Visible-Light-Induced Difunctionalization of the C-C Bond of Alkylidenecyclopropanes with Acyl Chlorides
Abstract
1. Introduction
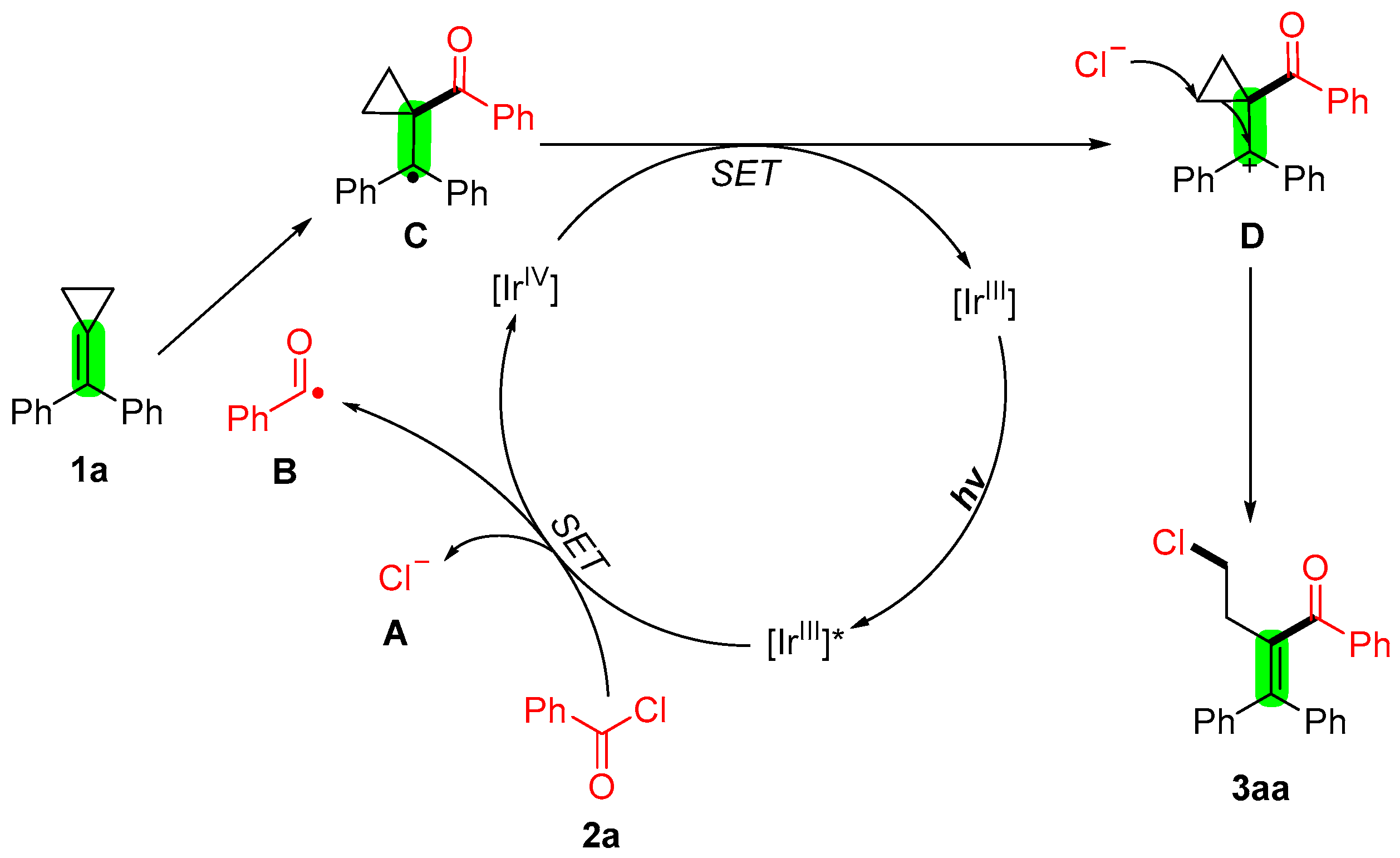
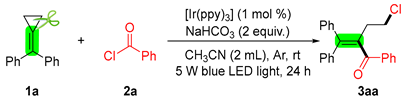
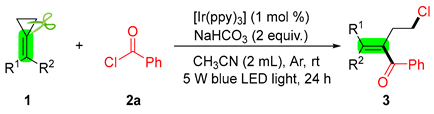
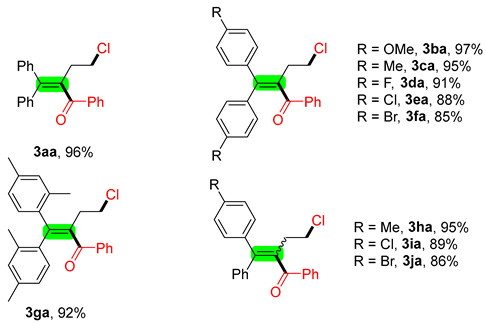
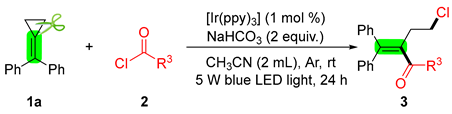
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of 3
3.3. Characterization Data for 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cohen, Y.; Cohen, A.; Marek, I. Creating Stereocenters within Acyclic Systems by C-C Bond Cleavage of Cyclopropanes. Chem. Rev. 2021, 121, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, G.; Stanton, S.; Bower, J.F. Recent Methodologies That Exploit C-C Single-Bond Cleavage of Strained Ring Systems by Transition Metal Complexes. Chem. Rev. 2017, 117, 9404–9432. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zang, W.; Bird, M.J.; Hyland, C.J.T.; Shi, M. Gold-Catalyzed Conversion of Highly Strained Compounds. Chem. Rev. 2021, 121, 8685–8755. [Google Scholar] [CrossRef] [PubMed]
- Pirenne, V.; Muriel, B.; Waser, J. Catalytic Enantioselective Ring-Opening Reactions of Cyclopropanes. Chem. Rev. 2021, 121, 227–263. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.O.; Bower, J.F. Selective Carbon-Carbon Bond Cleavage of Cyclopropylamine Derivatives. Chem. Rev. 2021, 121, 80–109. [Google Scholar] [CrossRef]
- Wang, J.; Blaszczyk, S.A.; Li, X.; Tang, W. Transition Metal-Catalyzed Selective Carbon-Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions. Chem. Rev. 2021, 121, 110–139. [Google Scholar] [CrossRef]
- Brandi, A.; Cicchi, S.; Cordero, F.M.; Goti, A. Heterocycles from Alkylidenecyclopropanes. Chem. Rev. 2003, 103, 1213–1270. [Google Scholar] [CrossRef]
- Brandi, A.; Cicchi, S.; Cordero, F.M.; Goti, A. Progress in the Synthesis and Transformations of Alkylidenecyclopropanes and Alkylidenecyclobutanes. Chem. Rev. 2014, 114, 7317–7420. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in the synthesis and reactivity of methylene- and alkylidenecyclopropane derivatives. Tetrahedron 2014, 70, 4991–5031. [Google Scholar] [CrossRef]
- Yu, L.-Z.; Chen, K.; Zhu, Z.-Z.; Shi, M. Recent advances in the chemical transformations of functionalized alkylidenecyclopropanes (FACPs). Chem. Commun. 2017, 53, 5935–5945. [Google Scholar] [CrossRef]
- Yu, L.-Z.; Shi, M. The Construction of Molecular Complexity from Functionalized Alkylidenecyclopropanes (FACPs). Chem.—Eur. J. 2019, 25, 7591–7606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Li, P.-H.; Shi, M. Fluorination of Alkylidenecyclopropanes. Asian J. Org. Chem. 2018, 7, 1924–1933. [Google Scholar] [CrossRef]
