Construction of NiFe-Layered Double Hydroxides Arrays as Robust Electrocatalyst for Oxygen Evolution Reaction
Abstract
:1. Introduction
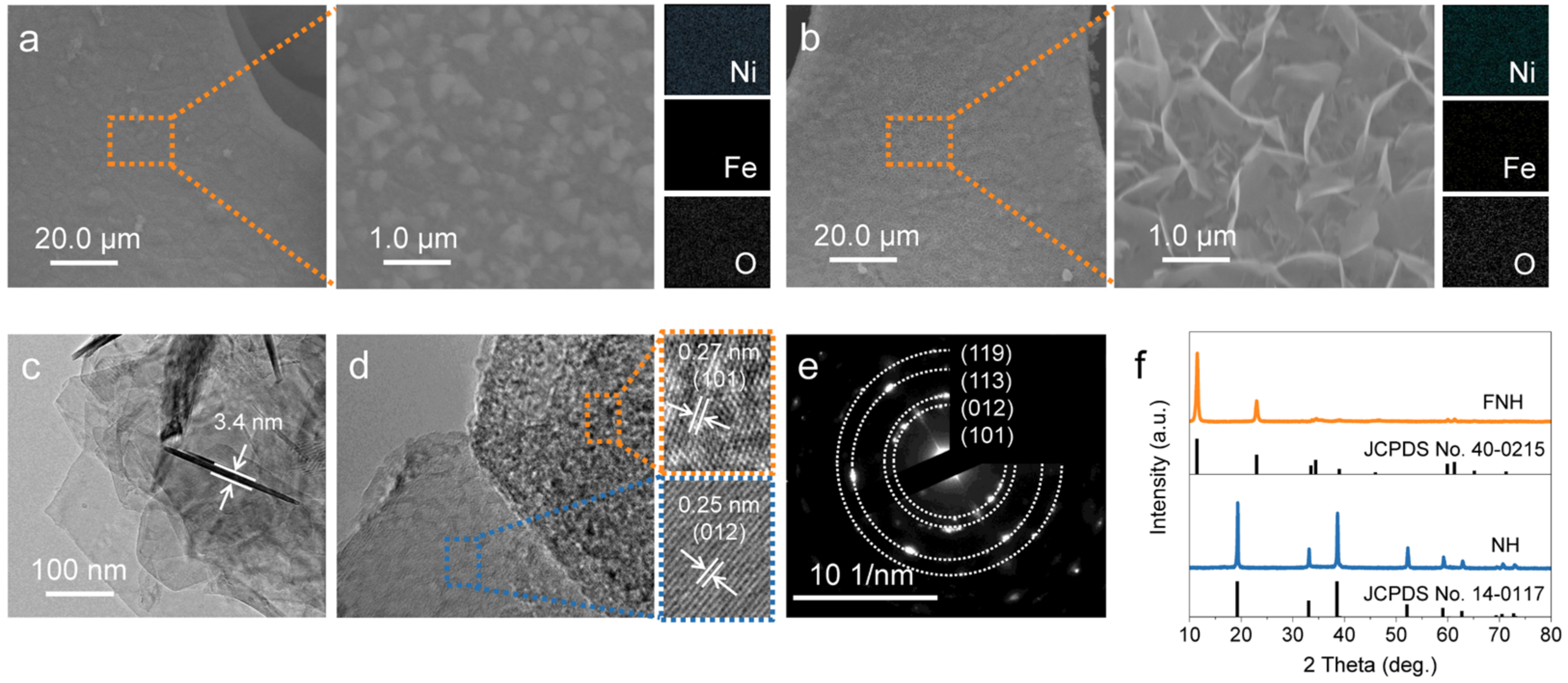
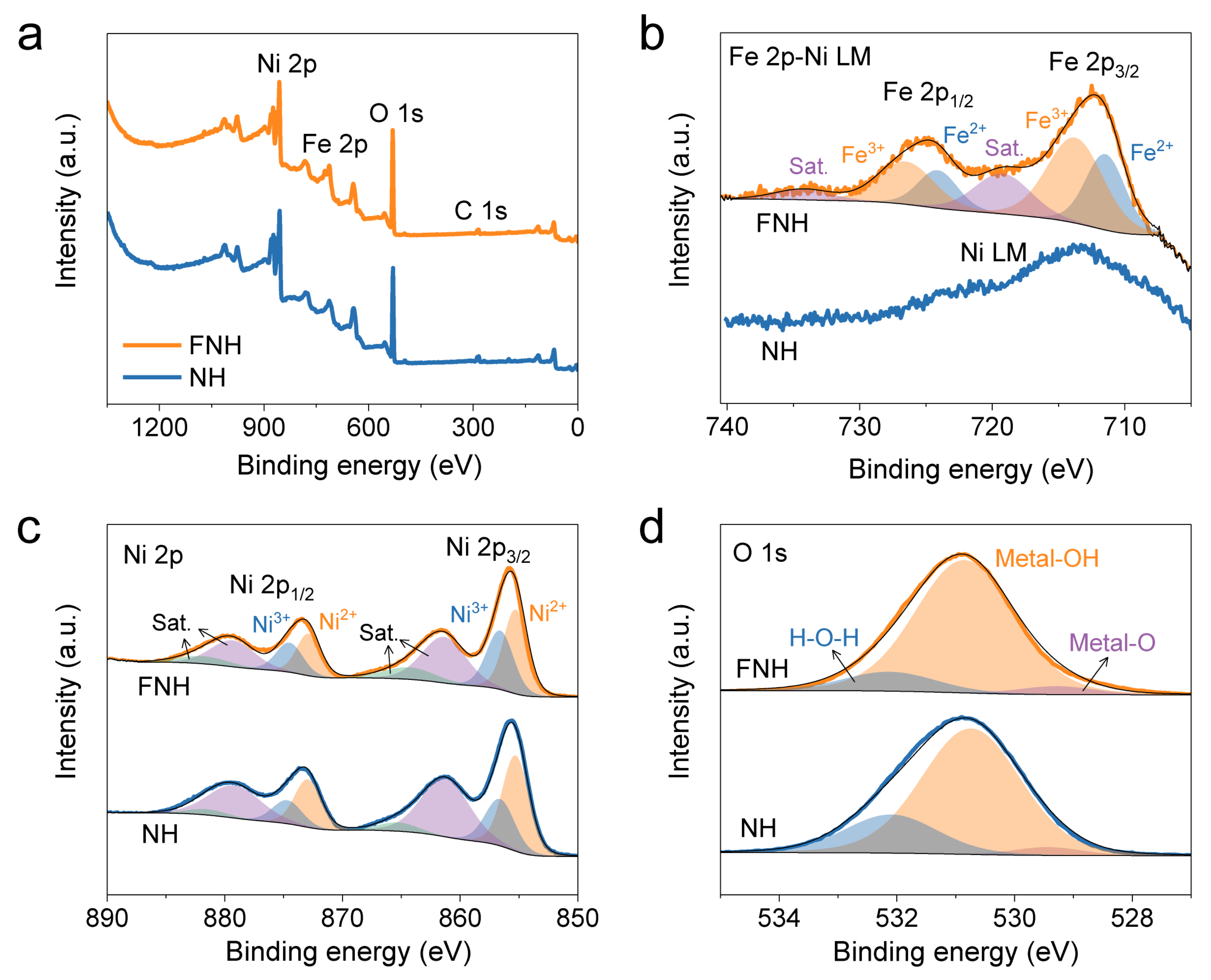
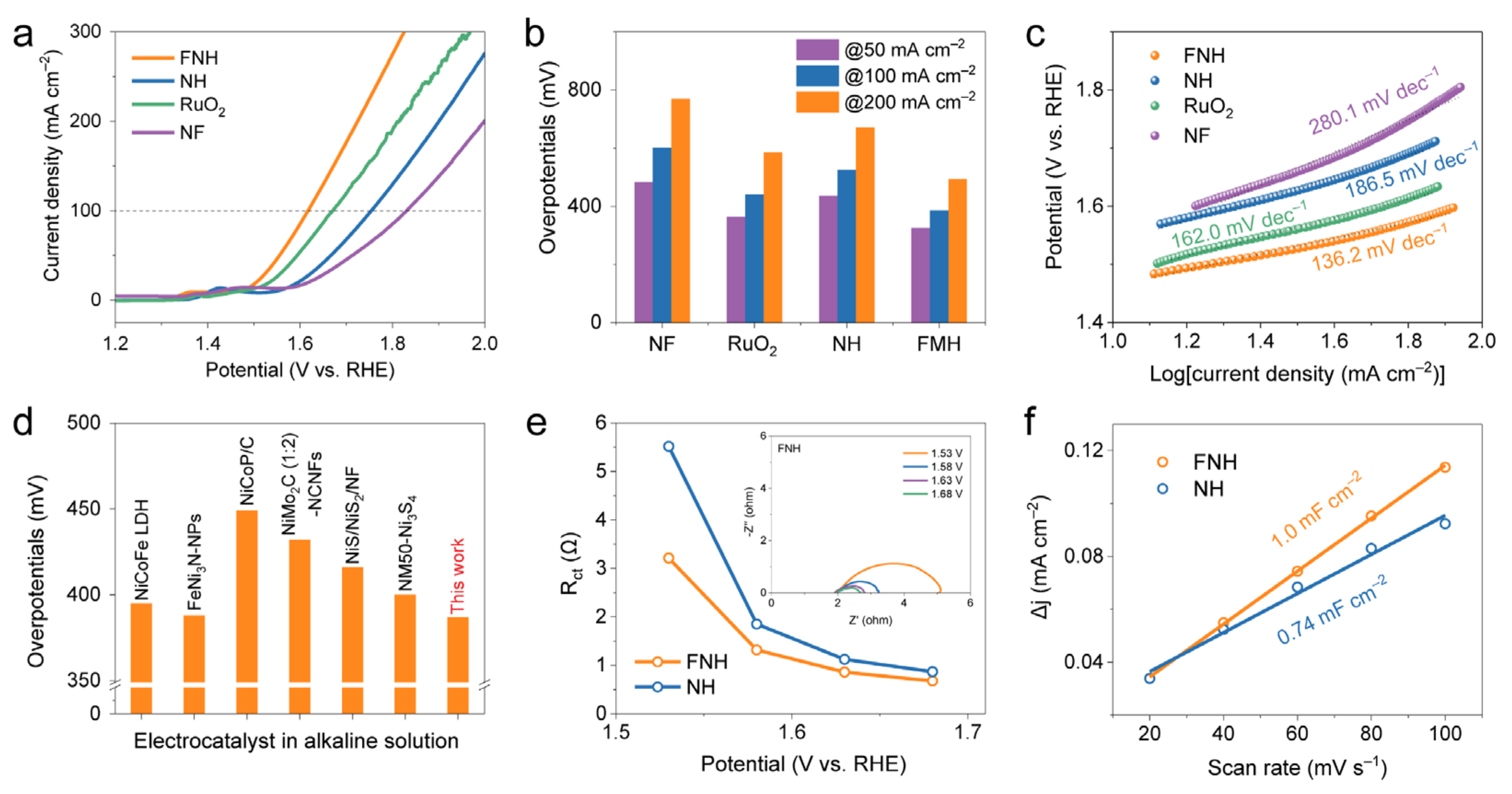
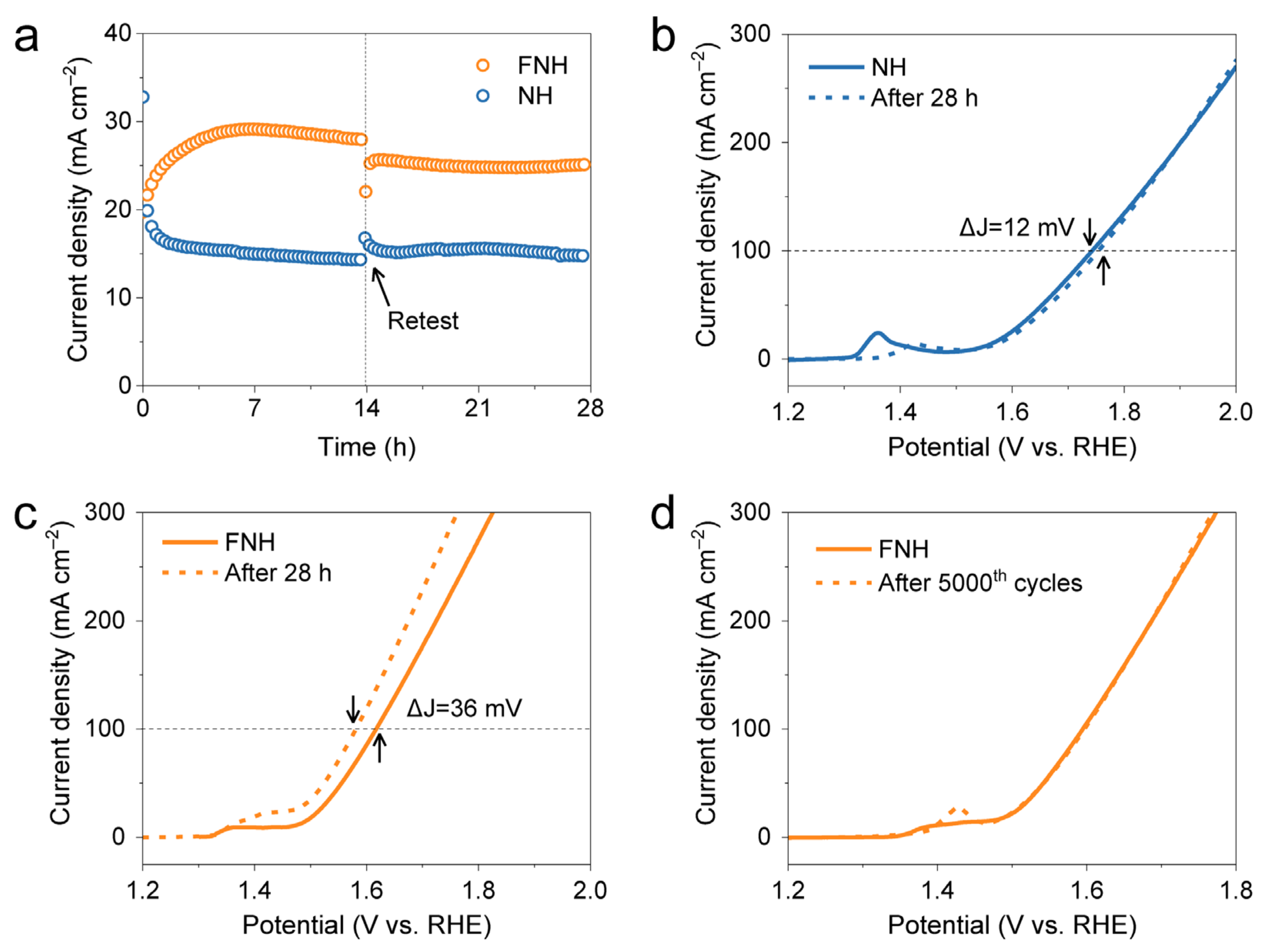
2. Results
3. Materials and Methods
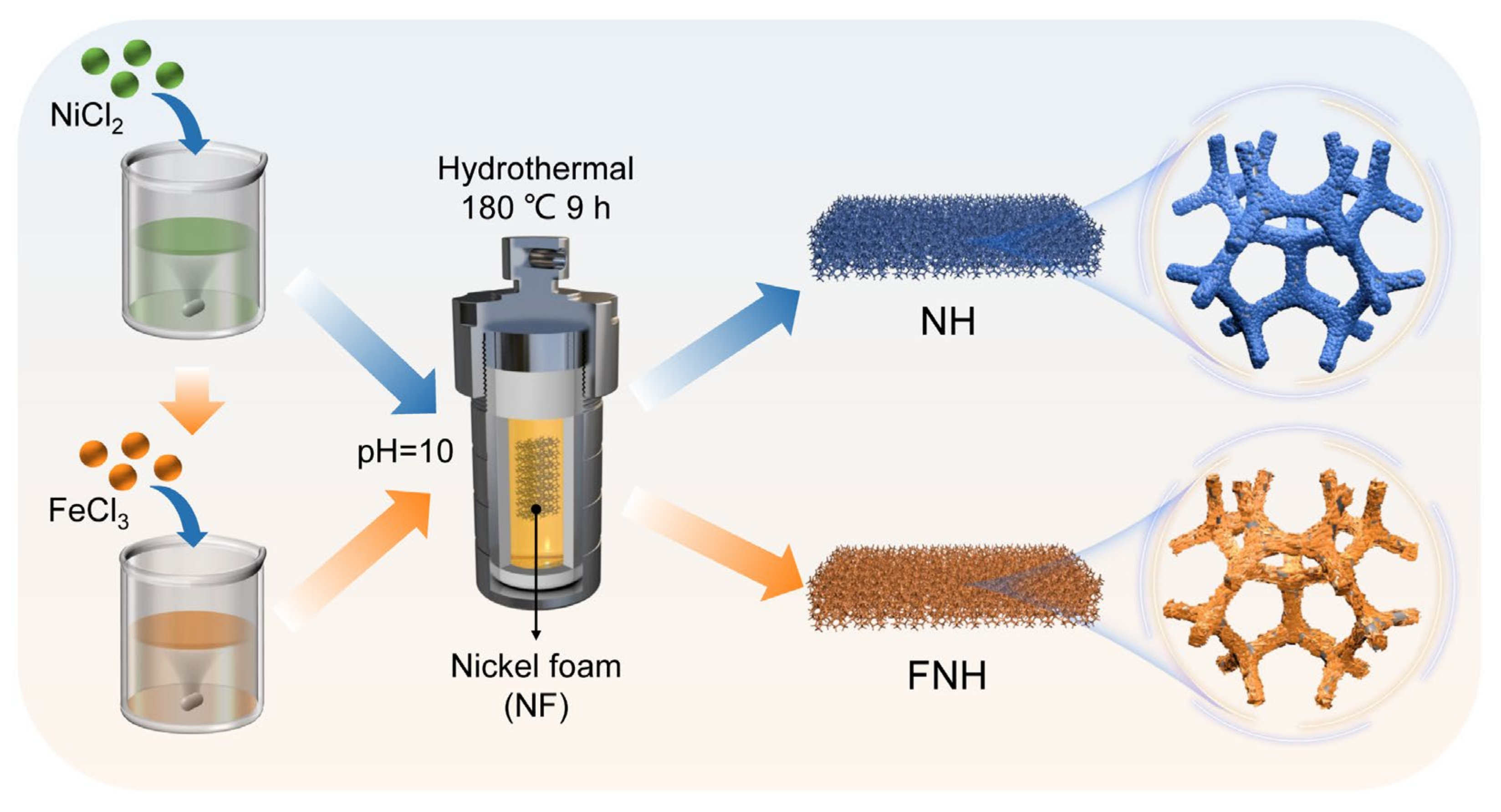
3.1. Synthesis of NH and FNH Catalysts
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, X.; Li, J.; Xing, Z.; Lu, B.; Liang, S.; Zhou, J. Biocompatible zinc battery with programmable electro-cross-linked electrolyte. Natl. Sci. Rev. 2023, 10, nwac281. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ruan, P.; Mao, C.; Chang, Y.; Wang, L.; Dai, L.; Zhou, P.; Lu, B.; Zhou, J.; He, Z. Metal-organic frameworks functionalized separators for robust aqueous zinc-ion batteries. Nano-Micro Lett. 2022, 14, 218. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Xie, X.; Li, Z.; Wang, P.; Lu, B.; Liang, S.; Tang, Y.; Zhou, J. A functionalized separator enables dendrite-free Zn anode via metal-polydopamine coordination chemistry. InfoMat. 2022, e12374. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B 2023, 325, 122388. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef]
