrossRef]
- Xiao, J.; Wei, J. Diffusion of Hydrocarbons Theory. Chem. Eng. Sci. 1992, 47, 1123–1141. [Google Scholar] [CrossRef]
- Xiao, J.; Wei, J. Diffusion mechanism of hydrocarbons in zeolites-II. Analysis of experimental observations. Chem. Eng. Sci. 1992, 47, 1143–1159. [Google Scholar] [CrossRef]
- Sharbini Kamaluddin, H.; Gong, X.; Ma, P.; Narasimharao, K.; Dutta Chowdhury, A.; Mokhtar, M. Influence of zeolite ZSM-5 synthesis protocols and physicochemical properties in the methanol-to-olefin process. Mater. Today Chem. 2022, 26, 101061. [Google Scholar] [CrossRef]
- Weissenberger, T.; Machoke, A.G.F.; Bauer, J.; Dotzel, R.; Casci, J.L.; Hartmann, M.; Schwieger, W. Hierarchical ZSM-5 Catalysts: The Effect of Different Intracrystalline Pore Dimensions on Catalyst Deactivation Behaviour in the MTO Reaction. ChemCatChem 2020, 12, 2461–2468. [Google Scholar] [CrossRef]
- Bai, R.; Song, Y.; Li, Y.; Yu, J. Creating Hierarchical Pores in Zeolite Catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Chen, L.H.; Sun, M.H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.L. Hierarchically structured zeolites: From design to application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef] [PubMed]
- Weckhuysen, B.M.; Fu, D.; Maris, J.J.E.; Stanciakova, K. Unravelling Channel Structure-Diffusivity Relationships in Zeolite ZSM-5 at the Single-Molecule Level. Angew. Chem. Int. Ed. 2022, 61, e202114388. [Google Scholar] [CrossRef]