- Fan, X.; Liu, R.; Wei, Y.; Shi, M. Rh-Catalyzed intramolecular decarbonylative cyclization of ortho-formyl group tethered alkylidenecyclopropanes (ACPs) for the construction of 2-methylindenes. Org. Chem. Front. 2019, 6, 2667–2671. [Google Scholar] [CrossRef]
- Lautens, M.; Klute, W.; Tam, W. Transition Metal-Mediated Cycloaddition Reactions. Chem. Rev. 1996, 96, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-S.; Lu, S.-C.; Chang, Z.-X.; Hao, L.; Li, F.-R.; Xia, C. Rhodium-Catalyzed Ring-Opening Hydroacylation of Alkylidenecyclopropanes with Chelating Aldehydes for the Synthesis of γ,δ-Unsaturated Ketones. Org. Lett. 2020, 22, 5145–5150. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Zeng, Y.-F.; Shu, B.; Zheng, Y.-C.; Xiao, L.; Chen, S.-Y.; Song, J.-L.; Zhang, X.; Zhang, S.-S. Rh(III)-Catalyzed dienylation and cyclopropylation of indoles at the C4 position with alkylidenecyclopropanes. Org. Chem. Front. 2022, 9, 4287–4293. [Google Scholar] [CrossRef]
- Xu, G.; Chen, Q.; Wu, F.; Bai, D.; Chang, J.; Li, X. Rh(III)-Catalyzed Chemodivergent Coupling of N-Phenoxyacetamides and Alkylidenecyclopropanes via C-H Activation. Org. Lett. 2021, 23, 2927–2932. [Google Scholar] [CrossRef]
- Yang, L.-M.; Zeng, H.-H.; Liu, X.-L.; Ma, A.-J.; Peng, J.-B. Copper catalyzed borocarbonylation of benzylidenecyclopropanes through selective proximal C-C bond cleavage: Synthesis of γ-boryl-γ,δ-unsaturated carbonyl compounds. Chem. Sci. 2022, 13, 7304–7309. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Tang, X.-Y.; Shi, M. Gold-Catalyzed Tandem Reactions of Methylenecyclopropanes and Vinylidenecyclopropanes. Acc. Chem. Res. 2014, 47, 913–924. [Google Scholar] [CrossRef]
- Hu, B.; Xing, S.; Wang, Z. Lewis Acid Catalyzed Ring-Opening Intramolecular Friedel-Crafts Alkylation of Methylenecyclopropane 1,1-Diesters. Org. Lett. 2008, 10, 5481–5484. [Google Scholar] [CrossRef]
- Rajamaki, S.; Kilburn, J.D. Lewis acid mediated endo-cyclisation of trimethylsilylmethylenecyclopropyl imines—A stereoselective route to indolizidines. Chem. Commun. 2005, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lu, J.-M.; Wei, Y.; Shao, L.-X. Rapid Generation of Molecular Complexity in the Lewis or Brønsted Acid-Mediated Reactions of Methylenecyclopropanes. Acc. Chem. Res. 2012, 45, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Xu, B.; Huang, J.-W. Lewis Acid-Mediated Cycloaddition of Methylenecyclopropanes with Aldehydes and Imines: A Facile Access to Indene, THF, and Pyrrolidine Skeletons via Homoallylic Rearrangement Protocol. Org. Lett. 2004, 6, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Y.; Shi, M. HOTf-Catalyzed Rearrangement of Methylenecyclopropane Aryl and Alkyl Alcohols. Eur. J. Org. Chem. 2010, 2010, 4106–4110. [Google Scholar] [CrossRef]
