Noble Metal Modified TiO2 Hierarchically Structured Microspheres with Enhanced Photocatalytic Activity
Abstract
1. Introduction
2. Results and Discussion
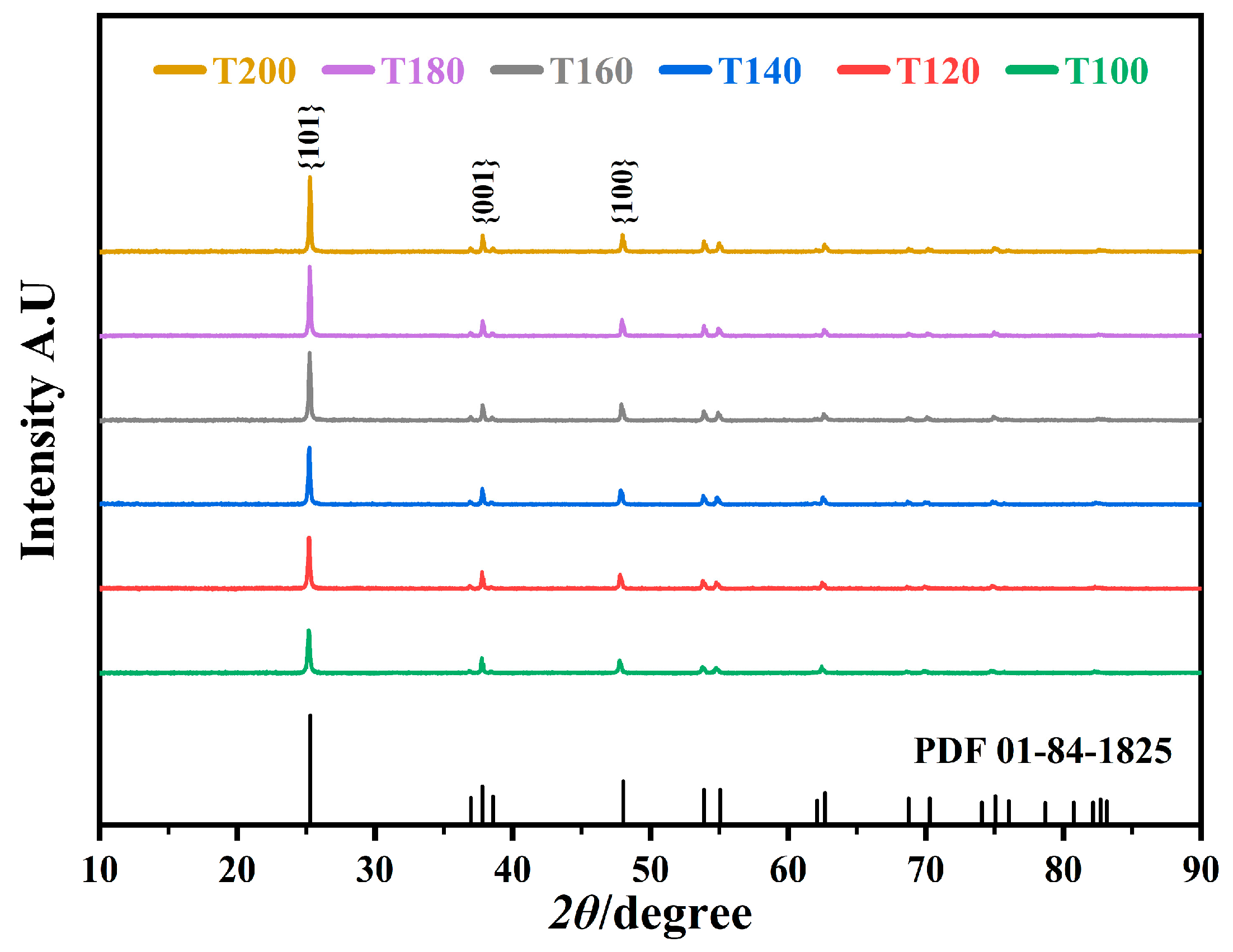
2.1. Structures and Morphology
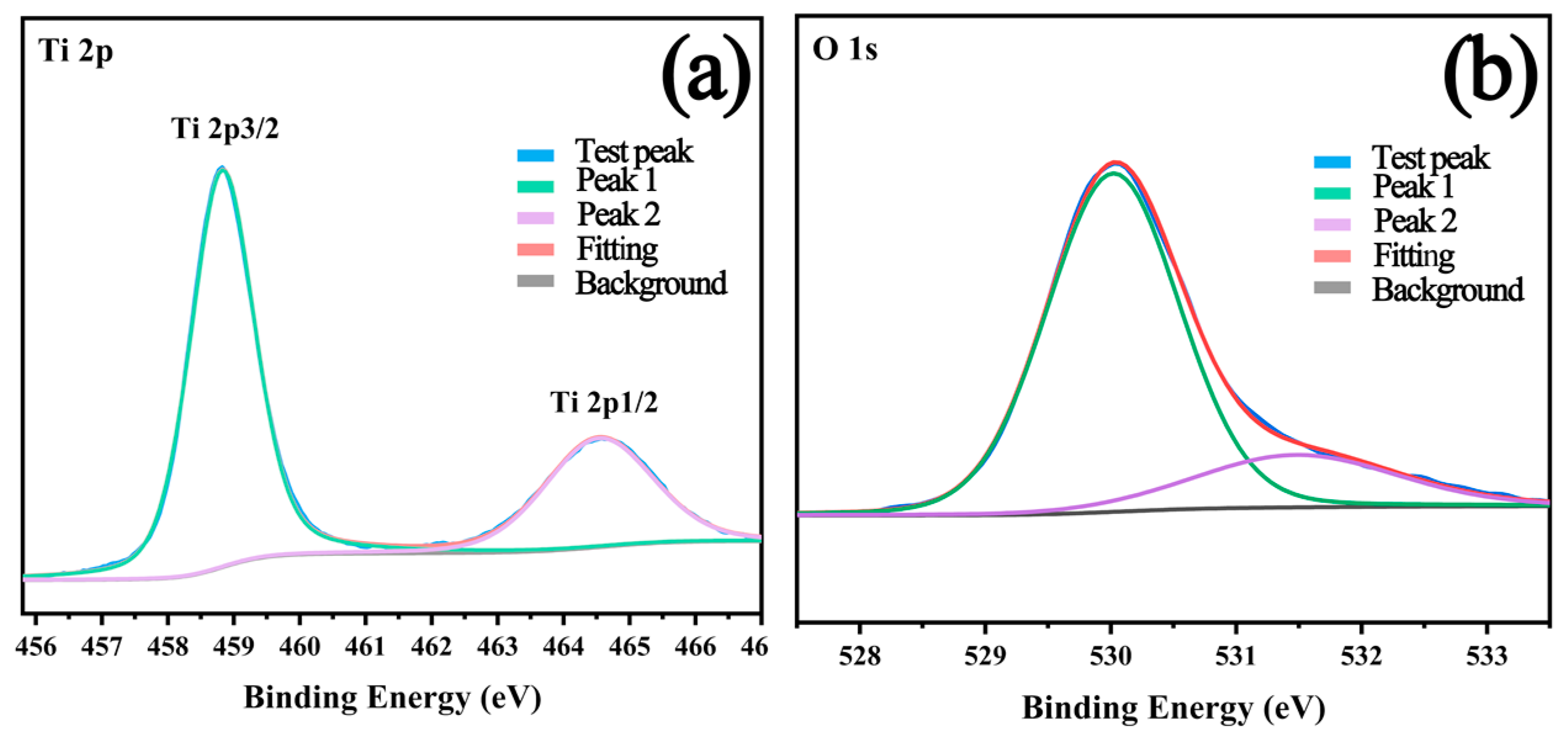
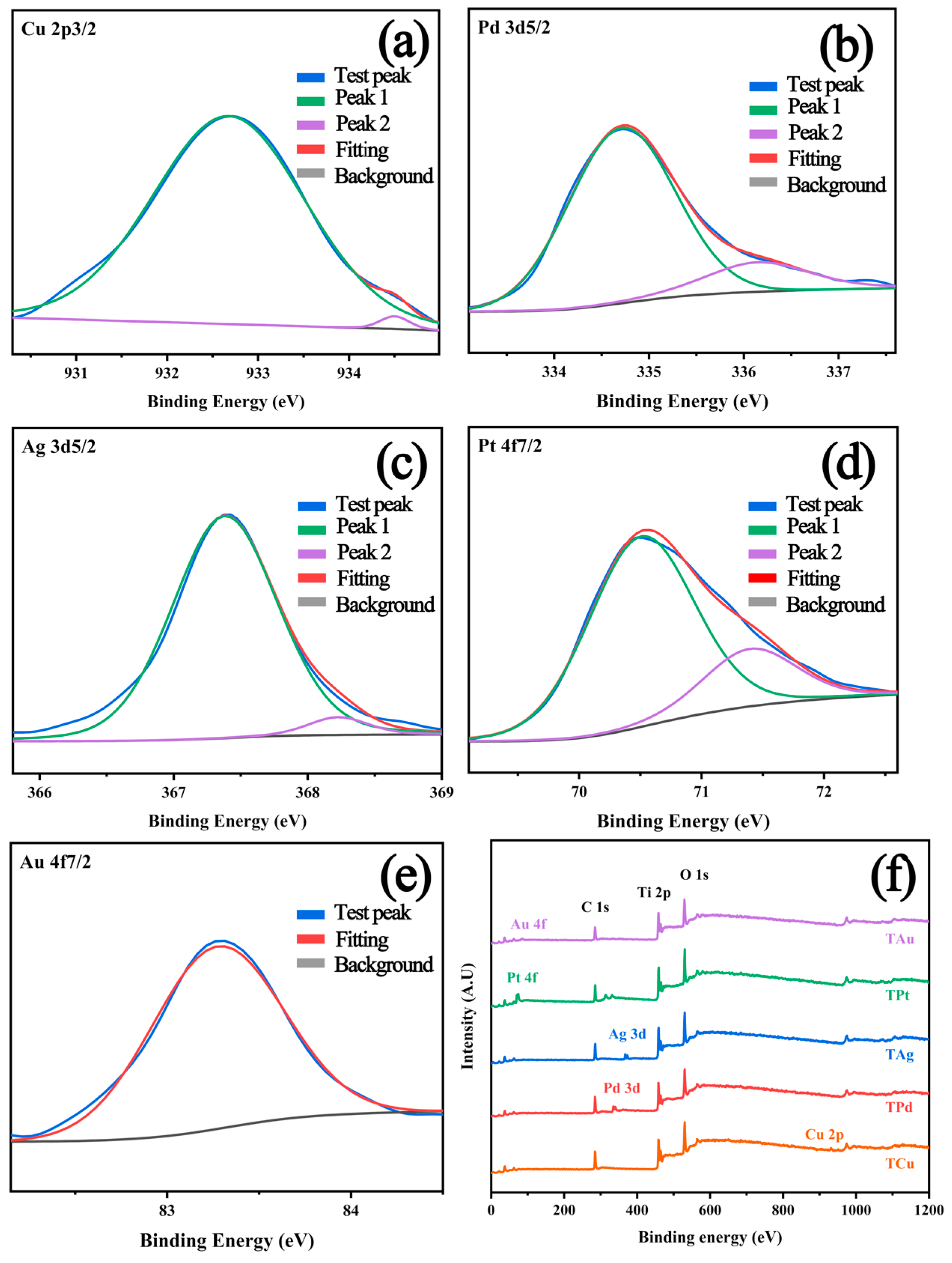
2.2. Surface Elements and Composition
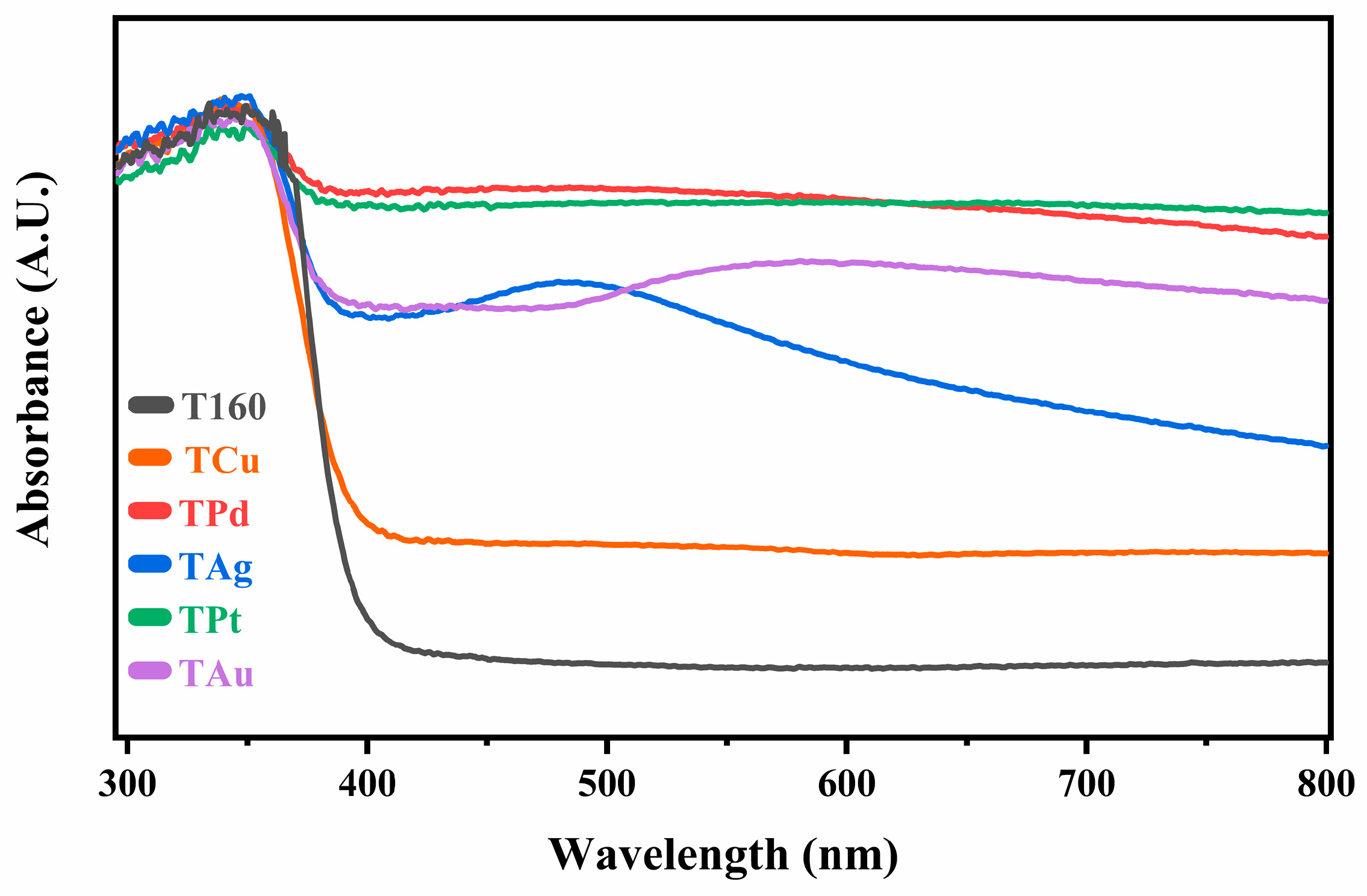
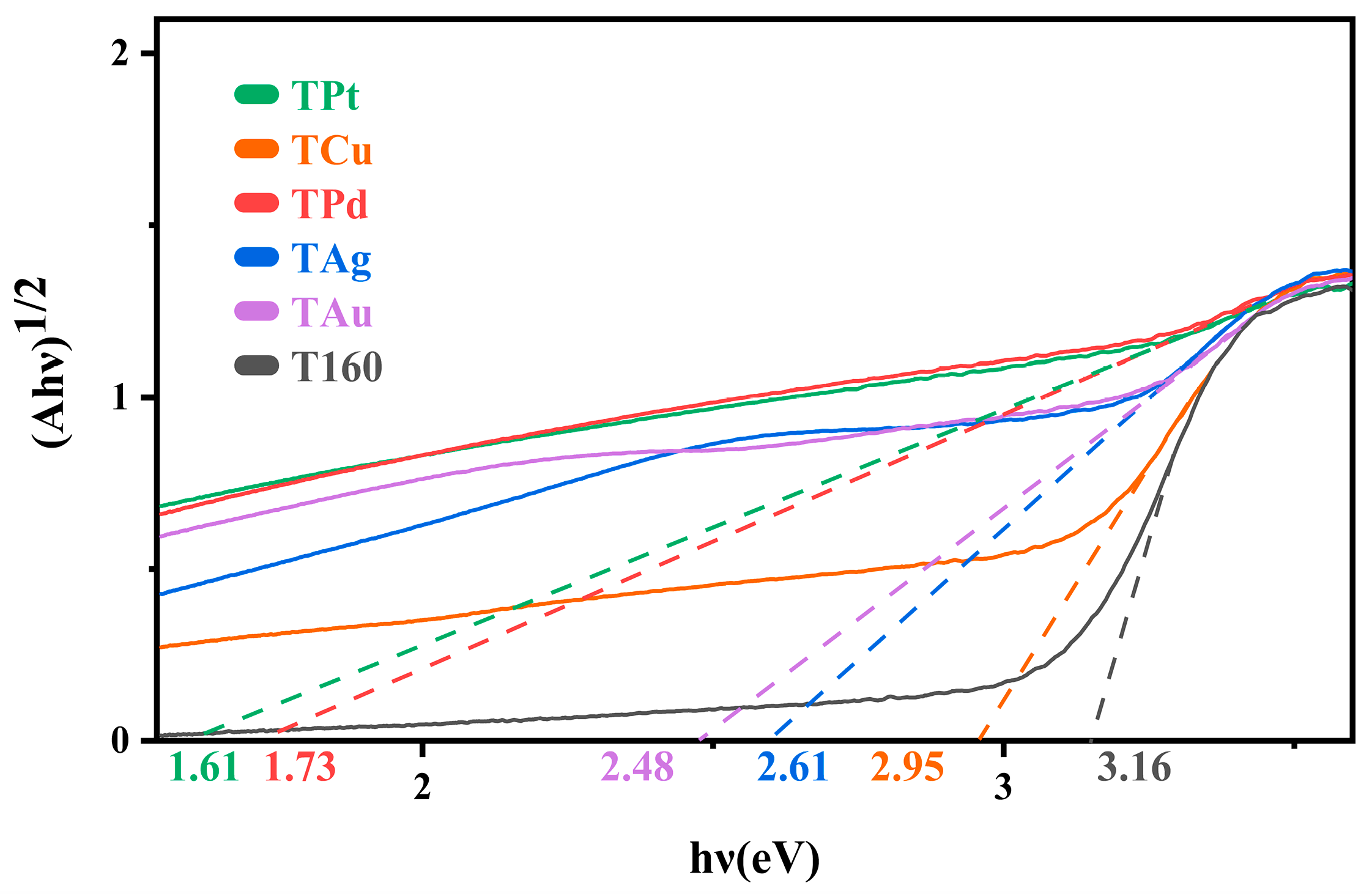
2.3. Visible and UV Absorption Properties
2.4. Evaluation of Photocatalytic Degradation Activity
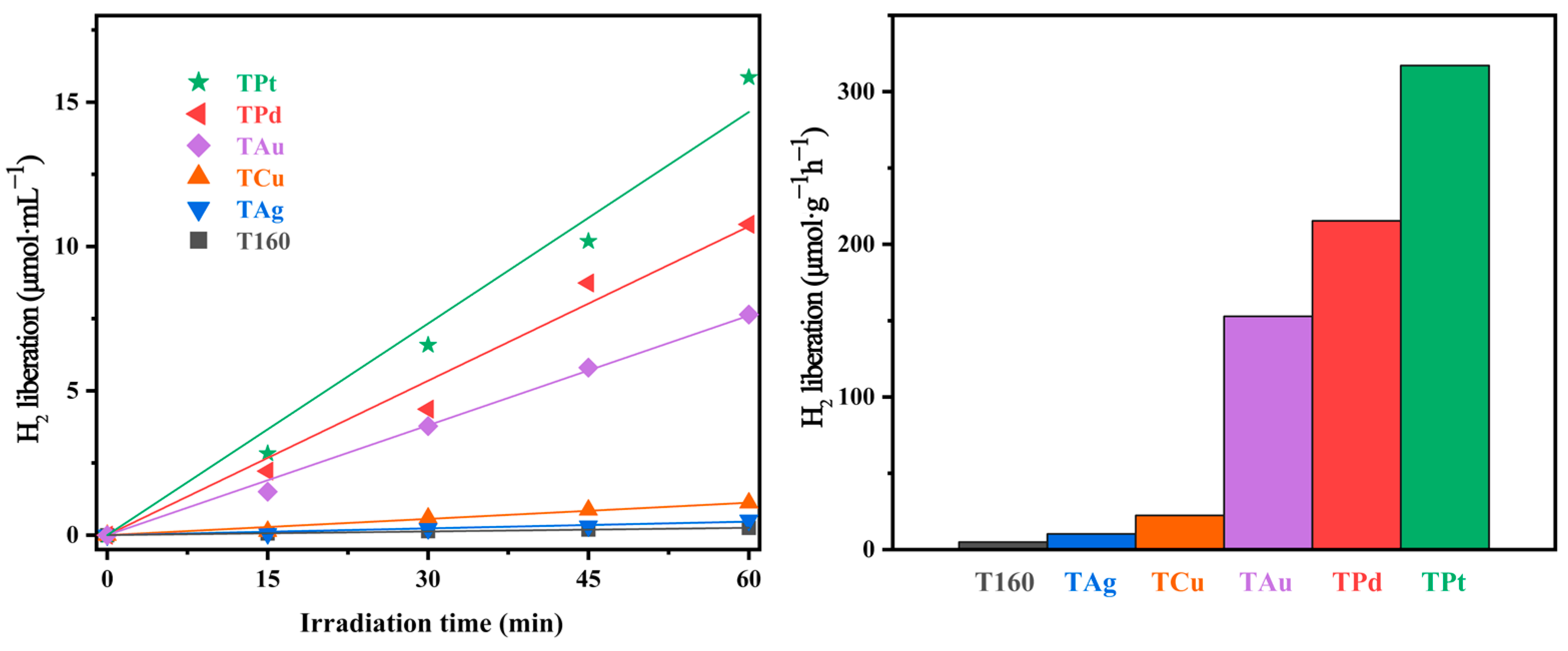
2.5. Evaluation of Photocatalytic Hydrogen Generation Activity
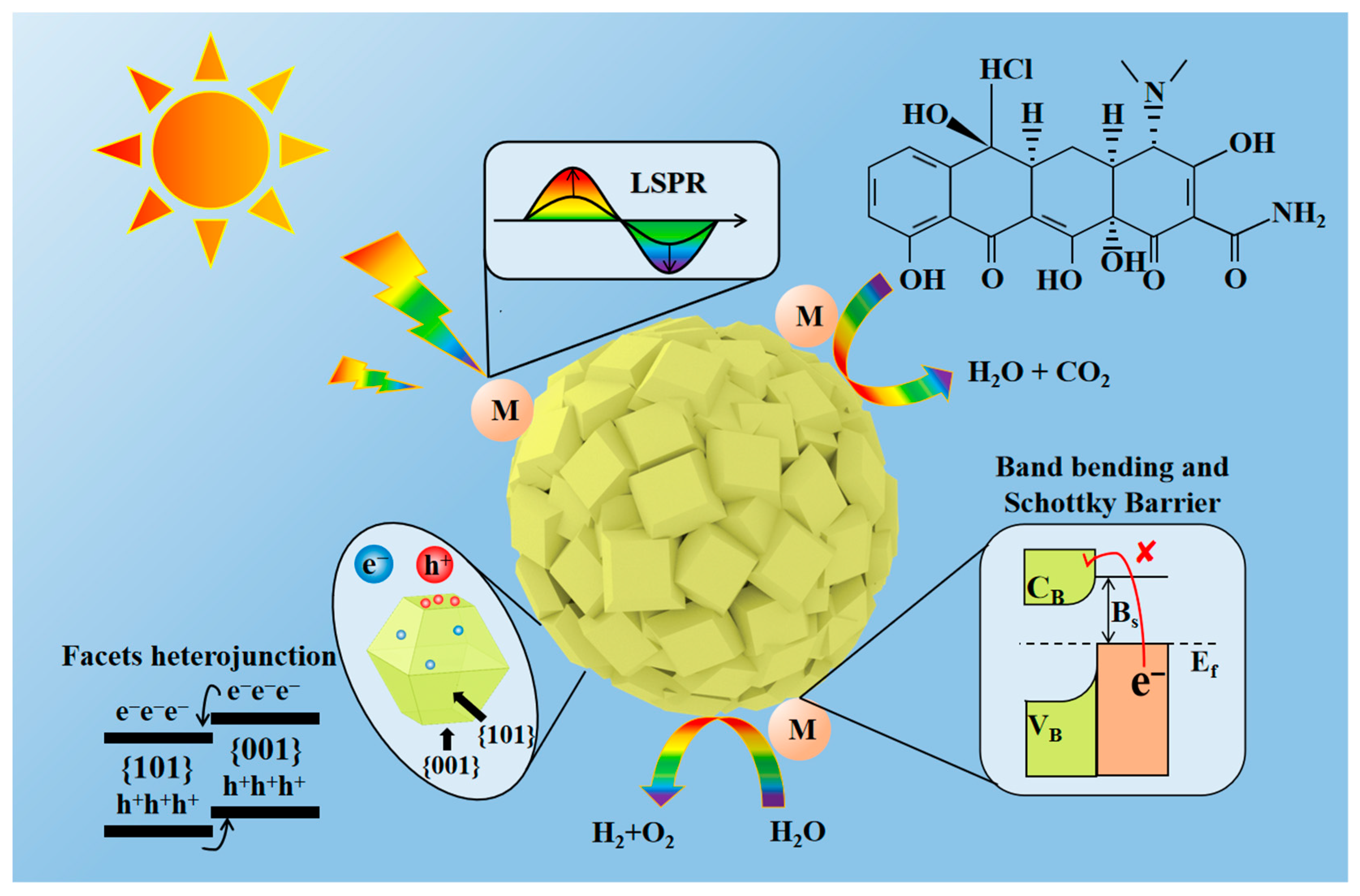
2.6. Photocatalytic Reaction Mechanism
3. Materials and Methods
3.1. Synthesis of Materials
3.2. Characterization of Materials
3.3. Photocatalytic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xin, Y.; Zhu, Q.; Gao, T.; Li, X.; Zhang, W.; Wang, H.; Ji, D.; Huang, Y.; Padervand, M.; Yu, F.; et al. Photocatalytic NO removal over defective Bi/BiOBr nanoflowers: The inhibition of toxic NO2 intermediate via high humidity. Appl. Catal. B Environ. 2023, 324, 122238. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Padervand, M.; Asgarpour, F.; Akbari, A.; Sis, B.E.; Lammel, G. Hexagonal Core–Shell SiO2[–MOYI]Cl–]Ag Nanoframeworks for Efficient Photodegradation of the Environmental Pollutants and Pathogenic Bacteria. J. Inorg. Organomet. Polym. 2019, 29, 1314–1323. [Google Scholar] [CrossRef]
