Production of Fructooligosaccharides Using a Commercial Heterologously Expressed Aspergillus sp. Fructosyltransferase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Sucrose Concentration—Initial Rate Measurements
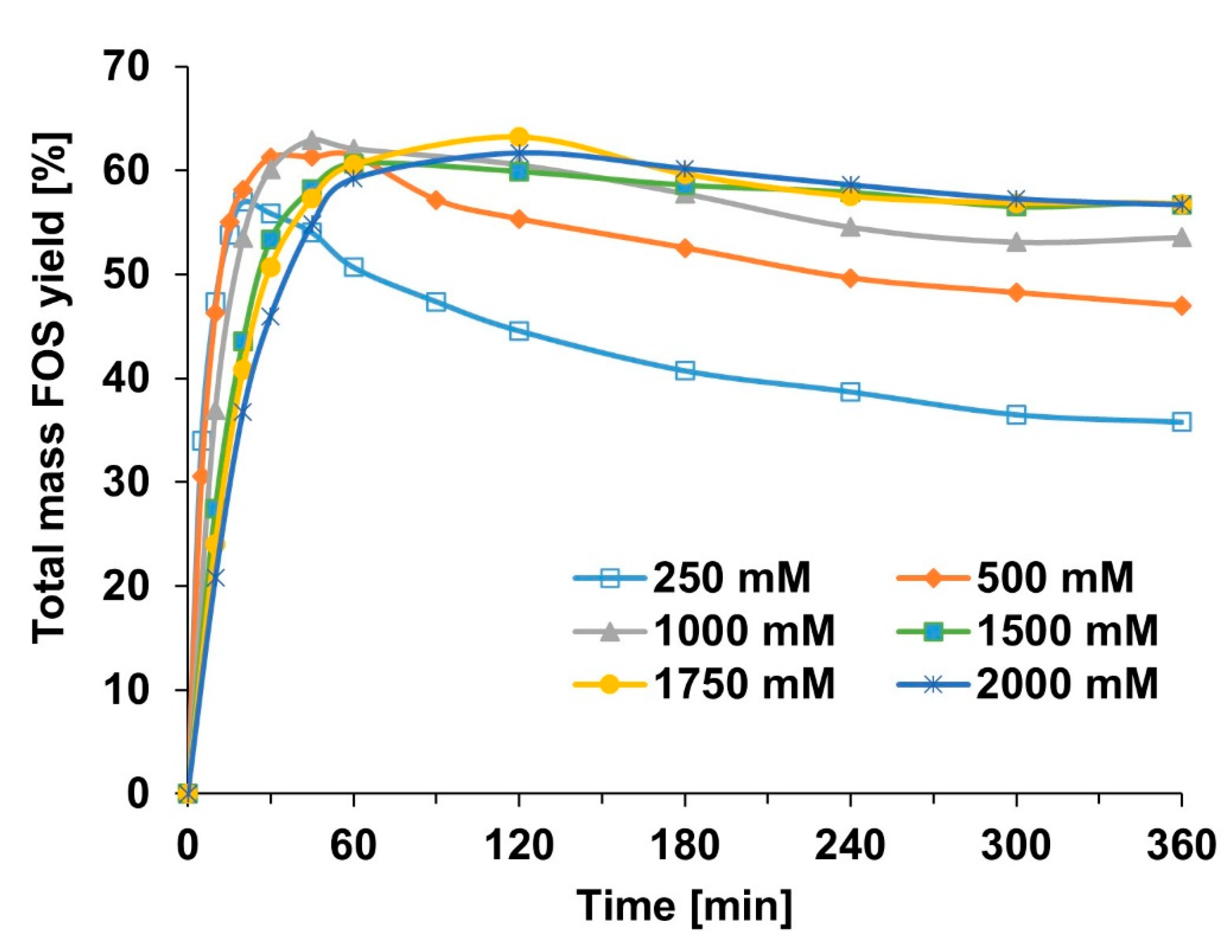
2.2. Effect of Sucrose Concentration—Progress Curve Measurements
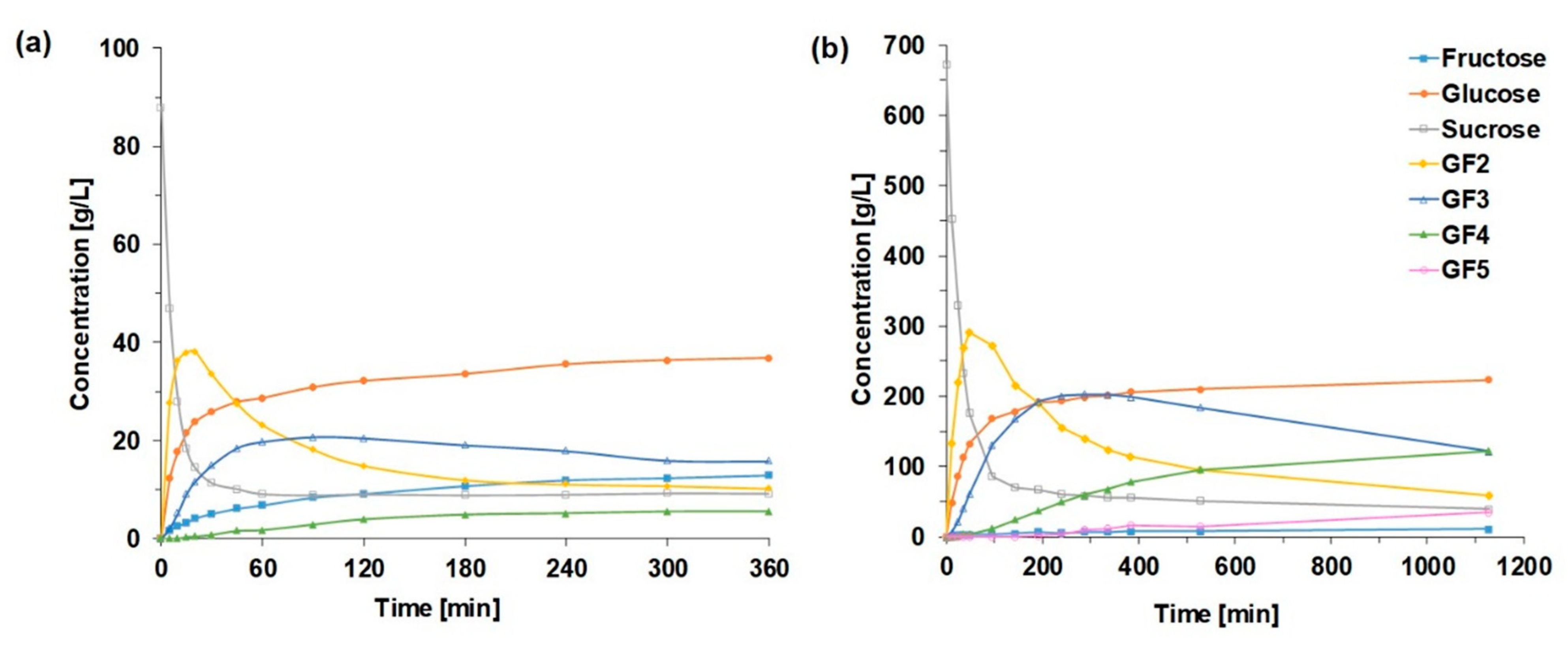
2.2.1. Saccharide Concentration Courses
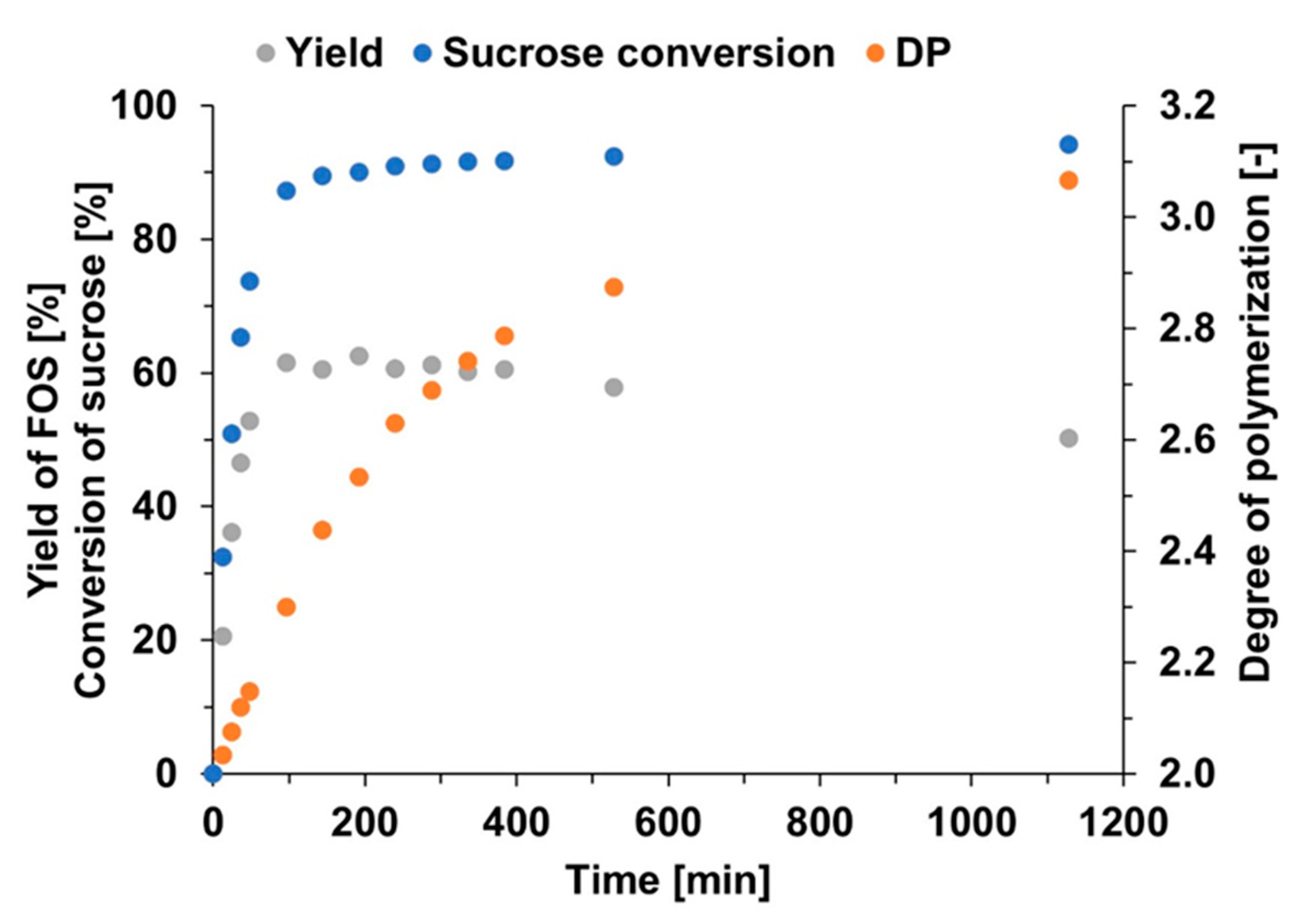
2.2.2. Courses of FOS Yield and Degree of Polymerization
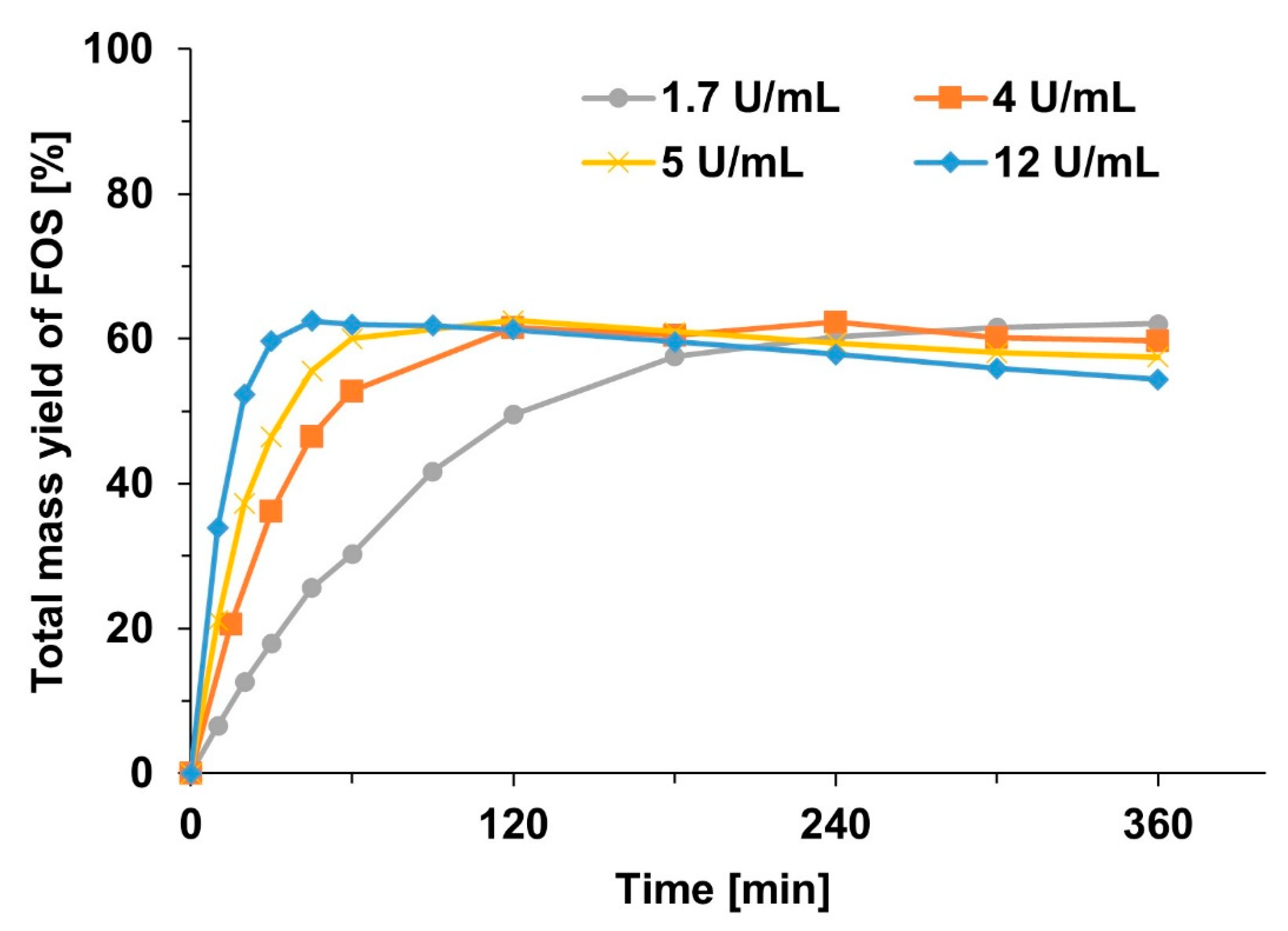
2.3. Effect of Enzyme Concentration
3. Materials and Methods
3.1. Materials
3.1.1. Enzyme
3.1.2. Chemicals
3.2. Analytical Methods
3.2.1. Enzyme Activity Assay
3.2.2. HPLC Analysis
3.3. Biocatalytic Experiments
3.3.1. Initial Rate Measurements
3.3.2. Progress Curve Measurements of Fructooligosaccharide Production
Effect of Initial Sucrose Concentration
Effect of Enzyme Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural Deep Eutectic Solvent Mediated Pretreatment of Rice Straw: Bioanalytical Characterization of Lignin Extract and Enzymatic Hydrolysis of Pretreated Biomass Residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.R.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Gonzalez-Herrera, S.M.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Fructosyltransferase Sources, Production, and Applications for Prebiotics Production. In Probiotics and Prebiotics in Human Nutrition and Health; InTech: Rijeka, Croatia, 2016; pp. 169–189. [Google Scholar]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary Fructooligosaccharides and Potential