- Huang, Z.-J.; Qin, J.-H.; Huang, M.-L.; Sun, Q.; Xie, H.-Y.; Li, Y.; Li, J.-H. Electrochemical dehydrogenative cyclization/aromatization of aniline-tethered alkylidenecyclopropanes: Facile access to benzo[c]carbazoles. Org. Chem. Front. 2023, 10, 1557–1563. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Shi, M. Visible light mediated synthesis of 4-aryl-1,2-dihydronaphthalene derivatives via single-electron oxidation or MHAT from methylenecyclopropanes. Org. Chem. Front. 2021, 8, 94–100. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-L.; Chen, Z.; Li, H.; Xiong, B.-Q.; Zhang, P.-L.; Tang, K.-W. Visible-light photoredox-catalyzed dual C-C bond cleavage: Synthesis of 2-cyanoalkylsulfonylated 3,4-dihydronaphthalenes through the insertion of sulfur dioxide. Chem. Commun. 2020, 56, 3011–3014. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-L.; Chen, Z.; Zhou, Q.; Li, H.; Zhou, C.-S.; Xiong, B.-Q.; Zhang, P.-L.; Tang, K.-W. Visible-Light-Catalyzed C-C Bond Difunctionalization of Methylenecyclopropanes with Sulfonyl Chlorides for the Synthesis of 3-Sulfonyl-1,2-dihydronaphthalenes. J. Org. Chem. 2019, 84, 2829–2839. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Y.; Chen, T.; Pan, Y.; Xu, Q. Direct Synthesis of Methylene-1,2-dichalcogenolanes via Radical [3 + 2] Cycloaddition of Methylenecyclopropanes with Elemental Chalcogens. Org. Lett. 2013, 15, 144–147. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, S.; Sun, Z.; Su, Y.; Ma, Q.; Yuan, Y.; Jia, X. Tris(4-bromophenyl)aminium Hexachloroantimonate-Initiated Oxidative Povarov-Type Reaction between Glycine Esters and (Cyclopropylidenemethyl)benzenes Using the Counterion as a Chlorine Donor. Org. Lett. 2020, 22, 6294–6298. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Ning, C.; Mao, B.; Wei, Y.; Shi, M. A visible-light mediated ring opening reaction of alkylidenecyclopropanes for the generation of homopropargyl radicals. Chem. Sci. 2021, 12, 9088–9095. [Google Scholar] [CrossRef]
- Armaly, A.M.; Bar, S.; Schindler, C.S. Acid Chlorides as Formal Carbon Dianion Linchpin Reagents in the Aluminum Chloride-Mediated Dieckmann Cyclization of Dicarboxylic Acids. Org. Lett. 2017, 19, 3962–3965. [Google Scholar] [CrossRef] [PubMed]
- Bousfield, T.W.; Pearce, K.P.R.; Nyamini, S.B.; Angelis-Dimakis, A.; Camp, J.E. Synthesis of amides from acid chlorides and amines in the bio-based solvent Cyrene™. Green Chem. 2019, 21, 3675–3681. [Google Scholar] [CrossRef]