- Takami, N.; Harada, Y.; Iwasaki, T.; Hoshina, K.; Yoshida, Y. Micro-size spherical TiO2(B) secondary particles as anode materials for high-power and long-life lithium-ion batteries. J. Power Sources 2015, 273, 923–930. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.L.; Tian, Y.; Lan, K.; Liu, Q.; Wang, C.Y.; Liu, Y.; Elzatahry, A.; Che, R.C.; Li, W.; et al. Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting. Chem. Sci. 2019, 10, 1664–1670. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, T.T.; Liu, C.; Yang, Y.; Pan, J.H.; Yao, J.X.; Hu, L.H.; Dai, S.Y. Shape-controlled synthesis of single-crystalline anatase TiO2 micro/nanoarchitectures for efficient dye-sensitized solar cells. Sustain. Energy Fuels 2017, 1, 520–528. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Han, X.G.; Kuang, Q.; Jin, M.S.; Xie, Z.X.; Zheng, L.S. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Z.; Zhou, J.Z.; Jiang, H.B.; Hu, Q.H.; Qiao, S.Z.; Yang, H.G. Synthesis of micro-sized titanium dioxide nanosheets wholly exposed with high-energy {001} and {100} facets. Chem. Commun. 2011, 47, 4400–4402. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001}and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, H. Novel Fe-doped anatase TiO2 nanosheet hierarchical spheres with 94%{001} facets for efficient visible light photodegradation of organic dye. RSC Adv. 2013, 3, 16255–16258. [Google Scholar] [CrossRef]
- Ji, L.; Liu, X.; Xu, T.; Gong, M.; Zhou, S. Preparation and photocatalytic properties of carbon/carbon-doped TiO2 double-layer hollow microspheres. J. Sol-Gel Sci. Technol. 2020, 93, 380–390. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D.; Chen, M.; Li, D.; Zhu, J.; Lü, X.; Yan, C. Preparation and characterization of monodisperse Ce-doped TiO2 microspheres with visible light photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 107–114. [Google Scholar] [CrossRef]
