Green Approach for Synthesizing Copper-Containing ZIFs as Efficient Catalysts for Click Chemistry
Abstract
:1. Introduction
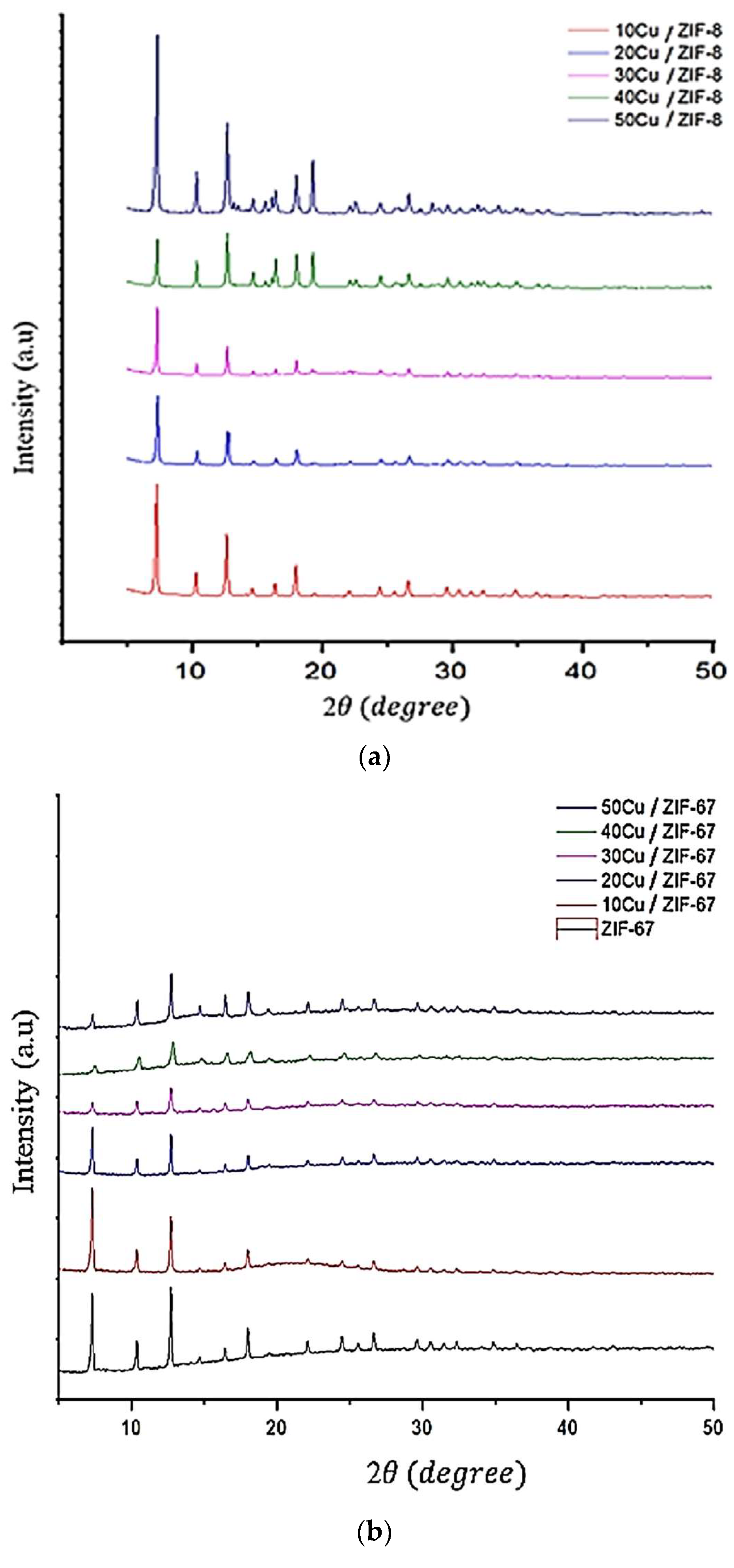
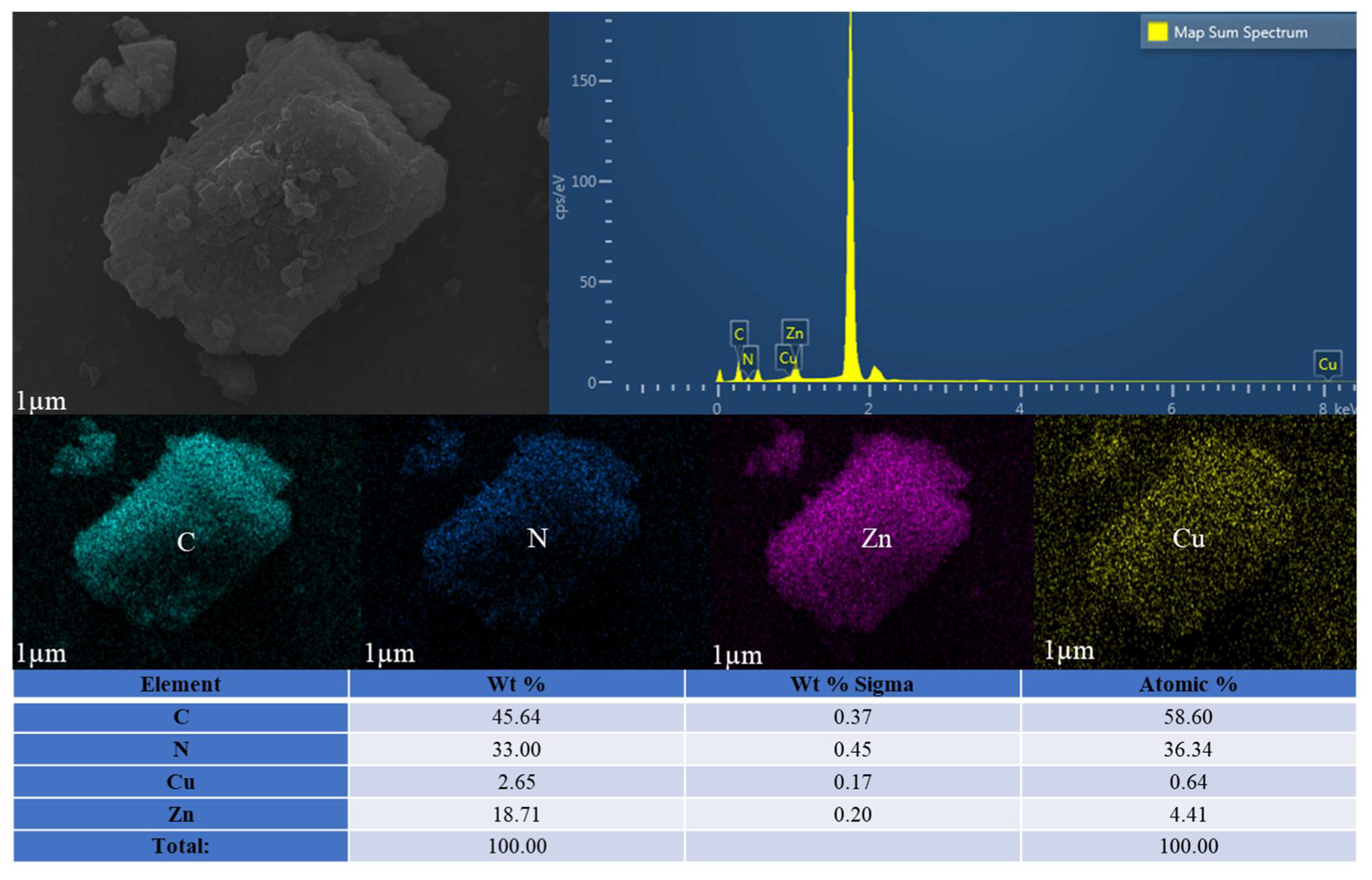
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Cu-ZIF-8 and Cu-ZIF-67
4.3. Characterization
4.4. Catalytic Reaction
4.5. CuAAC Mechanism Using Cu/ZIF-8 or Cu/ZIF-67 Catalysts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, C.A.; Timofeeva, T.V.; Settersten, T.B.; Patterson, B.D.; Liu, V.H.; Simmons, B.A.; Allendorf, M.D. Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks. J. Am. Chem. Soc. 2007, 129, 7136–7144. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaemchuen, S.; Kabir, N.A.; Zhou, K.; Verpoort, F. Metal-organic frameworks for upgrading biogas via CO2 adsorption to biogas green energy. Chem. Soc. Rev. 2013, 42, 9304–9332. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, D.; Henke, A.; Albrecht, K.; Moeller, M.; Nakagawa, K.; Kitagawa, S.; Groll, J. Rapid preparation of flexible porous coordination polymer nanocrystals with accelerated guest adsorption kinetics. Nat. Chem. 2010, 2, 410–416. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Jin, C.-X.; Shang, H.