- Magomedova, M.V.; Afokin, M.I.; Starozhitskaya, A.V.; Galanova, E.G. Pilot Test of Olefin Synthesis from Dimethyl Ether in a Synthesis Gas Atmosphere. Ind. Eng. Chem. Res. 2021, 60, 4602–4609. [Google Scholar] [CrossRef]
- Vu, X.H.; Armbruster, U.; Martin, A. Micro/mesoporous zeolitic composites: Recent developments in synthesis and catalytic applications. Catalysts 2016, 6, 183. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Knyazeva, E.E. Micro-mesoporous materials obtained by zeolite recrystallization: Synthesis, characterization and catalytic applications. Chem. Soc. Rev. 2013, 42, 3671–3688. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef]
- Shoinkhorova, T.; Dikhtiarenko, A.; Ramirez, A.; Dutta Chowdhury, A.; Caglayan, M.; Vittenet, J.; Bendjeriou-Sedjerari, A.; Ali, O.S.; Morales-Osorio, I.; Xu, W.; et al. Shaping of ZSM-5-Based Catalysts via Spray Drying: Effect on Methanol-to-Olefins Performance. ACS Appl. Mater. Interfaces 2019, 11, 44133–44143. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Shang, K.; Liang, L.; Chen, S.; Ouyang, J. Efficient and Stable Ni/ZSM-5@MCM-41 Catalyst for CO2 Methanation. ACS Sustain. Chem. Eng. 2022, 10, 12771–12782. [Google Scholar] [CrossRef]
- Millward, G.R.; Ramdas, S.; Thomas, J.M.; Barlow, M.T. Evidence for semi-regularly ordered sequences of mirror and inversion symmetry planes in ZSM-5/ZSM-11 shape-selective zeolitic catalysts. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1983, 79, 1075–1082. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Han, X.; Bao, X. Conversion of methanol to hydrocarbons over phosphorus-modified ZSM-5/ZSM-11 intergrowth zeolites. Catal. Lett. 2010, 134, 124–130. [Google Scholar] [CrossRef]