- Akple, M.S.; Low, J.; Qin, Z.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Liu, S. Nitrogen-doped TiO2 microsheets with enhanced visible light photocatalytic activity for CO2 reduction. Chin. J. Catal. 2015, 36, 2127–2134. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Qu, J.; Chen, Y.; Shao, Z. Nitrogen-doped TiO2 microspheres with hierarchical micro/nanostructures and richdual-phase junctions for enhanced photocatalytic activity. RSC Adv. 2016, 6, 40923–40931. [Google Scholar] [CrossRef]
- Park, B.-G. Photocatalytic Activity of TiO2-Doped Fe, Ag, and Ni with N under Visible Light Irradiation. Gels 2021, 8, 14. [Google Scholar] [CrossRef]
- Hu, K.; Lei, E.; Hu, C.; Zhao, D.; Zhu, M.; Wang, J.; Zhao, W. gC3N4/TiO2 composite microspheres: In situ growth and high visible light catalytic activity. CrystEngComm 2020, 22, 7104–7112. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Li, C.; Qin, F.; Wei, L.; Hu, C.; Hu, Q.; Duo, S. Nanoscaled Bi2O4 confined in firework-shaped TiO2 microspheres with enhanced visible light photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123757. [Google Scholar] [CrossRef]
- Wang, W.S.; Wang, D.H.; Qu, W.G.; Lu, L.Q.; Xu, A.W. Large ultrathin anatase TiO2 nanosheets with exposed {001} facets on graphene for enhanced visible light photocatalytic activity. J. Phys. Chem. C 2012, 116, 19893–19901. [Google Scholar] [CrossRef]
