Spinel LiMn2O4 as Electrocatalyst toward Solid-State Zinc–Air Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Structure Analysis
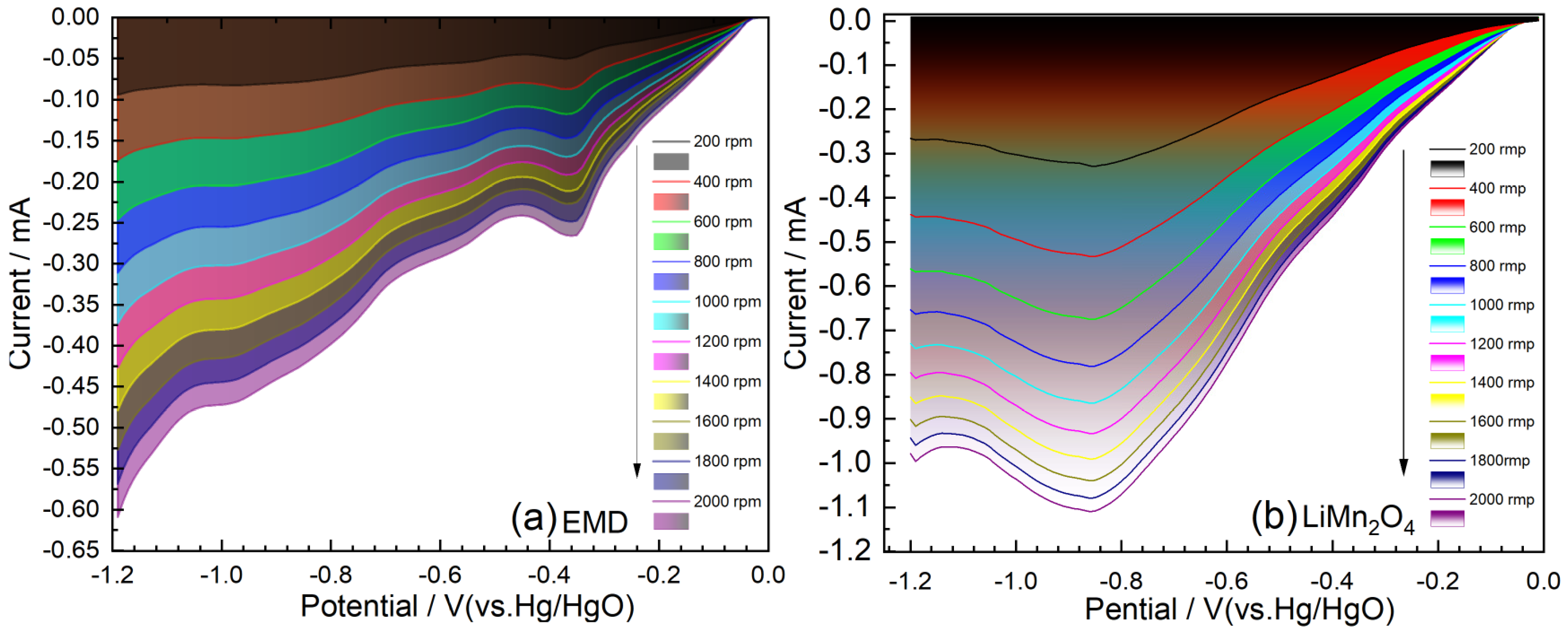
2.2. Polarization Characteristics of the Air Electrode
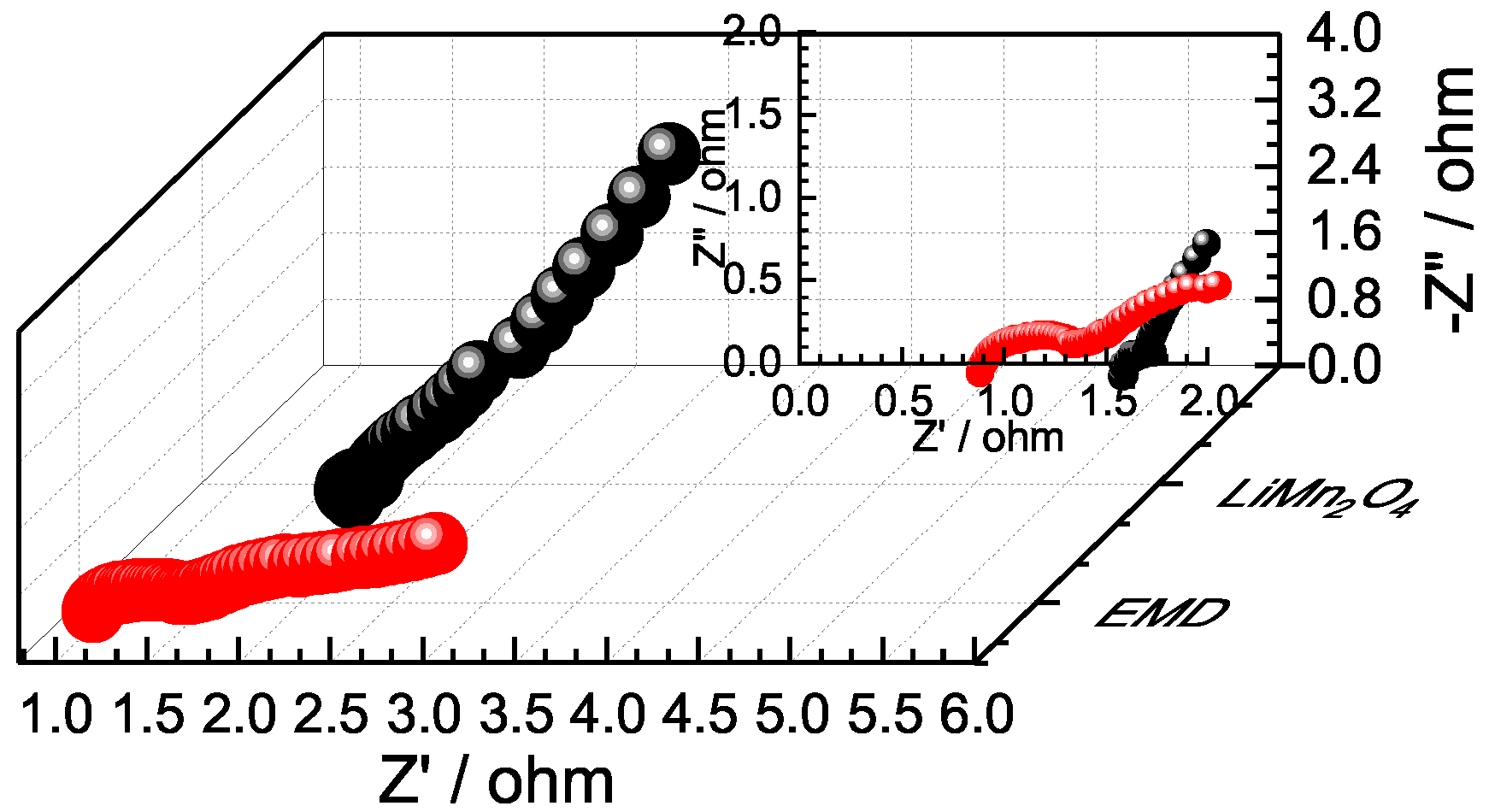
2.3. Electrochemical Impedance Spectroscopy (EIS) Study
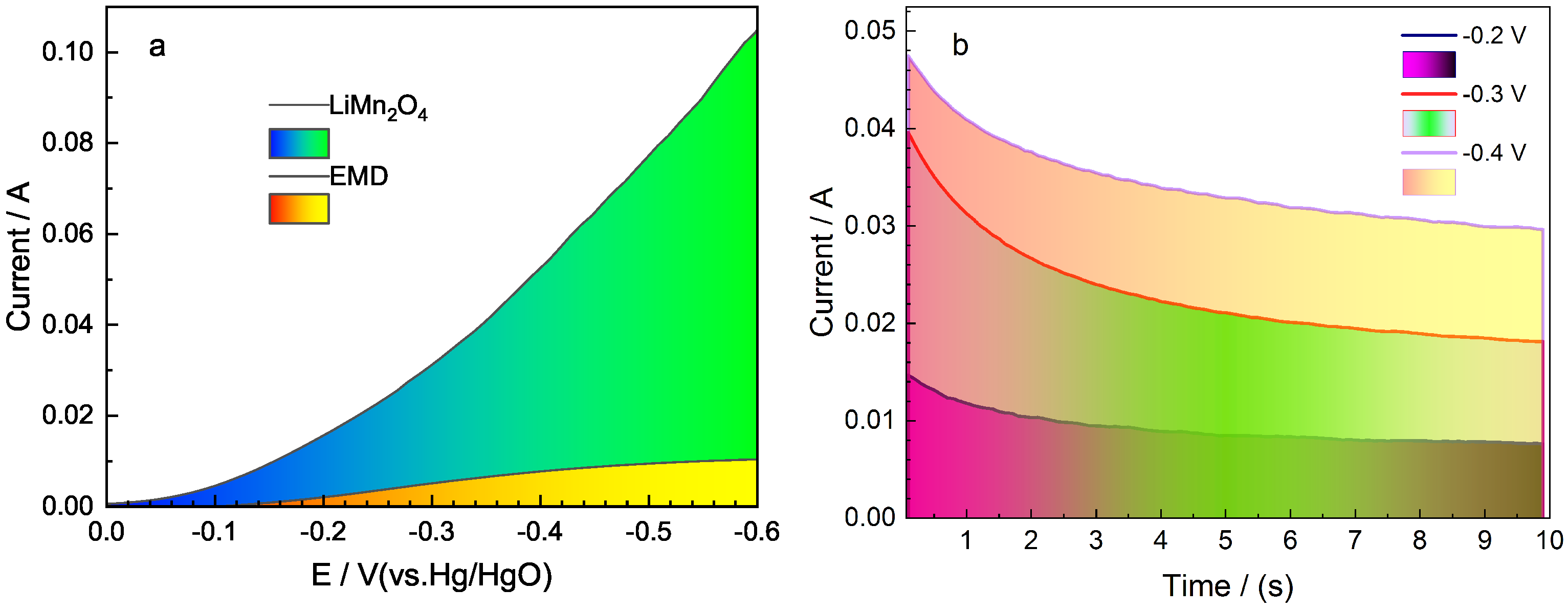
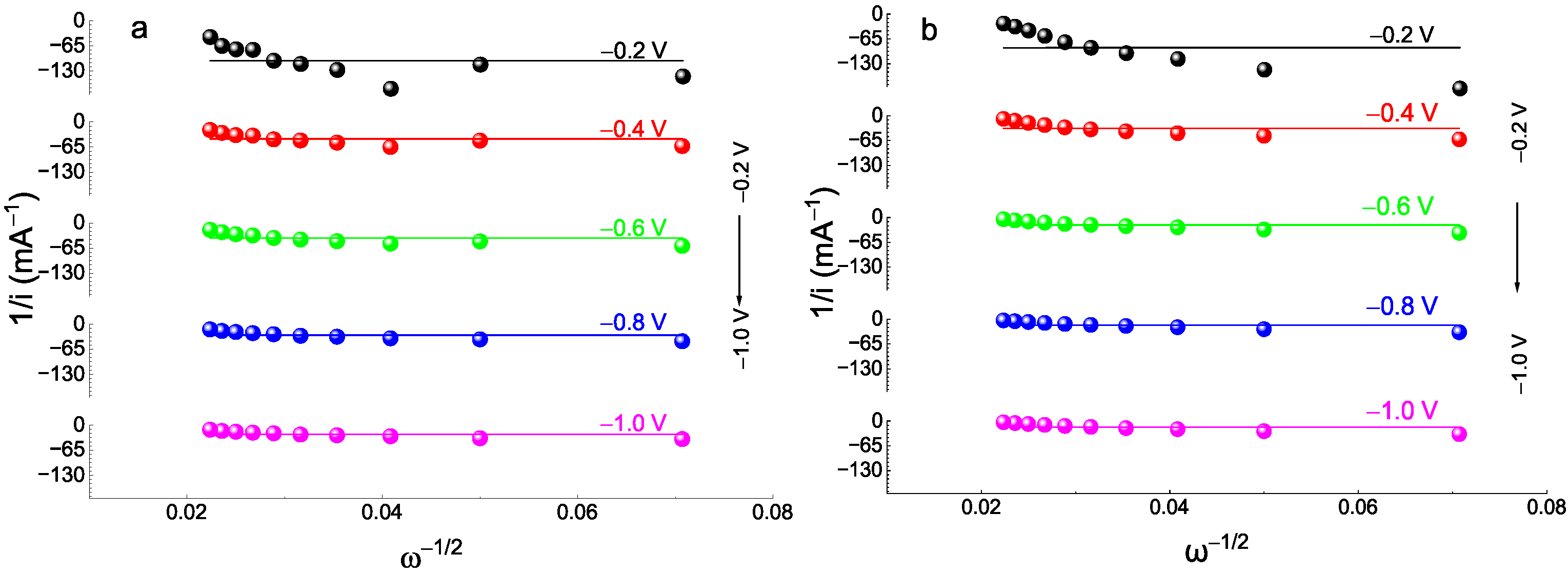
2.4. Electrochemical Catalytic Activity toward ORR
2.5. Discharge Characteristics of Solid-State Zinc–Air Cells
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xin, F.; Zhou, H.; Chen, X.; Zuba, M.; Chernova, N.; Zhou, G.; Whittingham, M.S. Li–Nb–O Coating/Substitution Enhances the Electrochemical Performance of the LiNi0.8Mn0.1Co0.1O2 (NMC 811) Cathode. ACS Appl. Mater. Interfaces 2019, 11, 34889–34894. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Xu, M.; Zheng, Y.; Li, Z.; Wen, G.; Zhang, Z.; Yang, L.; Ma, Q.; Yu, A.; Luo, D.; et al. Bioinspired Tough Solid-State Electrolyte for Flexible Ultralong-Life Zinc-Air Battery. Adv. Mater. 2022, 34, 2110585. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.; Wang, W.; Wang, G.J.N.; Rastak, R.; Molina-Lopez, F.; Chung, J.W.; Niu, S.; Feig, V.R.; Lopez, J.; et al. Skin Electronics from Scalable Fabrication of an Intrinsically Stretchable Transistor Array. Nature 2018, 555, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xia, D.; Li, M.; Cheng, D.; Wang, K.; Meng, Y.S.; Chen, Z.; Bae, J. Self-Healing and Anti-CO2 Hydrogels for Flexible Solid-State Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 