Preparation of Mn-Co-MCM-41 Molecular Sieve with Thermosensitive Template and Its Degradation Performance for Rhodamine B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Reagents and Instruments
2.2. Synthesis of the Thermosensitive Template PNxDy
2.3. Preparation of the Bimetallic (Mn-Co)-Doped MCM-41 Molecular Sieve with PNxDy
2.4. Characterization of the Molecular Sieve
2.5. Degradation Performance of RhB by the Molecular Sieve
3. Results and Discussion
3.1. Characterization of the Thermosensitive Polymer PN100D4
3.2. Characterization of the Bimetallic Mn-Co Doped MCM-41 Molecular Sieve
3.2.1. FTIR
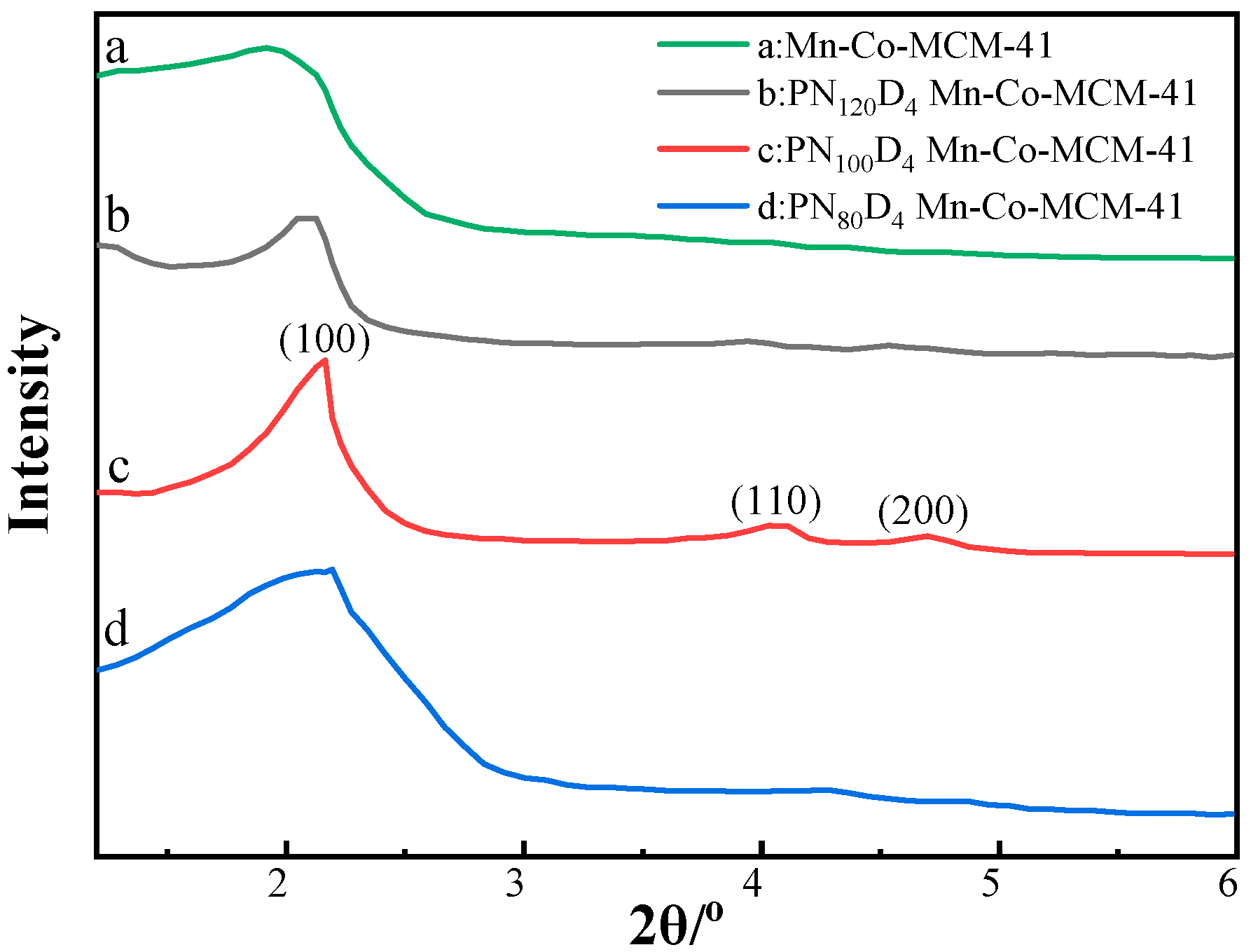
3.2.2. Small-Angle XRD
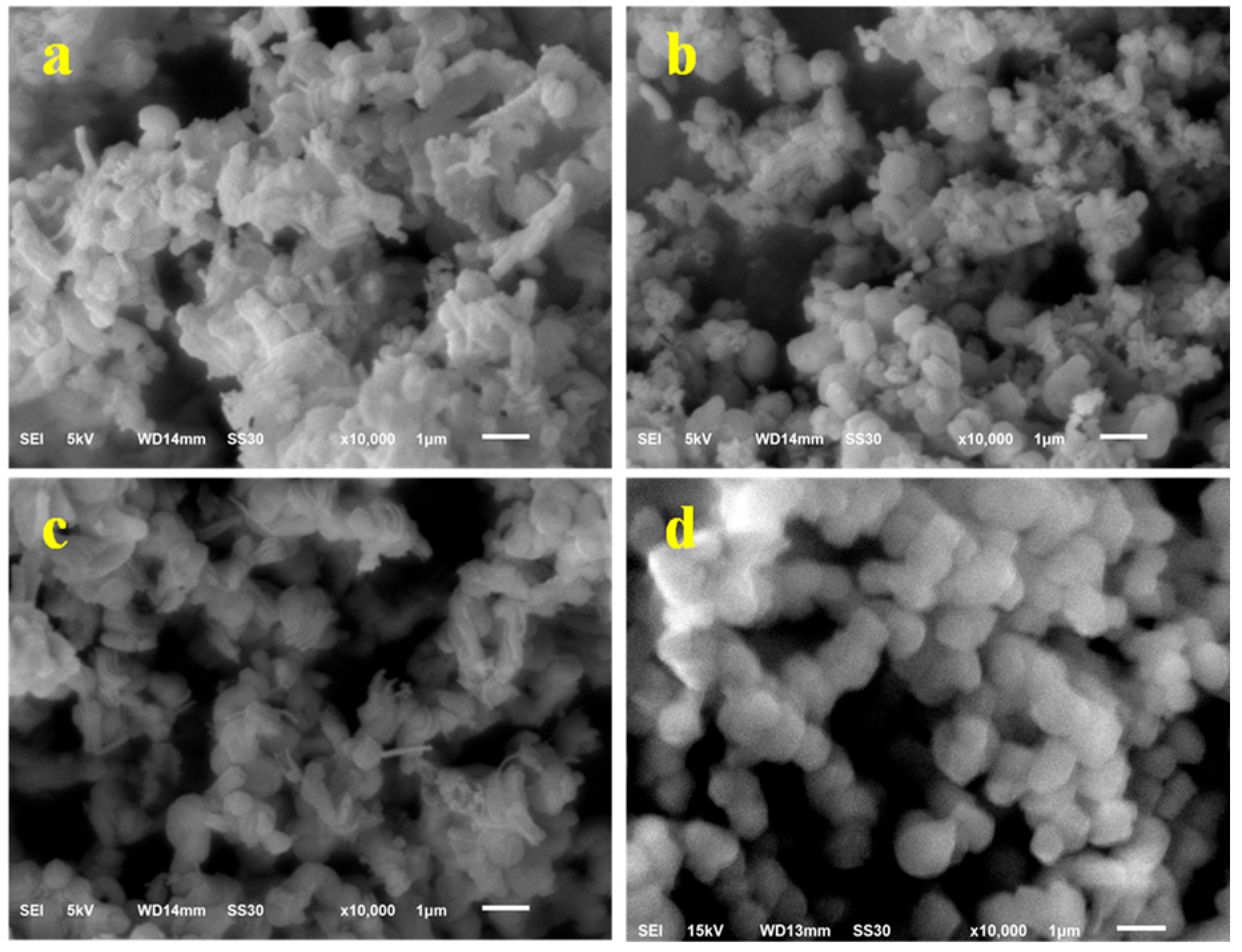
3.2.3. SEM
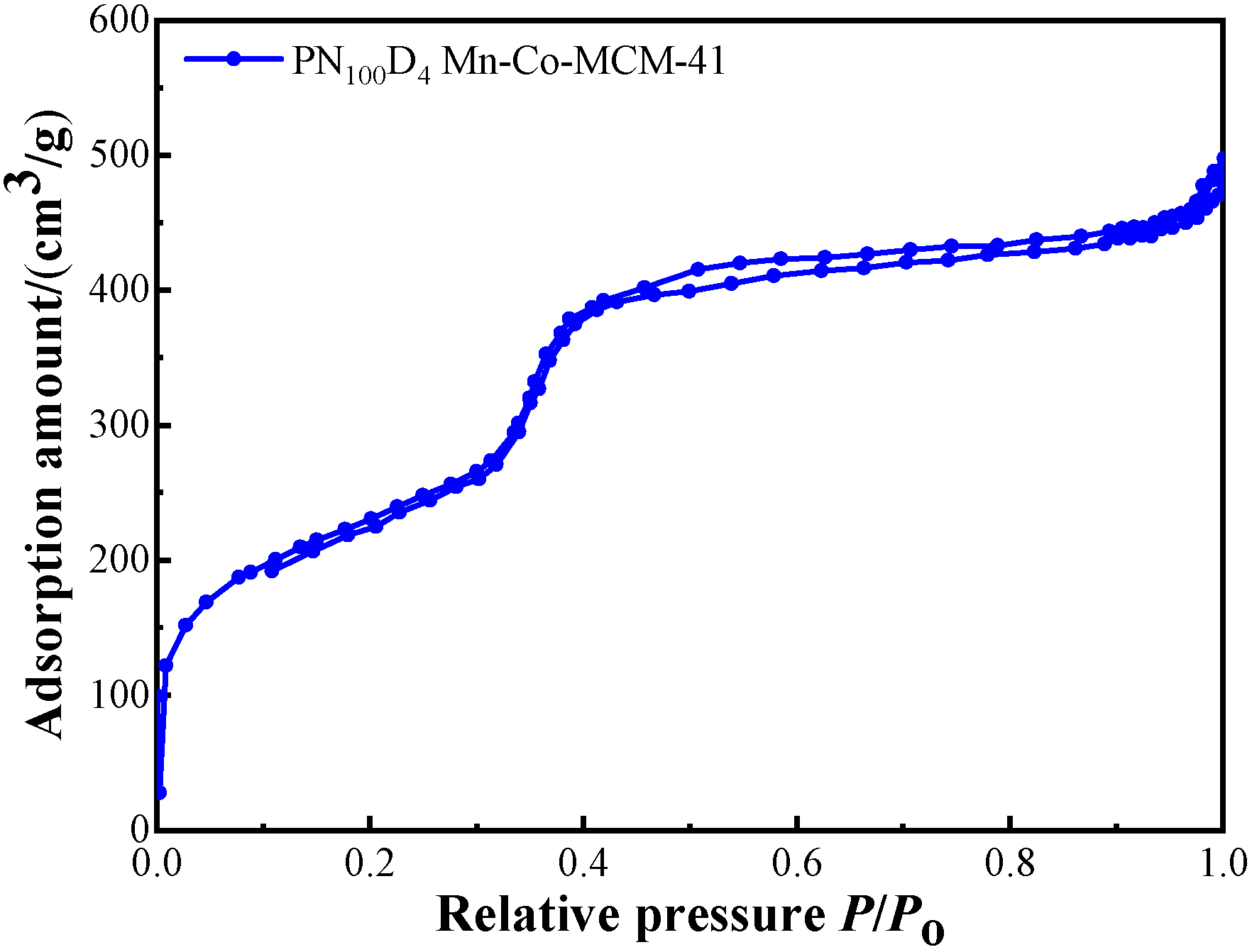
3.2.4. N2 Adsorption–Desorption
3.3. Performance of Catalyst-Activated PMS for RhB Degradation
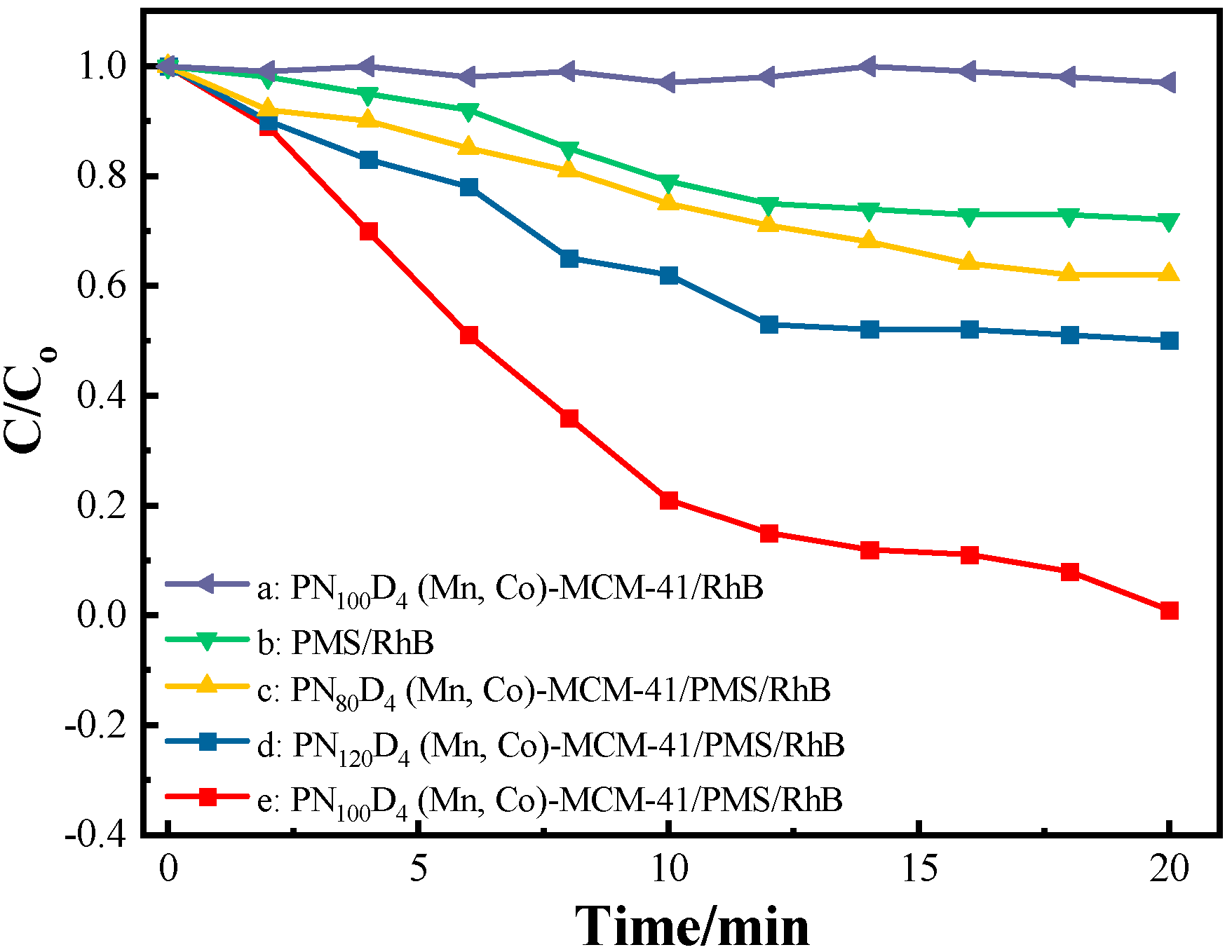
3.3.1. Influence of Catalyst Type
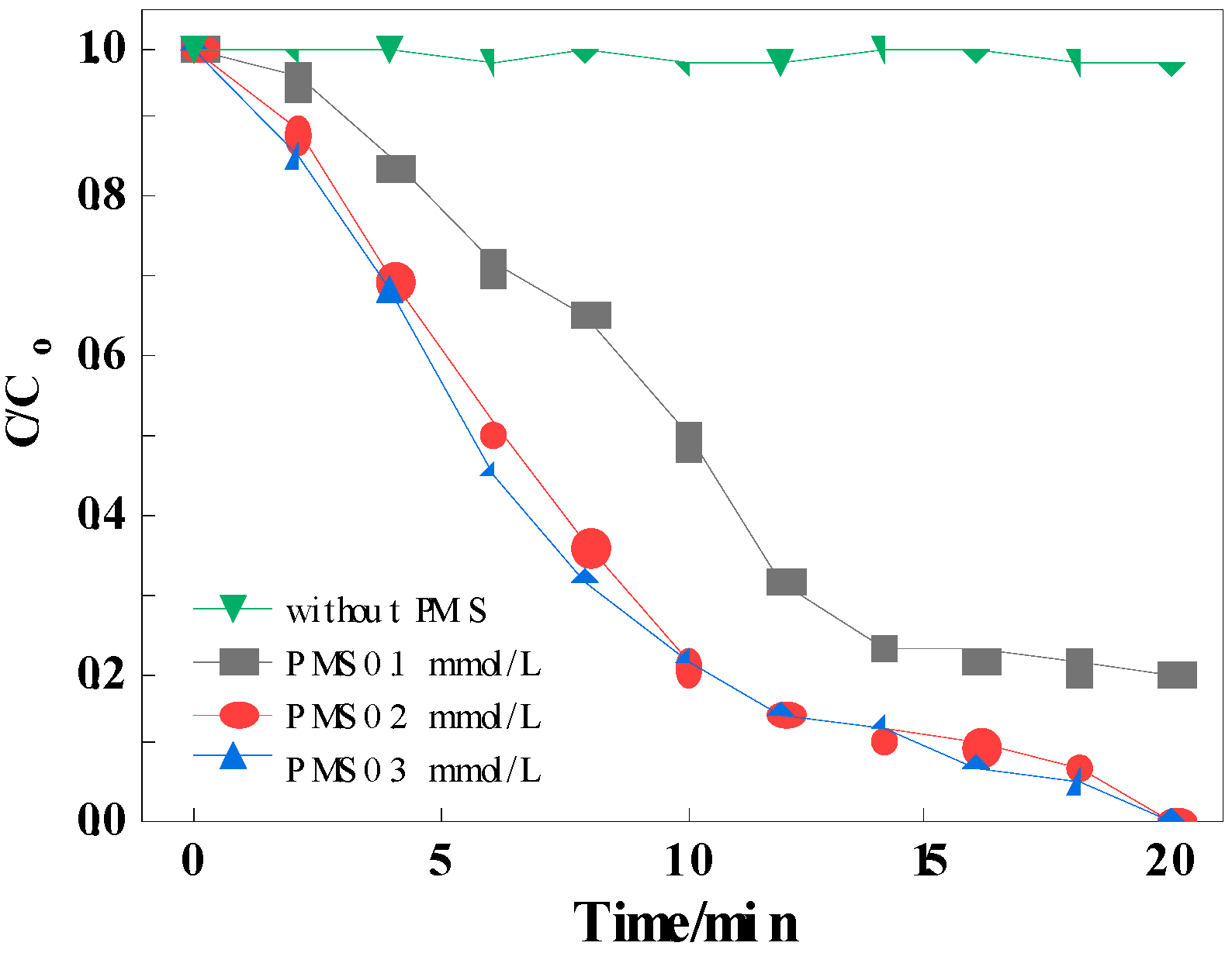
3.3.2. Influence of PMS Concentration
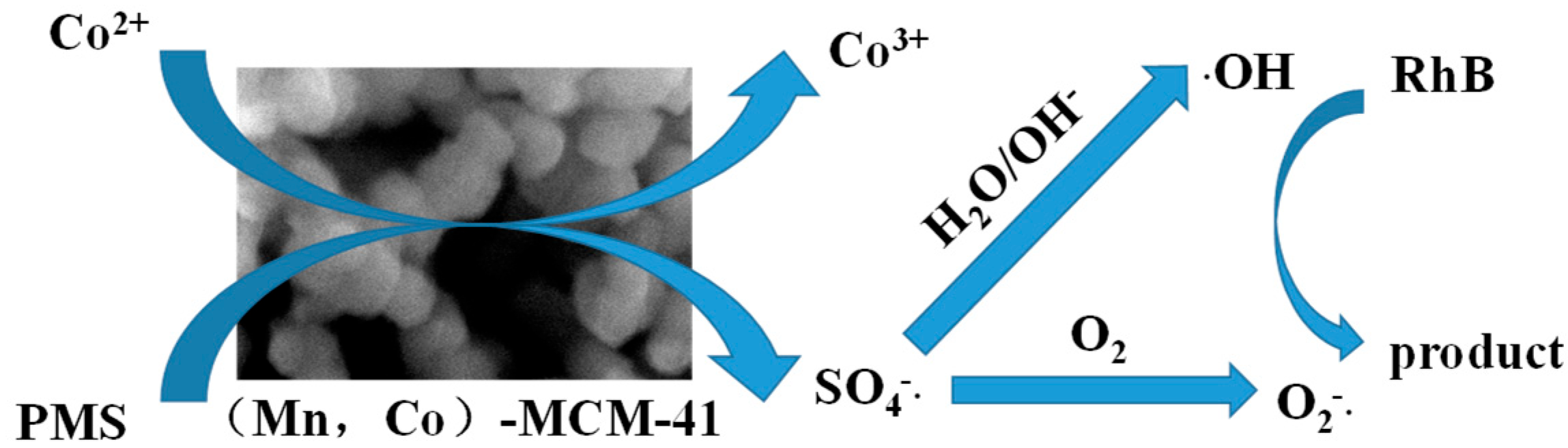
3.3.3. Reaction Mechanism of Mn-Co-MCM-41(PN100D4)-Activated PMS for RhB Degradation
3.4. Catalyst Recycling Performance
4. Conclusions
- The Mn-Co-MCM-41 molecular sieves obtained by bimetallic doping with the 12 hermos-sensitive templating PN100D4 had uniform pore channels and regular morphology.
- Bimetallic (Mn-Co) doping did not destroy the skeletal structure of MCM-41.
- The MCM-41 molecular sieve was loaded with bimetallic (Mn-Co) doping using the thermosensitive polymer material PN100D4 as a templating agent to better activate PMS for RhB degradation. The degradation rate of RhB could reach 98% with a 20 min reaction by Mn-Co-MCM-41 (PN100D4).