Benefits on Health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.C.; Gomez-Zavaglia, A. Technological Aspects of the Production of Fructo and Galacto-Oligosaccharides. Enzymatic Synthesis and Hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Tochio, T.; Kadota, Y.; Tanaka, T.; Koga, Y. 1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium Prausnitzii as Well as Bifidobacteria in Humans. Foods 2018, 7, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Aiba, Y.; Takeda, H.; Fukumori, Y.; Koga, Y. Superiority of 1-Kestose, the Smallest Fructo-Oligosaccharide, to a Synthetic Mixture of Fructo-Oligosaccharides in the Selective Stimulating Activity on Bifidobacteria. Biosci. Microflora 2006, 25, 109–116. [Google Scholar] [CrossRef]
- Antošová, M.; Polakovič, M. Fructosyltransferases: The Enzymes Catalyzing Production of Fructooligosaccharides. Chem. Pap. 2001, 55, 350–358. [Google Scholar]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Production of Fructo-Oligosaccharides by Fructosyl Transferase from Aspergillus oryzae CFR 202 and Aureobasidium pullulans CFR 77. Process Biochem. 2004, 39, 755–760. [Google Scholar] [CrossRef]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic Oligosaccharides: Special Focus on Fructooligosaccharides, Its Biosynthesis and Bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef]
- Ganaie, M.A.; Lateef, A.; Gupta, U.S. Enzymatic Trends of Fructooligosaccharides Production by Microorganisms. Appl. Biochem. Biotechnol. 2014, 172, 2143–2159. [Google Scholar] [CrossRef]