- Kim, J.J.; Jeong, D.J.; Jung, H.S.; Hur, Y.G.; Choung, J.W.; Baik, J.H.; Park, M.J.; Chung, C.H.; Bae, J.W. Dimethyl ether conversion to hydrocarbons on the closely interconnected FER@ZSM-5 nanostructures. Microporous Mesoporous Mater. 2022, 340, 112034. [Google Scholar] [CrossRef]
- Liu, C.; Uslamin, E.A.; van Vreeswijk, S.H.; Yarulina, I.; Ganapathy, S.; Weckhuysen, B.M.; Kapteijn, F.; Pidko, E.A. An integrated approach to the key parameters in methanol-to-olefins reaction catalyzed by MFI/MEL zeolite materials. Chin. J. Catal. 2022, 43, 1879–1893. [Google Scholar] [CrossRef]
- Magomedova, M.; Galanova, E.; Davidov, I.; Afokin, M.; Maximov, A. Dimethyl Ether to Olefins over Modified ZSM-5 Based Catalysts Stabilized by Hydrothermal Treatment. Catalysts 2019, 9, 485. [Google Scholar] [CrossRef]
- Bin, F.; Song, C.; Lv, G.; Song, J.; Wu, S.; Li, X. Selective catalytic reduction of nitric oxide with ammonia over zirconium-doped copper/ZSM-5 catalysts. Appl. Catal. B Environ. 2014, 150–151, 532–543. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Chu, P.; Lawton, S.L.; Meier, W.M. Synthesis and structure of synthetic zeolite ZSM-11. Nature 1978, 275, 119–120. [Google Scholar] [CrossRef]