- This type of catalyst can be conveniently recycled and recovered for efficient reuse, which is expected to realize industrial applications.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nandigana, P.; Mahato, S.; Dhandapani, M.; Pradhan, B.; Subramanian, B.; Panda, S.K. Lyophilized tin-doped MoS2 as an efficient photocatalyst for overall degradation of Rhodamine B dye. J. Alloys Compd. 2022, 907, 164470. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lin, J.; Han, S.; Lei, L. Azo dye treatment with simultaneous electricity production in an anaerobic-aerobic sequential reactor and microbial fuel cell coupled system. Bioresour. Technol. 2010, 101, 4440–4445. [Google Scholar] [CrossRef] [PubMed]
- Olaru, P. Synthesis and testing of cellulose acetate nicotinate as adsorbent for rhodamine B dye. J. Appl. Polym. Sci. 2019, 136, 47772. [Google Scholar] [CrossRef]
- Saeed, M.; Ahmad, A.; Boddula, R.; Inamuddin; Haq, A.U.; Azhar, A. Ag@MnxOy: An effective catalyst for photo-degradation of rhodamine B dye. Env. Chem. Lett. 2017, 16, 287–294. [Google Scholar] [CrossRef]
- Singh, S.; Parveen, N.; Gupta, H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: A biosorbent. Environ. Technol. Innov. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Hira, S.A.; Yusuf, M.; Annas, D.; Hui, H.S.; Park, K.H. Biomass-derived activated carbon as a catalyst for the effective degradation of rhodamine B dye. Processes 2020, 8, 926. [Google Scholar] [CrossRef]
- Gunnagol, R.M.; Rabinal, M. TiO2-graphene nanocomposites for effective photocatalytic degradation of rhodamine-B dye. Chemistryselect 2018, 3, 2578–2585. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Moghaddam, M.R.A.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef]