- Fu, L.; You, J.; Nishihara, Y. Palladium-catalyzed decarbonylative and decarboxylative cross-coupling of acyl chlorides with potassium perfluorobenzoates affording unsymmetrical biaryls. Chem. Commun. 2021, 57, 3696–3699. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Denton, E.H.; Morandi, B. Palladium-catalysed carboformylation of alkynes using acid chlorides as a dual carbon monoxide and carbon source. Nat. Chem. 2021, 13, 123–130. [Google Scholar] [CrossRef]
- Pan, F.; Boursalian, G.B.; Ritter, T. Palladium-Catalyzed Decarbonylative Difluoromethylation of Acid Chlorides at Room Temperature. Angew. Chem. Int. Ed. 2018, 57, 16871–16876. [Google Scholar] [CrossRef]
- Boudjelel, M.; Sadek, O.; Mallet-Ladeira, S.; García-Rodeja, Y.; Sosa Carrizo, E.D.; Miqueu, K.; Bouhadir, G.; Bourissou, D. Phosphine-Borane Ligands Induce Chemoselective Activation and Catalytic Coupling of Acyl Chlorides at Palladium. ACS Catal. 2021, 11, 3822–3829. [Google Scholar] [CrossRef]
- Cherney, A.H.; Kadunce, N.T.; Reisman, S.E. Catalytic Asymmetric Reductive Acyl Cross-Coupling: Synthesis of Enantioenriched Acyclic α,α-Disubstituted Ketones. J. Am. Chem. Soc. 2013, 135, 7442–7445. [Google Scholar] [CrossRef]
- Ding, D.; Wang, C. Nickel-Catalyzed Reductive Electrophilic Ring Opening of Cycloketone Oxime Esters with Aroyl Chlorides. ACS Catal. 2018, 8, 11324–11329. [Google Scholar] [CrossRef]
- Huang, Y.; Smith, K.B.; Brown, M.K. Copper-Catalyzed Borylacylation of Activated Alkenes with Acid Chlorides. Angew. Chem. Int. Ed. 2017, 56, 13314–13318. [Google Scholar] [CrossRef]
- Panferova, L.I.; Miloserdov, F.M.; Lishchynskyi, A.; Martínez Belmonte, M.; Benet-Buchholz, J.; Grushin, V.V. Well-Defined CuC2F5 Complexes and Pentafluoroethylation of Acid Chlorides. Angew. Chem. Int. Ed. 2015, 54, 5218–5222. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Wu, X.; Huang, W.; Qu, J.; Chen, Y. Recent advances in transition metal-catalyzed reactions of carbamoyl chlorides. Org. Chem. Front. 2021, 8, 4024–4045. [Google Scholar] [CrossRef]
- de Pedro Beato, E.; Mazzarella, D.; Balletti, M.; Melchiorre, P. Photochemical generation of acyl and carbamoyl radicals using a nucleophilic organic catalyst: Applications and mechanism thereof. Chem. Sci. 2020, 11, 6312–6324. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Banerjee, A.; Shah, J.A.; Mukherjee, U.; Frederiks, N.C.; Johnson, C.J.; Ngai, M.-Y. Excited-State Copper-Catalyzed [4 + 1] Annulation Reaction Enables Modular Synthesis of α,β-Unsaturated-γ-Lactams. J. Am. Chem. Soc. 2022, 144, 20884–20894. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Banerjee, A.; Yao, W.; Patterson, E.V.; Ngai, M.-Y. Photocatalytic Radical Aroylation of Unactivated Alkenes: Pathway to β-Functionalized 1,4-, 1,6-, and 1,7-Diketones. ACS Catal. 2019, 9, 10358–10364. [Google Scholar] [CrossRef]
