Giving New Life to Waste Cigarette Butts: Transformation into Platinum Group Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Acid, Neutral and Alkaline Environment
Abstract
:1. Introduction
2. Results and Discussion
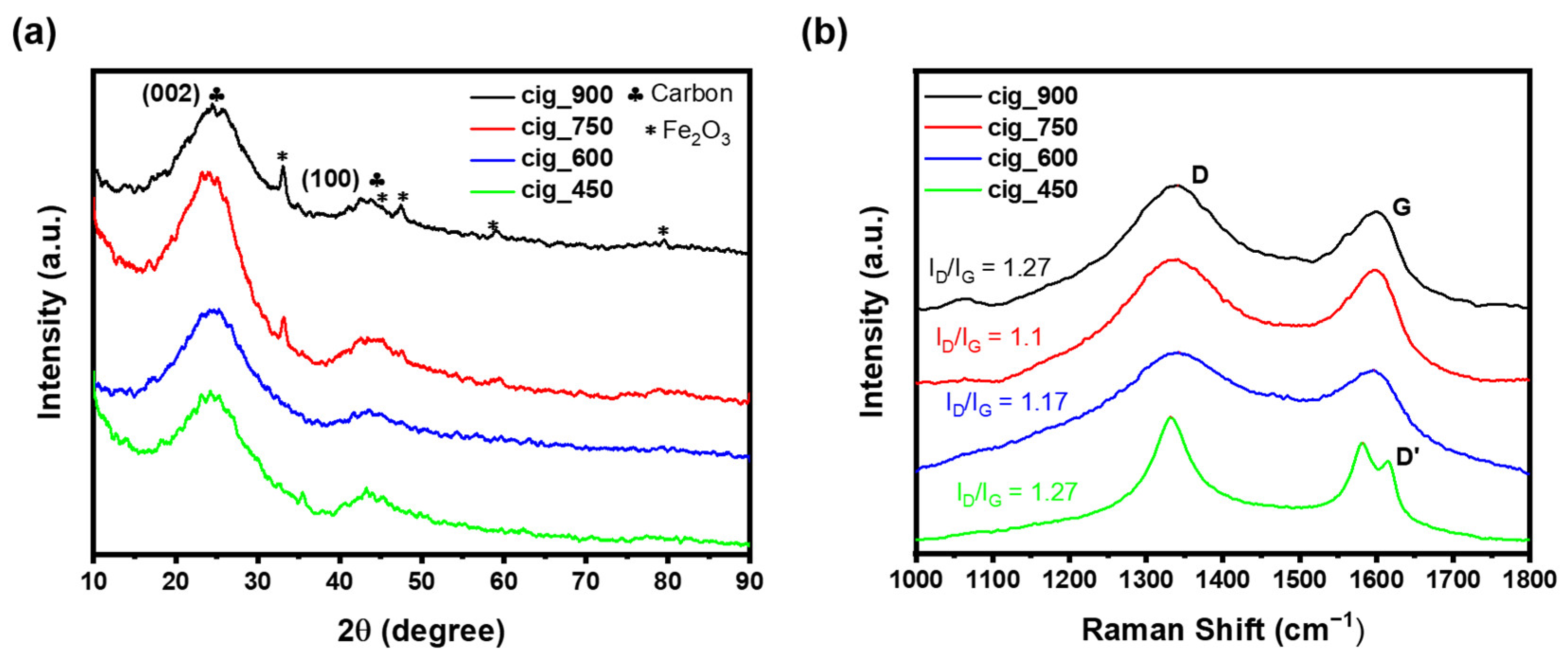
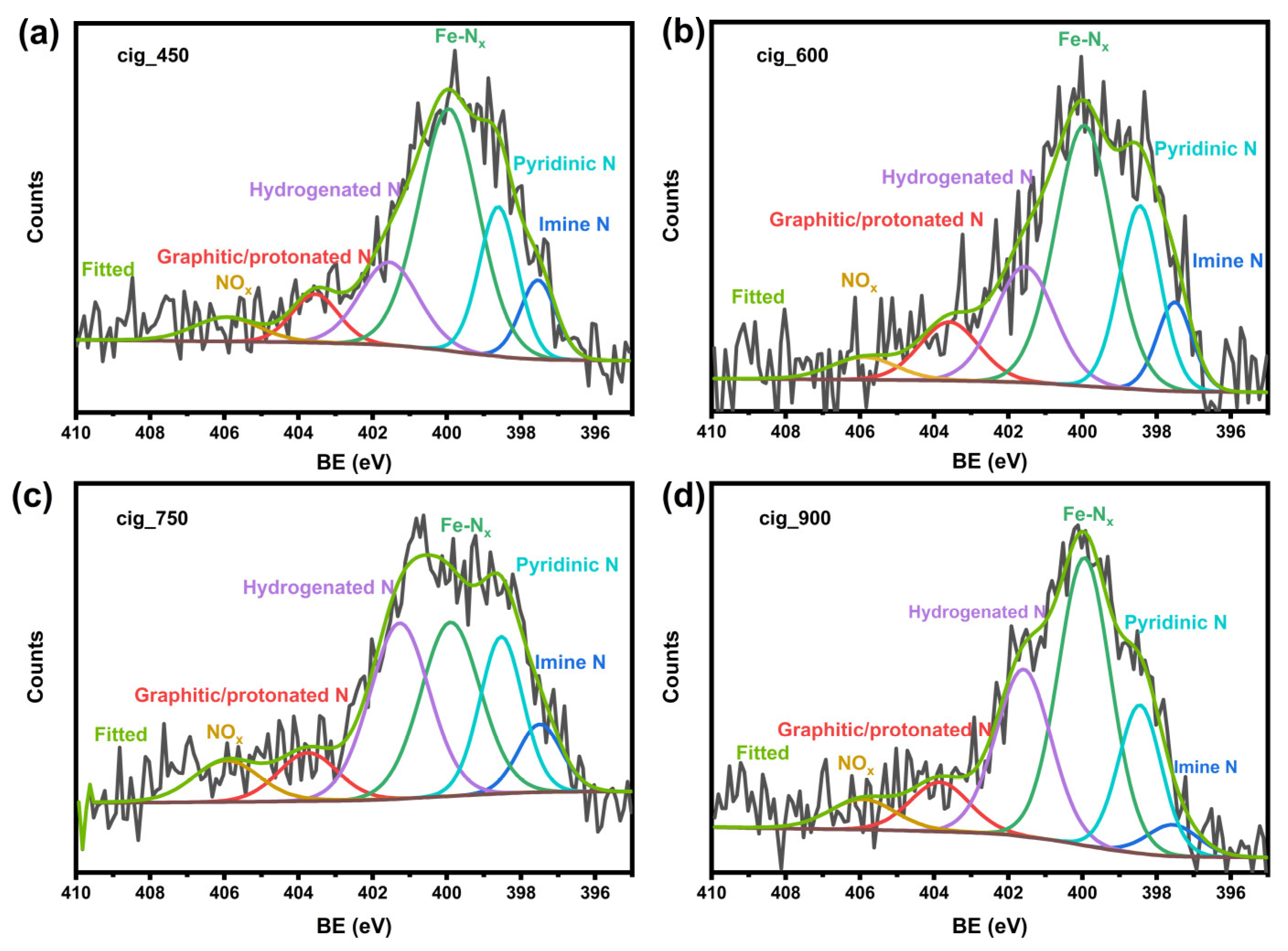
2.1. Structural and Morphological Results
2.2. Electrochemical Performance of the Cigarette-Butt-Derived Electrocatalysts
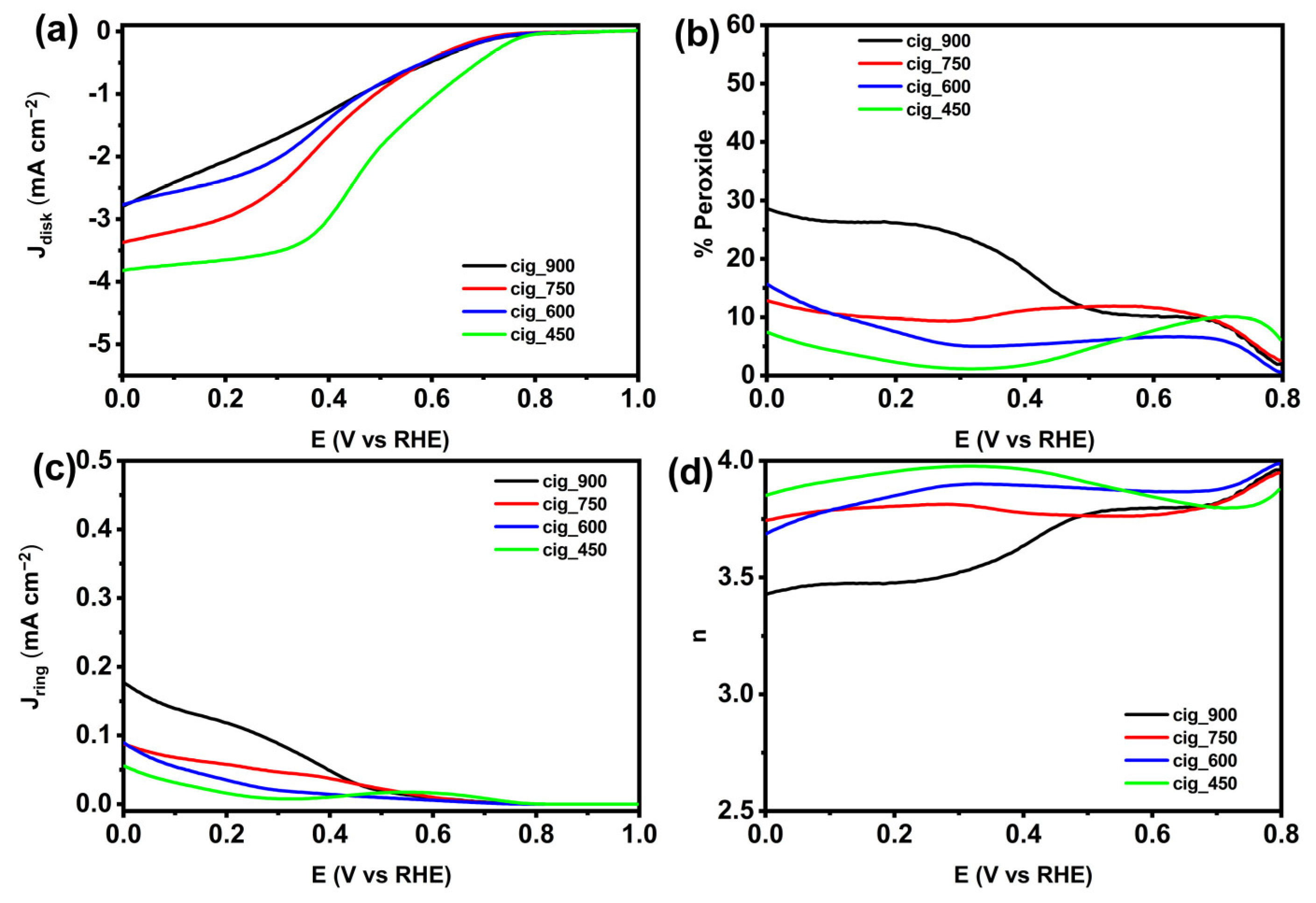
2.2.1. Electrocatalytic Activity in Acidic Media
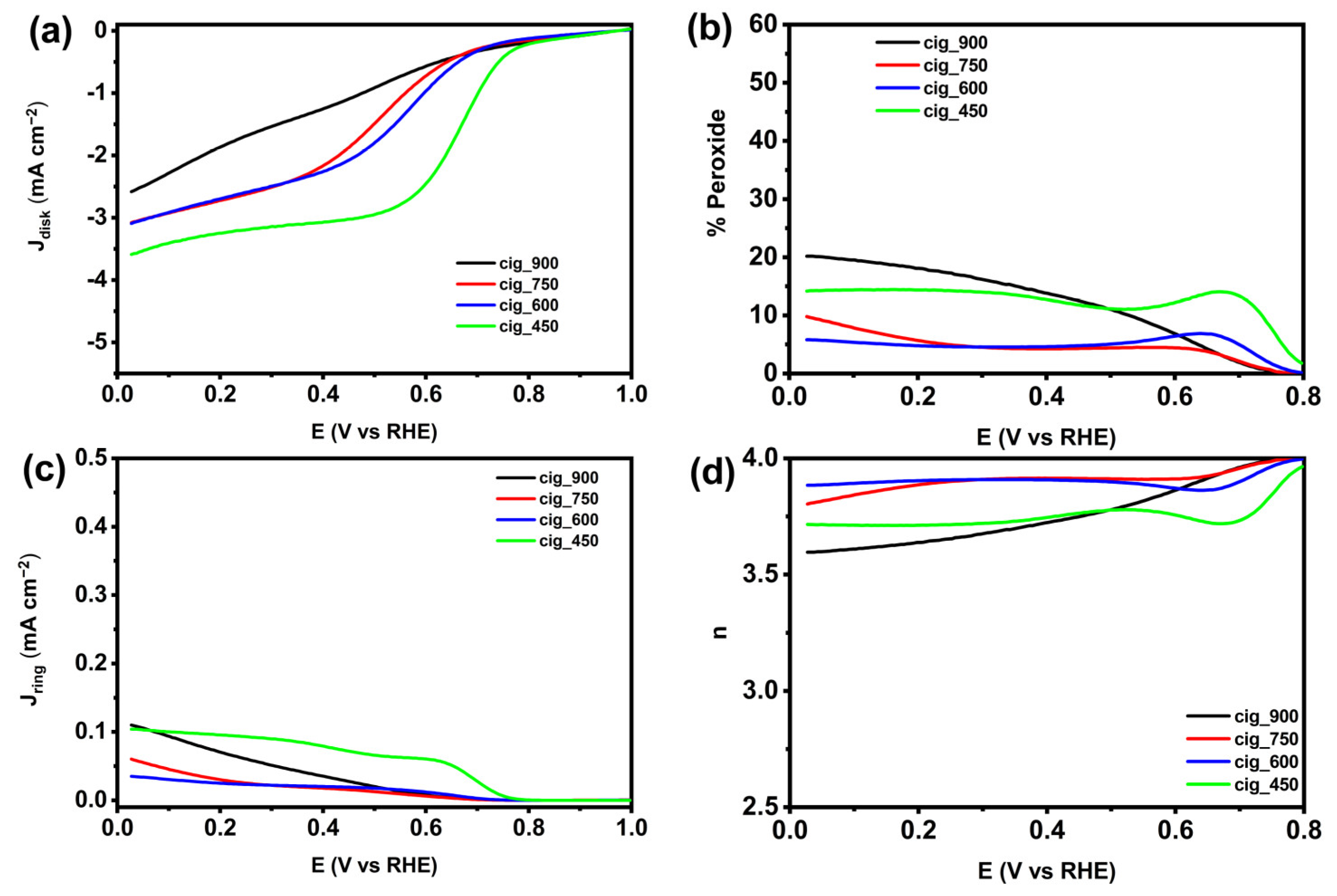
2.2.2. Electrocatalytic Activity in Neutral Media
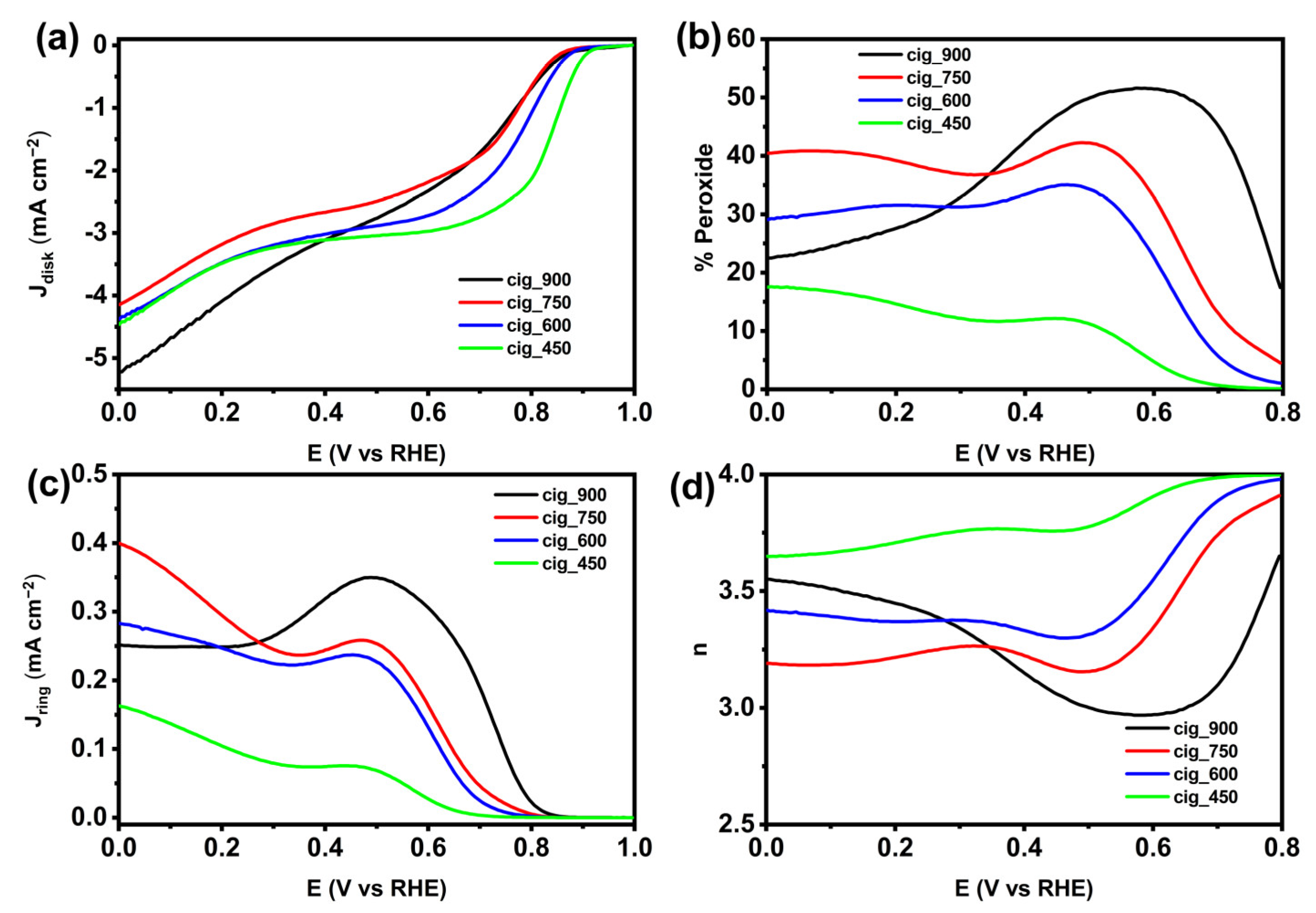
2.2.3. Electrocatalytic Activity in Alkaline Media
2.3. Novelty and Perspectives
3. Material and Methods
3.1. Synthesis Processes
3.2. Morphological and Surface Chemistry Investigations
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zupančič, M.; Možic, V.; Može, M.; Cimerman, F.; Golobič, I. Current Status and Review of Waste-to-Biogas Conversion for Selected European Countries and Worldwide. Sustainability 2022, 14, 1823. [Google Scholar] [CrossRef]
- Li, J.; Song, G.; Cai, M.; Bian, J.; Sani Mohammed, B. Green Environment and Circular Economy: A State-of-the-Art Analysis. Sustain. Energy Technol. Assess. 2022, 52, 102106. [Google Scholar] [CrossRef]