- Kang, N.; Zhou, W.; Qi, Z.; Li, Y.; Wang, Z.; Li, Q.; Lv, K. Recent progress of natural mineral materials in environmental remediation. Catalysts 2022, 12, 996. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical water splitting: Bridging the gaps between fundamental research and industrial applications. Energy Environ. Sci. 2022, e12441. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liang, X.; Gu, L.; Zhang, Y.; Li, G.D.; Zou, X.; Chen, J.S. Corrosion engineering towards efficient oxygen evolution electrodes with stable catalytic activity for over 6000 hours. Nat. Commun. 2018, 9, 2609. [Google Scholar] [CrossRef] [Green Version]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Diaz-Morales, O.; Kolb, M.; Rao, R.R.; Frydendal, R.; Qiao, L.; Wang, X.R.; Halck, N.B.; Rossmeisl, J.; Hansen, H.A.; et al. Orientation-dependent oxygen evolution on RuO2 without lattice exchange. ACS Energy Lett. 2017, 2, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zheng, D.; Deng, T.; Chen, Q.; Zhu, C.; Pei, C.; Li, H.; Wu, F.; Shi, W.; Yang, S.W.; et al. Boosting electrocatalytic activity of 3D-block metal (hydro)oxides by ligand-induced conversion. Angew. Chem. Int. Ed. 2021, 60, 10614–10619. [Google Scholar] [CrossRef]