- Gupta, H. Photocatalytic degradation of phenanthrene in the presence of akaganeite nano-rods and the identification of degradation products. Rsc. Adv. 2016, 6, 112721–112727. [Google Scholar] [CrossRef]
- Mishra, S.; Maiti, A. The efficiency of Eichhornia crassipes in the removal of organic and inorganic pollutants from wastewater: A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 7921–7937. [Google Scholar] [CrossRef]
- Ai, X. Ag/AgCl-GO: A composite for degradation of Rhodamine B in dye wastewater. Adv. Powder Technol. Int. J. Soc. Powder Technol. Jpn. 2019, 30, 3193–3202. [Google Scholar] [CrossRef]
- Sachdeva, S.; Kumar, A. Preparation of nanoporous composite carbon membrane for separation of rhodamine B dye. J. Membr. Sci. 2009, 329, 2–10. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; Ul, H.A.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 101846. [Google Scholar] [CrossRef]
- AbdulRazak, A.A.; Rohani, S. Sodium dodecyl sulfate-modified Fe2O3/molecular sieves for removal of rhodamine B dyes. Adv. Mater. Sci. Eng. 2018, 2018, 3849867. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Hui, B.; Dong, Y.; Sheng, Q.; Li, X.; Hao, Q.; Liu, C. Distributions of Ni in MCM-41 for the hydrogenation of N-ethylcarbazole. Fuel 2022, 324, 124405. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, H.; Tay-Agbozo, S.; Kispert, L.D. Photo-induced electron transfer of carotenoids in mesoporous sieves (MCM-41) and surface modified MCM-41: The role of hydrogen bonds on the electron transfer. J. Photochem. Photobiol. A Chem. 2017, 341, 1–11. [Google Scholar] [CrossRef]
- Lin, K.A.; Chen, B.; Chen, C. Evaluating Prussian blue analogues MII3[MIII (CN)6]2 (MII=Co, Cu, Fe, Mn, Ni; MIII=Co, Fe) as activators for peroxymonosulfate in water. Rsc. Adv. 2016, 6, 92923–92933. [Google Scholar] [CrossRef]