- Dugkhuntod, P.; Imyen, T.; Wannapakdee, W.; Yutthalekha, T.; Salakhum, S.; Wattanakit, C. Synthesis of hierarchical ZSM-12 nanolayers for levulinic acid esterification with ethanol to ethyl levulinate. RSC Adv. 2019, 9, 18087–18097. [Google Scholar] [CrossRef]
- Conte, M.; Xu, B.; Davies, T.E.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Khalid, K.; Hutchings, G.J. Enhanced selectivity to propene in the methanol to hydrocarbons reaction by use of ZSM-5/11 intergrowth zeolite. Microporous Mesoporous Mater. 2012, 164, 207–213. [Google Scholar] [CrossRef]
- Maksimov, A.L.; Magomedova, M.V.; Afokin, M.I.; Tsaplin, D.E.; Kulikov, L.A.; Ionin, D.A. Method for Producing an HZSM-Type Zeolite (Variants) and Method for Producing Aromatic Hydrocarbons of the C6-C11 Fraction. RU Patent 2753263C1, 8 December 2021. [Google Scholar]
- Lemus-Yegres, L.J.; Such-Basáñez, I.; Román-Martínez, M.C.; de Lecea, C.S.M. Catalytic properties of a Rh-diamine complex anchored on activated carbon: Effect of different surface oxygen groups. Appl. Catal. A Gen. 2007, 331, 26–33. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Z.-M.; Cao, C.-Y.; Song, W.-G. 0.3 Å Makes the Difference: Dramatic Changes in Methanol-to-Olefin Activities between H-ZSM-12 and H-ZSM-22 Zeolites. J. Phys. Chem. C 2011, 115, 24987–24992. [Google Scholar] [CrossRef]
- Karge, H.G. Comparative Measurements on Acidity of Zeolites. Stud. Surf. Sci. Catal. 1991, 65, 133–156. [Google Scholar] [CrossRef]
- Lónyi, F.; Valyon, J. On the interpretation of the NH3-TPD patterns of. Microporous Mesoporous Mater. 2001, 47, 293–301. [Google Scholar] [CrossRef]