- Moysiadou, A.; Lee, S.; Hsu, C.S.; Chen, H.M.; Hu, X. Mechanism of oxygen evolution catalyzed by cobalt oxyhydroxide: Cobalt superoxide species as a key intermediate and dioxygen release as a rate-determining step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhang, M.; Zhou, Y.; Rao, D.; Zhong, C.; Zhang, J.; Han, X.; Hu, W.; Zhang, Y.; et al. Lattice-strain engineering of homogeneous NiS0.5Se0.5 core-shell nanostructure as a highly efficient and robust electrocatalyst for overall water splitting. Adv. Mater. 2020, 32, 2000231. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Xie, J.; Lv, M.; Luo, J.; Yang, F.; Yu, Y.; Lu, X. Iron-induced electron modulation of nickel hydroxide/carbon nanotubes composite to effectively boost the oxygen evolution activity. Chem. Eng. J. 2023, 452, 139369. [Google Scholar] [CrossRef]
- Bao, W.; Li, Y.; Zhang, J.; Ai, T.; Yang, C.; Feng, L. Interface engineering of the NiCo2O4@MoS2/TM heterostructure to realize the efficient alkaline oxygen evolution reaction. Int. J. Hydrogen. Energy 2023. [Google Scholar] [CrossRef]
- Yu, M.; Budiyanto, E.; Tuysuz, H. Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar]
- Ali, A.; Long, F.; Shen, P.K. Innovative strategies for overall water splitting using nanostructured transition metal electrocatalysts. Electrochem. Energy Rev. 2022, 5, 1. [Google Scholar] [CrossRef]
