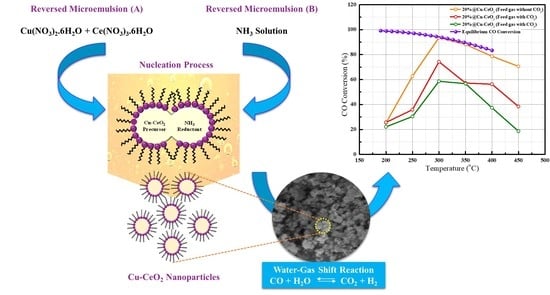
Modification of Copper-Ceria Catalyst via Reverse Microemulsion Method and Study of the Effects of Surfactant on WGS Catalyst Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction (XRD) Analysis
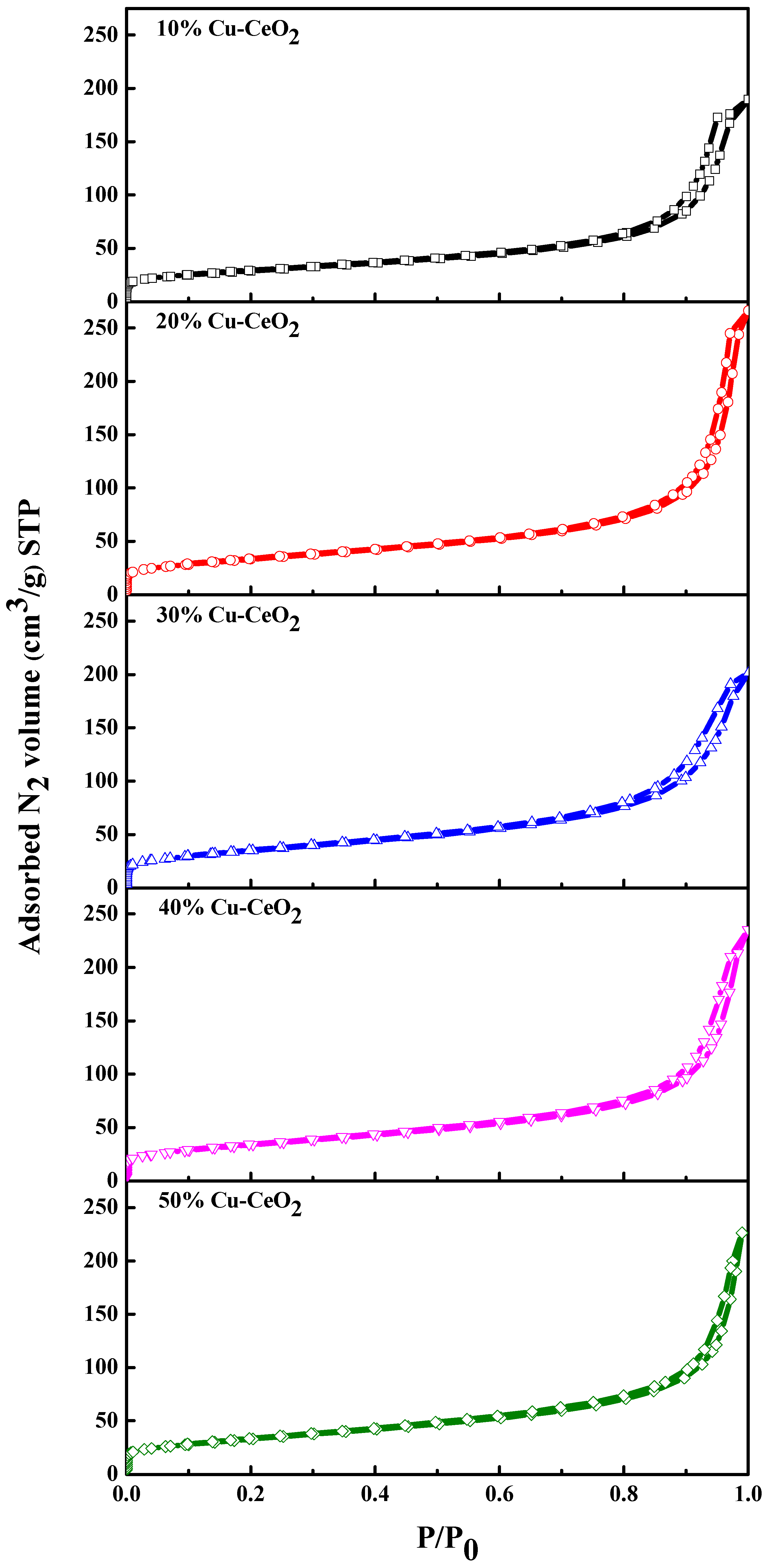
2.2. Pore Characteristics and Braunauer–Emmett–Teller (BET) Surface Area Analysis
2.3. Temperature Programmed Reduction by Hydrogen (H2-TPR) Analysis
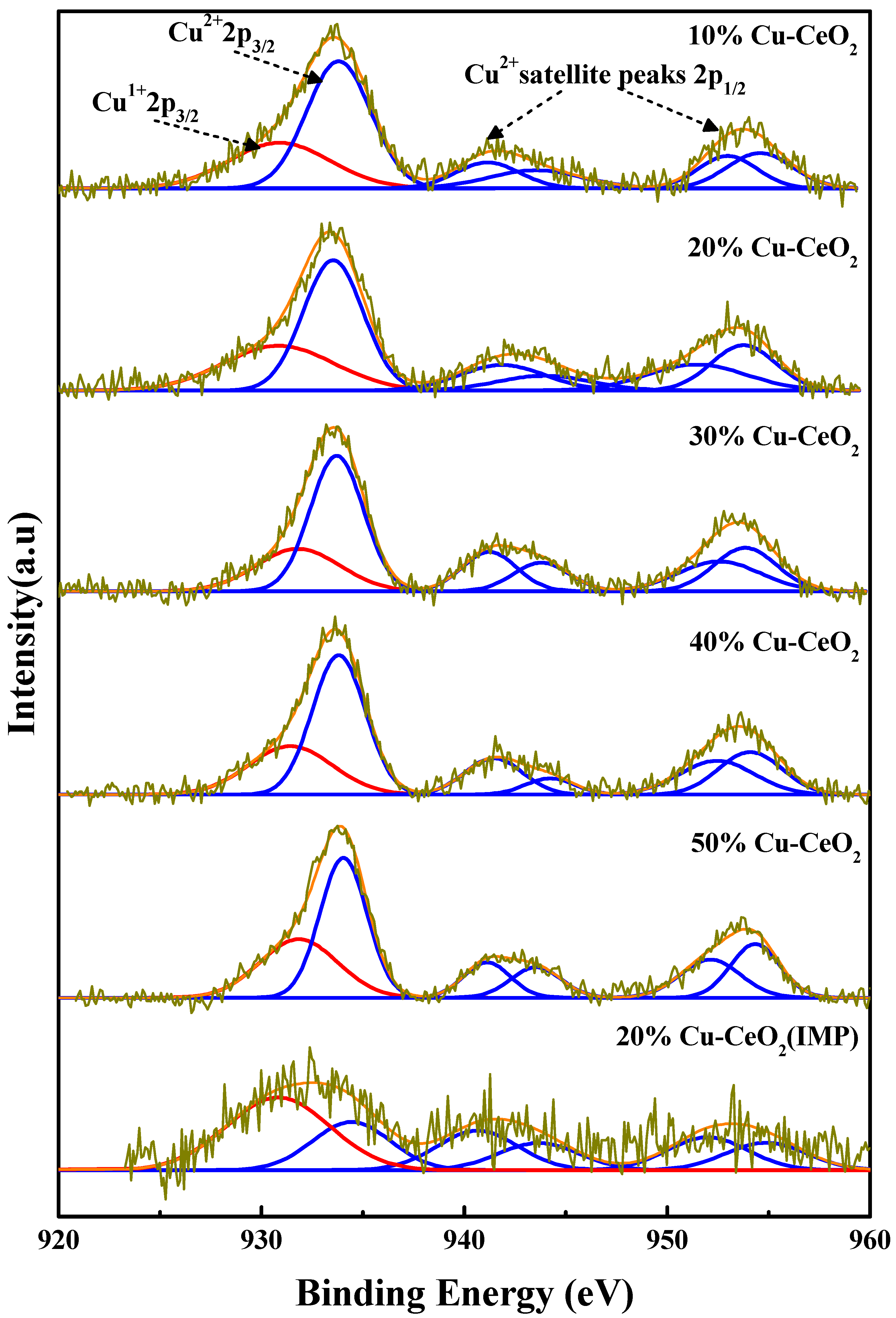
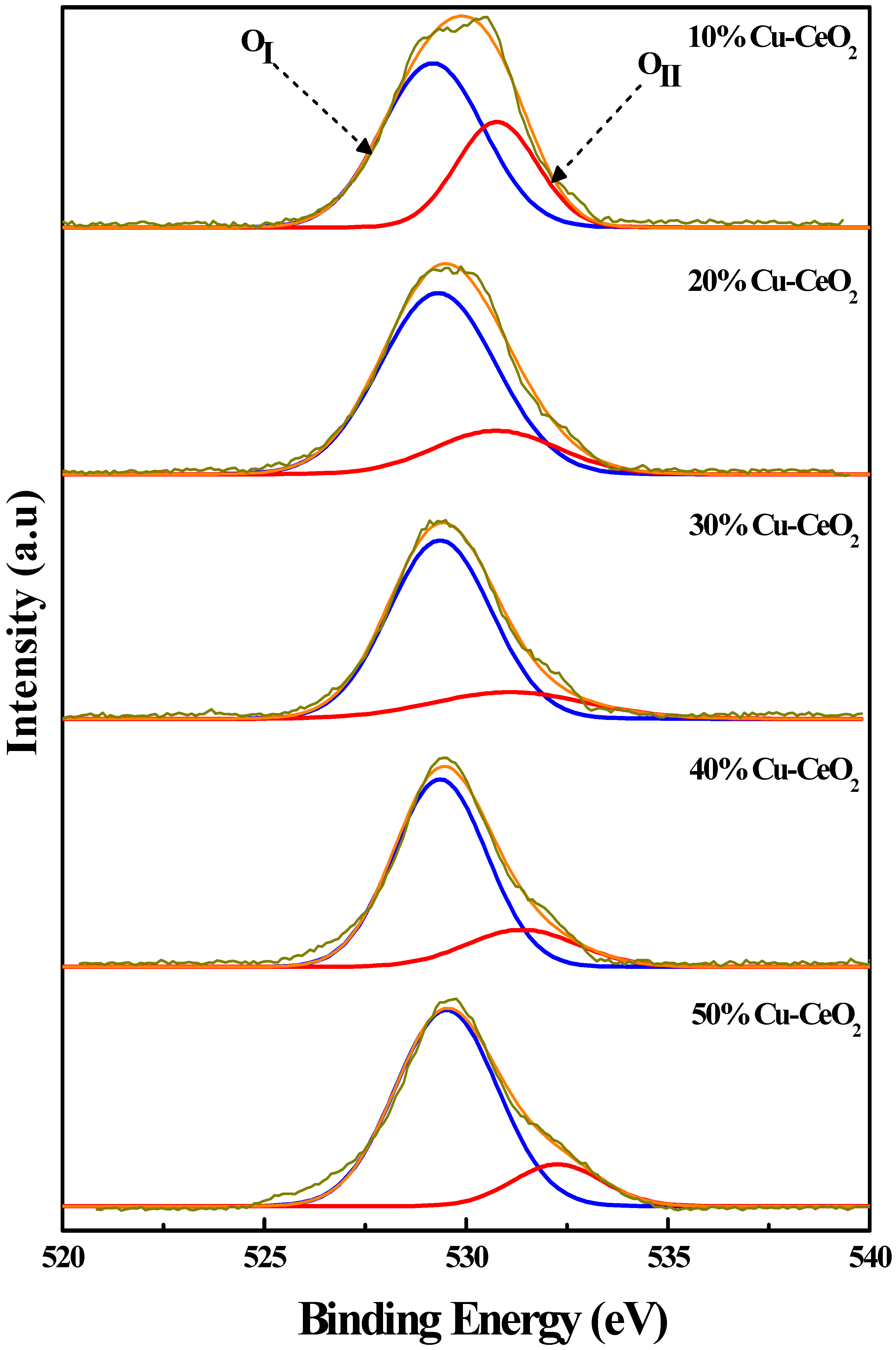
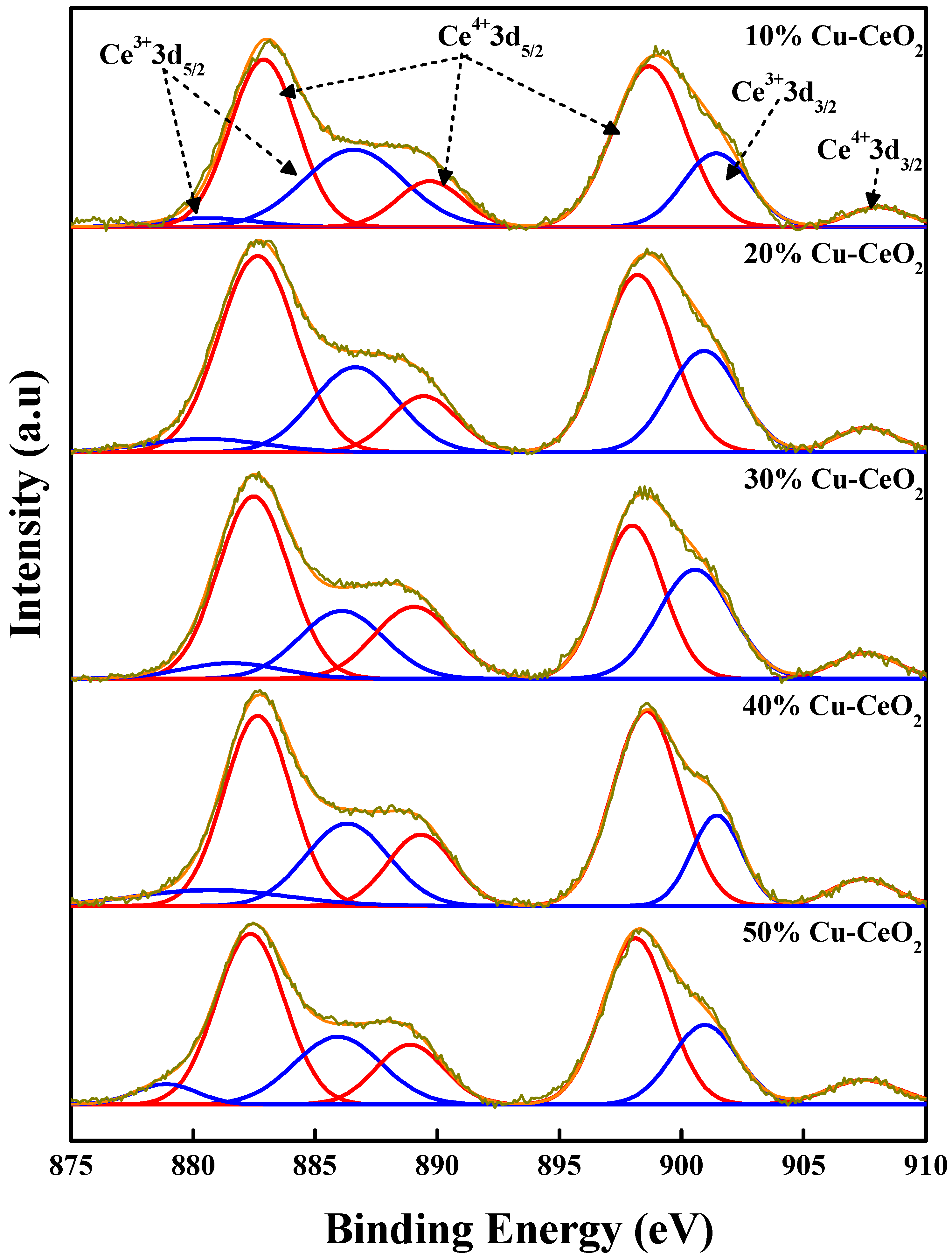
2.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
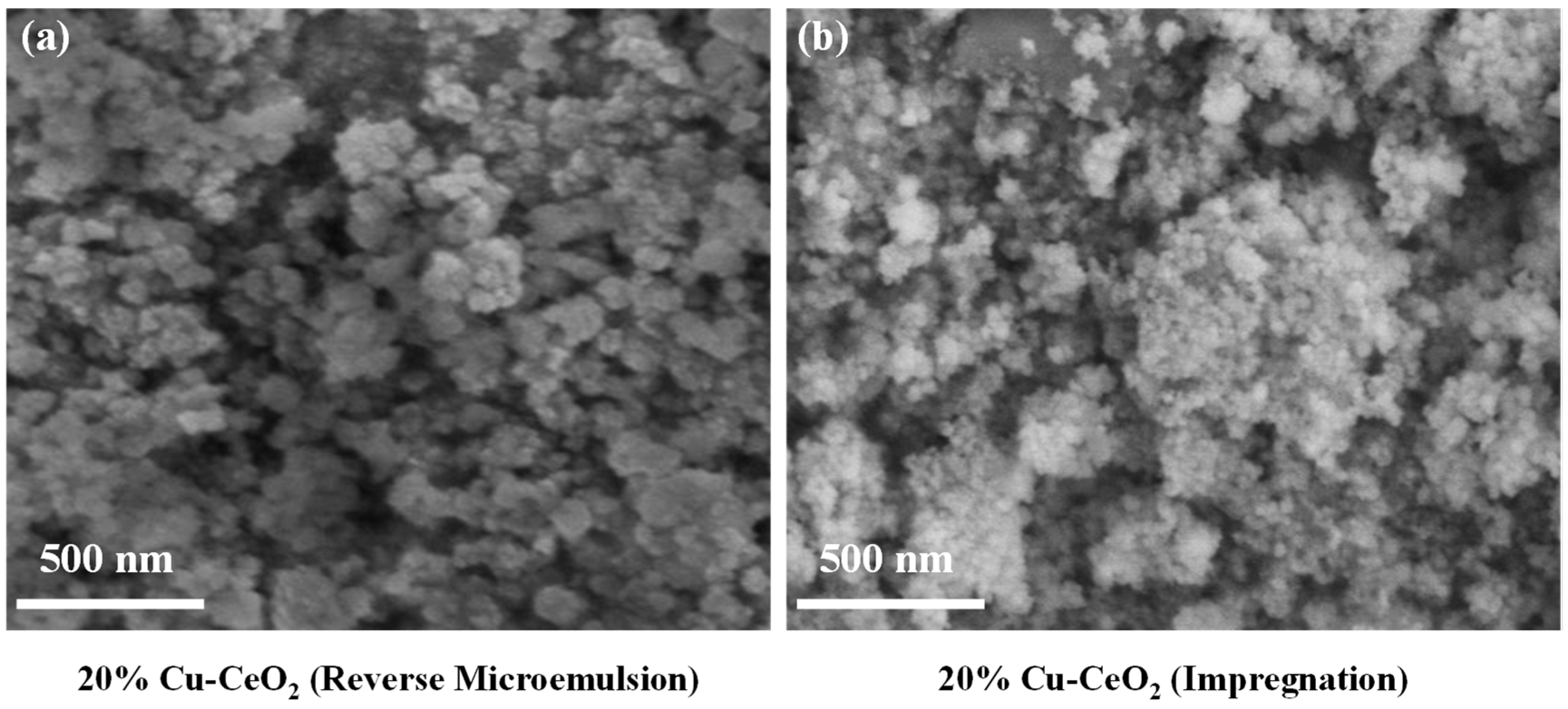
2.5. Scanning Electron Microscope (SEM) Analysis
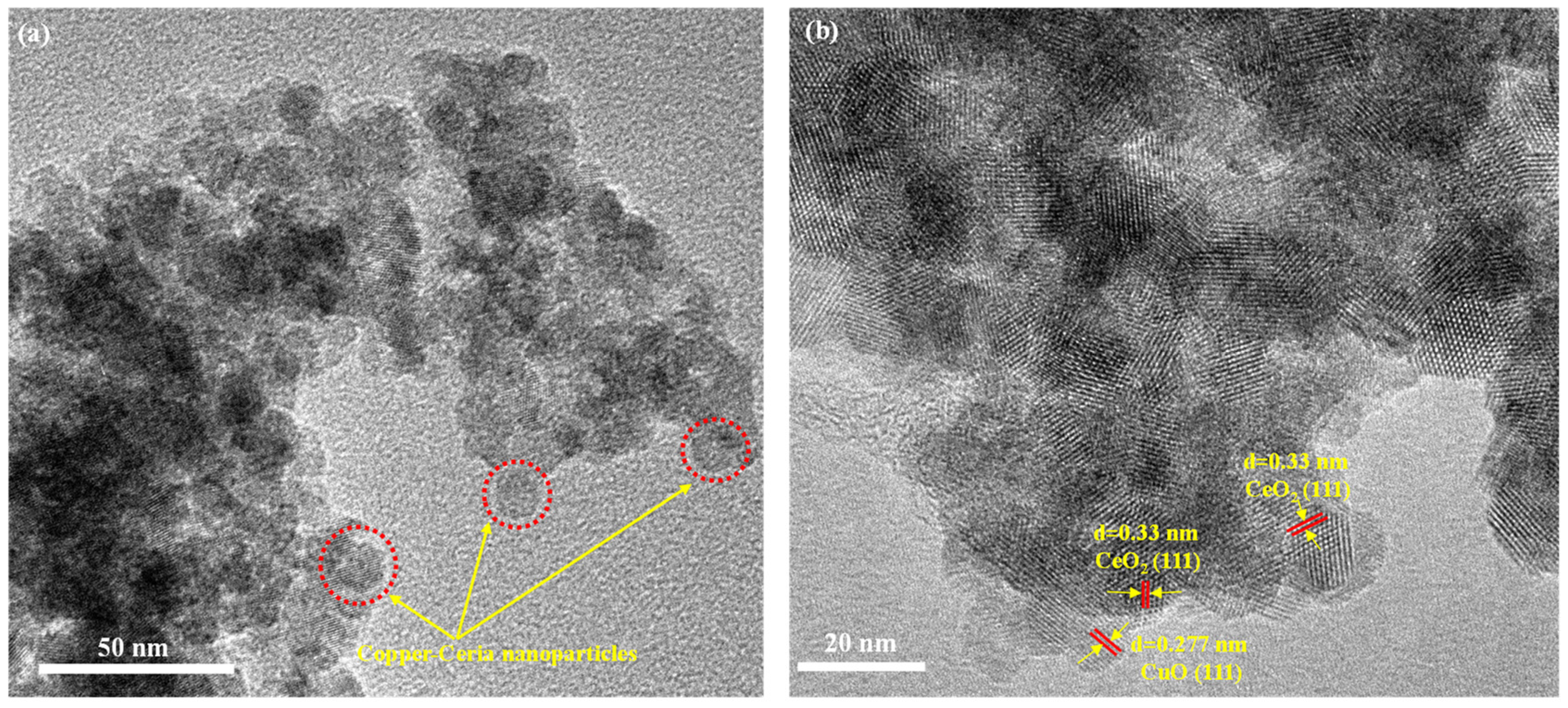
2.6. High-Resolution Transmission Electron Microscopy (HR-TEM) Analysis
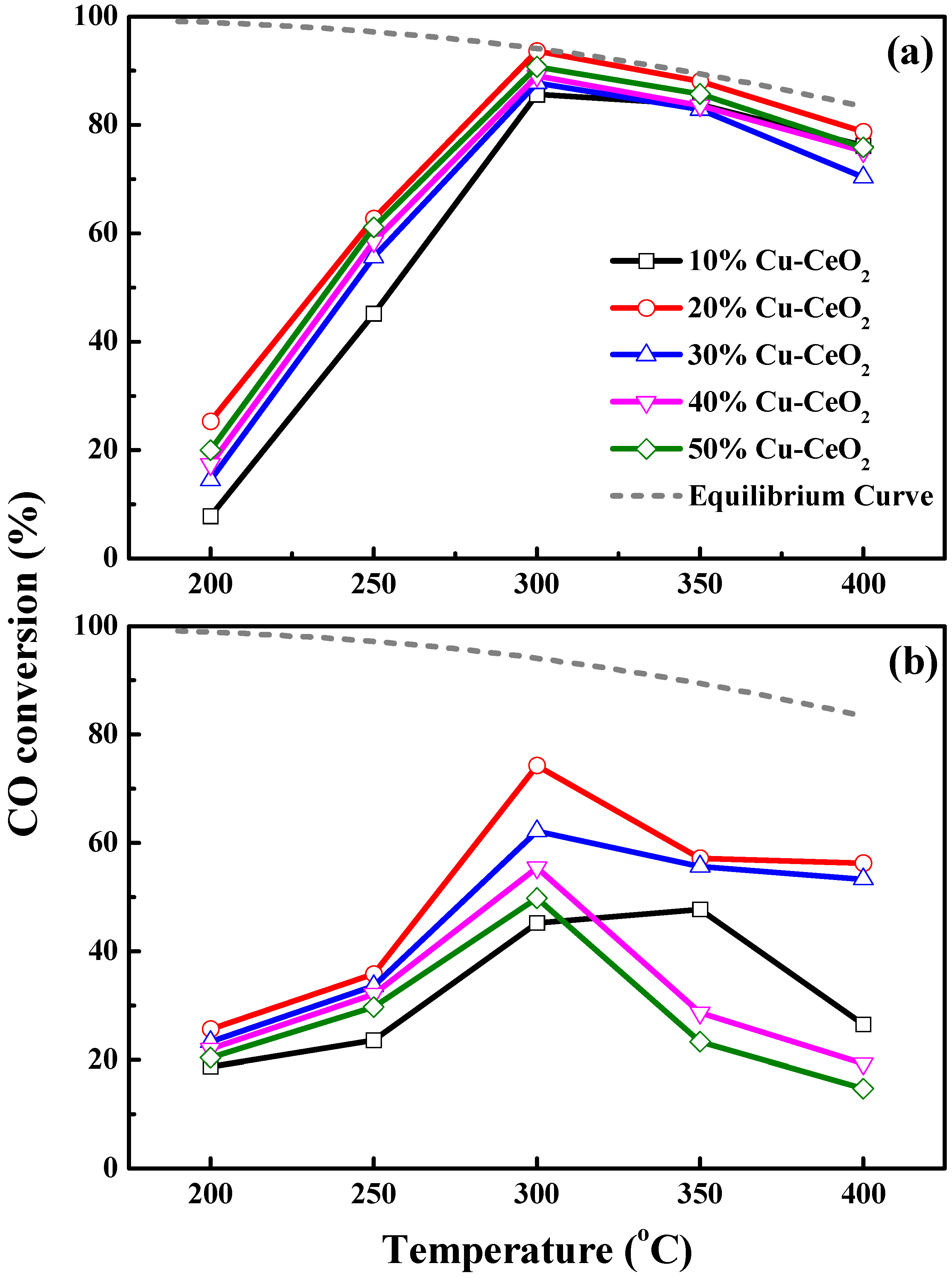
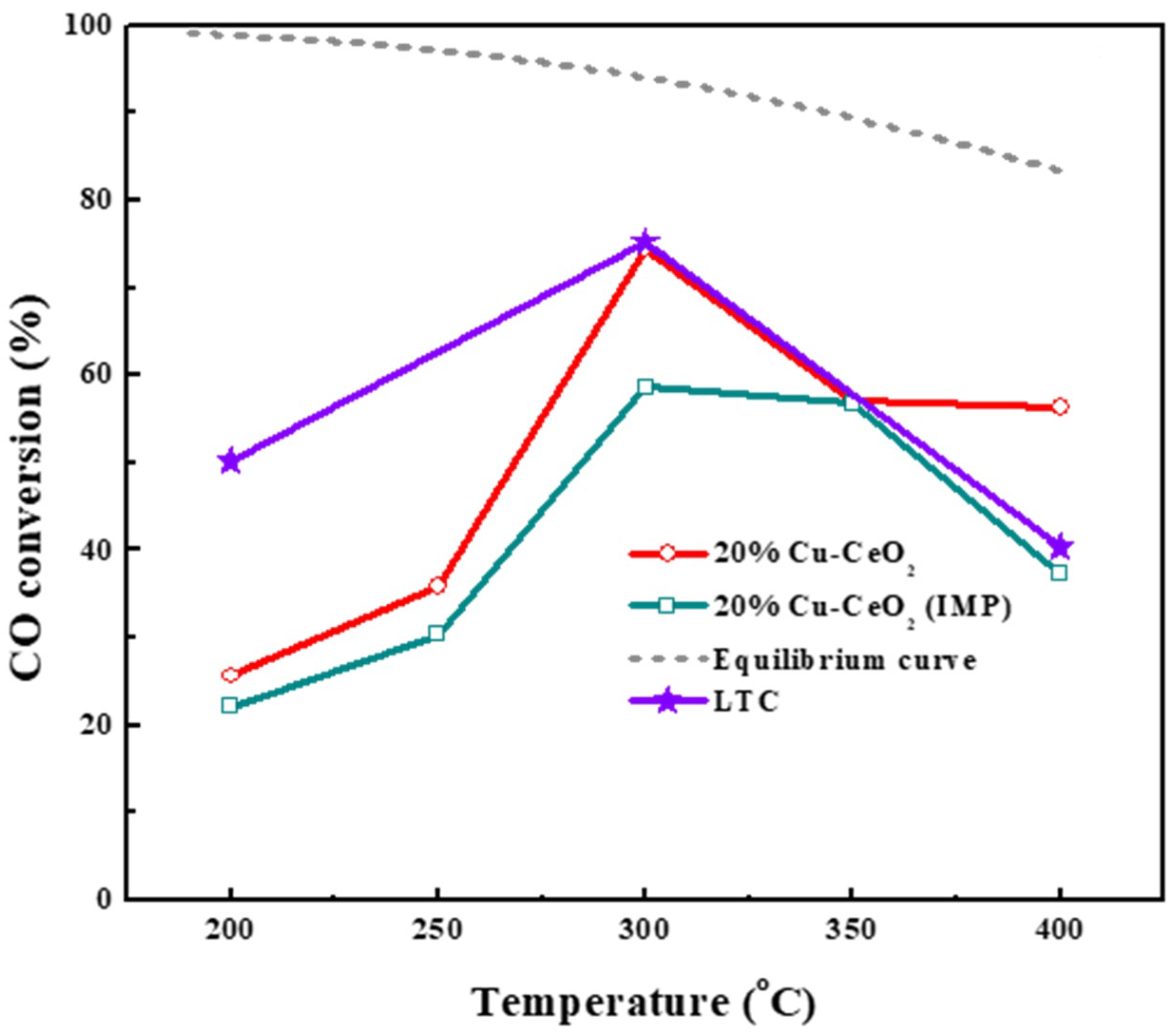
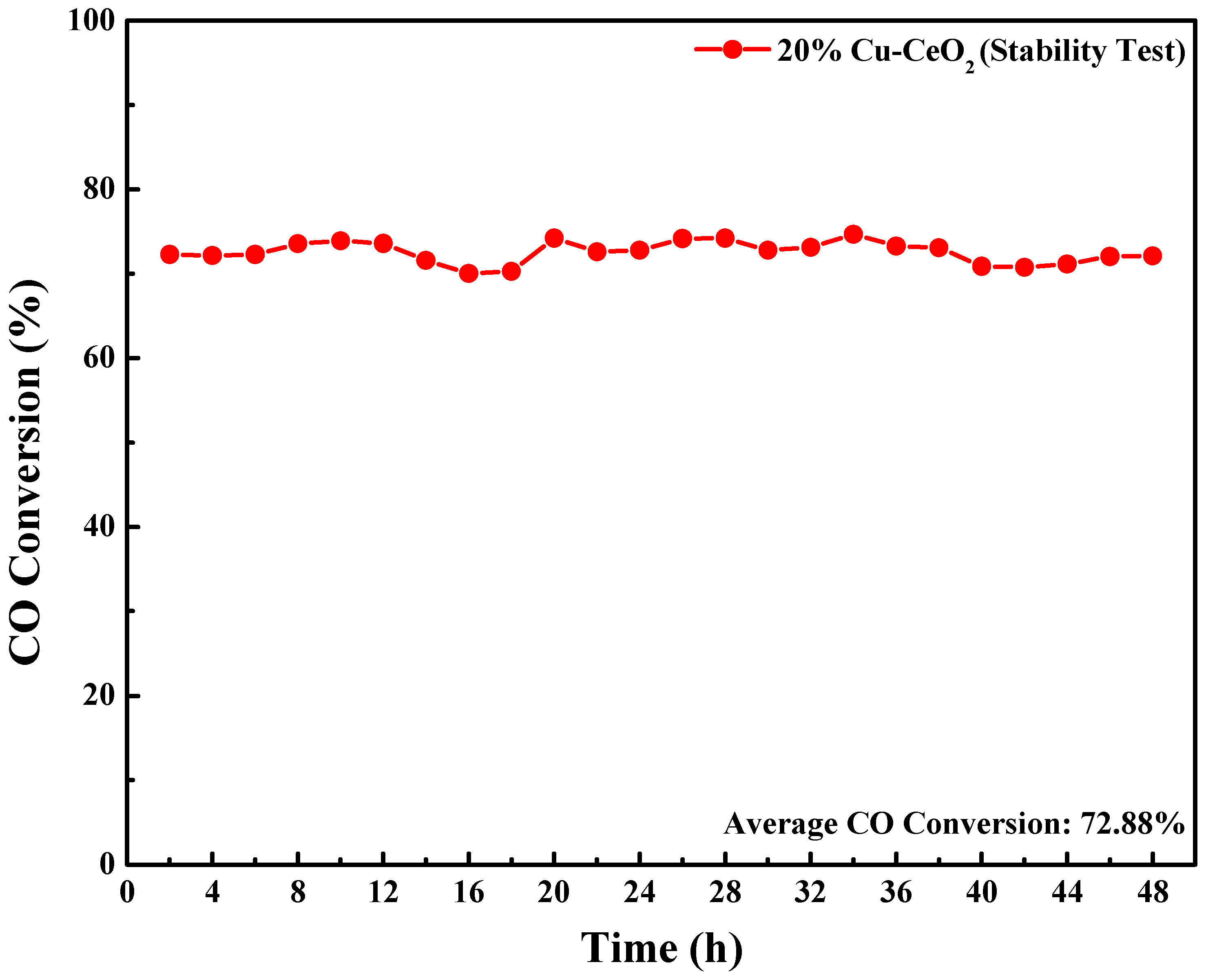
2.7. Evaluation of Catalytic Performances in Water Gas Shift Reaction
3. Materials and Methods
3.1. Preparation of Cu-CeO2 Catalysts by Reverse Microemulsion (RME) Method
3.2. Preparation of Cu-CeO2 Catalysts by Impregnation Method
3.3. Characterization of Catalysts
3.4. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Newell, R.; Raimi, D.; Villanueva, S.; Prest, B. Global Energy Outlook 2021: Pathways from Paris. Resour. Future 2021, 8, 3–12. [Google Scholar]
- Smil, V. Energy Transitions: Global and National Perspectives, 2nd ed.; ABC-CLIO: Santa Barbara, CA, USA, 2016. [Google Scholar]
- Dudley, B. Bp Statistical Review of World Energy 2018. Energy Economic, Centre for Energy Economics Research and Policy. British Petroleum. 2018. Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy/electricity.html (accessed on 7 February 2023).
- Lee, Y.L.; Lee, K.; Ko, C.H.; Roh, H.S. Optimization of Nano-catalysts for Application in Compact Reformers. Chem. Eng. J. 2022, 431, 134299. [Google Scholar] [CrossRef]