- Zhai, Q. Study on SBA-15 as an effective sorbent for dye butyl rhodamine B. J. Sol-Gel Sci. Technol. 2020, 96, 34–46. [Google Scholar] [CrossRef]
- Albayati, T.M.; Alwan, G.M.; Mahdy, O.S. High performance methyl orange capture on magnetic nanoporous MCM-41 prepared by incipient wetness impregnation method. Korean J. Chem. Eng. 2017, 34, 259–265. [Google Scholar] [CrossRef]
- Sar, Y.; Lmaz, M.; Dere Zdemir, Z.; Pi Kin, S. Synthesis and characterization of MCM-41 with different methods and adsorption of Sr2+ on MCM-41. Res. Chem. Intermediat. 2013, 41, 199–211. [Google Scholar]
- Selvaraj, M.; Pandurangan, A.; Seshadri, K.S.; Sinha, P.K.; Lal, K.B. Synthesis, characterization and catalytic application of MCM-41 mesoporous molecular sieves containing Zn and Al. Appl. Catal. A Gen. 2003, 242, 347–364. [Google Scholar] [CrossRef]
- Huang, W.; Liu, J.; Rao, N.; Fan, G.; Yan, J.; Cheng, Q.; Song, G. Influence of surfactant on CO2 adsorption of amine-functionalized MCM-41. Env. Technol. 2021, 43, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.J.T.; Coriolano, A.C.F.; Silva, J.B.; Castro, F.L.; Fernandes, V.J.; Araujo, A.S. Synthesis characterization and acid properties of niobium-containing MCM-41. J. Therm. Anal. Calorim. 2018, 131, 691–695. [Google Scholar] [CrossRef]
- Kaur, T.S.; Prakash, K.J.; Kumar, S.V. Adsorptive interaction of 4-aminobiphenyl with mesoporous MCM-41. Phys. Chem. Liq. 2018, 57, 720–732. [Google Scholar]
- Molaei, S.; Tamoradi, T.; Ghadermazi, M.; Ghorbani-Choghamarani, A. Highly Efficient Oxidative Coupling of Thiols and Oxidation of Sulfides in the Presence of MCM-41@Tryptophan-Cd and MCM-41@Tryptophan-Hg as Novel and Recoverable Nanocatalysts. Catal. Lett. 2018, 148, 1834–1847. [Google Scholar] [CrossRef]