- Wang, C.; Humayun, M.; Debecker, D.P.; Wu, Y. Electrocatalytic water oxidation with layered double hydroxides confining single atoms. Coord. Chem. Rev. 2023, 478, 214973. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, M.Y.; Li, X.; Bao, W.W.; Jin, C.Q.; Feng, X.H.; Liu, G.; Yang, C.M.; Zhang, N.N. Chromium-modified ultrathin CoFe LDH as high-efficiency electrode for hydrogen evolution reaction. Nanomaterials 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Yang, C.; Ai, T.; Zhang, J.; Zhou, L.; Li, Y.; Wei, X.; Zou, X.; Wang, Y. Modulating interfacial charge distribution of NiSe nanoarrays with NiFe-LDH nanosheets for boosting oxygen evolution reaction. Fuel 2023, 332, 126227. [Google Scholar] [CrossRef]
- Dang Van, C.; Kim, S.; Kim, M.; Lee, M.H. Effect of rare-earth element doping on NiFe-layered double hydroxides for water oxidation at ultrahigh current densities. ACS Sustain. Chem. Eng. 2023, 11, 1333–1343. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, C.; Wang, H.-F.; Li, B.-Q.; Zhang, Q.; Li, C.; Yang, C.; Wei, F. Monolithic-structured ternary hydroxides as freestanding bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2016, 4, 7245–7250. [Google Scholar] [CrossRef]
- Duan, M.; Qiu, M.; Sun, S.; Guo, X.; Liu, Y.; Zheng, X.; Cao, F.; Kong, Q.; Zhang, J. Intercalating assembly of NiFe LDH nanosheets/CNTs composite as high-performance electrocatalyst for oxygen evolution reaction. Appl. Clay Sci. 2022, 216, 106360. [Google Scholar] [CrossRef]