-B. Synthetic methods, properties and controlling roles of synthetic parameters of zeolite imidazole framework-8: A review. J. Solid State Chem. 2021, 297, 122040. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry and catalysts. J. Mater. Chem. A 2018, 6, 1887–1899. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Zhou, K.; Mousavi, B.; Ghadamyari, M.; Heynderickx, P.M.; Zhuiykov, S.; Yusubov, M.S.; Verpoort, F. Spray drying of zeolitic imidazolate frameworks: Investigation of crystal formation and properties. Cryst. Eng. Commun. 2018, 20, 3601–3608. [Google Scholar] [CrossRef]
- Lorignon, F.; Gossard, A.; Carboni, M. Hierarchically porous monolithic MOFs: An ongoing challenge for industrial-scale effluent treatment. Chem. Eng. J. 2020, 393, 124765. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Luo, Z.; Zhou, K.; Mousavi, B.; Phatanasri, S.; Jaroniec, M.; Verpoort, F. Defect formation in metal–organic frameworks initiated by the crystal growth-rate and effect on catalytic performance. J. Catal. 2017, 354, 84–91. [Google Scholar] [CrossRef]
- Wang, J.; Chaemchuen, S.; Klomkliang, N.; Verpoort, F. In Situ Thermal Solvent-Free Synthesis of Zeolitic Imidazolate Frameworks with High Crystallinity and Porosity for Effective Adsorption and Catalytic Applications. Cryst. Growth Des. 2021, 21, 5349–5359. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Ozin, G.A.; Cademartiri, L. Nanochemistry: What Is Next? Small 2009, 5, 1240–1244. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Cole-Hamilton, D.J. Homogeneous Catalysis—New Approaches to Catalyst Separation, Recovery, and Recycling. Science 2003, 299, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Grigorjeva, L.; Daugulis, O. Cobalt-Catalyzed Direct Carbonylation of Aminoquinoline Benzamides. Org. Lett. 2014, 16, 4688–4690. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W.A.; Chen, G. Efficient Alkyl Ether Synthesis via Palladium-Catalyzed, Picolinamide-Directed Alkoxylation of Unactivated C(sp3)–H and C(sp2)–H Bonds at Remote Positions. J. Am. Chem. Soc. 2012, 134, 7313–7316. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, M.; Mousa, M.; Hussein, A.; Hammouti, B.; Hadda, T.B.; Warad, I. Copper (II)-oxide nanostructures: Synthesis, characterizations and their applications-review. J. Mater. Environ. Sci. 2013, 4, 792–797. [Google Scholar] [CrossRef]
- Min, C.; Sanchawala, A.; Seidel, D. Dual C–H functionalization of N-aryl amines: Synthesis of polycyclic amines via an oxidative povarov approach. Org. Lett. 2014, 16, 2756–2759. [Google Scholar] [CrossRef] [PubMed]