- Fiorio, J.L.; Gothe, M.L.; Kohlrausch, E.C.; Zardo, M.L.; Tanaka, A.A.; de Lima, R.B.; da Silva, A.G.M.; Garcia, M.A.S.; Vidinha, P.; Machado, G. Nanoengineering of Catalysts for Enhanced Hydrogen Production. Hydrogen 2022, 3, 218–254. [Google Scholar] [CrossRef]
- Johnston, B.; Mayo, M.C.; Khare, A. Hydrogen: The Energy Source for the 21st century. Technovation 2005, 25, 569–585. [Google Scholar] [CrossRef]
- Canton, H. International Energy Agency-IEA. In The Europa Directory of International Organizations 2021, 23rd ed.; Europa Publications, Ed.; Routledge: London, UK, 2021; pp. 684–686. [Google Scholar] [CrossRef]
- U.S.E.I Adminstration, Hydrogen Explained: Production of Hydrogen. Available online: https://www.eia.gov/energyexplained/hydrogen/production-of-hydrogen.php (accessed on 21 January 2022).
- IRENA, Green Hydrogen: A Guide to Policy Making. Available online: https://www.irena.org/publications/2020/Nov/Green-hydrogen (accessed on 25 November 2020).
- Sadik-Zada, E.R. Political Economy of Green Hydrogen Rollout: A Global Perspective. Sustainability 2021, 13, 13464. [Google Scholar] [CrossRef]
- Lee, Y.L.; Kim, K.J.; Hong, G.R.; Roh, H.S. Target-oriented water–gas shift reactions with customized reaction conditions and catalysts. Chem. Eng. J. 2023, 458, 141422. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- El Gemayel, J.; Macchi, A.; Hughes, R.; Anthony, E.J. Simulation of the Integration of a Bitumen Upgrading Facility and an IGCC Process with Carbon Capture. Fuel 2014, 117, 1288–1297. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.S.; Park, J.; Kang, B.M.; Cho, C.H.; Lim, H.; Won, W. Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production. Appl. Sci. 2021, 11, 6021. [Google Scholar] [CrossRef]
- Coletta, V.C.; Gonçalves, R.V.; Bernardi, M.I.; Hanaor, D.A.; Assadi, M.H.; Marcos, F.C.; Nogueira, F.G.; Assaf, E.M.; Mastelaro, V.R. Cu-Modified SrTiO3 Perovskites Toward Enhanced Water–Gas Shift Catalysis: A Combined Experimental and Computational Study. ACS Appl. Energy Mater. 2020, 4, 452–461. [Google Scholar] [CrossRef]