- Serrano, D.P.; García, R.A.; Vicente, G.; Linares, M.; Procházková, D.; Čejka, J. Acidic and catalytic properties of hierarchical zeolites and hybrid ordered mesoporous materials assembled from MFI protozeolitic units. J. Catal. 2011, 279, 366–380. [Google Scholar] [CrossRef]
- Malinowski, P.J.; Derzsi, M.; Mazej, Z.; Jagličić, Z.; Gaweł, B.; ŁAsocha, W.; Grochala, W. AgIIso4: A genuine sulfate of divalent silver with anomalously strong one-dimensional antiferromagnetic Interactions. Angew. Chem. Int. Ed. 2010, 49, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Valyon, J.; Lo, F. A TPD and IR study of the surface species formed from ammonia on zeolite H-ZSM-5, H-mordenite, and H-beta. Thermochim. Acta 2001, 373, 53–57. [Google Scholar]
- Corma, A.; Fornés, V.; Navarro, M.T.; Pérez-Pariente, J. Acidity and stability of MCM-41 crystalline aluminosilicates. J. Catal. 1994, 148, 569–574. [Google Scholar] [CrossRef]
- Kosslick, H.; Lischke, G.; Parlitz, B.; Storek, W.; Fricke, R. Acidity and active sites of Al-MCM-41. Appl. Catal. A Gen. 1999, 184, 49–60. [Google Scholar] [CrossRef]
- Jentys, A.; Pham, N.H.; Vinek, H. Nature of hydroxy groups in MCM-41. J. Chem. Soc. Faraday Trans. 1996, 92, 3287–3291. [Google Scholar] [CrossRef]
- Hunger, M.; Schenk, U.; Breuninger, M.; Gläser, R.; Weitkamp, J. Characterization of the acid sites in MCM-41-type materials by spectroscopic and catalytic techniques. Microporous Mesoporous Mater. 1999, 27, 261–271. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Li, H.-X.; Davis, M.E. Studies on mesoporous materials. Microporous Mater. 1993, 2, 17–26. [Google Scholar] [CrossRef]