- Antošová, M.; Illeová, V.; Vandáková, M.; Družkovská, A.; Polakovič, M. Chromatographic Separation and Kinetic Properties of Fructosyltransferase from Aureobasidium pullulans. J. Biotechnol. 2008, 135, 58–63. [Google Scholar] [CrossRef]
- Madlová, A.; Antošova, M.; Baráthová, M.; Polakovič, M.; Štefúca, V.; Báleš, V. Screening of Microorganisms for Transfructosylating Activity and Optimization of Biotransformation of Sucrose to Fructooligosaccharides. Chem. Pap.-Slovak Acad. Sci. 1999, 53, 366–369. [Google Scholar]
- Coetzee, G.; van Rensburg, E.; Görgens, J.F. Evaluation of the Performance of an Engineered β-Fructofuranosidase from Aspergillus fijiensis to Produce Short-Chain Fructooligosaccharides from Industrial Sugar Streams. Biocatal. Agric. Biotechnol. 2020, 23, 101484. [Google Scholar] [CrossRef]
- Ganaie, M.A.; Gupta, U.S.; Kango, N. Screening of Biocatalysts for Transformation of Sucrose to Fructooligosaccharides. J. Mol. Catal. B Enzym. 2013, 97, 12–17. [Google Scholar] [CrossRef]
- Vega-Paulino, R.J.; Zúniga-Hansen, M.E. Potential Application of Commercial Enzyme Preparations for Industrial Production of Short-Chain Fructooligosaccharides. J. Mol. Catal. B Enzym. 2012, 76, 44–51. [Google Scholar] [CrossRef]
- Hollá, V.; Antošová, M.; Karkeszová, K.; Mastihuba, V.; Polakovič, M. Screening of Commercial Enzymes for Transfructosylation of Tyrosol: Effect of Process Conditions and Reaction Network. Biotechnol. J. 2019, 14, 1800571. [Google Scholar] [CrossRef]
- Hollá, V.; Karkeszová, K.; Antošová, M.; Polakovič, M. Transglycosylation Properties of a Kluyveromyces Lactis Enzyme Preparation: Production of Tyrosol β-Fructoside Using Free and Immobilized Enzyme. Process Biochem. 2021, 110, 168–175. [Google Scholar] [CrossRef]