- Callaghan, C.A. Kinetics and Catalysis of the Water-Gas-Shift Reaction: A Microkinetic and Graph Theoretic Approach. Doctoral Dissertation, Worcester Polytechnic Institute, Worcester, MA, USA, 31 March 2006. [Google Scholar]
- Jain, R.; Maric, R. Synthesis of nano-Pt onto ceria support as catalyst for water–gas shift reaction by Reactive Spray Deposition Technology. Appl. Catal. A Gen. 2014, 475, 461–468. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Stacchiola, D.; Rodriguez, J.A. Unique Properties of Ceria Nanoparticles Supported on Metals: Novel Inverse Ceria/Copper Catalysts for CO Oxidation and the Water-Gas Shift Reaction. ACC Chem. Res. 2013, 46, 1702–1711. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Wu, K.; Sun, L.D.; Yan, C.H. Recent progress in well-controlled synthesis of ceria-based nanocatalysts towards enhanced catalytic performance. Adv. Energy Mater. 2016, 6, 1600501. [Google Scholar] [CrossRef]
- Chen, A.; Yu, X.; Zhou, Y.; Miao, S.; Li, Y.; Kuld, S.; Sehested, J.; Liu, J.; Aoki, T.; Hong, S.; et al. Structure of the catalytically active copper–ceria interfacial perimeter. Nat. Catal. 2019, 2, 334–341. [Google Scholar] [CrossRef]
- Yao, S.Y.; Xu, W.Q.; Johnston-Peck, A.C.; Zhao, F.Z.; Liu, Z.Y.; Luo, S.; Senanayake, S.D.; Martínez-Arias, A.; Liu, W.J.; Rodriguez, J.A. Morphological effects of the nanostructured ceria support on the activity and stability of CuO/CeO2 catalysts for the water-gas shift reaction. Phys. Chem. Chem. Phys. 2014, 16, 17183–17195. [Google Scholar] [CrossRef]
- Jia, M.L.; Zhang, Y.P.; Bao, Y.S.; Wang, J.; Xu, A.J. Recyclable CuMgAl hydrotalcite for oxidative esterification of aldehydes with alkylbenzenes. Green Chem. Lett. Rev. 2018, 11, 230–236. [Google Scholar] [CrossRef]
- Reina, T.R.; Ivanova, S.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A. WGS and CO-PrOx reactions using gold promoted copper-ceria catalysts: “Bulk CuOCeO2 vs. CuOCeO2/Al2O3 with low mixed oxide content”. Appl. Catal. B Environ. 2016, 197, 62–72. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Yu, Y.; Simakov, D.S.A. Enhancing the surface area stability of the cerium oxide reverse water gas shift nanocatalyst via reverse microemulsion synthesis. Catal. Today 2023, 407, 230–243. [Google Scholar] [CrossRef]