- Zhai, Y.; Ren, X.; Sun, Y.; Li, D.; Wang, B.; Liu, S. Synergistic effect of multiple vacancies to induce lattice oxygen redox in NiFe-layered double hydroxide OER catalysts. Appl. Catal. B 2023, 323, 122091. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Shen, X.; Xie, M.; Zhu, G.; Ji, Z.; Zhou, H.; Xu, K.; Yue, X.; Yuan, A.; Zhu, J.; et al. Nickel@nitrogen-doped carbon@MoS2 nanosheets: An efficient electrocatalyst for hydrogen evolution reaction. Small 2019, 15, 1804545. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.F.; Xia, X.H.; Wu, J.B.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Nickel foam-supported porous Ni(OH)2/NiOOH composite film as advanced pseudocapacitor material. Electrochim. Acta 2011, 56, 2627–2632. [Google Scholar] [CrossRef]
- Hu, H.; Wageh, S.; Al-Ghamdi, A.A.; Yang, S.; Tian, Z.; Cheng, B.; Ho, W. NiFe-LDH nanosheet/carbon fiber nanocomposite with enhanced anionic dye adsorption performance. Appl. Surf. Sci. 2020, 511, 145570. [Google Scholar] [CrossRef]
- Liu, D.; Yang, Y.; Zhang, J.; Wang, L.; Ma, Z.; Ren, L.; Wang, J.; Xue, B.; Li, F. Improved OER catalytic performance of NiFe-LDH with hydrothermal carbonization microspheres. J. Alloys Compd. 2023, 941, 168994. [Google Scholar] [CrossRef]