- L’Hocine, L.; Wang, Z.; Jiang, B.; Xu, S. Purification and Partial Characterization of Fructosyltransferase and Invertase from Aspergillus niger AS0023. J. Biotechnol. 2000, 81, 73–84. [Google Scholar] [CrossRef]
- Yoshikawa, J.; Amachi, S.; Shinoyama, H.; Fujii, T. Multiple β-Fructofuranosidases by Aureobasidium pullulans DSM2404 and Their Roles in Fructooligosaccharide Production. FEMS Microbiol. Lett. 2006, 265, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Kurakake, M.; Ogawa, K.; Sugie, M.; Takemura, A.; Sugiura, K.; Komaki, T. Two Types of β-Fructofuranosidases from Aspergillus oryzae KB. J. Agric. Food Chem. 2008, 56, 591–596. [Google Scholar] [CrossRef]
- Choukade, R.; Kango, N. Production, Properties, and Applications of Fructosyltransferase: A Current Appraisal. Crit. Rev. Biotechnol. 2021, 41, 1178–1193. [Google Scholar] [CrossRef]
- Mao, S.; Liu, Y.; Yang, J.; Ma, X.; Zeng, F.; Zhang, Z.; Wang, S.; Han, H.; Qin, H.M.; Lu, F. Cloning, Expression and Characterization of a Novel Fructosyltransferase from: Aspergillus niger and Its Application in the Synthesis of Fructooligosaccharides. RSC Adv. 2019, 9, 23856–23863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Guo, W.; Han, L.; Shen, W.; Chen, X.; Yang, H. Significant Improvement of Both Catalytic Efficiency and Stability of Fructosyltransferase from Aspergillus niger by Structure-Guided Engineering of Key Residues in the Conserved Sequence of the Catalytic Domain. J. Agric. Food Chem. 2022, 70, 7202–7210. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Zhang, L.; Shen, W. Heterologous Expression and Enzymatic Characterization of Fructosyltransferase from Aspergillus niger in Pichia Pastoris. New Biotechnol. 2016, 33, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yang, H.; Qiang, S.; Fan, Y.; Shen, W.; Chen, X. Overproduction, Purification, and Property Analysis of an Extracellular Recombinant Fructosyltransferase. Eur. Food Res. Technol. 2016, 242, 1159–1168. [Google Scholar] [CrossRef]
- Trollope, K.M.; van Wyk, N.; Kotjomela, M.A.; Volschenk, H. Sequence and Structure-Based Prediction of Fructosyltransferase Activity for Functional Subclassification of Fungal GH32 Enzymes. FEBS J. 2015, 282, 4782–4796. [Google Scholar] [CrossRef]
- Olarte-Avellaneda, S.; Rodríguez-López, A.; Patiño, J.D.; Alméciga-Díaz, C.J.; Sánchez, O.F. In Silico Analysis of the Structure of Fungal Fructooligosaccharides-Synthesizing Enzymes. Interdiscip. Sci. 2018, 10, 53–67. [Google Scholar] [CrossRef]
- Alvarado-Obando, M.; Contreras, N.; León, D.; Botero, L.; Beltran, L.; Díaz, D.; Rodríguez-López, A.; Reyes, L.H.; Alméciga-Díaz, C.J.; Sánchez, O.F. Engineering a Heterologously Expressed Fructosyltransferase from Aspergillus oryzae N74 in Komagataella Phaffii (Pichia pastoris) for Kestose Production. New Biotechnol. 2022, 69, 18–27. [Google Scholar] [CrossRef]
- Biocatalyst for Sucrose Conversion into Fructo-Oligosaccharides. Available online: https://www.seqens.com/en/biocatalyst-for-sucrose-conversion-into-fructo-oligosaccharides/ (accessed on 27 March 2023).