- Meshkani, F.; Rezaei, M. Preparation of mesoporous nanocrystalline alkali promoted chromium free catalysts (Fe2O3–Al2O3–NiO) for a high temperature water gas shift reaction. RSC Adv. 2015, 5, 9955–9964. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Ce3+-ion, Surface Oxygen Vacancy, and Visible Light-induced Photocatalytic Dye Degradation and Photocapacitive Performance of CeO2-Graphene Nanostructures. Sci. Rep. 2017, 7, 5928. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.K.; Khalid, N.R.; Tanveer, M.; Kebaili, I.; Alrobei, H. Fabrication of CuO/MoO3 p-n heterojunction for enhanced dyes degradation and hydrogen production from water splitting. Int. J. Hydrogen Energy 2022, 47, 15491–15504. [Google Scholar] [CrossRef]
- Siddiqui, H.; Parra, M.R.; Qureshi, M.S.; Malik, M.M.; Haque, F.Z. Studies of structural, optical, and electrical properties associated with defects in sodium-doped copper oxide (CuO/Na) nanostructures. J. Mater. Sci. 2018, 53, 8826–8843. [Google Scholar] [CrossRef]
- Ning, J.; Zhou, Y.; Chen, A.; Li, Y.; Miao, S.; Shen, W. Dispersion of copper on ceria for the low-temperature water-gas shift reaction. Catal. Today 2020, 357, 460–467. [Google Scholar] [CrossRef]
- Paschalidou, P.; Theocharis, C.R. Surface properties of ceria synthesised using Triton-X based reverse microemulsions. RSC Adv. 2019, 9, 7025–7031. [Google Scholar] [CrossRef]
- Housaindokht, M.R.; Nakhaei Pour, A. Size Control of Iron Oxide Nanoparticles Using Reverse Microemulsion Method: Morphology, Reduction, and Catalytic Activity in CO Hydrogenation. J. Chem. 2013, 2013, 781595. [Google Scholar] [CrossRef]