- Bhubalan, K.; Tamothran, A.M.; Kee, S.H.; Foong, S.Y.; Lam, S.S.; Ganeson, K.; Vigneswari, S.; Amirul, A.A.; Ramakrishna, S. Leveraging Blockchain Concepts as Watermarkers of Plastics for Sustainable Waste Management in Progressing Circular Economy. Environ. Res. 2022, 213, 113631. [Google Scholar] [CrossRef]
- Marinello, S.; Lolli, F.; Gamberini, R.; Rimini, B. A Second Life for Cigarette Butts? A Review of Recycling Solutions. J. Hazard. Mater. 2020, 384, 121245. [Google Scholar] [CrossRef]
- Araújo, M.C.B.; Costa, M.F. A Critical Review of the Issue of Cigarette Butt Pollution in Coastal Environments. Environ. Res. 2019, 172, 137–149. [Google Scholar] [PubMed]
- Dai, X.; Gakidou, E.; Lopez, A.D. Evolution of the Global Smoking Epidemic over the Past Half Century: Strengthening the Evidence Base for Policy Action. Tob. Control 2022, 31, 129–137. [Google Scholar] [CrossRef]
- George, M.; Khadtar, R. Review on Recycling of Microplastics in Cigarette Butts. IOP Conf. Ser. Earth Environ. Sci. 2022, 1084, 012027. [Google Scholar] [CrossRef]
- Shen, M.; Li, Y.; Song, B.; Zhou, C.; Gong, J.; Zeng, G. Smoked Cigarette Butts: Unignorable Source for Environmental Microplastic Fibers. Sci. Total Environ. 2021, 791, 148384. [Google Scholar] [PubMed]
- Dobaradaran, S.; Mutke, X.A.M.; Schmidt, T.C.; Swiderski, P.; De-la-Torre, G.E.; Jochmann, M.A. Aromatic Amines Contents of Cigarette Butts: Fresh and Aged Cigarette Butts vs Unsmoked Cigarette. Chemosphere 2022, 301, 134735. [Google Scholar] [CrossRef] [PubMed]