- Arguelles Arias, A. Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; FORMATEX: Budapest, Hungary, 2011; pp. 668–674. [Google Scholar]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraji, M.; Amini, M.; Anbari, A.P. Preparation and characterization of TiO2-nanotube/Ti plates loaded Cu2O nanoparticles as a novel heterogeneous catalyst for the azide–alkyne cycloaddition. Catal. Commun. 2016, 76, 72–75. [Google Scholar] [CrossRef]
- Lim, Y.-F.; Choi, J.J.; Hanrath, T. Facile synthesis of colloidal CuO nanocrystals for light-harvesting applications. J. Nanomater. 2012, 2012, 4. [Google Scholar] [CrossRef] [Green Version]
- Muller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Hung, M.-H.; Farnham, W.B.; Feiring, A.E.; Rozen, S. Functional Fluorpolymers and Fluoropolymers. Fluoropolym. 1 Synth. 2002, 51–66. [Google Scholar] [CrossRef]
- Furuya, T.; Benitez, D.; Tkatchouk, E.; Strom, A.E.; Tang, P.; Goddard, W.A., III; Ritter, T. Mechanism of C−F reductive elimination from palladium (IV) fluorides. J. Am. Chem. Soc. 2010, 132, 3793–3807. [Google Scholar] [CrossRef] [Green Version]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem 2004, 5, 570–589. [Google Scholar] [CrossRef]