- Ricciardi, L.; Martini, M.; Tillement, O.; Sancey, L.; Perriat, P.; Ghedini, M.; Szerb, E.I.; Yadav, Y.J.; La Deda, M. Multifunctional material based on ionic transition metal complexes and gold–silica nanoparticles: Synthesis and photophysical characterization for application in imaging and therapy. J. Photochem. Photobiol. B Biol. 2014, 140, 396–404. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Flytzani-Stephanopoulos, M. Low-temperature water-gas shift reaction over Cu- and Ni-loaded cerium oxide catalysts. Appl. Catal. B 2000, 27, 179–191. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Effect of synthesis parameters on catalytic properties of CuO-CeO2. Appl. Catal. B 2006, 67, 1–11. [Google Scholar] [CrossRef]
- Gunawardana, P.V.D.S.; Lee, H.C.; Kim, D.H. Performance of copper–ceria catalysts for water gas shift reaction in medium temperature range. Int. J. Hydrogen Energy 2009, 34, 1336–1341. [Google Scholar] [CrossRef]
- Kang, W.; Ozgur, D.O.; Varma, A. Solution Combustion Synthesis of High Surface Area CeO2 Nanopowders for Catalytic Applications: Reaction Mechanism and Properties. ACS Appl. Nano Mater. 2018, 1, 675–685. [Google Scholar] [CrossRef]
- Bernard, P.; Stelmachowski, P.; Broś, P.; Makowski, W.; Kotarba, A. Demonstration of the influence of specific surface area on reaction rate in heterogeneous catalysis. J. Chem. Educ. 2021, 98, 935–940. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.; Luque, R.; Clark, J.H.; Macquarrie, D.J. Tuneable porous carbonaceous materials from renewable resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef] [PubMed]
- Broekhoff, J.C.P. Mesopore Determination from Nitrogen Sorption Isotherms: Fundamentals, Scope, Limitations. Stud. Surf. Sci. Catal. 1979, 3, 663–684. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Li, Y.; Xu, Y.; Xin, Q.; Shen, W. Carbon monoxide oxidation over CuO/CeO2 catalysts. Catal. Today 2004, 93, 191–198. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Park, J.; Baek, J.H.; Jo, G.H.; Rasheed, H.U.; Yi, K.B. Catalytic Characteristics of Water-Treated Cu/ZnO/MgO/Al2O3 Catalyst for LT-WGS Reaction. Trans. Korean Hydrog. New Energy Soc. 2019, 30, 95–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, N.; Wang, K.; Zhang, S.; Wu, J. Effect of Copper Nanoparticles Dispersion on Catalytic Performance of Cu/SiO2 Catalyst for Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. J. Nanomater. 