- Farzadkia, M.; Salehi Sedeh, M.; Ghasemi, A.; Alinejad, N.; Samadi Kazemi, M.; Jafarzadeh, N.; Torkashvand, J. Estimation of the Heavy Metals Released from Cigarette Butts to Beaches and Urban Environments. J. Hazard. Mater. 2022, 425, 127969. [Google Scholar] [CrossRef]
- Hazbehiean, M.; Mokhtarian, N.; Hallajisani, A. Converting the Cigarette Butts into Valuable Products Using the Pyrolysis Process. Glob. J. Environ. Sci. Manag. 2022, 8, 133–150. [Google Scholar] [CrossRef]
- Xiong, S.; Peng, Y.; Chen, K.; Lu, S.; Jiang, W.; Li, X.; Wang, F.; Cen, K. Phase Distribution, Migration and Relationship of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans and Heavy Metals in a Large-Scale Hazardous Waste Incinerator. J. Clean. Prod. 2022, 341, 130764. [Google Scholar] [CrossRef]
- van der Hulst, M.K.; Ottenbros, A.B.; van der Drift, B.; Ferjan, Š.; van Harmelen, T.; Schwarz, A.E.; Worrell, E.; van Zelm, R.; Huijbregts, M.A.J.; Hauck, M. Greenhouse Gas Benefits from Direct Chemical Recycling of Mixed Plastic Waste. Resour. Conserv. Recycl. 2022, 186, 106582. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M.; Rosaldo, P. Energy. Available online: https://ourworldindata.org/energy (accessed on 2 January 2023).