- Wang, D.; Ackermann, L. Three-component carboacylation of alkenes via cooperative nickelaphotoredox catalysis. Chem. Sci. 2022, 13, 7256–7263. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Huang, H.; Mao, G.; Deng, G.-J. Bromine radical-enhanced HAT activity leading to stoichiometric couplings of methylarenes with acid chlorides. Green Chem. 2022, 24, 8324–8329. [Google Scholar] [CrossRef]
- Xu, J.; Lu, F.; Sun, L.; Huang, M.; Jiang, J.; Wang, K.; Ouyang, D.; Lu, L.; Lei, A. Electrochemical reductive cross-coupling of acyl chlorides and sulfinic acids towards the synthesis of thioesters. Green Chem. 2022, 24, 7350–7354. [Google Scholar] [CrossRef]
- Xu, S.-M.; Chen, J.-Q.; Liu, D.; Bao, Y.; Liang, Y.-M.; Xu, P.-F. Aroyl chlorides as novel acyl radical precursors via visible-light photoredox catalysis. Org. Chem. Front. 2017, 4, 1331–1335. [Google Scholar] [CrossRef]
- Abdtawfeeq, T.H.; Mahmood, E.A.; Azimi, S.B.; Kadhim, M.M.; Kareem, R.T.; Charati, F.R.; Vessally, E. Direct selenosulfonylation of unsaturated compounds: A review. RSC Adv. 2022, 12, 30564–30576. [Google Scholar] [CrossRef]
- Bouchet, D.; Varlet, T.; Masson, G. Strategies toward the Difunctionalizations of Enamide Derivatives for Synthesizing α,β-Substituted Amines. Acc. Chem. Res. 2022, 55, 3265–3283. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Z.-M. The Alkynylative Difunctionalization of Alkenes. Chem.—Eur. J. 2022, 28, e202201519. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 2020, 49, 1790–1811. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Yin, Z.; Liu, J.; Sun, H.; Han, J. Recent Advances on the Electrochemical Difunctionalization of Alkenes/Alkynes. Chin. J. Chem. 2019, 37, 292–301. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Laru, S.; Hajra, A. Remote difunctionalization of 2H-indazoles using Koser’s reagents. Chem. Commun. 2022, 58, 981–984. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Wang, L.; Niu, Y.; Guo, M.; Ren, X.; Zhao, W.; Tang, X.; Wang, G. Slicing and Splicing of Bromodifluoro-N-arylacetamides: Dearomatization and Difunctionalization of Pyridines. Org. Lett. 2020, 22, 6610–6616. [Google Scholar] [CrossRef]