2013, 2013, 629375. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Di Benedetto, A.; Lisi, L. Synergy between ceria and metals (Ag or Cu) in catalytic diesel particulate filters: Effect of the metal content and of the preparation method on the regeneration performance. Top. Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Carboneras, M.; Viejo, F.; Arrabal, R.; Munoz, J. Influence of Cu and Sn content in the corrosion of AISI 304 and 316 stainless steels in H2SO4. Corros. Sci. 2006, 48, 1075–1092. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Y.; Yin, L.; Yuan, Z.; Lan, Y.; Xu, D.; Yang, C.; Yang, K. Improved corrosion resistance and biofilm inhibition ability of copper-bearing 304 stainless steel against oral microaerobic Streptococcus mutans. J. Mater. Sci. Technol. 2021, 66, 112–120. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, A.; Ning, J.; Shen, W. Electronic and geometric structure of the copper-ceria interface on Cu/CeO2 catalysts. Chin. J. Catal. 2020, 41, 928–937. [Google Scholar] [CrossRef]
- Szabová, L.; Camellone, M.F.; Huang, M.; Matolín, V.; Fabris, S. Thermodynamic, electronic and structural properties of Cu/CeO2 surfa ces and interfaces from first-principles DFT+ U calculations. J. Chem. Phys. 2010, 133, 234705. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, W.; Zhang, L.; Zheng, Y.; Wang, Z. Insights into the Surface-Defect Dependence of Photoreactivity over CeO2 Nanocrystals with Well-Defined Crystal Facets. ACS Catal. 2015, 5, 4851–4858. [Google Scholar] [CrossRef]
- Jeong, D.W.; Jang, W.J.; Na, H.S.; Shim, J.O.; Jha, A.; Roh, H.S. Comparative study on cubic and tetragonal Cu–CeO2–ZrO2 catalysts for water gas shift reaction. J. Ind. Eng. Chem. 2015, 27, 35–39. [Google Scholar] [CrossRef]