- Hagio, T.J.Y.Y. Facile Hydrothermal Synthesis of EAB-Type Zeolite under Static Synthesis Conditions. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2021, 56, 2000163. [Google Scholar] [CrossRef]
- Pradhan, A.; Sahoo, M.; Bellamkonda, S.; Parida, K.M.; Rao, G.R. Enhanced photodegradation of dyes and mixed dyes by heterogeneous mesoporous Co-Fe/Al2O3-MCM-41 nanocomposites: Nanoparticles formation, semiconductor behavior and mesoporosity. Rsc. Adv. 2016, 6, 94263–94277. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.H. Selective Hydrogenation of Furfural: Pure Silica Supported Metal Catalysts. Chemistryselect 2022, 7, e202200013. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Ji, J.; Li, X.; Yuan, X.; Duan, A.; Guan, X.; Jiang, L.; Li, Y. Recycling of waste power lithium-ion batteries to prepare nickel/cobalt/manganese-containing catalysts with inter-valence cobalt/manganese synergistic effect for peroxymonosulfate activation. J. Colloid. Interf. Sci. 2022, 626, 564–580. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, R.; Zhao, G.; Luo, X.; Xing, C.; Yin, D. Thermo-responsive self-assembled metallomicelles accelerate asymmetric sulfoxidation in water. J. Catal. 2016, 335, 62–71. [Google Scholar] [CrossRef]
- Ramallo-López, J.M.; Lede, E.J.; Requejo, F.G.; Rodriguez, J.A.; Kim, J.; Rosas-Salas, R.; Domínguez, J.M. XANES Characterization of Extremely Nanosized Metal-Carbonyl Subspecies (Me = Cr, Mn, Fe, and Co) Confined into the Mesopores of MCM-41 Materials. J. Phys. Chem. B 2004, 108, 20005–20010. [Google Scholar] [CrossRef]