- Fathy, A.; Elaziz, M.A.; Sayed, E.T.; Olabi, A.G.; Rezk, H. Optimal Parameter Identification of Triple-Junction Photovoltaic Panel Based on Enhanced Moth Search Algorithm. Energy 2019, 188, 116025. [Google Scholar] [CrossRef]
- Konstantinidis, E.I.; Botsaris, P.N. Wind Turbines: Current Status, Obstacles, Trends and Technologies. IOP Conf. Ser. Mater. Sci. Eng. 2016, 161, 012079. [Google Scholar] [CrossRef]
- Wilberforce, T.; El Hassan, Z.; Durrant, A.; Thompson, J.; Soudan, B.; Olabi, A.G. Overview of Ocean Power Technology. Energy 2019, 175, 165–181. [Google Scholar] [CrossRef] [Green Version]
- Olabi, A.G.; Mahmoud, M.; Soudan, B.; Wilberforce, T.; Ramadan, M. Geothermal Based Hybrid Energy Systems, toward Eco-Friendly Energy Approaches. Renew. Energy 2020, 147, 2003–2012. [Google Scholar] [CrossRef]
- Sutikno, T.; Arsadiando, W.; Wangsupphaphol, A.; Yudhana, A.; Facta, M. A Review of Recent Advances on Hybrid Energy Storage System for Solar Photovoltaics Power Generation. IEEE Access 2022, 10, 42346–42364. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A Review on Hydrogen Production and Utilization: Challenges and Opportunities. Int. J. Hydrog. Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A. Fuel Cell Application in the Automotive Industry and Future Perspective. Energy 2021, 214, 118955. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, D.; Li, Y. A Fundamental Comprehension and Recent Progress in Advanced Pt-based ORR Nanocatalysts. SmartMat 2021, 2, 56–75. [Google Scholar] [CrossRef]
- He, Y.; Wu, G. PGM-Free Oxygen-Reduction Catalyst Development for Proton-Exchange Membrane Fuel Cells: Challenges, Solutions, and Promises. Acc. Mater. Res. 2022, 3, 224–236. [Google Scholar] [CrossRef]
- Tosoni, S.; di Liberto, G.; Matanovic, I.; Pacchioni, G. Modelling Single Atom Catalysts for Water Splitting and Fuel Cells: A Tutorial Review. J. Power Sources 2023, 556, 232492. [Google Scholar] [CrossRef]
- Arbizzani, C.; Righi, S.; Soavi, F.; Mastragostino, M. Graphene and Carbon Nanotube Structures Supported on Mesoporous Xerogel Carbon as Catalysts for Oxygen Reduction Reaction in Proton-Exchange-Membrane Fuel Cells. Int. J. Hydrog. Energy 2011, 36, 5038–5046. [Google Scholar] [CrossRef]
- Xu, H.; Wang, D.; Yang, P.; Liu, A.; Li, R.; Li, Y.; Xiao, L.; Ren, X.; Zhang, J.; An, M. Atomically Dispersed M-N-C Catalysts for the Oxygen Reduction Reaction. J. Mater. Chem. A Mater. 2020, 8, 23187–23201. [Google Scholar] [CrossRef]
- Wang, H.Y.; Weng, C.C.; Yuan, Z.Y. Insights into Efficient Transition Metal-Nitrogen/Carbon Oxygen Reduction Electrocatalysts. J. Energy Chem. 2021, 56, 470–485. [Google Scholar] [CrossRef]
- Cousins, I.T.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Patton, S.; Scheringer, M.; Trier, X.; Vierke, L.; et al. The Concept of Essential Use for Determining When Uses of PFASs Can Be Phased Out. Environ. Sci. Process. Impacts 2019, 21, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Bangma, J.; Guillette, T.C.; Strynar, M.; Lindstrom, A.; McCord, J.; Hill, D.; Lau, C.; Chernoff, N.; Lang, J.R. A Rapid Assessment Bioaccumulation Screening (RABS) Study Design for Emerging per-and Polyfluoroalkyl Substances in Mice Exposed to Industrially Impacted Surface Water. Chemosphere 2022, 308, 136159. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Lavacchi, A.; Mustarelli, P.; Di Noto, V.; Elbaz, L.; Dekel, D.R.; Jaouen, F. What Is Next in Anion-Exchange Membrane Water Electrolyzers? Bottlenecks, Benefits, and Future. ChemSusChem 2022, 15, e202200027. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A Comprehensive Review on Microbial Fuel Cell Technologies: Processes, Utilization, and Advanced Developments in Electrodes and Membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent Advances in the Use of Different Substrates in Microbial Fuel Cells toward Wastewater Treatment and Simultaneous Energy Recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Artyushkova, K.; Atanassov, P. Platinum Group Metal-Free Oxygen Reduction Electrocatalysts Used in Neutral Electrolytes for Bioelectrochemical Reactor Applications. Curr. Opin. Electrochem. 2020, 23, 106–113. [Google Scholar] [CrossRef]
- Katz, E.; Bollella, P. Fuel Cells and Biofuel Cells: From Past to Perspectives. Isr. J. Chem. 2021, 61, 68–84. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I.; Umar, K. Utilizing Biomass-Based Graphene Oxide–Polyaniline–Ag Electrodes in Microbial Fuel Cells to Boost Energy Generation and Heavy Metal Removal. Polymers 2022, 14, 845. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Shahdost-Fard, F.; Salehzadeh, H.; Ganjali, M.R.; Iman, M.; Rahimi-Nasrabadi, M.; Ahmadi, F. A Nanocomposite Prepared from Reduced Graphene Oxide, Gold Nanoparticles and Poly(2-Amino-5-Mercapto-1,3,4-Thiadiazole) for Use in an Electrochemical Sensor for Doxorubicin. Microchim. Acta 2019, 186, 641. [Google Scholar] [CrossRef]
- Santoro, C.; Bollella, P.; Erable, B.; Atanassov, P.; Pant, D. Oxygen Reduction Reaction Electrocatalysis in Neutral Media for Bioelectrochemical Systems. Nat. Catal. 2022, 5, 473–484. [Google Scholar] [CrossRef]
- Cosenza, A.; Delafontaine, L.; Ly, A.; Wang, H.; Murphy, E.; Liu, Y.; Specchia, S.; Atanassov, P. Novel Acid-Free Process Intensification for the Synthesis of Non-Precious Metal-Nitrogen-Carbon Electrocatalysts for Oxygen Reduction Reaction. J. Power Sources 2023, 556, 232382. [Google Scholar] [CrossRef]
- Berretti, E.; Longhi, M.; Atanassov, P.; Sebastián, D.; lo Vecchio, C.; Baglio, V.; Serov, A.; Marchionni, A.; Vizza, F.; Santoro, C.; et al. Platinum Group Metal-Free Fe-Based (Fe–N–C) Oxygen Reduction Electrocatalysts for Direct Alcohol Fuel Cells. Curr. Opin. Electrochem. 2021, 29, 100756. [Google Scholar] [CrossRef]