- Silaghi, M.C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Pérez-Uriarte, P.; Ateka, A.; Aguayo, A.T.; Gayubo, A.G.; Bilbao, J. Kinetic model for the reaction of DME to olefins over a HZSM-5 zeolite catalyst. Chem. Eng. J. 2016, 302, 801–810. [Google Scholar] [CrossRef]
- Naik, S.P.; Bui, V.; Ryu, T.; Miller, J.D.; Zmierczak, W. Al-MCM-41 as methanol dehydration catalyst. Appl. Catal. A Gen. 2010, 381, 183–190. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, H.; Zheng, Y.; Wang, J.; Li, H.; Zhang, J. Catalytic dehydration of methanol to dimethyl ether over micro-mesoporous ZSM-5/MCM-41 composite molecular sieves. Appl. Catal. A Gen. 2012, 413–414, 36–42. [Google Scholar] [CrossRef]
- Sun, X.; Mueller, S.; Liu, Y.; Shi, H.; Haller, G.L.; Sanchez-Sanchez, M.; Van Veen, A.C.; Lercher, J.A. On reaction pathways in the conversion of methanol to hydrocarbons on HZSM-5. J. Catal. 2014, 317, 185–197. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, Y.; Zhang, L.; Liu, Y.; Dou, T.; Xu, J.; Deng, F. Hydrothermal treatment on ZSM-5 extrudates catalyst for methanol to propylene reaction: Finely tuning the acidic property. Fuel Process. Technol. 2015, 129, 130–138. [Google Scholar] [CrossRef]
- Galanova, E.G.; Magomedova, M.V.; Afokin, M.I.; Starozhitskaya, A.V.; Maximov, A.L. Synthesis of olefins from dimethyl ether in a synthesis gas atmosphere. Catal. Commun. 2021, 153, 106297. [Google Scholar] [CrossRef]