- Yu, H.; Ding, D.; Zhao, S.; Faheem, M.; Mao, W.; Yang, L.; Chen, L.; Cai, T. Co/N co-doped porous carbon as a catalyst for the degradation of RhB by efficient activation of peroxymonosulfate. Env. Sci. Pollut. R 2023, 30, 10969–10981. [Google Scholar] [CrossRef]
- Liu, J.; Sui, H.; Sheng, L. Hydrothermal synthesis, characterization and catalytic performance of Mn-MCM-41 mesoporous molecular sieve. Mod. Chem. Ind. 2018, 38, 93–97. [Google Scholar]
- Kong, Y.; Zhu, H.; Yang, G.; Guo, X.; Hou, W.; Yan, Q.; Gu, M.; Hu, C. Investigation of the structure of MCM-41 samples with a high copper content. Adv. Funct. Mater. 2004, 14, 816–820. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, X.; Chen, B. Magnetic biochar supported α-MnO2 nanorod for adsorption enhanced degradation of 4-chlorophenol via activation of peroxydisulfate. Sci. Total Env. 2020, 724, 138278. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Mosleh, N. CdS quantum dots encapsulated within the mesopores of MCM-41 and interlayers of montmorillonite as photocatalysts for rhodamine-B degradation in aqueous solution. Env. Sci. Pollut. R 2021, 28, 4615–4622. [Google Scholar] [CrossRef]
- Zhao, C.; Cai, L.; Wang, K.; Li, B.; Yuan, S.; Zeng, Z.; Zhao, L.; Wu, Y.; He, Y. Novel Bi2WO6/ZnSnO3 heterojunction for the ultrasonic-vibration-driven piezocatalytic degradation of RhB. Env. Pollut. 2023, 319, 120982. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, H.; Bai, K.; Chen, X.; Li, E.; Shen, Y.; Wang, M. Fabrication of a g-C3N4/MoS2 photocatalyst for enhanced RhB degradation. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 144, 115361. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, H.; Liu, W. The characteristics of degradation of rhodamine B by quinone-activated persulfate process. China Environ. Sci. 2016, 36, 1732–1737. [Google Scholar]
