Techno-Economic Analysis of Electrocatalytic CO2 Reduction into Methanol: A Comparative Study between Alkaline Flow Cell and Neutral Membrane Electrode Assembly
Abstract
:1. Introduction
2. Model for CO2 Electrolyzer System
3. Economic Cost Analysis
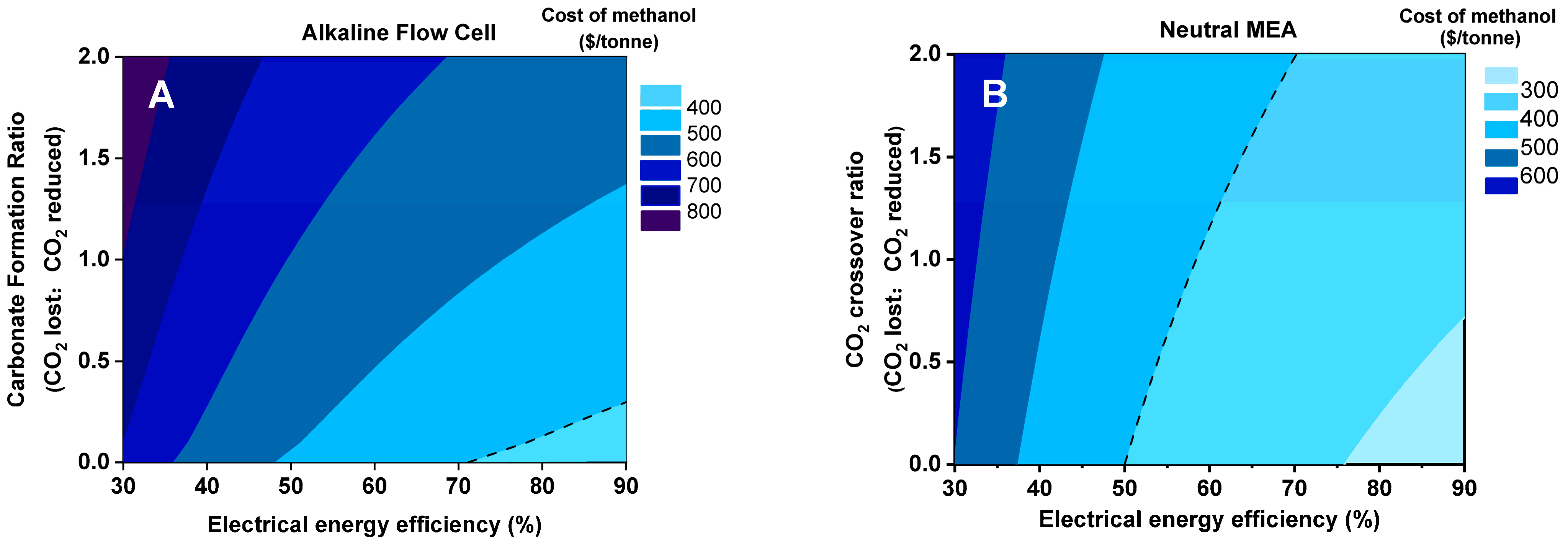
3.1. Sensitivity Analysis
3.2. Comparative Analysis of the Cost of Methanol Production between Two Types of Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, K.; Ashworth, P.; Zhang, S.; Liang, X.; Sun, Y.; Angus, D. China’s carbon capture, utilization and storage (CCUS) policy: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109601. [Google Scholar] [CrossRef]
- Wu, D.; He, B.; Wang, Y.; Lv, P.; Ma, D.; Jia, Y. Double-atom catalysts for energy-related electrocatalysis applications: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 203001. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity inelectrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochem. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Yavari, Z.; Meshginkhoud, A.; Barjahri, R.M.; Kerman, K.; Noroozifar, M. CH3OH electrooxidation by nanosized Pd loaded on porous LaMnO3. Mater. Today Chem. 2021, 19, 100398. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2020, 57, 2165–2177. [Google Scholar] [CrossRef]
- Adnan, M.A.; Kibria, M.G. Comparative techno-economic and life-cycle assessment of power-to-methanol synthesis pathways. Appl. Energy 2020, 278, 115614. [Google Scholar] [CrossRef]
- Brushett, M.O.S. A general techno-economic model for evaluating emerging electrolytic processes. Energy Technol. 2020, 11, 1900994. [Google Scholar]