- Yu, J.; Lu, K.; Wang, C.; Wang, Z.; Fan, C.; Bai, G.; Wang, G.; Yu, F. Modification of NiFe layered double hydroxide by lanthanum doping for boosting water splitting. Electrochim. Acta 2021, 390, 138824. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, L.; Liao, Y.; Cao, X.; Liu, X.; Yu, Y.; Wang, Z.; Lu, X.; Wang, J. Ultrathin-FeOOH-coated MnO2 sonosensitizers with boosted reactive oxygen species yield and remodeled tumor microenvironment for efficient cancer therapy. Adv. Sci. 2022, 9, 2200005. [Google Scholar] [CrossRef]
- Wang, K.; Du, H.; He, S.; Liu, L.; Yang, K.; Sun, J.; Liu, Y.; Du, Z.; Xie, L.; Ai, W.; et al. Kinetically controlled, scalable synthesis of γ-FeOOH nanosheet arrays on nickel foam toward efficient oxygen evolution: The key role of in-situ-generated γ-NiOOH. Adv. Mater. 2021, 33, 2005587. [Google Scholar] [CrossRef]
- Shang, W.; Yu, W.; Tan, P.; Chen, B.; Xu, H.; Ni, M. A high-performance Zn battery based on self-assembled nanostructured NiCo2O4 electrode. J. Power Sources 2019, 421, 6–13. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, X.; Zhou, L.; Lin, S.; Cao, X.; Jiang, J.; Lu, X. Tuning the interfacial electronic coupling of NiO via CeO2 and nitrogen co-decoration for highly efficient oxygen evolution reaction. Chem. Eng. J. 2022, 432, 134255. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, M.; Li, Y.; Wang, S. Tuning surface electronic configuration of NiFe LDHs nanosheets by introducing cation vacancies (Fe or Ni) as highly efficient electrocatalysts for oxygen evolution reaction. Small 2018, 14, 1800136. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Chu, J.; Niu, S.; Wang, J.; Du, Y.; Li, Z.; Han, X.; Xu, P. Understanding the phase-induced electrocatalytic oxygen evolution reaction activity on FeOOH nanostructures. ACS Catal. 2019, 9, 10705–10711. [Google Scholar] [CrossRef]
- Cao, L.-M.; Wang, J.-W.; Zhong, D.-C.; Lu, T.-B. Template-directed synthesis of sulphur doped NiCoFe layered double hydroxide porous nanosheets with enhanced electrocatalytic activity for the oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 3224–3230. [Google Scholar] [CrossRef]
- He, P.; Yu, X.Y.; Lou, X.W. Carbon-incorporated nickel-cobalt mixed metal phosphide nanoboxes with enhanced electrocatalytic activity for oxygen evolution. Angew. Chem. Int. Ed. 2017, 56, 3897–3900. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, Y.; Chen, G.; Shang, L.; Shi, R.; Kang, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Ni3FeN nanoparticles derived from ultrathin NiFe-layered double hydroxide nanosheets: An efficient overall water splitting electrocatalyst. Adv. Energy Mater. 2016, 6, 1502585. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Wang, H.; Wang, C.; Pinna, N.; Lu, X. Ni strongly coupled with Mo2C encapsulated in nitrogen-doped carbon nanofibers as robust bifunctional catalyst for overall water splitting. Adv. Energy Mater. 