- Chen, X.; Liu, L. PMS activation over MoS2/Co0.75Mo3S3.75 for RhB pollutant oxidation removal in fuel cell system. J. Environ. Chem. Eng. 2022, 10, 107449. [Google Scholar] [CrossRef]
- Liu, C.; Pan, D.; Tang, X.; Hou, M.; Zhou, Q.; Zhou, J. Degradation of Rhodamine B by the α-MnO2/Peroxymonosulfate System. Water Air Soil Pollut. 2016, 227, 92. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Env. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Wang, J.; Zhai, Y.; Chen, P.; Ning, T.; Shi, C.; Yang, H.; Bao, Y.; Gao, Q.; Zhu, S. Efficient activation of peroxymonosulfate mediated by Co(II)-CeO2 as a novel heterogeneous catalyst for the degradation of refractory organic contaminants: Degradation pathway, mechanism and toxicity assessment. J. Hazard. Mater. 2022, 435, 129013. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yin, S.; Li, M.; Kang, X.; Wu, Z.; Zhao, S.; Liao, F. The synthesis of dandelion-like CuO nanoflowers and photocatalytic degradation of RhB. Colloid. Polym. Sci. 2017, 295, 1797–1803. [Google Scholar] [CrossRef]
- Shimizu, A.; Tokumura, M.; Nakajima, K.; Kawase, Y. Phenol removal using zero-valent iron powder in the presence of dissolved oxygen: Roles of decomposition by the Fenton reaction and adsorption/precipitation. J. Hazard. Mater. 2012, 201–202, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, A.H.; Arayesh, M.A.; Momeni, M.M. Degradation of MB and RhB by modified ZrO2 nanoparticles via sunlight. Appl. Phys. A 2021, 127, 158. [Google Scholar] [CrossRef]
- Frindy, S.; Sillanpää, M. Synthesis and application of novel α-Fe2O3/graphene for visible-light enhanced photocatalytic degradation of RhB. Mater. Des. 2020, 188, 108461. [Google Scholar] [CrossRef]