- De Luna, P.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 2019, 364, 3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, J.; Seo, B.; Kim, J.; Lee, C.W.; Lee, H.; Hwang, Y.J.; Min, B.K.; Lee, D.K.; Oh, H.-S.; Lee, U. General technoeconomic analysis for electrochemical coproduction coupling carbon dioxide reduction with organic oxidation. Nat. Commun. 2019, 10, 5193. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Hamasaki, Y.; Kim, C.; Huang, W.; Lu, S.; Jhong, H.-R.M.; Gewirth, A.A.; Fujigaya, T.; Nakashima, N.; Kenis, P.J.A. Insights into the Low Overpotential Electroreduction of CO2 to CO on a Supported Gold Catalyst in an Alkaline Flow Electrolyzer. ACS Energy Lett. 2018, 3, 193–198. [Google Scholar] [CrossRef]
- Leverett, J.; Khan, M.H.A.; Tran-Phu, T.; Tricoli, A.; Hocking, R.K.; Yun, S.L.J.; Dai, L.; Daiyan, R.; Amal, R. Renewable Power for Electrocatalytic Gener-ation of Syngas: Tuning the Syngas Ratio by Manipulating the Active Sites and System Design. ChemCatChem 2022, 14, 4317–4327. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, L.; Hensen, E.J.M.; Hofmann, J.P. Evaluating the Stability of Co2P Electrocatalysts in the Hydrogen Evolution Reaction for Both Acidic and Alkaline Electrolytes. ACS Energy Lett. 2018, 3, 1360–1365. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Seifitokaldani, A.; Saidaminov, M.I.; Tan, C.S.; Quan, L.N.; Proppe, A.; Kibria, M.G.; et al. 2D Metal Oxyhalide-Derived Catalysts for Efficient CO2 Electroreduction. Adv. Mater. 2018, 30, 1802858. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Li, H.; Lin, L.; Gao, D.; Zhang, X.; Gong, H.; Qing, G.; Cai, R.; Wang, G.; Bao, X. CO2 electrolysis at industrial current densities using anion exchange membrane based electrolyzers. Sci. China Chem. 2020, 63, 1711–1715. [Google Scholar] [CrossRef]