12033–12041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, M.; Zhang, Y.; Jin, Z.; Peng, Z.; Liu, C.; Chen, S.; Ge, J.; Xing, W. Metal–Organic Framework-Induced Synthesis of Ultrasmall Encased NiFe Nanoparticles Coupling with Graphene as an Efficient Oxygen Electrode for a Rechargeable Zn–Air Battery. ACS Catal. 2016, 6, 6335–6342. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, C.; Amal, R.; Dai, L.; Lu, X. Heteroatom-doped Carbon Catalysts for Zinc–air Batteries: Progress, Mechanism, and Opportunities. Energy Environ. Sci. 2020, 13, 4536–4563. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Nath, K.; Kumar, S.; Tiwari, R.N.; Kemp, K.C.; Le, N.H.; Youn, D.H.; Lee, J.S.; Kim, K.S. Stable Platinum Nanoclusters on Genomic DNA–graphene Oxide with a High Oxygen Reduction Reaction Activity. Nat. Commun. 2013, 4, 2221. [Google Scholar] [CrossRef]
- Nesselberger, M.; Roefzaad, M.; Fayçal Hamou, R.; Ulrich Biedermann, P.; Schweinberger, F.F.; Kunz, S.; Schloegl, K.; Wiberg, G.K.H.; Ashton, S.; Heiz, U.; et al. The Effect of Particle Proximity on the Oxygen Reduction Rate of Size-selected Platinum Clusters. Nat. Mater. 2013, 12, 919–924. [Google Scholar] [CrossRef]
- Chung, T.D.; Anson, F.C. Catalysis of the Electroreduction of O2 by Cobalt 5,10,15,20-tetraphenylporphyrin Dissolved in Thin Layers of Benzonitrile on Graphite Electrodes. J. Electroanal. Chem. 2001, 508, 115–122. [Google Scholar] [CrossRef]
- Zeng, K.; Zheng, X.; Li, C.; Yan, J.; Tian, J.; Jin, C.; Strasser, P.; Yang, R. Recent Advances in Non-Noble Bifunctional Oxygen Electrocatalysts toward Large-Scale Production. Adv. Funct. Mater. 2020, 30, 2000503. [Google Scholar] [CrossRef]