- Jin, S.; Byun, H.; Lee, C.H. Enhanced oxygen mobility of nonreducible MgO-supported Cu catalyst by defect engineering for improving the water-gas shift reaction. J. Catal. 2021, 400, 195–211. [Google Scholar] [CrossRef]
- Dongil, A.B.; Bachiller-Baeza, B.; Castillejos, E.; Escalona, N.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. The promoter effect of potassium in CuO/CeO2 systems supported on carbon nanotubes and graphene for the CO-PROX reaction. Catal. Sci. Technol. 2016, 6, 6118–6127. [Google Scholar] [CrossRef]
- Chen, Z.; Kronawitter, C.X.; Yang, X.; Yeh, Y.W.; Yao, N.; Koel, B.E. The promoting effect of tetravalent cerium on the oxygen evolution activity of copper oxide catalysts. Phys. Chem. Chem. Phys. 2017, 19, 31545–31552. [Google Scholar] [CrossRef]
- Shen, H.; Tang, Z.; Xiao, X.; Wu, H.; Zhou, H.; Fang, P.; Zhu, D.; Ge, J. Catalytic Oxidation of NO by Ozone over Mn-Ce/Al2O3/TiO2 Catalyst. Processes 2022, 10, 1946. [Google Scholar] [CrossRef]
- Wang, L.; Zhuang, L.; Xin, H.; Huang, Y.; Wang, D. Semi-Quantitative Estimation of Ce3+/Ce4+ Ratio in YAG: Ce3+ Phosphor under Different Sintering Atmosphere. Open J. Inorg. Chem. 2015, 5, 12. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Hu, W.; Huang, X.; Li, Q.; Xu, Y.; Zuo, Y.; Li, G. Anchoring High-Concentration Oxygen Vacancies at Interfaces of CeO2–x/Cu toward Enhanced Activity for Preferential CO Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 22999–23007. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, J.D.; Kim, K.; Yun, H.J.; Jung, W.; Bae, J. Negative Effects of Dopants on Copper–Ceria Catalysts for CO Preferential Oxidation Under the Presence of CO2 and H2O. Catal. Lett. 2017, 147, 2987–3003. [Google Scholar] [CrossRef]
- Pradhan, S.; Reddy, A.S.; Devi, R.N.; Chilukuri, S. Copper-based catalysts for water gas shift reaction: Influence of support on their catalytic activity. Catal. Today 2009, 141, 72–76. [Google Scholar] [CrossRef]



| Sample | Average Crystallite Size (nm) |
|---|---|
| 10% Cu-CeO2 | 9.92 |
| 20% Cu-CeO2 | 9.70 |
| 30% Cu-CeO2 | 10.19 |