- Giordano, E.; Berretti, E.; Capozzoli, L.; Lavacchi, A.; Muhyuddin, M.; Santoro, C.; Gatto, I.; Zaffora, A.; Santamaria, M. Boosting DMFC Power Output by Adding Sulfuric Acid as a Supporting Electrolyte: Effect on Cell Performance Equipped with Platinum and Platinum Group Metal-Free Cathodes. J. Power Sources 2023, 563, 232806. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, X.; Yang, H.; Chen, S. Optimization Strategies of Preparation of Biomass-Derived Carbon Electrocatalyst for Boosting Oxygen Reduction Reaction: A Minireview. Catalysts 2020, 10, 1472. [Google Scholar] [CrossRef]
- Du, L.; Zhang, G.; Liu, X.; Hassanpour, A.; Dubois, M.; Tavares, A.C.; Sun, S. Biomass-Derived Nonprecious Metal Catalysts for Oxygen Reduction Reaction: The Demand-Oriented Engineering of Active Sites and Structures. Carbon Energy 2020, 2, 561–581. [Google Scholar] [CrossRef]
- Li, S.; Ho, S.H.; Hua, T.; Zhou, Q.; Li, F.; Tang, J. Sustainable Biochar as an Electrocatalysts for the Oxygen Reduction Reaction in Microbial Fuel Cells. Green Energy Environ. 2021, 6, 644–659. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1703691. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Yang, H.; Ku, W.; Yang, S.; Liu, Z.; Lu, G. Carbon-Based Electrocatalysts Derived From Biomass for Oxygen Reduction Reaction: A Minireview. Front. Chem. 2020, 8, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi-Maleh, H.; Karaman, C.; Karaman, O.; Karimi, F.; Vasseghian, Y.; Fu, L.; Baghayeri, M.; Rouhi, J.; Senthil Kumar, P.; Show, P.L.; et al. Nanochemistry Approach for the Fabrication of Fe and N Co-Decorated Biomass-Derived Activated Carbon Frameworks: A Promising Oxygen Reduction Reaction Electrocatalyst in Neutral Media. J. Nanostruct. Chem. 2022, 12, 429–439. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Friedman, A.; Poli, F.; Petri, E.; Honig, H.; Basile, F.; Fasolini, A.; Lorenzi, R.; Berretti, E.; Bellini, M.; et al. Lignin-Derived Bimetallic Platinum Group Metal-Free Oxygen Reduction Reaction Electrocatalysts for Acid and Alkaline Fuel Cells. J. Power Sources 2023, 556, 232416. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Sylla, N.F.; Bubu, A.; Ndiaye, N.M.; Santoro, C.; Brilloni, A.; Poli, F.; Manyala, N.; Soavi, F. Valorization of Biodigestor Plant Waste in Electrodes for Supercapacitors and Microbial Fuel Cells. Electrochim. Acta 2021, 391, 138960. [Google Scholar] [CrossRef]
- Müller-Hülstede, J.; Schonvogel, D.; Schmies, H.; Wagner, P.; Dyck, A.; Wark, M. Incorporation of Activated Biomasses in Fe-N-C Catalysts for Oxygen Reduction Reaction with Enhanced Stability in Acidic Media. ACS Appl. Energy Mater. 2021, 4, 6912–6922. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, H.; Yu, H.; Peng, F. From Chicken Feather to Nitrogen and Sulfur Co-Doped Large Surface Bio-Carbon Flocs: An Efficient Electrocatalyst for Oxygen Reduction Reaction. Electrochim. Acta 2016, 213, 273–282. [Google Scholar] [CrossRef]
- Zago, S.; Bartoli, M.; Muhyuddin, M.; Vanacore, G.M.; Jagdale, P.; Tagliaferro, A.; Santoro, C.; Specchia, S. Engineered Biochar Derived from Pyrolyzed Waste Tea as a Carbon Support for Fe-N-C Electrocatalysts for the Oxygen Reduction Reaction. Electrochim. Acta 2022, 412, 140128. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Zhang, J.; Kou, Z.; Liu, X.; Asare, O.K.; Zhou, H.; Cheng, K.; Zhang, H.; Mai, L.; Pan, M.; et al. Self-Organized 3D Porous Graphene Dual-Doped with Biomass-Sponsored Nitrogen and Sulfur for Oxygen Reduction and Evolution. ACS Appl. Mater. Interfaces 2016, 8, 29408–29418. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Zocche, N.; Lorenzi, R.; Ferrara, C.; Poli, F.; Soavi, F.; Santoro, C. Valorization of the Inedible Pistachio Shells into Nanoscale Transition Metal and Nitrogen Codoped Carbon-Based Electrocatalysts for Hydrogen Evolution Reaction and Oxygen Reduction Reaction. Mater. Renew. Sustain. Energy 2022, 11, 131–141. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Mustarelli, P.; Santoro, C. Recent Advances in Waste Plastic Transformation into Valuable Platinum-Group Metal-Free Electrocatalysts for Oxygen Reduction Reaction. ChemSusChem 2021, 14, 3785–3800. [Google Scholar] [CrossRef]
- Veksha, A.; Yin, K.; Moo, J.G.S.; da Oh, W.; Ahamed, A.; Chen, W.Q.; Weerachanchai, P.; Giannis, A.; Lisak, G. Processing of Flexible Plastic Packaging Waste into Pyrolysis Oil and Multi-Walled Carbon Nanotubes for Electrocatalytic Oxygen Reduction. J. Hazard. Mater. 2020, 387, 121256. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Quibén, J.; Bailón-García, E.; Moral-Rodríguez, A.I.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Recycling and Valorization of LDPE: Direct Transformation into Highly Ordered Doped-Carbon Materials and Their Application as Electro-Catalysts for the Oxygen Reduction Reaction. Catal. Sci. Technol. 2022, 12, 1187–1201. [Google Scholar] [CrossRef]
- Daniel, G.; Kosmala, T.; Dalconi, M.C.; Nodari, L.; Badocco, D.; Pastore, P.; Lorenzetti, A.; Granozzi, G.; Durante, C. Upcycling of Polyurethane into Iron-Nitrogen-Carbon Electrocatalysts Active for Oxygen Reduction Reaction. Electrochim. Acta 2020, 362, 137200. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Filippi, J.; Zoia, L.; Bonizzoni, S.; Lorenzi, R.; Berretti, E.; Capozzoli, L.; Bellini, M.; Ferrara, C.; Lavacchi, A.; et al. Waste Face Surgical Mask Transformation into Crude Oil and Nanostructured Electrocatalysts for Fuel Cells and Electrolyzers. ChemSusChem 2022, 15, e202102351. [Google Scholar] [CrossRef]
- Mahto, A.; Halakarni, M.A.; Maraddi, A.; D’Souza, G.; Samage, A.A.; Thummar, U.G.; Mondal, D.; Nataraj, S.K. Upcycling Cellulose Acetate from Discarded Cigarette Butts: Conversion of Contaminated Microfibers into Loose-Nanofiltration Membranes for Selective Separation. Desalination 2022, 535, 115807. [Google Scholar] [CrossRef]