2019, 9, 1803185. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Han, C.; Ma, X.; Lu, Q.; Xing, Z.; Yang, X. Construction of amorphous interface in an interwoven NiS/NiS2 structure for enhanced overall water splitting. J. Mater. Chem. A 2018, 6, 8233–8237. [Google Scholar] [CrossRef]
- Wan, K.; Luo, J.; Zhou, C.; Zhang, T.; Arbiol, J.; Lu, X.; Mao, B.W.; Zhang, X.; Fransaer, J. Hierarchical porous Ni3S4 with enriched high-valence Ni sites as a robust electrocatalyst for efficient oxygen evolution reaction. Adv. Funct. Mater. 2019, 29, 1900315. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wang, Y.; Lu, X. Construction of NiFe-Layered Double Hydroxides Arrays as Robust Electrocatalyst for Oxygen Evolution Reaction. Catalysts 2023, 13, 586. https://doi.org/10.3390/catal13030586
Liu Q, Wang Y, Lu X. Construction of NiFe-Layered Double Hydroxides Arrays as Robust Electrocatalyst for Oxygen Evolution Reaction. Catalysts. 2023; 13(3):586. https://doi.org/10.3390/catal13030586
Chicago/Turabian StyleLiu, Qiyu, Yi Wang, and Xihong Lu. 2023. "Construction of NiFe-Layered Double Hydroxides Arrays as Robust Electrocatalyst for Oxygen Evolution Reaction" Catalysts 13, no. 3: 586. https://doi.org/10.3390/catal13030586