- Tsaplin, D.E.; Kulikov, L.A.; Maksimov, A.L.; Karakhanov, E.A. Method for Obtaining a Composite material with a Hierarchical Structure. RU Patent 2773945C1, 14 June 2022. [Google Scholar]
- Khadzhiev, S.N.; Kolesnichenko, N.V.; Gorjainova, T.I.; Birjukova, E.N.; Kulumbegov, R.V. Catalyst and Method of Producing Olefins from Dimethyl Ether in Its Presence. RU Patent 2445158 C2, 20 May 2012. [Google Scholar]
- Kolesnichenko, N.V.; Bukina, Z.M.; Jashina, O.V.; Zavalishin, I.N.; Markova, N.A.; Khadzhiev, S.N.; Lin, G.I.; Rozovskij, A.J.; Kitaev, L.E. Catalyst and Method to Manufacture Olefines from Dimethyl Ether in the Presence Thereof. RU Patent 2323777 C1, 10 May 2008. [Google Scholar]
- Hubbard, C.R.; Snyder, R.L. RIR—Measurement and Use in Quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef]
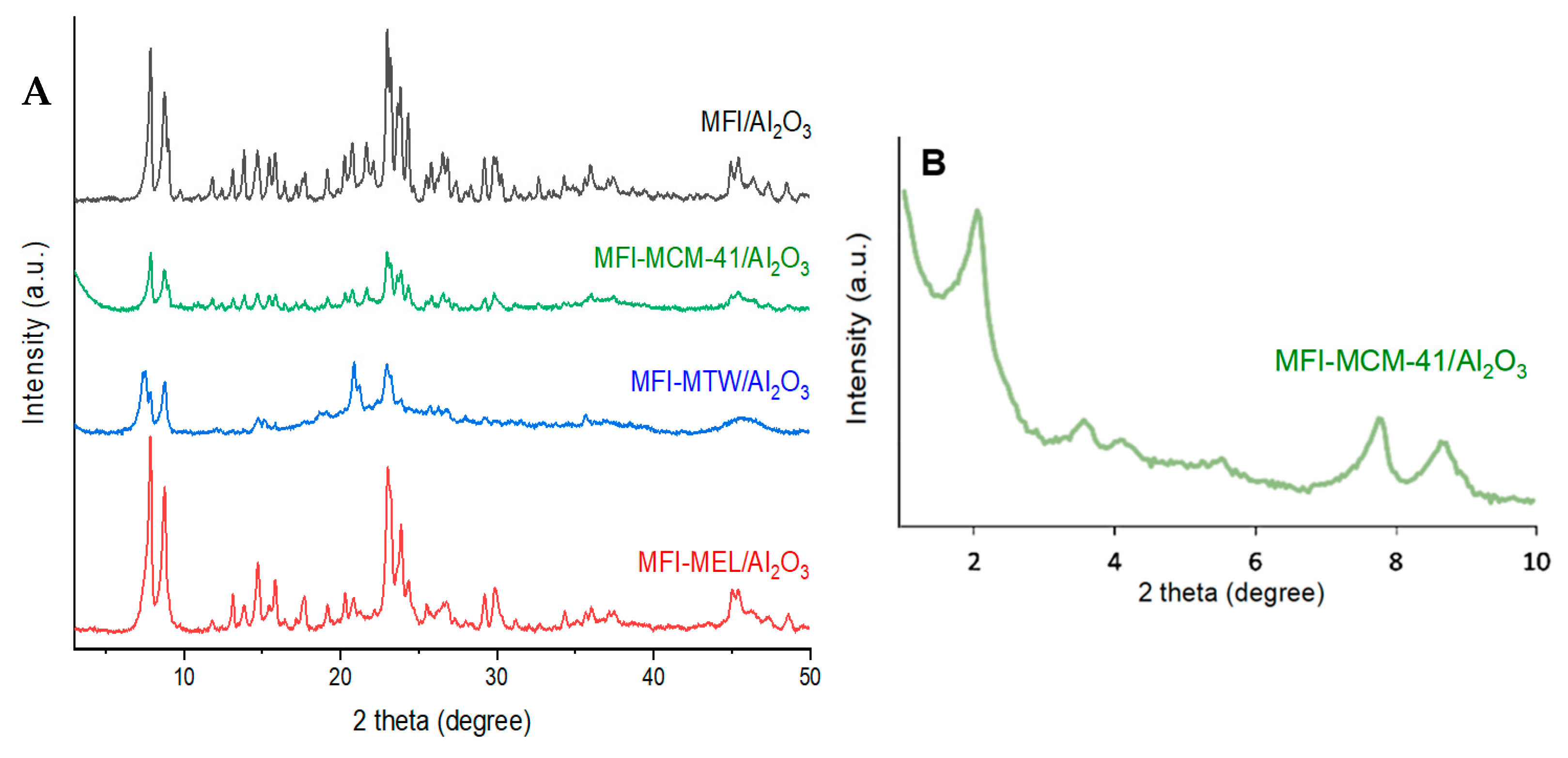
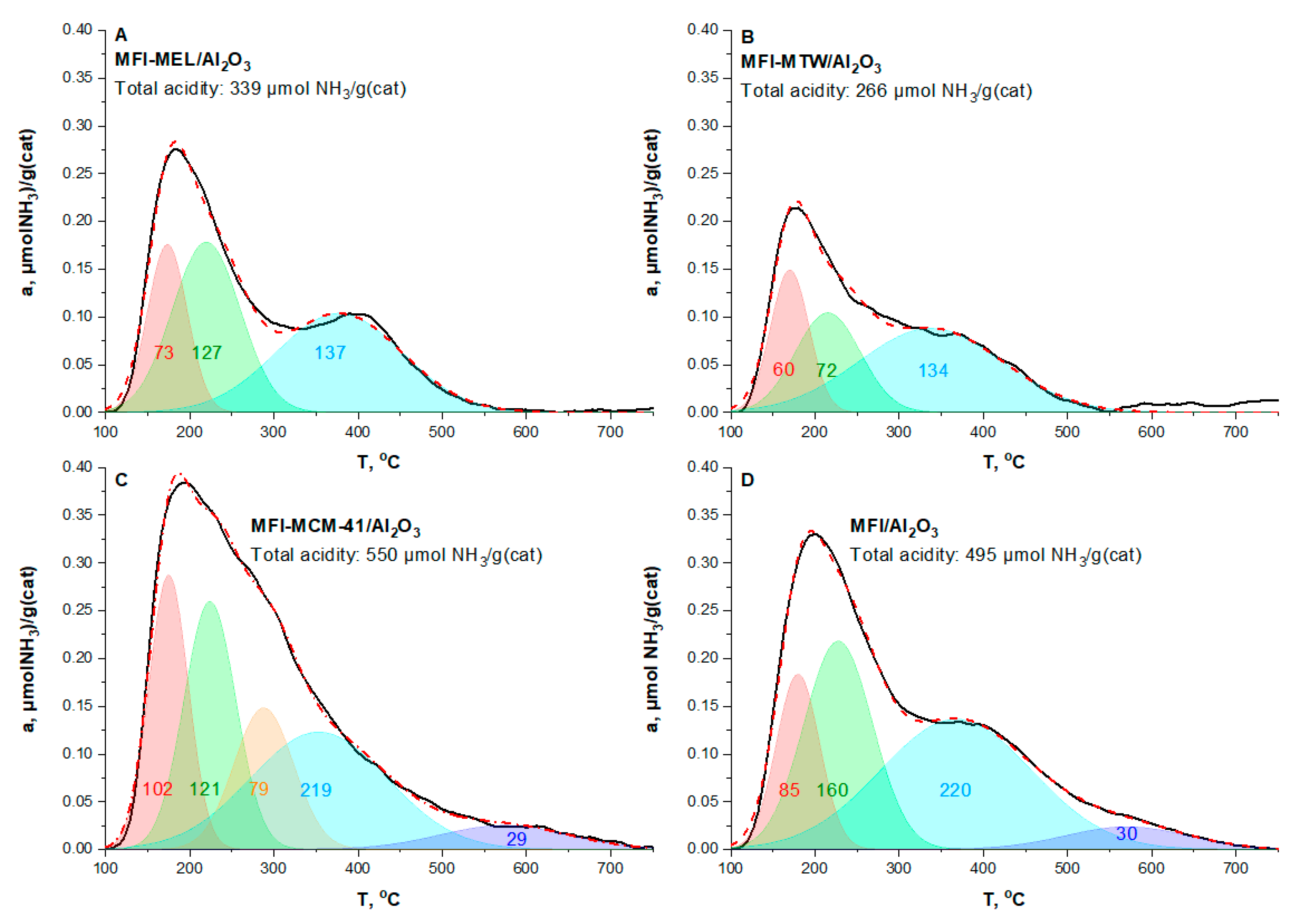
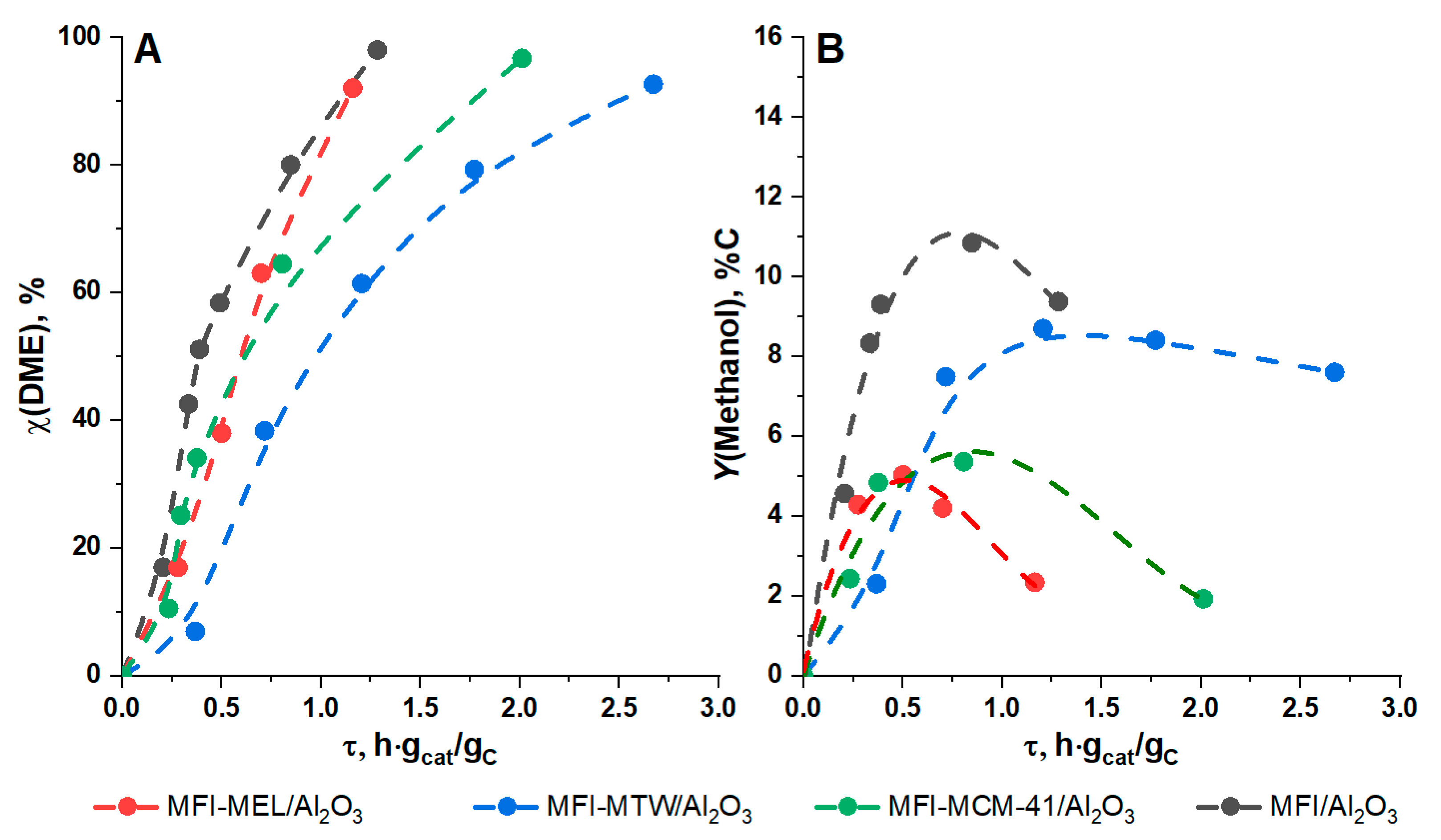
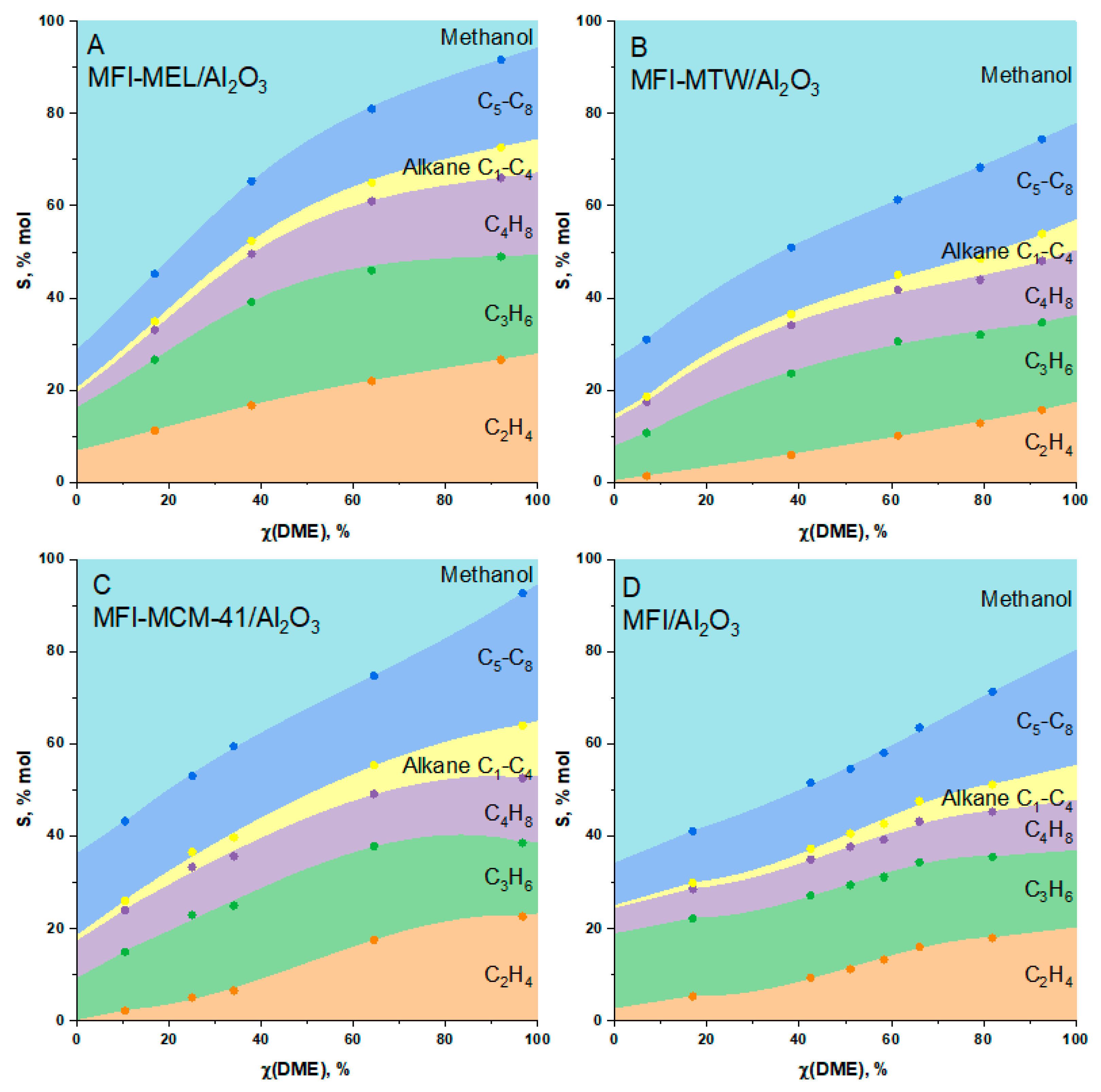
| No | Catalyst | SBET, m2/g(Cat) | Smicro, m2/g(Cat) | V(pore), cm3/g(Cat) | ||
|---|---|---|---|---|---|---|
| Total | Micro | Meso | ||||
| 1 | MFI-MEL/Al2O3 | 361 | 250 | 0.286 | 0.088 (30.8%) | 0.198 (69.2%) |
| 2 | MFI-MTW/Al2O3 | 179 | 11 | 0.443 | 0.012 (2.7%) | 0.431 (97.3%) |
| 3 | MFI-MCM-41/Al2O3 | 250 | 42 | 0.340 | 0.040 (11.8%) | 0.300 (88.2%) |
| 4 | MFI/Al2O3 | 293 | 181 | 0.198 | 0.057 (28.7%) | 0.142 (71.3%) |