- McCallum, C.; Gabardo, C.M.; O’Brien, C.P.; Edwards, J.P.; Wicks, J.; Xu, Y.; Sargent, E.H.; Sinton, D. Reducing the crossover of carbonate and liquid products during carbon dioxide electroreduction. Cell Rep. Phys. Sci. 2021, 2, 100522. [Google Scholar] [CrossRef]
- Blommaert, M.A.; Subramanian, S.; Yang, K.; Smith, W.A.; Vermaas, D.A. High Indirect Energy Consumption in AEM-Based CO2 Electrolyzers Demonstrates the Potential of Bipolar Membranes. ACS Appl. Mater. Interfaces 2022, 14, 557–563. [Google Scholar] [CrossRef]
- Gao, F.Y.; Bao, R.C.; Gao, M.R.; Yu, S.H. Electrochemical CO2-to-CO conversion: Electrocatalysts, electrolytes, and electrolyzers. J. Mater. Chem. A Mater. Energy Sustain. 2020, 8, 15458–15478. [Google Scholar] [CrossRef]
- Lin, R.; Guo, J.; Li, X.; Patel, P.; Seifitokaldani, A. Electrochemical Reactors for CO2 Conversion. Catalysts 2020, 10, 473. [Google Scholar] [CrossRef]
- Sisler, J.; Khan, S.; Ip, A.H.; Schreiber, M.W.; Jaffer, S.A.; Bobicki, E.R.; Dinh, C.T.; Sargent, E.H. Ethylene electrosynthesis: A comparative techno-economic analysis of alkaline vs membrane electrode assembly vs CO2–CO–C2H4 tandems. ACS Energy Lett. 2021, 6, 997. [Google Scholar] [CrossRef]