- Hagmann, W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Kirsch, P. Modern Fluoroorganic Chemistry; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-3-527-60419-7. [Google Scholar]
- Schultz, M.J.; Sigman, M.S. Recent advances in homogeneous transition metal-catalyzed aerobic alcohol oxidations. Tetrahedron 2006, 35, 8227–8241. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Jiao, N. Copper-catalyzed direct transformation of simple alkynes to alkenyl nitriles via aerobic oxidative N-incorporation. Chem. Sci. 2015, 6, 6355–6360. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.D.; Popov, I.; Daugulis, O. Copper-promoted sulfenylation of sp2 C–H bonds. J. Am. Chem. Soc. 2012, 134, 18237–18240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, M.; Pourvahabi Anbari, A.; Ramezani, S.; Gautam, S.; Hwa Chae, K. Copper (II) oxide nanoparticles as an efficient catalyst in the azide–alkynecycloaddition. ChemistrySelect 2016, 1, 4607–4612. [Google Scholar] [CrossRef]
- Amini, M.; Ramezani, S.; Anbari, A.P.; Beheshti, A.; Gautam, S.; Chae, K.H. Simple preparation of cuprous oxide nanoparticles for catalysis of azide–alkyne cycloaddition. J. Chem. Res. 2018, 42, 166–169. [Google Scholar] [CrossRef]