- Blankenship, T.S.; Mokaya, R. Cigarette Butt-Derived Carbons Have Ultra-High Surface Area and Unprecedented Hydrogen Storage Capacity. Energy Environ. Sci. 2017, 10, 2552–2562. [Google Scholar] [CrossRef]
- Masoudi Soltani, S.; Yazdi, S.K.; Hosseini, S. Effects of Pyrolysis Conditions on the Porous Structure Construction of Mesoporous Charred Carbon from Used Cigarette Filters. Appl. Nanosci. 2014, 4, 551–569. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wei, S.; Bai, P.; Liu, W.; Yang, C.; Xu, L. Temperature versus Type: Which Is the Determining Factor in Biomass-Based Electrocatalyst Performance? Appl. Catal. B 2023, 325, 122391. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef] [Green Version]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.A. FTIR, TGA and DC Electrical Conductivity Studies of Phthalocyanine and Its Complexes. J. Mol. Struct. 2005, 753, 119–126. [Google Scholar] [CrossRef]
- de Fenzo, A.; Giordano, M.; Sansone, L. A Clean Process for Obtaining High-Quality Cellulose Acetate from Cigarette Butts. Materials 2020, 13, 4710. [Google Scholar] [CrossRef]
- Shao, N.; Xue, F.; Hou, L.; Li, D.; Gao, Y.; Zhu, X. Effects of Ternary Potassium Containing Intumescent Flame Retardant Coating on the Combustion and Thermal Degradation Properties of Reconstituted Tobacco Sheet. Thermochim. Acta 2019, 678, 178310. [Google Scholar] [CrossRef]
- Gaworski, C.L.; Lemus-Olalde, R.; Carmines, E.L. Toxicological Evaluation of Potassium Sorbate Added to Cigarette Tobacco. Food Chem. Toxicol. 2008, 46, 339–351. [Google Scholar] [CrossRef]
- Yang, H.; Shang, L.; Zhang, Q.; Shi, R.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. A Universal Ligand Mediated Method for Large Scale Synthesis of Transition Metal Single Atom Catalysts. Nat. Commun. 2019, 10, 4585. [Google Scholar] [CrossRef] [Green Version]
- Soflaee, F.; Farahmandjou, M.; Firoozabadi, T.P. Polymer-Mediated Synthesis of Iron Oxide (Fe2O3) Nanorod. Chin. J. Phys. 2015, 53, 178–186. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying Disorder in Graphite-Based Systems by Raman Spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Kagkoura, A.; Tagmatarchis, N. Carbon Nanohorn-Based Electrocatalysts for Energy Conversion. Nanomaterials 2020, 10, 1407. [Google Scholar] [CrossRef]
- Xu, X.; Shi, C.; Li, Q.; Chen, R.; Chen, T. Fe-N-Doped Carbon Foam Nanosheets with Embedded Fe2O3 Nanoparticles for Highly Efficient Oxygen Reduction in Both Alkaline and Acidic Media. RSC Adv. 2017, 7, 14382–14388. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B Condens Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Hasdeo, E.H.; Nugraha, A.R.T.; Dresselhaus, M.S.; Saito, R. Breit-Wigner-Fano Line Shapes in Raman Spectra of Graphene. Phys. Rev. B Condens. Matter Mater. Phys. 2014, 90, 245140. [Google Scholar] [CrossRef] [Green Version]
- Xing, T.; Li, L.H.; Hou, L.; Hu, X.; Zhou, S.; Peter, R.; Petravic, M.; Chen, Y. Disorder in Ball-Milled Graphite Revealed by Raman Spectroscopy. Carbon N. Y. 2013, 57, 515–519. [Google Scholar] [CrossRef]
- Artyushkova, K. Misconceptions in Interpretation of Nitrogen Chemistry from X-ray Photoelectron Spectra. J. Vac. Sci. Technol. A 2020, 38, 031002. [Google Scholar] [CrossRef] [Green Version]
- Asset, T.; Atanassov, P. Iron-Nitrogen-Carbon Catalysts for Proton Exchange Membrane Fuel Cells. Joule 2020, 4, 33–44. [Google Scholar] [CrossRef]
- Dzara, M.J.; Artyushkova, K.; Shulda, S.; Strand, M.B.; Ngo, C.; Crumlin, E.J.; Gennett, T.; Pylypenko, S. Characterization of Complex Interactions at the Gas-Solid Interface with in Situ Spectroscopy: The Case of Nitrogen-Functionalized Carbon. J. Phys. Chem. C 2019, 123, 9074–9086. [Google Scholar] [CrossRef]
- Matanovic, I.; Artyushkova, K.; Atanassov, P. Understanding PGM-Free Catalysts by Linking Density Functional Theory Calculations and Structural Analysis: Perspectives and Challenges. Curr. Opin. Electrochem. 2018, 9, 137–144. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Role of Nitrogen Moieties in N-Doped 3D-Graphene Nanosheets for Oxygen Electroreduction in Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 11623–11632. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the Active Center Structure of Nitrogen-Doped Graphene-Based Catalysts for Oxygen Reduction Reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Rojas-Carbonell, S.; Artyushkova, K.; Serov, A.; Santoro, C.; Matanovic, I.; Atanassov, P. Effect of PH on the Activity of Platinum Group Metal-Free Catalysts in Oxygen Reduction Reaction. ACS Catal. 2018, 8, 3041–3053. [Google Scholar] [CrossRef]
- Malko, D.; Kucernak, A.; Lopes, T. In Situ Electrochemical Quantification of Active Sites in Fe-N/C Non-Precious Metal Catalysts. Nat. Commun. 2016, 7, 13285. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Huang, Y.; Xu, M.; Asset, T.; Yan, X.; Artyushkova, K.; Kodali, M.; Murphy, E.; Ly, A.; Pan, X.; et al. Catalysts by Pyrolysis: Direct Observation of Transformations during Re-Pyrolysis of Transition Metal-Nitrogen-Carbon Materials Leading to State-of-the-Art Platinum Group Metal-Free Electrocatalyst. Mater. Today 2022, 53, 58–70. [Google Scholar] [CrossRef]
- Santoro, C.; Rojas-Carbonell, S.; Awais, R.; Gokhale, R.; Kodali, M.; Serov, A.; Artyushkova, K.; Atanassov, P. Influence of Platinum Group Metal-Free Catalyst Synthesis on Microbial Fuel Cell Performance. J. Power Sources 2018, 375, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G.; Kosmala, T.; Brombin, F.; Mazzucato, M.; Facchin, A.; Dalconi, M.C.; Badocco, D.; Pastore, P.; Granozzi, G.; Durante, C. Highly Graphitized Fe–N–C Electrocatalysts Prepared from Chitosan Hydrogel Frameworks. Catalysts 2021, 11, 390. [Google Scholar] [CrossRef]