- Atkinson, S. Recent developments in polymer electrolyte membrane-based fuel cells. Membr. Technol. 2004, 2004, 7–9. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, P.; Jiang, Q.; Pan, Y.; Liang, W.; Stavitski, E.; Alshareef, H.N.; Wang, H. Continuous production of pure liquid fuel solutions via electro-catalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 2019, 4, 776. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, Q.; Dai, L.; Qin, R.; Zhao, X.; Chen, X.; Ou, D.; Chen, J.; Chuong, T.T.; Wu, B.; et al. Electrochemical Reduction of Carbon Dioxide to Methanol on Hierarchical Pd/SnO2 Nanosheets with Abundant Pd-O-Sn Interfaces. Angew. Chem. Int. Ed. 2018, 57, 9475–9479. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, S.; Tan, X.; Wu, R.; Yan, X.; Chen, C.; Zhu, Q.; Zheng, L.; Ma, J.; Zhang, J.; et al. Highly Efficient CO2 Electroreduction to Methanol through Atomically Dispersed Sn Coupled with Defective CuO Catalysts. Angew. Chem. Int. Ed. 2021, 60, 21979–21987. [Google Scholar] [CrossRef]
- Ji, L.; Chang, L.; Zhang, Y.; Mou, S.; Wang, T.; Luo, Y.; Wang, Z.; Sun, X. Electrocatalytic CO2 Reduction to Alcohols with High Selectivity over a Two-Dimensional Fe2P2S6 Nanosheet. ACS Catal. 2019, 9, 9721–9725. [Google Scholar] [CrossRef]
- Kong, S.; Lv, X.; Wang, X.; Liu, Z.; Li, Z.; Jia, B.; Sun, D.; Yang, C.; Liu, L.; Guan, A.; et al. Delocalization state-induced selective bond breaking for efficient methanol electrosynthesis from CO2. Nat. Catal. 2023, 6, 6–15. [Google Scholar] [CrossRef]
- Bagchi, D.; Raj, J.; Singh, A.K.; Cherevotan, A.; Roy, S.; Manoj, K.S.; Vinod, C.P.; Peter, S.C. Structure-tailored surface oxide on Cu-Ga intermetallics enhances CO2 reduction selectivity to methanol at ultralow potential. Adv. Mater. 2022, 34, e2109426. [Google Scholar] [CrossRef]