- Rouquet, G.; Chatani, N. Catalytic functionalization of C (sp2)—H and C (sp3)—H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jang, M.-S.; Kwon, H.-J.; Ahn, W.-S. Zeolitic imidazolate frameworks: Synthesis, functionalization, and catalytic/adsorption applications. Catal. Surv. Asia 2014, 18, 101–127. [Google Scholar] [CrossRef]
- Pan, H.; Chu, H.; Wang, X.; Li, Y.; Zhao, S.; Li, G.; Li, D. Optical nonlinearity of zeolitic imidazolate framework-67 in the near-infrared region. Mater. Chem. Front. 2020, 4, 2081–2088. [Google Scholar] [CrossRef]
- Mousavi, B.; Chaemchuen, S.; Moosavi, B.; Luo, Z.; Gholampour, N.; Verpoort, F. Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the conversion of CO2 to cyclic carbonates. New J. Chem. 2016, 40, 5170–5176. [Google Scholar] [CrossRef]
- Cravillon JMu nzer, S.; Lohmeier, S.J.; Feldhoff, A.; Huber, K.; Wiebcke, M. Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework. Chem. Mater. 2009, 21, 1410–1412. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Liu, R.; Qu, J.; Wang, H.; Zhang, X. Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. RSC Adv. 2015, 5, 48433–48441. [Google Scholar] [CrossRef]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Bergaoui, M.; Khalfaoui, M.; Awadallah, F.A.; Al-Muhtaseb, S. A review of the features and applications of ZIF-8 and its derivatives for separating CO2 and isomers of C3- and C4- hydrocarbons. J. Nat. Gas Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of high crystalline ZIF-8 in an aqueous solution. Cryst. Eng. Commun. 2013, 15, 1794–1801. [Google Scholar] [CrossRef]