- Watanabe, M.; Tryk, D.A.; Wakisaka, M.; Yano, H.; Uchida, H. Overview of Recent Developments in Oxygen Reduction Electrocatalysis. Electrochim. Acta 2012, 84, 187–201. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, L.; Yan, X.; Yao, X. Recent Progress in Oxygen Electrocatalysts for Zinc-Air Batteries. Small Methods 2017, 1, 1700209. [Google Scholar] [CrossRef]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly Efficient Nonprecious Metal Catalysts towards Oxygen Reduction Reaction Based on Three-dimensional Porous Carbon Nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced Zinc-Air Batteries Based on High-Performance Hybrid Electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef]
- Joo, H.; Lee, J.; Yoon, J. Short Review: Timeline of the Electrochemical Lithium Recovery System Using the Spinel LiMn2O4 as a Positive Electrode. Energies 2020, 13, 6235. [Google Scholar] [CrossRef]
- Iskandar Radzi, Z.; Helmy Arifin, K.; Zieauddin Kufian, M.; Balakrishnan, V.; Rohani Sheikh Raihan, S.; Abd Rahim, N.; Subramaniam, R. Review of Spinel LiMn2O4 Cathode Materials under High Cut-off Voltage in Lithium-ion Batteries: Challenges and Strategies. J. Electroanal. Chem. 2022, 920, 116623. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, W.; Li, Y.; Chen, Q.; Liu, Y. Spinel LiMn2O4 nanoparticles dispersed on nitrogen-doped reduced graphene oxide nanosheets as an efficient electrocatalyst for aluminium-air battery. Int. J. Hydrog. Energy 2015, 40, 9225–9234. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Zeng, X.; Song, H.; Shu, T.; Wang, H.; Ren, J.; Liao, S. Spinel LiMn2O4 Nanoparticles Grown in Situ on Nitrogen-Doped Reduced Graphene Oxide as an Efficient Cathode for a Li-O2/Li-Ion Twin Battery. ACS Sustain. Chem. Eng. 2018, 7, 430–439. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef]