- Herranz, J.; Pătru, A.; Fabbri, E.; Schmidt, T.J. Co-electrolysis of CO2 and H2O: From electrode reactions to cell-level development. Curr. Opin. Electrochem. 2020, 23, 89–95. [Google Scholar] [CrossRef]
- Gabardo, C.M.; O’Brien, C.P.; Edwards, J.P.; McCallum, C.; Xu, Y.; Dinh, C.-T.; Li, J.; Sargent, E.H.; Sinton, D. Continuous Carbon Dioxide Electroreduction to Concentrated Multi-carbon Products Using a Membrane Electrode Assembly. Joule 2019, 3, 2777–2791. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Lang, C.C.; Gao, M.R.; Chen, Y.; Fu, Q.Q.; Duan, Y.; Yu, S.H. Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energy Environ. Sci. 2018, 11, 1890. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 2020, 19, 1140–1150. [Google Scholar] [CrossRef]
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 2020, 5, 378. [Google Scholar] [CrossRef]
- Ozden, A.; Wang, Y.; Li, F.; Luo, M.; Sisler, J.; Thevenon, A.; Rosas-Hernández, A.; Burdyny, T.; Lum, Y.; Yadegari, H.; et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 2021, 5, 706–719. [Google Scholar] [CrossRef]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.-J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef]
- Kutz, R.B.; Chen, Q.; Yang, H.; Sajjad, S.D.; Liu, Z.; Masel, I.R. Sustainion Imidazolium-Functionalized Polymers for Carbon Dioxide Electrolysis. Energy Technol. 2017, 5, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, C.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What should we make with CO2 and how can we make it? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Spurgeon, J.M.; Kumar, B. A comparative technoeconomic analysis of pathways for commercial electrochemical CO2 reduction to liquid products. Energy Environ. Sci. 2018, 11, 1536–1551. [Google Scholar] [CrossRef]