- He, H.Y.; Lin, J.H.; Fu, W.; Wang, X.L.; Wang, H.; Zeng, Q.S.; Gu, Q.; Li, Y.M.; Yan, C.; Tay, B.K.; et al. MoS2/TiO2 edge-on heterostructure for efficient photocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1600464. [Google Scholar] [CrossRef]
- Li, G.; Chen, J.; Wu, S.; Yu, Y.; Li, Y.; Gao, J. The relationship between the mesostructure of WO3/TiO2 hollow microsphere and its property. Surf. Innov. 2017, 6, 37–46. [Google Scholar] [CrossRef]
- Wang, A.; Wu, S.; Dong, J.; Wang, R.; Wang, J.; Zhang, J.; Zhong, S.; Bai, S. Interfacial facet engineering on the Schottky barrier between plasmonic Au and TiO2 in boosting the photocatalytic CO2 reduction under ultraviolet and visible light irradiation. Chem. Eng. J. 2021, 404, 127145. [Google Scholar] [CrossRef]
- Wanbayor, R.; Ruangpornvisuti, V. A periodic DFT study on binding of Pd, Pt and Au on the anatase TiO2(0 0 1) surface and adsorption of CO on the TiO2 surface-supported Pd, Pt and Au. Appl. Surf. Sci. 2012, 258, 3298–3301. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Shi, Z.; Lin, L.; Chen, R.; Yan, L. Adsorption of CO molecules on anatase TiO2 (001) loaded with noble metals M (M = Ir/Pd/Pt): A study from DFT calculations. Mater. Today Commun. 2021, 28, 102699. [Google Scholar] [CrossRef]
- Tong, R.; Liu, C.; Xu, Z.; Kuang, Q.; Xie, Z.; Zheng, L. Efficiently Enhancing Visible Light Photocatalytic Activity of Faceted TiO2 Nanocrystals by Synergistic Effects of Core-Shell Structured Au@CdS Nanoparticles and Their Selective Deposition. ACS Appl. Mater. Interfaces 2016, 8, 21326–21333. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Tunable photocatalytic selectivity of TiO2 films consisted of flower-like microspheres with exposed {001} facets. Chem. Commun. 2011, 47, 4532–4534. [Google Scholar] [CrossRef]