| 40% Cu-CeO2 | 10.47 |
| 50% Cu-CeO2 | 10.55 |
| 20% Cu-CeO2(IMP) | 11.60 |
| Sample | BET Surface Area (m2 g−1) | Average Pore Diameter (nm) |
|---|---|---|
| 10% Cu-CeO2 | 100.2 | 10.8 |
| 20% Cu-CeO2 | 123.1 | 13.3 |
| 30% Cu-CeO2 | 120.5 | 12.0 |
| 40% Cu-CeO2 | 118.3 | 11.6 |
| 50% Cu-CeO2 | 116.4 | 9.6 |
| Sample | Reduction Temperature (°C) | |
|---|---|---|
| Reduction of Finely Dispersed CuO | Reduction of Cu-Ox-Ce Interaction | |
| 10% Cu-CeO2 | 140.0 | 168.4 |
| 20% Cu-CeO2 | 110.2 | 143.5 |
| 30% Cu-CeO2 | 133.6 | 168.7 |
| 40% Cu-CeO2 | 128.9 | 162.1 |
| 50% Cu-CeO2 | 133.8 | 168.2 |
| 20% Cu-CeO2(IMP) | 130.0 | 160.4 |
| Sample | Atomic Composition (%) * | |||||
|---|---|---|---|---|---|---|
| Cu1+ | Cu2+ | Ce3+ | Ce4+ | Lattice O2 | O-Defect | |
| 10% Cu-CeO2 | 22.68 | 77.32 | 21.09 | 78.91 | 76.39 | 23.61 |
| 20% Cu-CeO2 | 24.77 | 75.23 | 28.51 | 71.49 | 66.37 | 33.63 |
| 30% Cu-CeO2 | 22.27 | 77.73 | 26.40 | 73.60 | 79.75 | 20.25 |
| 40% Cu-CeO2 | 20.78 | 79.22 | 25.23 | 74.77 | 81.32 | 18.68 |
| 50% Cu-CeO2 | 18.08 | 81.92 | 25.13 | 74.87 | 81.80 | 18.20 |
| 20% Cu-CeO2(IMP) | 17.43 | 82.57 | 21.07 | 78.93 | 83.33 | 16.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oo, W.; Park, J.H.; Sonia, Z.A.; Win, M.Z.; Cho, D.; Yi, K.B. Modification of Copper-Ceria Catalyst via Reverse Microemulsion Method and Study of the Effects of Surfactant on WGS Catalyst Activity. Catalysts 2023, 13, 951. https://doi.org/10.3390/catal13060951
Oo W, Park JH, Sonia ZA, Win MZ, Cho D, Yi KB. Modification of Copper-Ceria Catalyst via Reverse Microemulsion Method and Study of the Effects of Surfactant on WGS Catalyst Activity. Catalysts. 2023; 13(6):951. https://doi.org/10.3390/catal13060951
Chicago/Turabian StyleOo, Wathone, Ji Hye Park, Zakia Akter Sonia, May Zaw Win, Dooyong Cho, and Kwang Bok Yi. 2023. "Modification of Copper-Ceria Catalyst via Reverse Microemulsion Method and Study of the Effects of Surfactant on WGS Catalyst Activity" Catalysts 13, no. 6: 951. https://doi.org/10.3390/catal13060951