- El-Shafie, O.A.; El-Maghraby, R.M.; Albo, J.; Fateen, S.-E.K.; Abdelghany, A. Modeling and numerical investigation of the performance of gas diffusion electrodes for the electrochemical reduction of carbon dioxide to methanol. Ind. Eng. Chem. 2020, 59, 20929–20942. [Google Scholar] [CrossRef]
- Jones, J.P.; Prakash, G.K.S.; Olah, G.A. Electrochemical CO2 reduction: Recent advances and current trends. Isr. J. Chem. 2014, 54, 1451–1466. [Google Scholar] [CrossRef]
- Ma, W.; Xie, S.; Liu, T.; Fan, Q.; Ye, J.; Sun, F.; Jiang, Z.; Zhang, Q.; Cheng, J.; Wang, Y. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 2020, 3, 478–487. [Google Scholar] [CrossRef]
- Ruth, M.F.; Mayyas, A.T.; Mann, M.K. Manufacturing competitiveness analysis for PEM and alkaline water electrolysis systems. In Proceedings of the Manufacturing Competitiveness Analysis for PEM and Alkaline Water Electrolysis Systems, Long Beach, CA, USA, 6–9 November 2017. [Google Scholar]
- Paturska, A.; Repele, M.; Bazbauers, G. Economic assessment of biomethane supply system based on natural gas infrastructure. Energy Procedia 2015, 72, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Keith, D.W. Why capture CO2 from the atmosphere? Science 2009, 325, 1654–1655. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Lopatnikov, A. Scaling laws: Uses and misuses in industrial plant and equipment replacement cost estimates. MTS J. 2017, 33, 38–44. [Google Scholar]