- Han, S.; Niu, Q.; Qin, N.; Gu, X.; Zhang, Y.N.; Hua, G. In situ growth of M-{001} TiO2/Ti photoelectrodes: Synergetic dominant {001} facets and ratio-optimal surface junctions for the effective oxidation of environmental pollutants. Chem. Commun. 2020, 56, 1337–1340. [Google Scholar] [CrossRef]
- Chang, Y.; He, P.; Wei, Z.; Chen, Y.; Wang, H.; Wu, C.; Zhou, Z.; Huang, H.; Kowalska, E.; Dong, S. Three-dimensional monodispersed TiO2 microsphere network formed by a sub-zero sol-gel method. Mater. Lett. 2020, 268, 127592. [Google Scholar] [CrossRef]
- Feng, N.D.; Lin, H.W.; Song, H.; Yang, L.X.; Tang, D.M.; Deng, F.; Ye, J.H. Efficient and selective photocatalytic CH4 conversion to CH3OH with O2 by controlling overoxidation on TiO2. Nat. Commun. 2021, 12, 4652. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K.; Lee, C.H. The advanced removal of benzene from aerosols by photocatalytic oxidation and adsorption of Cu-TiO2/PU under visible light irradiation. Appl. Catal. B Environ. 2016, 182, 172–183. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, X.F.; Li, Z.C.; Ye, X.Y.; Liu, Y.; Chen, Y.; Yang, L.; Chen, M.; Zhang, D.Q.; Li, G.S.; et al. Cooperation between inside and outside of TiO2: Lattice Cu+ accelerates carrier migration to the surface of metal copper for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2020, 264, 118515. [Google Scholar] [CrossRef]