- Mazzucato, M.; Daniel, G.; Mehmood, A.; Kosmala, T.; Granozzi, G.; Kucernak, A.; Durante, C. Effects of the Induced Micro- and Meso-Porosity on the Single Site Density and Turn over Frequency of Fe-N-C Carbon Electrodes for the Oxygen Reduction Reaction. Appl. Catal. B 2021, 291, 120068. [Google Scholar] [CrossRef]
- Daniel, G.; Foltran, E.; Brandiele, R.; Nodari, L.; Pilot, R.; Menna, E.; Rizzi, G.A.; Ahmed Isse, A.; Durante, C.; Gennaro, A. Platinum-Free Electrocatalysts for Oxygen Reduction Reaction: Fe-Nx Modified Mesoporous Carbon Prepared from Biosources. J. Power Sources 2018, 402, 434–446. [Google Scholar] [CrossRef]
- Javed, R.; Khan, M.A.; Ye, D.; Zhao, Y.; Shah, L.A.; Zhang, J.; Zhao, H. Boosting Oxygen Reduction Catalysis Through Electronic Reconfiguration of Fe–N–C Induced by P Doping. Electrocatalysis 2021, 12, 747–758. [Google Scholar] [CrossRef]
- Lv, M.; Guo, H.; Shen, H.; Wang, J.; Wang, J.; Shimakawa, Y.; Yang, M. Fe3C Cluster-Promoted Single-Atom Fe, N Doped Carbon for Oxygen-Reduction Reaction. Phys. Chem. Chem. Phys. 2020, 22, 7218–7223. [Google Scholar] [CrossRef]
- Sgarbi, R.; Kumar, K.; Jaouen, F.; Zitolo, A.; Ticianelli, E.A.; Maillard, F. Oxygen Reduction Reaction Mechanism and Kinetics on M-NxCy and M@N-C Active Sites Present in Model M-N-C Catalysts under Alkaline and Acidic Conditions. J. Solid State Electrochem. 2021, 25, 45–56. [Google Scholar] [CrossRef]
- Bhuvanendran, N.; Ravichandran, S.; Xu, Q.; Maiyalagan, T.; Su, H. A Quick Guide to the Assessment of Key Electrochemical Performance Indicators for the Oxygen Reduction Reaction: A Comprehensive Review. Int. J. Hydrog. Energy 2022, 47, 7113–7138. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ben Belgacem, I.; Emori, W.; Uzoma, P.C. Nafion Degradation Mechanisms in Proton Exchange Membrane Fuel Cell (PEMFC) System: A Review. Int. J. Hydrog. Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-Free Catalysts for Proton Exchange Membrane Fuel Cells: Stability Challenges and Material Solutions. Adv. Mater. 2021, 33, 1908232. [Google Scholar] [CrossRef] [PubMed]
- Primbs, M.; Sun, Y.; Roy, A.; Malko, D.; Mehmood, A.; Sougrati, M.T.; Blanchard, P.Y.; Granozzi, G.; Kosmala, T.; Daniel, G.; et al. Establishing Reactivity Descriptors for Platinum Group Metal (PGM)-Free Fe-N-C Catalysts for PEM Fuel Cells. Energy Environ. Sci. 2020, 13, 2480–2500. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Testa, D.; Lorenzi, R.; Vanacore, G.M.; Poli, F.; Soavi, F.; Specchia, S.; Giurlani, W.; Innocenti, M.; Rosi, L.; et al. Iron-Based Electrocatalysts Derived from Scrap Tires for Oxygen Reduction Reaction: Evolution of Synthesis-Structure-Performance Relationship in Acidic, Neutral and Alkaline Media. Electrochim. Acta 2022, 433, 141254. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; Almomani, F.; Al-Ghouti, M.; Hassan, M.K. From Waste to Watts: Updates on Key Applications of Microbial Fuel Cells in Wastewater Treatment and Energy Production. Sustainability 2022, 14, 955. [Google Scholar] [CrossRef]
- Dhilllon, S.K.; Kundu, P.P.; Jain, R. Catalytic Advancements in Carbonaceous Materials for Bio-Energy Generation in Microbial Fuel Cells: A Review. Environ. Sci. Pollut. Res. 2022, 30, 24815–24841. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Villarrubia, C.W.N.; Stariha, S.; Babanova, S.; Artyushkova, K.; Schuler, A.J.; Atanassov, P. High Catalytic Activity and Pollutants Resistivity Using Fe-AAPyr Cathode Catalyst for Microbial Fuel Cell Application. Sci. Rep. 2015, 5, 16596. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, P.; Panwar, P. Perspective and Future Scope of Nanotechnology in Modification of Microbial Fuel Cell. Mater. Today Proc. 2022, 71, 179–185. [Google Scholar] [CrossRef]
- Rojas-Carbonell, S.; Santoro, C.; Serov, A.; Atanassov, P. Transition Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction Reaction in Neutral Electrolyte. Electrochem. Commun. 2017, 75, 38–42. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S.; Kuila, T.; Murmu, N.C.; Kundu, A. Biomass-Derived Advanced Carbon-Based Electrocatalysts for Oxygen Reduction Reaction. Biomass 2022, 2, 155–177. [Google Scholar] [CrossRef]
- Kaare, K.; Yu, E.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Dyck, A.; Niaura, G.; Tamasauskaite-Tamasiunaite, L.; Norkus, E.; Andrulevičius, M.; et al. Highly Active Wood-Derived Nitrogen-Doped Carbon Catalyst for the Oxygen Reduction Reaction. ACS Omega 2020, 5, 23578–23587. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Y.; Alnoush, W.; Hou, Y.; Higgins, D.; Wu, G. Tuning Two-Electron Oxygen-Reduction Pathways for H2O2 Electrosynthesis via Engineering Atomically Dispersed Single Metal Site Catalysts. Adv. Mater. 2022, 34, 2107954. [Google Scholar] [CrossRef]
- Brocato, S.; Serov, A.; Atanassov, P. PH Dependence of Catalytic Activity for ORR of the Non-PGM Catalyst Derived from Heat-Treated Fe-Phenanthroline. Electrochim. Acta 2013, 87, 361–365. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Influence of Inner- and Outer-Sphere Electron Transfer Mechanisms during Electrocatalysis of Oxygen Reduction in Alkaline Media. J. Phys. Chem. C 2011, 115, 18015–18026. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Alkaline Anion-Exchange Membrane Fuel Cells: Challenges in Electrocatalysis and Interfacial Charge Transfer. Chem. Rev. 2019, 119, 11945–11979. [Google Scholar] [CrossRef]
- Masa, J.; Zhao, A.; Wei, X.; Muhler, M.; Schuhmann, W. Metal-Free Catalysts for Oxygen Reduction in Alkaline Electrolytes: Influence of the Presence of Co, Fe, Mn and Ni Inclusions. Electrochim. Acta 2014, 128, 271–278. [Google Scholar] [CrossRef]