- Zhai, X.; Yang, W.; Li, M.; Lv, G.; Liu, J.; Zhang, X. Noncovalent hybrid of CoMn2O4 spinel nanocrystals and poly (diallyldimethylammonium chloride) functionalized carbon nanotubes as efficient electrocatalysts for oxygen reduction reaction. Carbon 2013, 65, 277–286. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Evans, D.G.; Yang, W. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale 2013, 5, 5312. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiang, L.; Qi, L.; Wang, E.; Sun, G. Electrocatalytic performance of Ni modified MnOx/C composites toward oxygen reduction reaction and their application in Zn-air battery. Int. J. Hydrog. Energy 2014, 39, 3423–3432. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Zhang, B.; Jin, J.; Su, D.S.; Wang, S.; Sun, G. Controllable Synthesis of Cobalt Monoxide Nanoparticles and the Size-Dependent Activity for Oxygen Reduction Reaction. ACS Catal. 2014, 4, 2998–3001. [Google Scholar] [CrossRef]
- Mandal, S.; Rojas, R.M.; Amarilla, J.M.; Calle, P.; Kosova, N.V.; Anufrienko, V.F.; Rojo, J.M. High Temperature Co-doped LiMn2O4-Based Spinels. Structural, Electrical, and Electrochemical Characterization. Chem. Mater. 2002, 14, 1598–1605. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M.; Rangan, S.; Lemal, M.; Guyomard, D. Study of Structural Defects in γ-MnO2 by Raman Spectroscopy. J. Raman Spectrosc. 2002, 33, 223–228. [Google Scholar] [CrossRef]