- DeSario, P.A.; Gordon, W.O.; Balboa, A.; Pennington, A.M.; Pitman, C.L.; McEntee, M.; Pietron, J.J. Photoenhanced degradation of sarin at Cu/TiO2 composite aerogels: Roles of bandgap excitation and surface plasmon excitation. ACS Appl. Mater. Interfaces 2021, 13, 12550–12561. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Yeo, I.S.; Kim, T.U.; Ki, H.C.; Gu, H.B. Surface plasmon resonance effect of silver nanoparticles on a TiO2 electrode for dye-sensitized solar cells. Appl. Surf. Sci. 2018, 432, 266–271. [Google Scholar] [CrossRef]
- Tanahashi, I.; Iwagishi, H.; Chang, G. Localized surface plasmon resonance sensing properties of photocatalytically prepared Au/TiO2 films. Mater. Lett. 2008, 62, 2714–2716. [Google Scholar] [CrossRef]
- Yao, G.Y.; Liu, Q.L.; Zhao, Z.Y. Studied localized surface plasmon resonance effects of Au nanoparticles on TiO2 by FDTD simulations. Catalysts 2018, 8, 236. [Google Scholar] [CrossRef]
- de Brito, J.F.; Tavella, F.; Genovese, C.; Ampelli, C.; Boldrin Zanoni, M.V.; Centi, G.; Perathoner, S. Role of CuO in the modification of the photocatalytic water splitting behavior of TiO2 nanotube thin films. Appl. Catal. B Environ. 2018, 224, 136–145. [Google Scholar] [CrossRef]
- Wei, Z.S.; Janczarek, M.; Endo, M.; Wang, K.L.; Balčytis, A.; Nitta, A.; Méndez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef]
- Ya, J.; Yang, N.N.; Hu, F.J.; Liu, Z.F.; Lei, E. Preparation and activity evaluation of TiO2/Cu-TiO2 composite catalysts. J. Sol-Gel Sci. Technol. 2015, 73, 322–331. [Google Scholar] [CrossRef]
- Li, Z.H.; Liu, J.W.; Wang, D.J.; Gao, Y.; Shen, J. Cu2O/Cu/TiO2 nanotube Ohmic heterojunction arrays with enhanced photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2012, 37, 6431–6437. [Google Scholar] [CrossRef]