- Fang, Y.-C.; Lin, H.-C.; Hsu, I.-J.; Lin, T.-S.; Mou, C.-Y. Bioinspired design of a Cu–Zn–imidazolate mesoporous silica catalyst system for superoxide dismutation. J. Phys. Chem. C 2011, 115, 20639–20652. [Google Scholar] [CrossRef]
- Shi, G.; Xu, W.; Wang, J.; Klomkliang, N.; Mousavi, B.; Chaemchuen, S. Thermochemical transformation in the single-step synthesis of zeolitic imidazole frameworks under solvent-free conditions. Dalton Trans. 2020, 49, 2811–2818. [Google Scholar] [CrossRef]

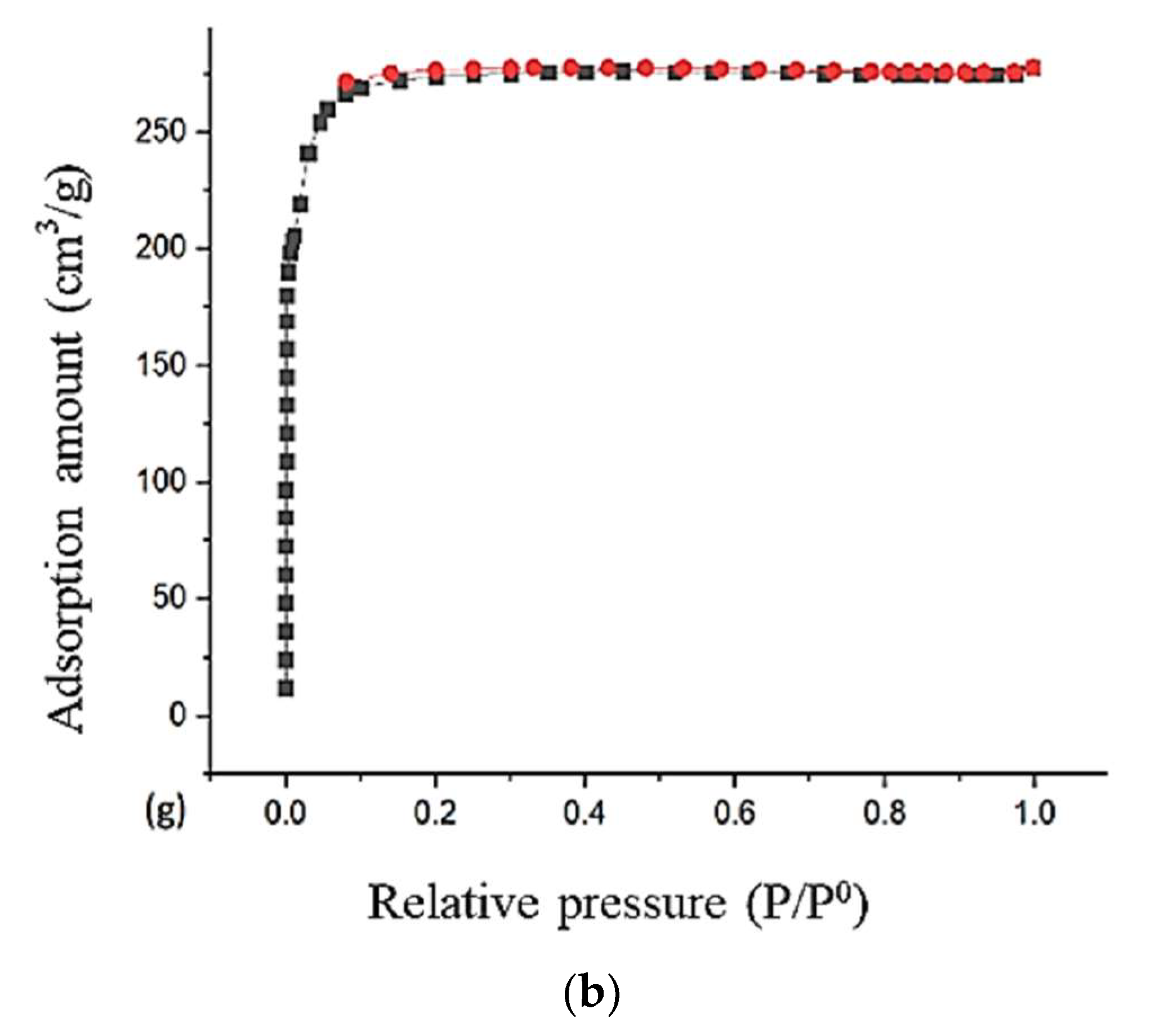

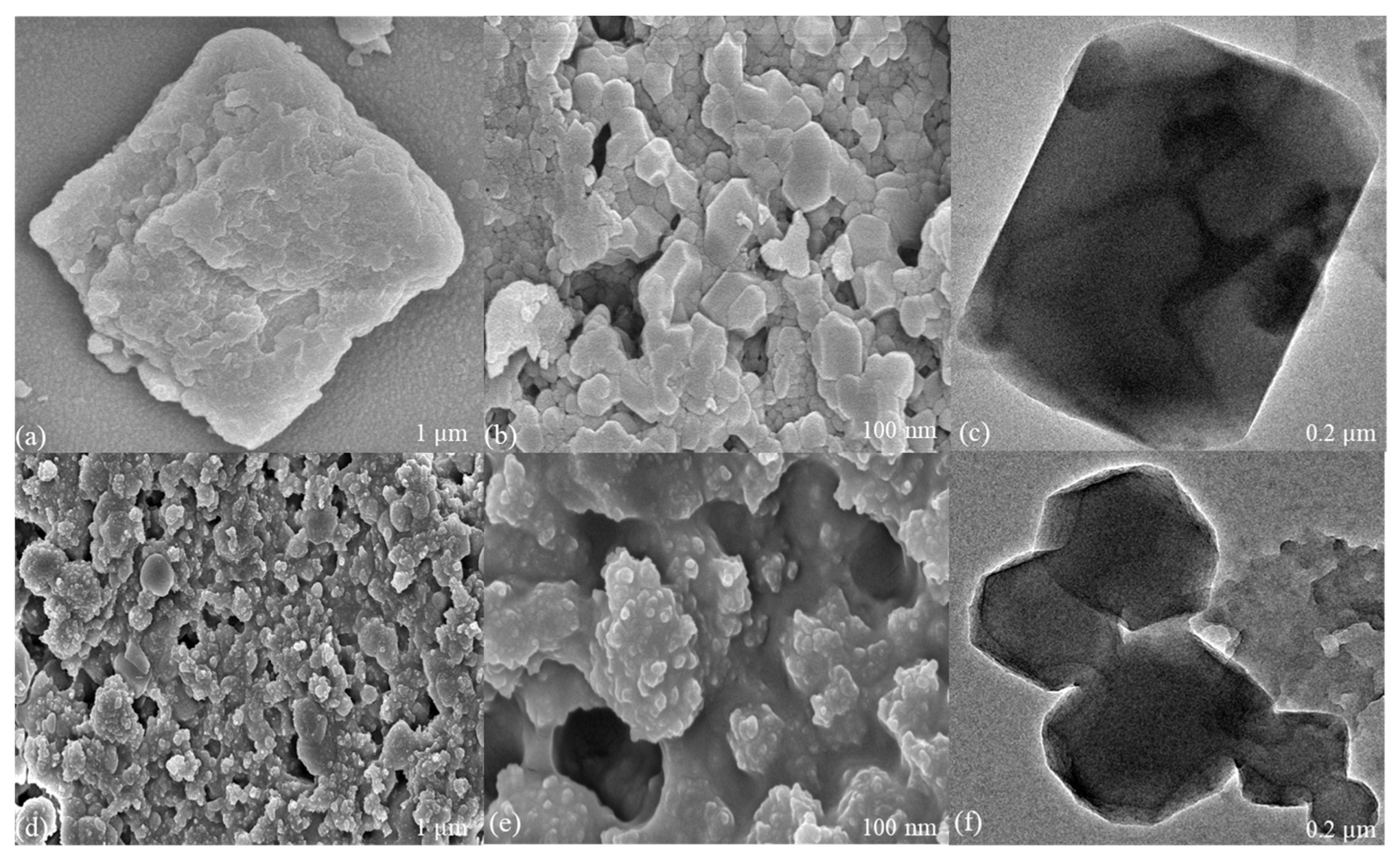
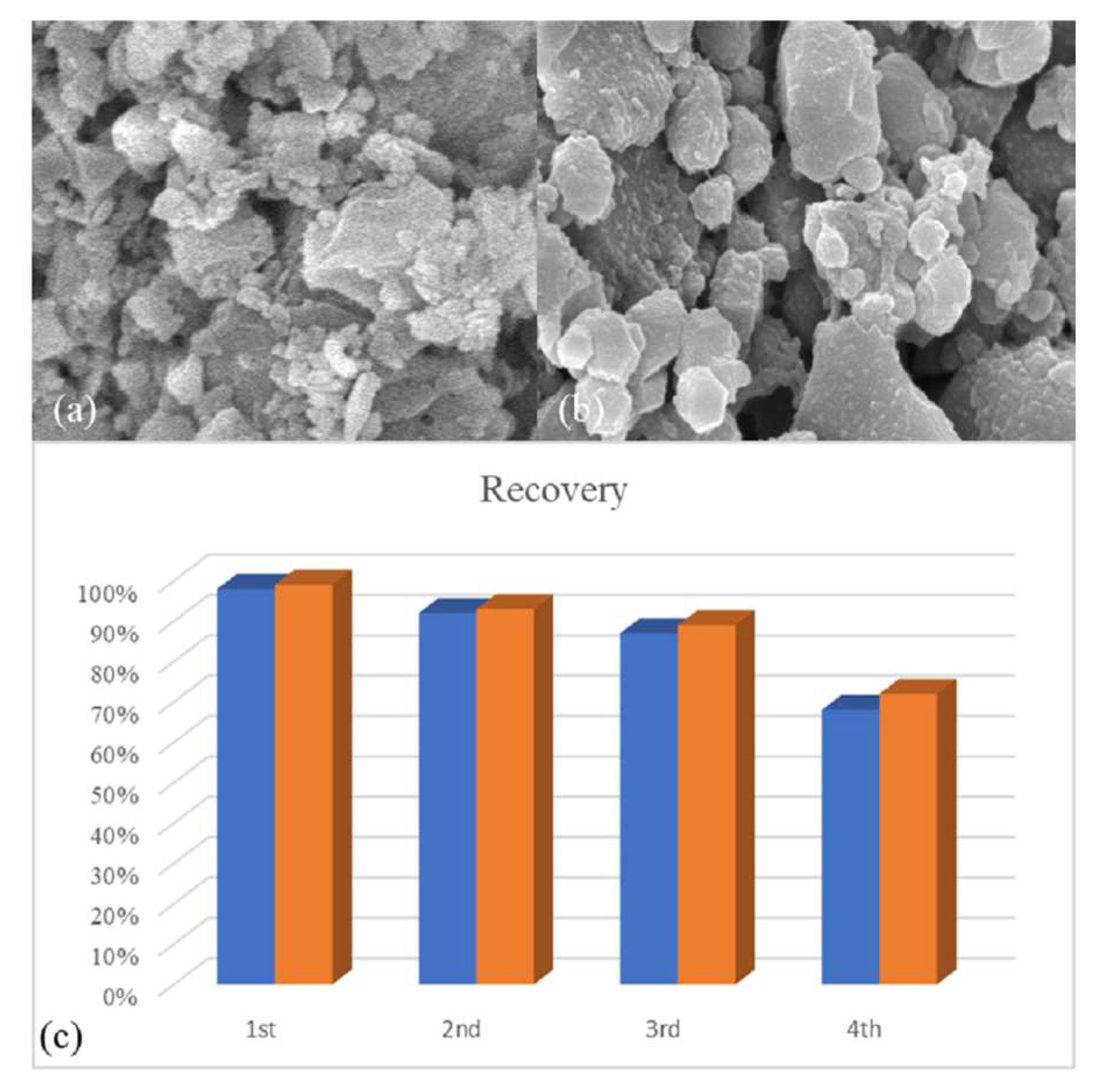

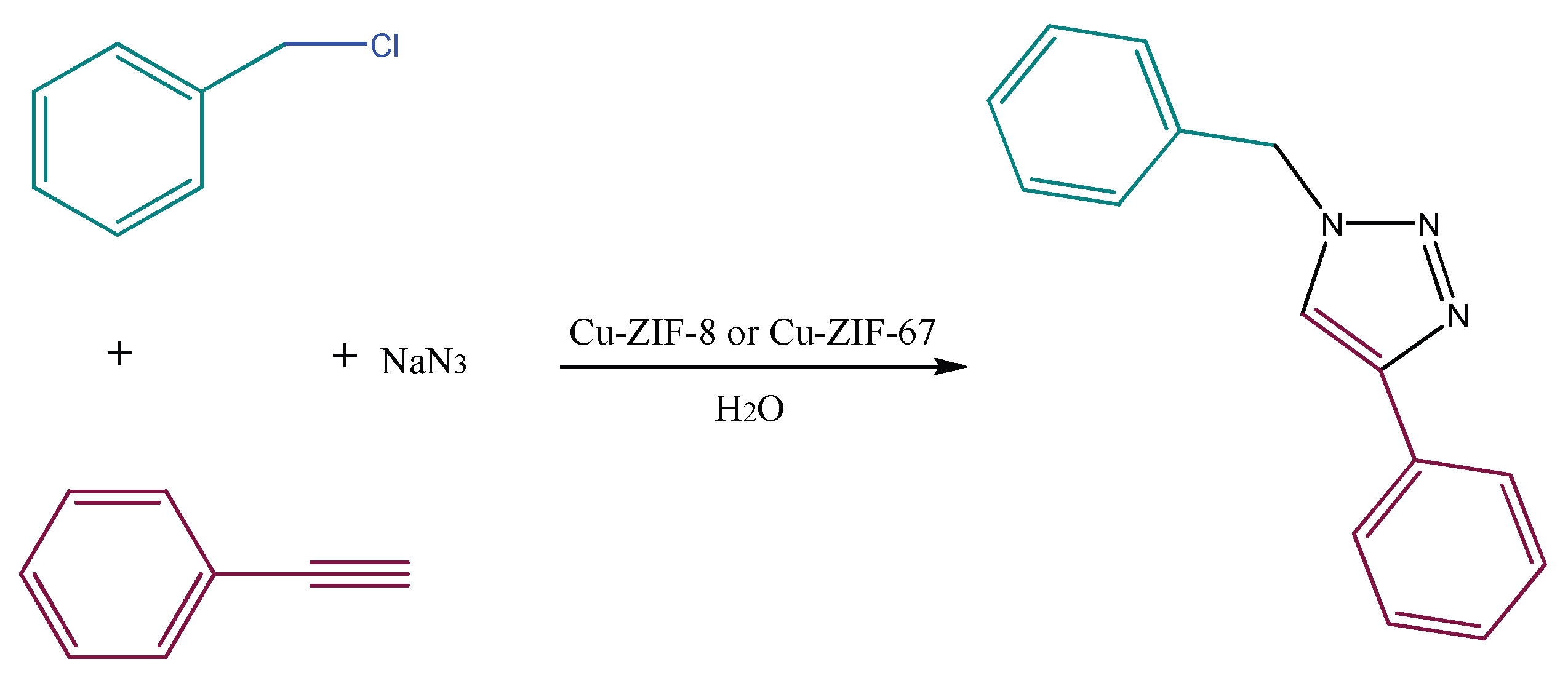
| Sample | BET | Langmuir | Pore Size (nm) | Pore Volume (cm3.g−1) |
|---|---|---|---|---|
| Cu10ZIF-8 | 1390.37 | 1477.82 | 1.437 | 0.499 |
| Cu20ZIF-67 | 1152.96 | 1256.79 | 1.490 | 0.429 |
| Samples | Co or Zn (%wt) | Cu (%wt) |
|---|---|---|
| Cu20ZIF-67 | 22.38 | 6.34 |
| Cu10ZIF-8 | 37.80 | 3.74 |
| Catalyst | Quantity | Time | Temperature | Solvent | Reusability | Substrate |
|---|---|---|---|---|---|---|