- Liu, L.; Sun, K.; Su, L.; Dong, J.; Cheng, L.; Zhu, X.; Au, C.-T.; Zhou, Y.; Yin, S.-F. Palladium-Catalyzed Regio- and Stereoselective Coupling-Addition of Propiolates with Arylsulfonyl Hydrazides: A Pattern for Difunctionalization of Alkynes. Org. Lett. 2018, 20, 4023–4027. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, Z.; Ma, N.; Wu, C.; Zhang, G.; Liu, Q.; Liu, T. Copper-Catalyzed Difunctionalization of Allenes with Sulfonyl Iodides Leading to (E)-α-Iodomethyl Vinylsulfones. Org. Lett. 2018, 20, 4318–4322. [Google Scholar] [CrossRef]
- Xun, X.; Zhao, M.; Xue, J.; Hu, T.; Zhang, M.; Li, G.; Hong, L. Difunctionalization of Alkenylpyridine N-Oxides by the Tandem Addition/Boekelheide Rearrangement. Org. Lett. 2019, 21, 8266–8269. [Google Scholar] [CrossRef]
- Engl, S.; Reiser, O. Copper-photocatalyzed ATRA reactions: Concepts, applications, and opportunities. Chem. Soc. Rev. 2022, 51, 5287–5299. [Google Scholar] [CrossRef]
- García-Santos, W.H.; Mateus-Ruiz, J.B.; Cordero-Vargas, A. Visible-Light Photocatalytic Preparation of 1,4-Ketoaldehydes and 1,4-Diketones from α-Bromoketones and Alkyl Enol Ethers. Org. Lett. 2019, 21, 4092–4096. [Google Scholar] [CrossRef]
- Hossain, A.; Engl, S.; Lutsker, E.; Reiser, O. Visible-Light-Mediated Regioselective Chlorosulfonylation of Alkenes and Alkynes: Introducing the Cu(II) Complex [Cu(dap)Cl2] to Photochemical ATRA Reactions. ACS Catal. 2019, 9, 1103–1109. [Google Scholar] [CrossRef]
- Li, D.; Mao, T.; Huang, J.; Zhu, Q. Copper-Catalyzed Bromodifluoroacetylation of Alkenes with Ethyl Bromodifluoroacetate. J. Org. Chem. 2018, 83, 10445–10452. [Google Scholar] [CrossRef]
- Wu, D.; Fan, W.; Wu, L.; Chen, P.; Liu, G. Copper-Catalyzed Enantioselective Radical Chlorination of Alkenes. ACS Catal. 2022, 12, 5284–5291. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Yang, S.; Zhu, C. Asymmetric Radical Cyclization of Alkenes by Stereospecific Homolytic Substitution of Sulfinamides. Angew. Chem. Int. Ed. 2022, 61, e202201027. [Google Scholar]
- Hu, D.; Zhou, Y.; Jiang, X. From aniline to phenol: Carbon-nitrogen bond activation via uranyl photoredox catalysis. Natl. Sci. Rev. 2021, 9, nwab156. [Google Scholar] [CrossRef]
- Jiang, X.; Jia, Y.; Xiao, W.-J.; Lu, L.-Q. Photoinduced palladium-catalyzed carbonylation of halides with weak nucleophiles. Sci. Bull. 2020, 65, 1696–1698. [Google Scholar] [CrossRef]
- Latrache, M.; Hoffmann, N. Photochemical radical cyclization reactions with imines, hydrazones, oximes and related compounds. Chem. Soc. Rev. 2021, 50, 7418–7435. [Google Scholar] [CrossRef]
- Luo, M.-J.; Xiao, Q.; Li, J.-H. Electro-/photocatalytic alkene-derived radical cation chemistry: Recent advances in synthetic applications. Chem. Soc. Rev. 2022, 51, 7206–7237. [Google Scholar] [CrossRef]