- Zhen, W.L.; Jiao, W.J.; Wu, Y.Q.; Jing, H.W.; Lu, G.X. The role of a metallic copper interlayer during visible photocatalytic hydrogen generation over a Cu/Cu2O/Cu/TiO2 catalyst. Catal. Sci. Technol. 2017, 7, 5028–5037. [Google Scholar] [CrossRef]
- He, C.; Shu, D.; Su, M.H.; Xia, D.H.; Asi, M.A.; Lin, L.; Xiong, Y. Photocatalytic activity of metal (Pt, Ag, and Cu)-deposited TiO2 photoelectrodes for degradation of organic pollutants in aqueous solution. Desalination 2010, 253, 88–93. [Google Scholar] [CrossRef]
- Bai, W.D.; Wu, M.B.; Du, X.L.; Gong, W.L.; Ding, Y.H.; Song, C.H.; Liu, L. Synergistic effect of multiple-phase rGO/CuO/Cu2O heterostructures for boosting photocatalytic activity and durability. Appl. Surf. Sci. 2019, 40, 105–113. [Google Scholar] [CrossRef]
- Derry, G.N.; Kern, M.E.; Worth, E.H. Recommended values of clean metal surface work functions. J. Vac. Sci. Technol. A 2015, 33, 060801. [Google Scholar] [CrossRef]
- Skriver, H.L.; Rosengaard, N.M. Surface energy and work function of elemental metals. Phys. Rev. B 1992, 46, 7157. [Google Scholar] [CrossRef]
- Hölzl, J.; Schulte, F.K. Work function of metals. In Solid Surface Physics; Springer Tracts in Modern Physics; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–150. [Google Scholar] [CrossRef]




| Volume (%) | 50.45 | 81.65 | 92.28 | 100 |
|---|---|---|---|---|
| Particle size range (μm) | 0.96–1.26 | 0.73–1.45 | 0.63–1.66 | 0.48–3.31 |
| Average particle size (μm) | 1.11 | 1.07 | 1.09 | 1.16 |
| Samples | TCu | TPd | TAg | TPt | TAu | |
|---|---|---|---|---|---|---|