- Vasiliev, V.P.; Manzhos, R.A.; Kochergin, V.K.; Krivenko, A.G.; Kabachkov, E.N.; Kulikov, A.V.; Shulga, Y.M.; Gutsev, G.L. A Facile Synthesis of Noble-Metal-Free Catalyst Based on Nitrogen Doped Graphene Oxide for Oxygen Reduction Reaction. Materials 2022, 15, 821. [Google Scholar] [CrossRef]
- Hossen, M.M.; Artyushkova, K.; Atanassov, P.; Serov, A. Synthesis and Characterization of High Performing Fe-N-C Catalyst for Oxygen Reduction Reaction (ORR) in Alkaline Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 214–221. [Google Scholar] [CrossRef]
- Conradi, M.; Sánchez-Moyano, J.E. Toward a Sustainable Circular Economy for Cigarette Butts, the Most Common Waste Worldwide on the Coast. Sci. Total Environ. 2022, 847, 157634. [Google Scholar] [CrossRef]
- Ghasemi, A.; Golbini Mofrad, M.M.; Parseh, I.; Hassani, G.; Mohammadi, H.; Hayati, R.; Alinejad, N. Cigarette Butts as a Super Challenge in Solid Waste Management: A Review of Current Knowledge. Environ. Sci. Pollut. Res. 2022, 29, 51269–51280. [Google Scholar] [CrossRef] [PubMed]
- Mirshokraee, S.A.; Muhyuddin, M.; Morina, R.; Poggini, L.; Berretti, E.; Bellini, M.; Lavacchi, A.; Ferrara, C.; Santoro, C. Upcycling of Waste Lithium-Cobalt-Oxide from Spent Batteries into Electrocatalysts for Hydrogen Evolution Reaction and Oxygen Reduction Reaction: A Strategy to Turn the Trash into Treasure. J. Power Sources 2023, 557, 232571. [Google Scholar] [CrossRef]
- Varela, A.S.; Ju, W.; Bagger, A.; Franco, P.; Rossmeisl, J.; Strasser, P. Electrochemical Reduction of CO2 on Metal-Nitrogen-Doped Carbon Catalysts. ACS Catal. 2019, 9, 7270–7284. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.P.; Varela, A.S.; Sinev, I.; Bon, V.; Roldan Cuenya, B.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding Activity and Selectivity of Metal-Nitrogen-Doped Carbon Catalysts for Electrochemical Reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.; Liu, Y.; Matanovic, I.; Guo, S.; Tieu, P.; Huang, Y.; Ly, A.; Das, S.; Zenyuk, I.; Pan, X.; et al. Highly Durable and Selective Fe- and Mo-Based Atomically Dispersed Electrocatalysts for Nitrate Reduction to Ammonia via Distinct and Synergized NO2-Pathways. ACS Catal. 2022, 12, 6651–6662. [Google Scholar] [CrossRef]
- Baghban, A.; Habibzadeh, S.; Zokaee Ashtiani, F. On the Evaluation of Hydrogen Evolution Reaction Performance of Metal-Nitrogen-Doped Carbon Electrocatalysts Using Machine Learning Technique. Sci. Rep. 2021, 11, 21911. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Peng, L.; Li, L.; Qiao, J.; Huang, H.; Shi, J. Metal–Nitrogen–Carbon Catalysts of Specifically Coordinated Configurations toward Typical Electrochemical Redox Reactions. Adv. Mater. 2021, 33, 2100997. [Google Scholar] [CrossRef]
- Morozan, A.; Goellner, V.; Nedellec, Y.; Hannauer, J.; Jaouen, F. Effect of the Transition Metal on Metal–Nitrogen–Carbon Catalysts for the Hydrogen Evolution Reaction. J. Electrochem. Soc. 2015, 162, H719–H726. [Google Scholar] [CrossRef]
- Miller, D.J.; Biesinger, M.C.; McIntyre, N.S. Interactions of CO2 and CO at Fractional Atmosphere Pressures with Iron and Iron Oxide Surfaces: One Possible Mechanism for Surface Contamination? Surf. Interface Anal. 2002, 33, 299–305. [Google Scholar] [CrossRef]


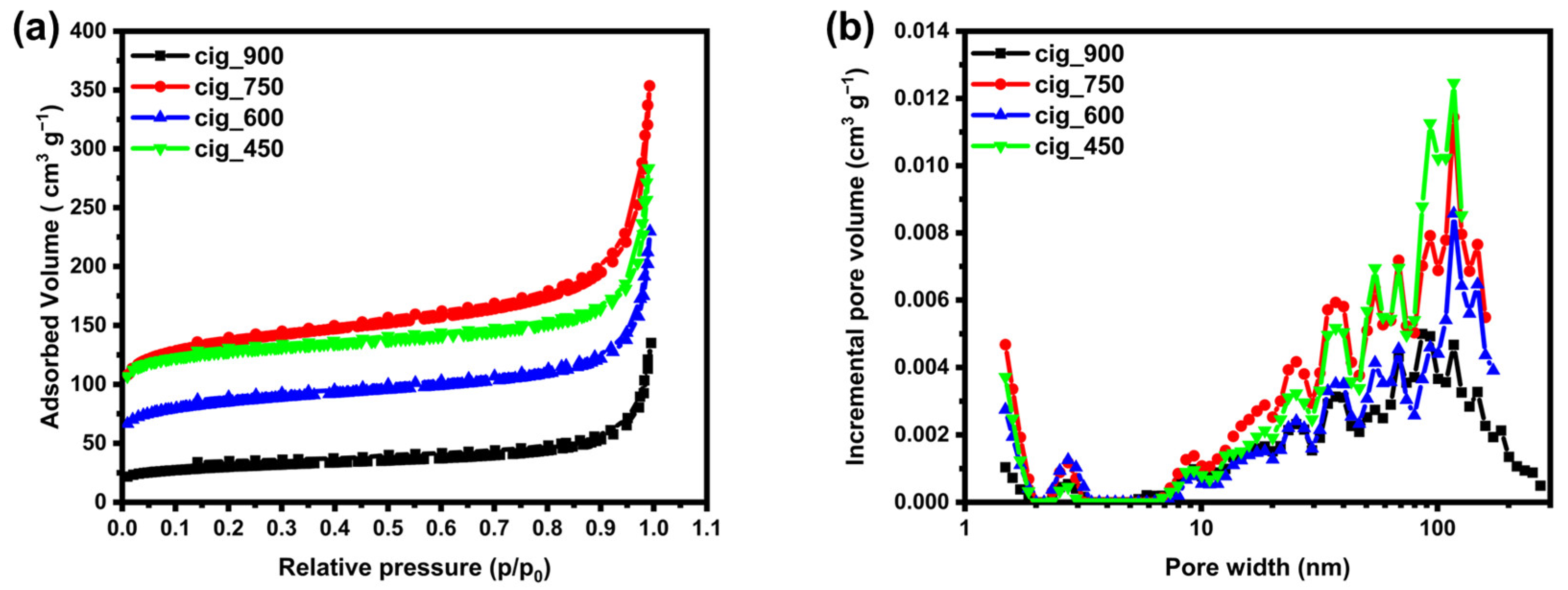
| Sample | SBET [m2 g−1] | Vmicro [cm3 g−1] | Vmeso [cm3 g−1] | Vtotal [cm3 g−1] |
|---|---|---|---|---|
| cig_450 | 490 | 0.15 | 0.06 | 0.31 (<127 nm) |
| cig_600 | 317 | 0.09 | 0.05 | 0.21 (<172 nm) |
| cig_750 | 513 | 0.14 | 0.08 | 0.32 (<179 nm) |
| cig_900 | 110 | 0.03 | 0.05 | 0.13 (<273 nm) |
| Acid | Neutral | Alkaline | ||||
|---|---|---|---|---|---|---|
| Sample | Eon [V vs. RHE] | E1/2 [V vs. RHE] | Eon [V vs. RHE] | E1/2 [V vs. RHE] | Eon [V vs. RHE] | E1/2 [V vs. RHE] |
| cig_900 | 0.88 | 0.50 | 0.73 | 0.41 | 0.88 | 0.76 |
| cig_750 | 0.86 | 0.53 | 0.71 | 0.37 | 0.86 | 0.79 |
| cig_600 | 0.83 | 0.59 | 0.73 | 0.37 | 0.88 | 0.81 |
| cig_450 | 0.88 | 0.68 | 0.77 | 0.43 | 0.91 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, D.; Zuccante, G.; Muhyuddin, M.; Landone, R.; Scommegna, A.; Lorenzi, R.; Acciarri, M.; Petri, E.; Soavi, F.; Poggini, L.; et al. Giving New Life to Waste Cigarette Butts: Transformation into Platinum Group Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Acid, Neutral and Alkaline Environment. Catalysts 2023, 13, 635. https://doi.org/10.3390/catal13030635
Testa D, Zuccante G, Muhyuddin M, Landone R, Scommegna A, Lorenzi R, Acciarri M, Petri E, Soavi F, Poggini L, et al. Giving New Life to Waste Cigarette Butts: Transformation into Platinum Group Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Acid, Neutral and Alkaline Environment. Catalysts. 2023; 13(3):635. https://doi.org/10.3390/catal13030635
Chicago/Turabian StyleTesta, Davide, Giovanni Zuccante, Mohsin Muhyuddin, Roberto Landone, Axel Scommegna, Roberto Lorenzi, Maurizio Acciarri, Elisabetta Petri, Francesca Soavi, Lorenzo Poggini, and et al. 2023. "Giving New Life to Waste Cigarette Butts: Transformation into Platinum Group Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Acid, Neutral and Alkaline Environment" Catalysts 13, no. 3: 635. https://doi.org/10.3390/catal13030635
APA StyleTesta, D., Zuccante, G., Muhyuddin, M., Landone, R., Scommegna, A., Lorenzi, R., Acciarri, M., Petri, E., Soavi, F., Poggini, L., Capozzoli, L., Lavacchi, A., Lamanna, N., Franzetti, A., Zoia, L., & Santoro, C. (2023). Giving New Life to Waste Cigarette Butts: Transformation into Platinum Group Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Acid, Neutral and Alkaline Environment. Catalysts, 13(3), 635. https://doi.org/10.3390/catal13030635