- Jen Duan, K.; Shin Chen, J.; Chyi Sheu, D. Kinetic Studies and Mathematical Model for Enzymatic Production of Fructooligosaccharides from Sucrose. Enzym. Microb. Technol. 1994, 16, 334–339. [Google Scholar] [CrossRef]
- Shearwin, K.E.; Winzor, D.J. Substrate as a Source of Thermodynamic Nonideality in Enzyme Kinetic Studies: Invertase-Catalyzed Hydrolysis of Sucrose. Arch. Biochem. Biophys. 1988, 260, 532–539. [Google Scholar] [CrossRef]

| Biocatalyst | Initial Sucrose Concentration (g/L) | Sucrose Conversion (%) | DP | Reference |
|---|---|---|---|---|
| 85 | 2.29 | |||
| Seqenzym® FT | 599 | 90 | 2.50 | This work |
| 92 | 2.73 | |||
| 85 | 2.23 | |||
| A. pullulans FTase | 600 | 90 | 2.36 | [11] |
| 92 | 2.43 | |||
| 85 | 2.29 | |||
| Pectinex Ultra SP-L | 536.2 | 90 | 2.41 | [15] |
| 92 | 2.50 | |||
| 85 | 2.31 | |||
| Rohapect CM | 536.2 | 90 | 2.46 | [15] |
| 92 | 2.55 | |||
| Viscozyme L | 536.2 | 85 | 2.25 | [15] |
| 90 | 2.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkeszová, K.; Polakovič, M. Production of Fructooligosaccharides Using a Commercial Heterologously Expressed Aspergillus sp. Fructosyltransferase. Catalysts 2023, 13, 843. https://doi.org/10.3390/catal13050843
Karkeszová K, Polakovič M. Production of Fructooligosaccharides Using a Commercial Heterologously Expressed Aspergillus sp. Fructosyltransferase. Catalysts. 2023; 13(5):843. https://doi.org/10.3390/catal13050843
Chicago/Turabian StyleKarkeszová, Klaudia, and Milan Polakovič. 2023. "Production of Fructooligosaccharides Using a Commercial Heterologously Expressed Aspergillus sp. Fructosyltransferase" Catalysts 13, no. 5: 843. https://doi.org/10.3390/catal13050843