- Sinha, M.; Gupta, H. Study of Zone-center Phonons in Lithium Manganese Oxide. Phys. B Condens. Matter 2002, 316–317, 166–169. [Google Scholar] [CrossRef]
- Ammundsen, B.; Burns, G.R.; Islam, M.S.; Kanoh, H.; Rozière, J. Lattice Dynamics and Vibrational Spectra of Lithium Manganese Oxides: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 1999, 103, 5175–5180. [Google Scholar] [CrossRef]
- Julien, C. Techniques for the Characterization of New Cathode Materials. In Trends in Materials Science; Narosa: New Delhi, India, 1997; pp. 44–63. [Google Scholar]
- Ramana, C.V.; Massot, M.; Julien, C.M. XPS and Raman Spectroscopic Characterization of LiMn2O4 Spinels. Surf. Interface Anal. 2005, 37, 412–416. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M. Raman Spectroscopic Studies of Lithium Manganates with Spinel Structure. J. Phys. Condens. Matter 2003, 15, 3151–3162. [Google Scholar] [CrossRef]
- Lu, J.; Zhan, C.; Wu, T.; Wen, J.; Lei, Y.; Kropf, A.J.; Wu, H.; Miller, D.J.; Elam, J.W.; Sun, Y.K.; et al. Effectively Suppressing Dissolution of Manganese from Spinel Lithium Manganate via a Nanoscale Surface-doping Approach. Nat. Commun. 2014, 5, 5693. [Google Scholar] [CrossRef]
- Goodenough, J.; Manthiram, A.; Wnetrzewski, B. Electrodes for Lithium Batteries. J. Power Sources 1993, 43, 269–275. [Google Scholar] [CrossRef]
- Hu, J.; Shi, Z.; Wang, X.; Qiao, H.; Huang, H. Silver-modified Porous 3D Nitrogen-doped Graphene Aerogel: Highly Efficient Oxygen Reduction Electrocatalyst for Zn-Air Battery. Electrochim. Acta 2019, 302, 216–224. [Google Scholar] [CrossRef]