| Content (at%) | O | 63.3 | 66.7 | 63.6 | 65.0 | 65.7 |
| Ti | 34.5 | 29.6 | 34.3 | 31.2 | 32.3 | |
| Cu | 2.2 | - | - | - | - | |
| Pd | - | 3.7 | - | - | - | |
| Ag | - | - | 2.1 | - | - | |
| Pt | - | - | - | 3.8 | - | |
| Au | - | - | - | - | 2.0 | |
| Samples | Degradation Rate in 2 h (%) | Increase Rate Compared with T160 (%) | K (Kinetic Linear Fitting) | R (k/k160) |
|---|---|---|---|---|
| T160 | 70.71 | 0.00 | 0.00991 | 1.00 |
| TCu | 91.96 | 21.25 | 0.02008 | 2.03 |
| TPd | 83.50 | 12.79 | 0.01461 | 1.47 |
| TAg | 73.28 | 2.57 | 0.01111 | 1.12 |
| TPt | 95.06 | 24.35 | 0.02450 | 2.47 |
| TAu | 78.40 | 7.69 | 0.01251 | 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Wang, J.; Kong, Q.; Xu, Y.; Wei, Z.; Chang, Y. Noble Metal Modified TiO2 Hierarchically Structured Microspheres with Enhanced Photocatalytic Activity. Catalysts 2023, 13, 995. https://doi.org/10.3390/catal13060995
Huang H, Wang J, Kong Q, Xu Y, Wei Z, Chang Y. Noble Metal Modified TiO2 Hierarchically Structured Microspheres with Enhanced Photocatalytic Activity. Catalysts. 2023; 13(6):995. https://doi.org/10.3390/catal13060995
Chicago/Turabian StyleHuang, Haisheng, Juan Wang, Qi Kong, Yao Xu, Zhishun Wei, and Ying Chang. 2023. "Noble Metal Modified TiO2 Hierarchically Structured Microspheres with Enhanced Photocatalytic Activity" Catalysts 13, no. 6: 995. https://doi.org/10.3390/catal13060995
APA StyleHuang, H., Wang, J., Kong, Q., Xu, Y., Wei, Z., & Chang, Y. (2023). Noble Metal Modified TiO2 Hierarchically Structured Microspheres with Enhanced Photocatalytic Activity. Catalysts, 13(6), 995. https://doi.org/10.3390/catal13060995