| Cu10ZIF-8 | 0.001 g | 5 h | 85 °C | water | 4 cycles | C8H6, C7H7Cl |
| Cu20ZIF-67 | 0.001 g | 2 h | 90 °C | water | 4 cycles | C8H6, C7H7Cl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anbari, A.P.; Delcheh, S.R.; Heynderickx, P.M.; Chaemcheun, S.; Zhuiykov, S.; Verpoort, F. Green Approach for Synthesizing Copper-Containing ZIFs as Efficient Catalysts for Click Chemistry. Catalysts 2023, 13, 1003. https://doi.org/10.3390/catal13061003
Anbari AP, Delcheh SR, Heynderickx PM, Chaemcheun S, Zhuiykov S, Verpoort F. Green Approach for Synthesizing Copper-Containing ZIFs as Efficient Catalysts for Click Chemistry. Catalysts. 2023; 13(6):1003. https://doi.org/10.3390/catal13061003
Chicago/Turabian StyleAnbari, Alireza Pourvahabi, Shima Rahmdel Delcheh, Philippe M. Heynderickx, Somboon Chaemcheun, Serge Zhuiykov, and Francis Verpoort. 2023. "Green Approach for Synthesizing Copper-Containing ZIFs as Efficient Catalysts for Click Chemistry" Catalysts 13, no. 6: 1003. https://doi.org/10.3390/catal13061003
APA StyleAnbari, A. P., Delcheh, S. R., Heynderickx, P. M., Chaemcheun, S., Zhuiykov, S., & Verpoort, F. (2023). Green Approach for Synthesizing Copper-Containing ZIFs as Efficient Catalysts for Click Chemistry. Catalysts, 13(6), 1003. https://doi.org/10.3390/catal13061003