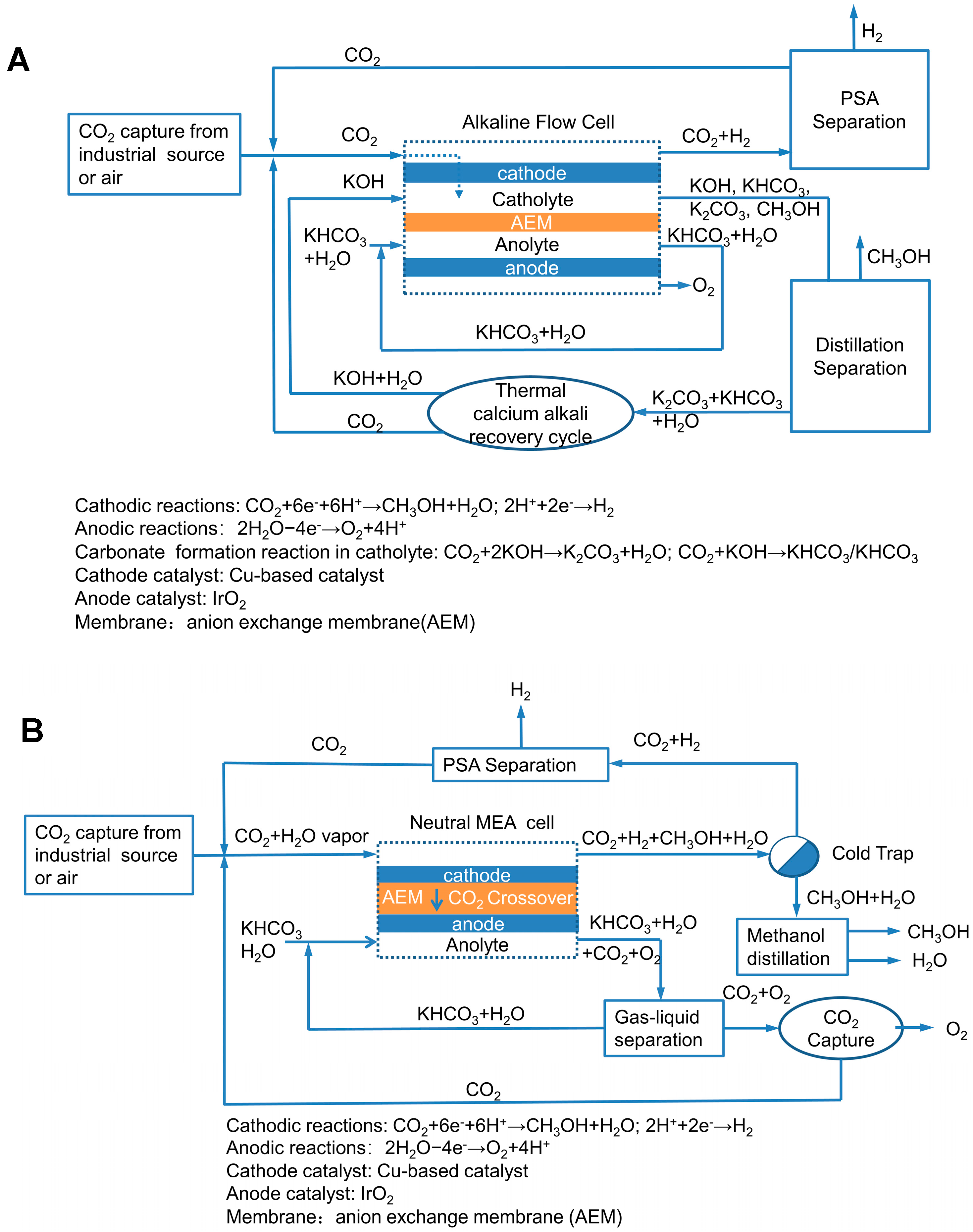
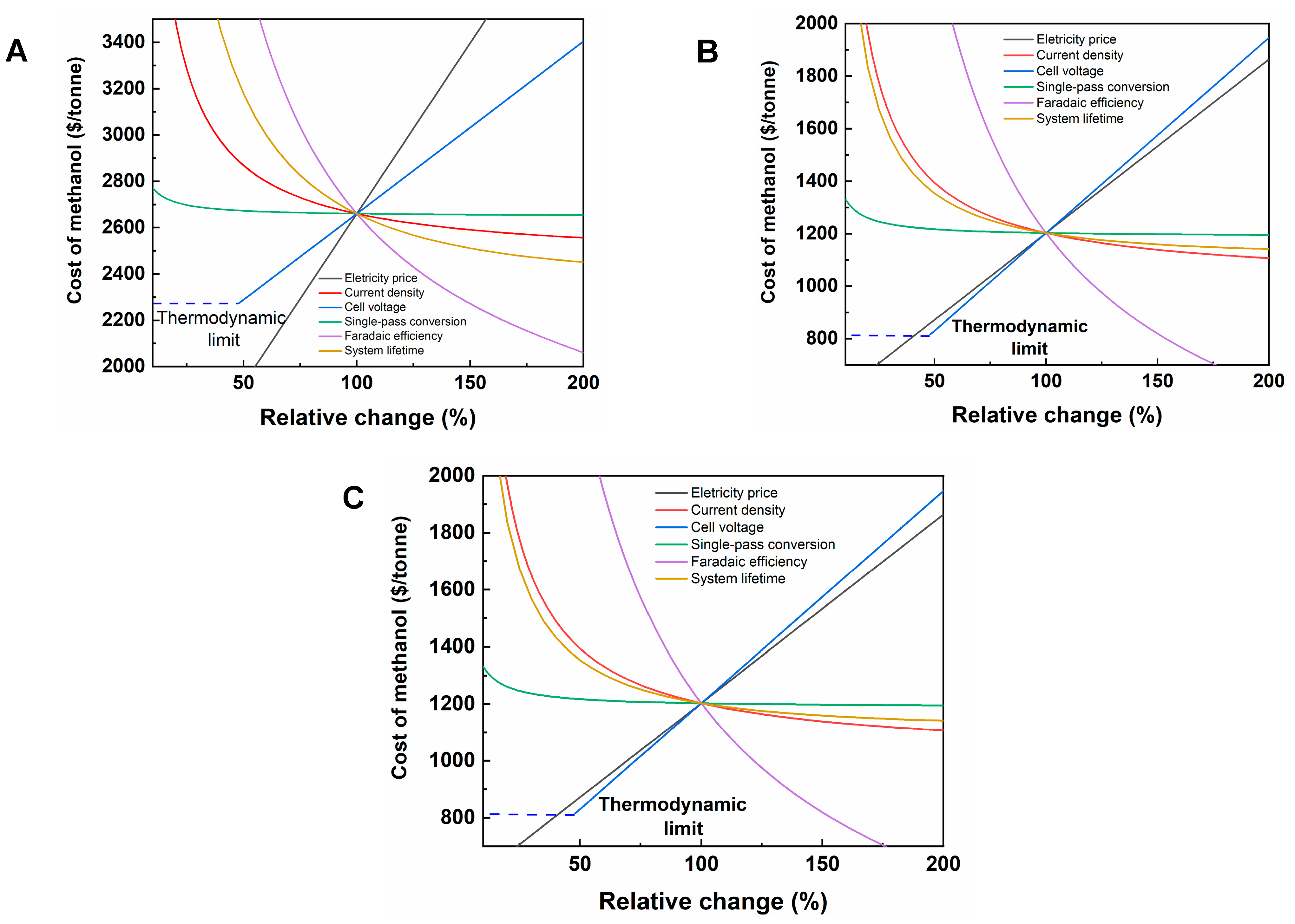
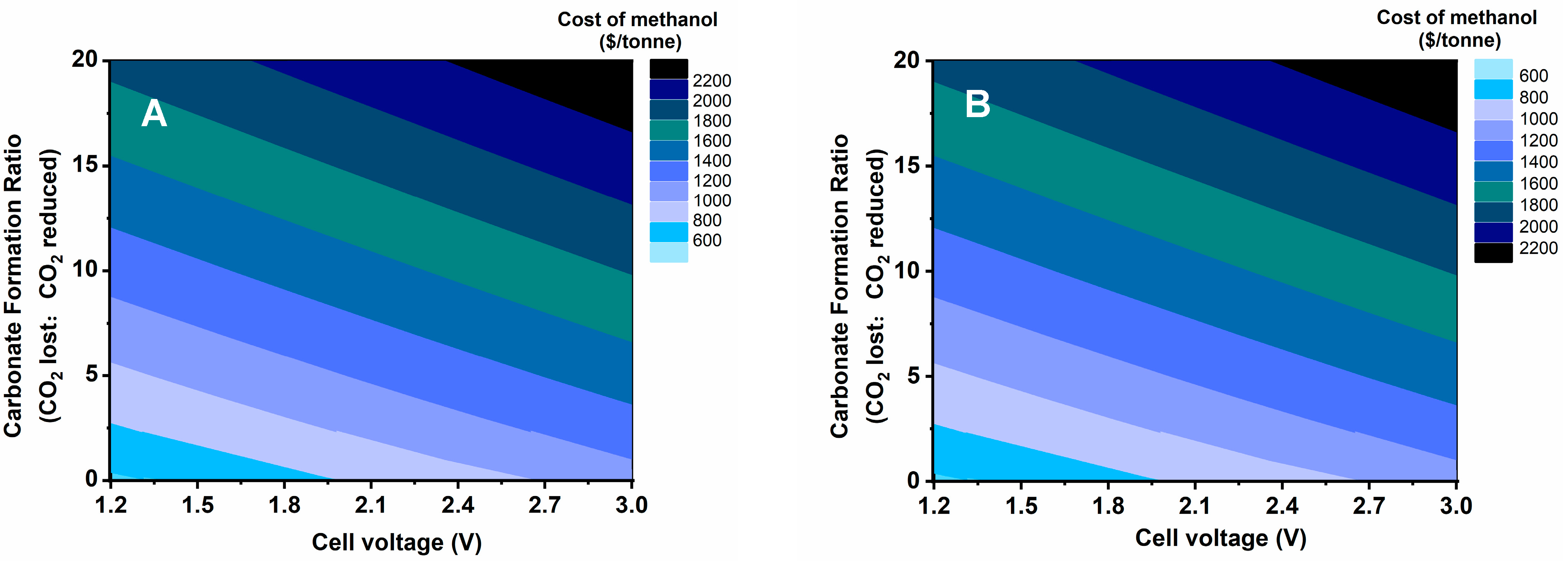
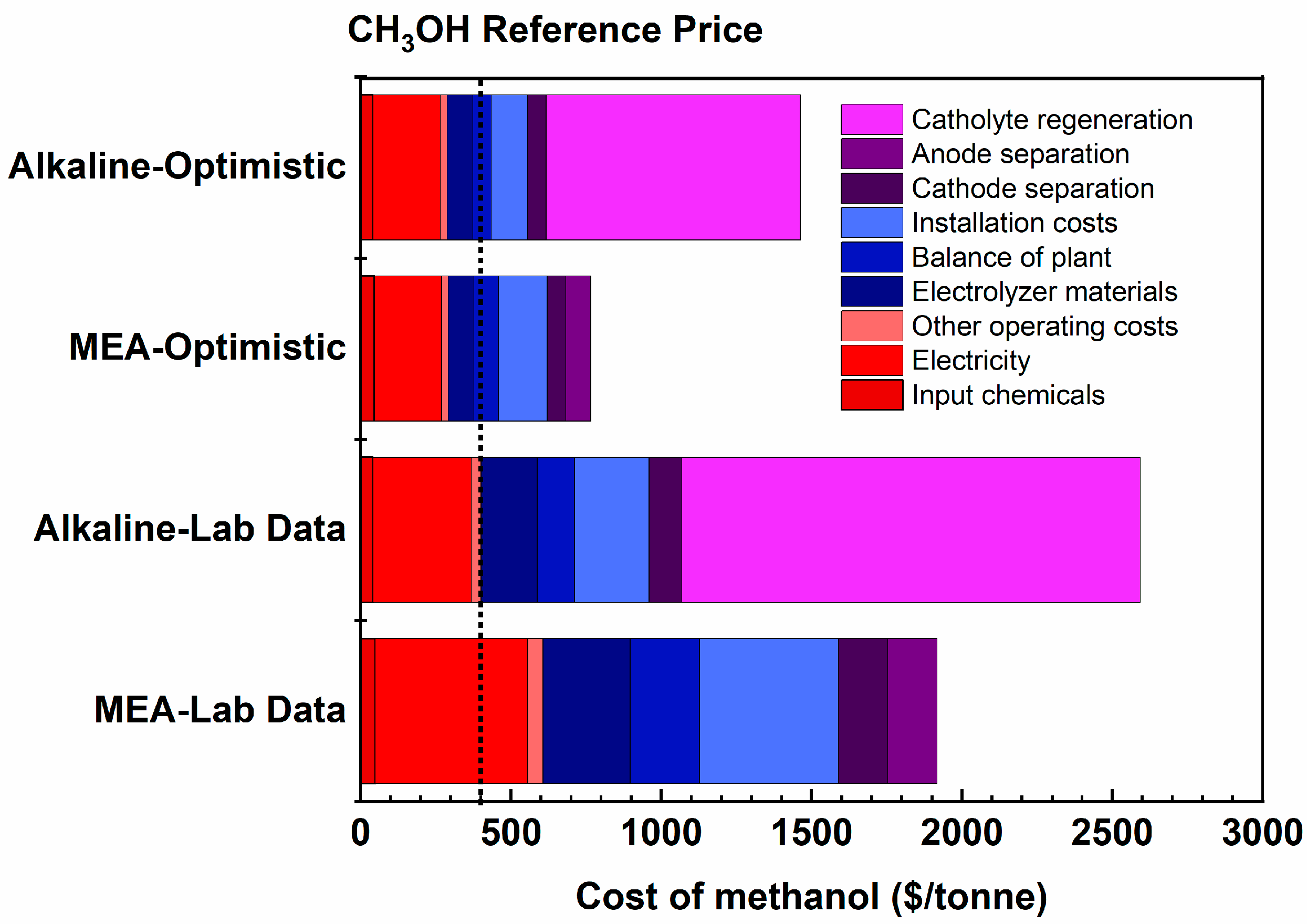
| Electrolyzer | Cell Voltage (V) | Faradaic Efficiency (%) | Current Density (mA/cm2) | Electrical Energy Efficiency (%) | Crossover/Carbonate Formation Ratio | Single-Pass Conversion (%) |
|---|---|---|---|---|---|---|
| Neutral MEA, laboratory data [16] | 3.4 | 70 | 100 | 24.7% | 4 | 30 |
| Alkaline flow cell, laboratory data [15] | 2.5 | 78 | 200 | 37.0% | 24 | 30 |
| Neutral MEA, optimized [12] | 2.0 | 90 | 1000 | 54.0% | 2 | 40 |
| Alkaline flow cell, optimized [12] | 2.0 | 90 | 1000 | 54.0% | 12 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Qu, T.; Li, Q.; Tan, S.; Chen, X. Techno-Economic Analysis of Electrocatalytic CO2 Reduction into Methanol: A Comparative Study between Alkaline Flow Cell and Neutral Membrane Electrode Assembly. Catalysts 2023, 13, 1005. https://doi.org/10.3390/catal13061005
Wang K, Qu T, Li Q, Tan S, Chen X. Techno-Economic Analysis of Electrocatalytic CO2 Reduction into Methanol: A Comparative Study between Alkaline Flow Cell and Neutral Membrane Electrode Assembly. Catalysts. 2023; 13(6):1005. https://doi.org/10.3390/catal13061005
Chicago/Turabian StyleWang, Ke, Tongxin Qu, Qiang Li, Shuting Tan, and Xiaoxiang Chen. 2023. "Techno-Economic Analysis of Electrocatalytic CO2 Reduction into Methanol: A Comparative Study between Alkaline Flow Cell and Neutral Membrane Electrode Assembly" Catalysts 13, no. 6: 1005. https://doi.org/10.3390/catal13061005