- Rao, C.S.; Gunasekaran, G. Cobalt-Lead-Manganese Oxides Combined Cathode Catalyst for Air Electrode in Zinc-air Battery. Electrochim. Acta 2015, 176, 649–656. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T. Handbook of Batteries; McGraw Hill Professional: New York, NY, USA, 2001. [Google Scholar]
- Cao, Y.; Yang, H.; Ai, X.; Xiao, L. The Mechanism of Oxygen Reduction on MnO2-catalyzed Air Cathode in Alkaline Solution. J. Electroanal. Chem. 2003, 557, 127–134. [Google Scholar] [CrossRef]
- Jiang, R.; Chu, D. Multiple Small Potential Steps at a Rotating Disk Electrode and Applications. Electrochim. Acta 2000, 45, 4025–4030. [Google Scholar] [CrossRef]
- Jiang, R.; Anson, F.C. The Origin of Inclined Plateau Currents in Steady-state Voltammograms for Electrode Processes Involving Electrocatalysis. J. Electroanal. Chem. Interfacial Electrochem. 1991, 305, 171–184. [Google Scholar] [CrossRef]
- Ohsaka, T.; Mao, L.; Arihara, K.; Sotomura, T. Bifunctional Catalytic Activity of Manganese Oxide toward O2 Reduction: Novel Insight into the Mechanism of Alkaline Air Electrode. Electrochem. Commun. 2004, 6, 273–277. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhang, P.; Kong, S.; Jin, B. Spinel LiMn2O4 as Electrocatalyst toward Solid-State Zinc–Air Batteries. Catalysts 2023, 13, 860. https://doi.org/10.3390/catal13050860
Zhang G, Zhang P, Kong S, Jin B. Spinel LiMn2O4 as Electrocatalyst toward Solid-State Zinc–Air Batteries. Catalysts. 2023; 13(5):860. https://doi.org/10.3390/catal13050860
Chicago/Turabian StyleZhang, Guoqing, Peng Zhang, Shuying Kong, and Binbin Jin. 2023. "Spinel LiMn2O4 as Electrocatalyst toward Solid-State Zinc–Air Batteries" Catalysts 13, no. 5: 860. https://doi.org/10.3390/catal13050860