- Liu, P.; Xing, L.; Lin, H.; Wang, H.; Zhou, Z.; Su, Z. Construction of porous covalent organic polymer as photocatalysts for RhB degradation under visible light. Sci. Bull. 2017, 62, 931–937. [Google Scholar] [CrossRef] [Green Version]
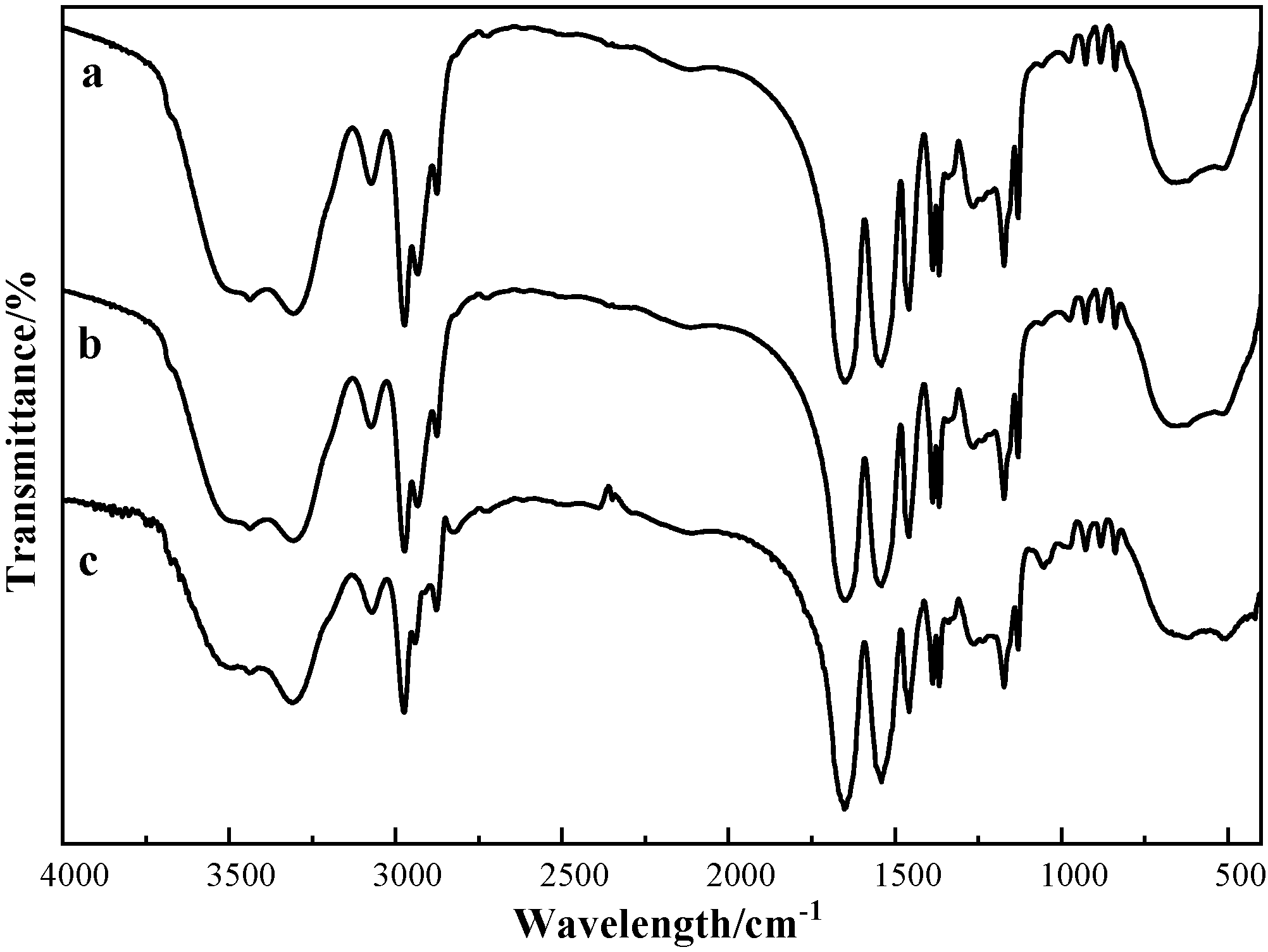
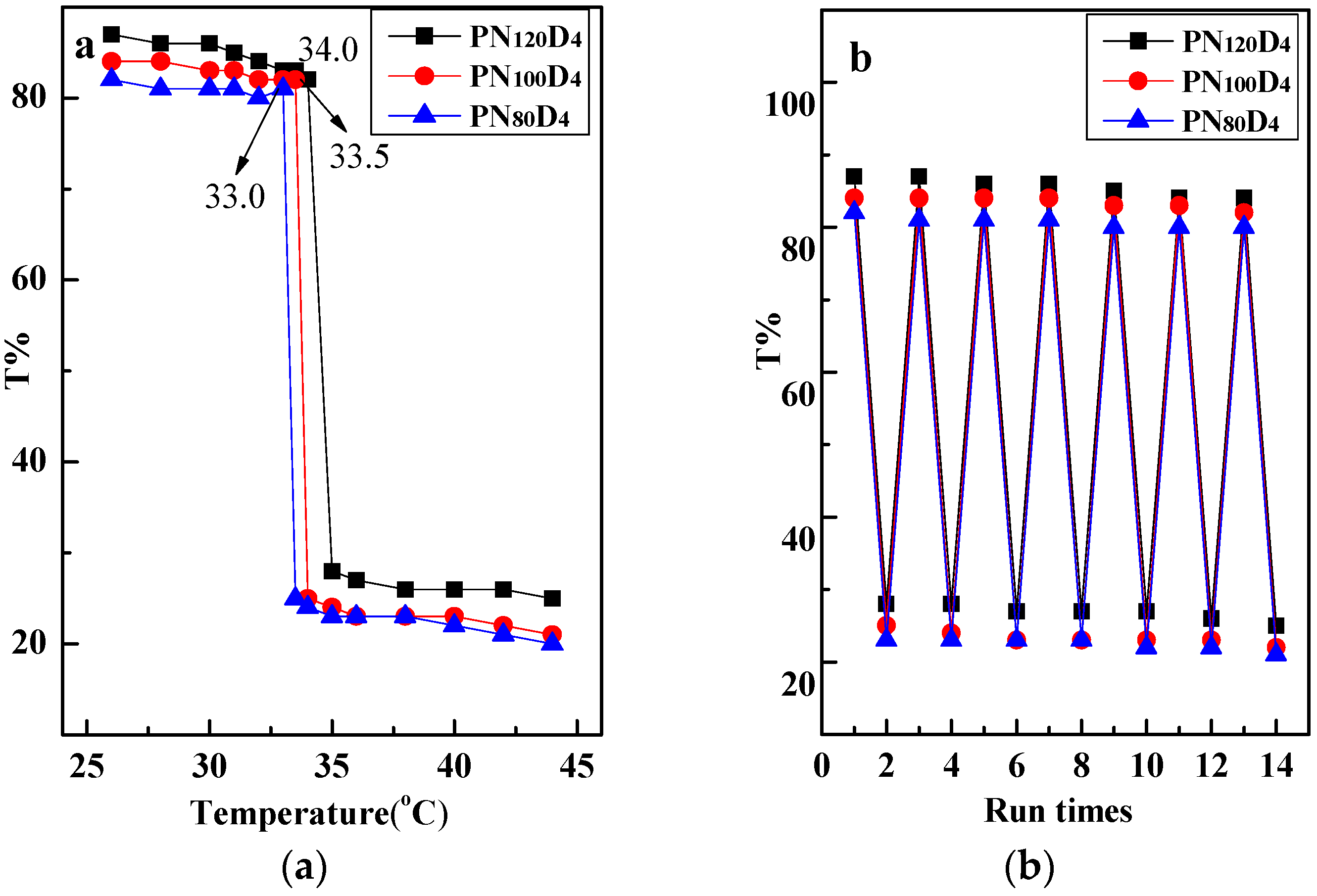
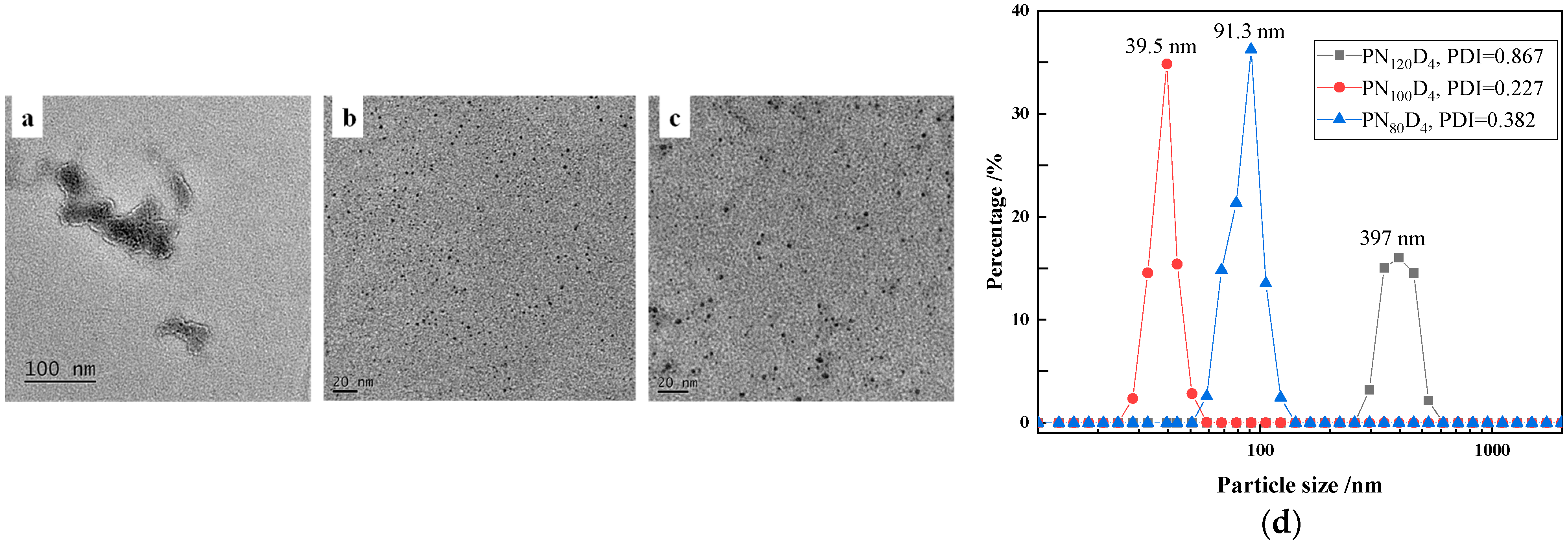
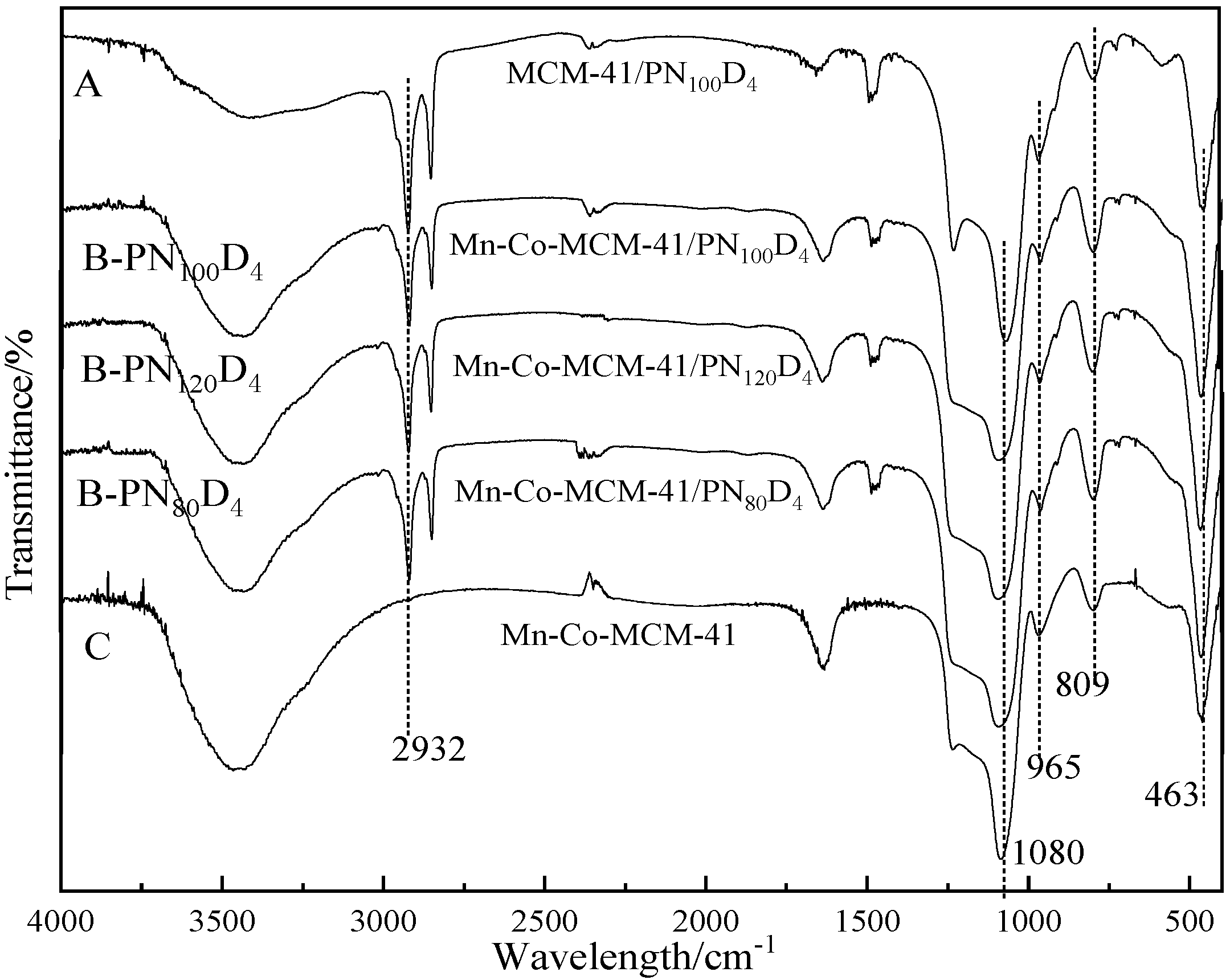




| Polymers | Hydrophilic/Hydrophobic Ratio | GPC | LCST(°C) | Morphology | Particle Size |
|---|---|---|---|---|---|
| PN120D4 | 30:1 | 17,624 | 34.0 | Random | / |
| PN100D4 | 25:1 | 16,121 | 33.5 | Nanoparticles | 2 nm |
| PN80D4 | 20:1 | 14,573 | 33.0 | Nanoparticles | 3 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Cai, L.; Lu, Y.; Zhang, Y. Preparation of Mn-Co-MCM-41 Molecular Sieve with Thermosensitive Template and Its Degradation Performance for Rhodamine B. Catalysts 2023, 13, 991. https://doi.org/10.3390/catal13060991
Peng W, Cai L, Lu Y, Zhang Y. Preparation of Mn-Co-MCM-41 Molecular Sieve with Thermosensitive Template and Its Degradation Performance for Rhodamine B. Catalysts. 2023; 13(6):991. https://doi.org/10.3390/catal13060991
Chicago/Turabian StylePeng, Wenju, Lixia Cai, Yani Lu, and Yaoyao Zhang. 2023. "Preparation of Mn-Co-MCM-41 Molecular Sieve with Thermosensitive Template and Its Degradation Performance for Rhodamine B" Catalysts 13, no. 6: 991. https://doi.org/10.3390/catal13060991
APA StylePeng, W., Cai, L., Lu, Y., & Zhang, Y. (2023). Preparation of Mn-Co-MCM-41 Molecular Sieve with Thermosensitive Template and Its Degradation Performance for Rhodamine B. Catalysts, 13(6), 991. https://doi.org/10.3390/catal13060991