- Qu, Z.; Tian, T.; Tan, Y.; Ji, X.; Deng, G.-J.; Huang, H. Redox-neutral ketyl radical coupling/cyclization of carbonyls with N-aryl acrylamides through consecutive photoinduced electron transfer. Green Chem. 2022, 24, 7403–7409. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Sun, Z.; Huang, H.; Mao, G.; Deng, G.-J. Stoichiometric couplings of methylarenes through visible-light-induced bromo radical formation from aryl halides. Green Chem. 2022, 24, 3293–3299. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Visible Light-Driven Radical-Mediated C-C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561. [Google Scholar] [CrossRef] [PubMed]
- The Cambridge Crystallographic Data Centre. CCDC 2262739 (3ad). Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 13 May 2023).


 | ||
|---|---|---|
| Entry | Variation from the Standard Conditions | Yield (%) 2 |
| 1 | None | 96 |
| 2 3 | [Ru(bpy)3Cl2] instead of [Ir(ppy)3] | 9 |
| 3 3 | Eosin Y instead of [Ir(ppy)3] | 0 |
| 4 3 | Without [Ir(ppy)3] | 0 |
| 5 3 | Without additional light | 0 |
| 6 4 | None | 68 |
| 7 5 | None | 91 |
| 8 6 | None | 86 |
| 9 | [Ir(ppy)3] (2 mol %) | 95 |
| 10 | [Ir(ppy)3] (0.5 mol %) | 88 |
| 11 | Na2CO3 instead of NaHCO3 | 75 |
| 12 | K2CO3 instead of NaHCO3 | 68 |
| 13 | Et3N instead of NaHCO3 | 24 |
| 14 | 2,6-Lutidine instead of NaHCO3 | 75 |
| 15 | Toluene instead of CH3CN | 87 |
| 16 | EtOAc instead of CH3CN | 90 |
| 17 | THF instead of CH3CN | 85 |
| 18 3 | DMF instead of CH3CN | <5 |
| 19 3 | DMSO instead of CH3CN | <5 |
| 20 | At 50 °C | 90 |
| 21 7 | None | 86 |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Huang, P.-F.; Xiong, B.; Tang, K.-W.; Liu, Y. Visible-Light-Induced Difunctionalization of the C-C Bond of Alkylidenecyclopropanes with Acyl Chlorides. Catalysts 2023, 13, 919. https://doi.org/10.3390/catal13060919
Ding C, Huang P-F, Xiong B, Tang K-W, Liu Y. Visible-Light-Induced Difunctionalization of the C-C Bond of Alkylidenecyclopropanes with Acyl Chlorides. Catalysts. 2023; 13(6):919. https://doi.org/10.3390/catal13060919
Chicago/Turabian StyleDing, Chuan, Peng-Fei Huang, Biquan Xiong, Ke-Wen Tang, and Yu Liu. 2023. "Visible-Light-Induced Difunctionalization of the C-C Bond of Alkylidenecyclopropanes with Acyl Chlorides" Catalysts 13, no. 6: 919. https://doi.org/10.3390/catal13060919
APA StyleDing, C., Huang, P.-F., Xiong, B., Tang, K.-W., & Liu, Y. (2023). Visible-Light-Induced Difunctionalization of the C-C Bond of Alkylidenecyclopropanes with Acyl Chlorides. Catalysts, 13(6), 919. https://doi.org/10.3390/catal13060919