| No | Sample | Acidity of Fresh Catalyst, μmol NH3/g(cat) | ||||
|---|---|---|---|---|---|---|
| Total | Weak Sites | Medium Sites | Strong Sites | |||
| T1 = 170–180 °C T2 = 215–230 °C | T3 = 290 °C | T4 = 350–380 °C | T5 = 570 °C | |||
| 1 | MFI-MEL/Al2O3 | 339 | 200 (59.0%) | - | 139 (41.0%) | - |
| 2 | MFI-MTW/Al2O3 | 266 | 132 (49.6%) | - | 134 (50.4%) | - |
| 3 | MFI-MCM-41/Al2O3 | 550 | 223 (40.5%) | 79 (14.4 %) | 219 (39.8%) | 29 (5.3%) |
| 4 | MFI/Al2O3 | 495 | 245 (49.5%) | - | 220 (44.4%) | 30 (6.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magomedova, M.V.; Starozhitskaya, A.V.; Davidov, I.A.; Tsaplin, D.E.; Maximov, A.L. Dimethyl Ether to Olefins on Hybrid Intergrowth Structure Zeolites. Catalysts 2023, 13, 570. https://doi.org/10.3390/catal13030570
Magomedova MV, Starozhitskaya AV, Davidov IA, Tsaplin DE, Maximov AL. Dimethyl Ether to Olefins on Hybrid Intergrowth Structure Zeolites. Catalysts. 2023; 13(3):570. https://doi.org/10.3390/catal13030570
Chicago/Turabian StyleMagomedova, Maria V., Anastasiya V. Starozhitskaya, Ilya A. Davidov, Dmitry E. Tsaplin, and Anton L. Maximov. 2023. "Dimethyl Ether to Olefins on Hybrid Intergrowth Structure Zeolites" Catalysts 13, no. 3: 570. https://doi.org/10.3390/catal13030570
APA StyleMagomedova, M. V., Starozhitskaya, A. V., Davidov, I. A., Tsaplin, D. E., & Maximov, A. L. (2023). Dimethyl Ether to Olefins on Hybrid Intergrowth Structure Zeolites. Catalysts, 13(3), 570. https://doi.org/10.3390/catal13030570







