A Concise Review of Multicomponent Reactions Using Novel Heterogeneous Catalysts under Microwave Irradiation
Abstract
:1. Introduction
1.1. What Are Microwaves?
1.2. How Are the Reactants Heated by Microwaves?
2. Applications of Microwave-Assisted Heterogeneous Catalysed Multi-Component Reactions
2.1. Synthesis of Nitrogen and Oxygen Containing Heterocyclic Compounds
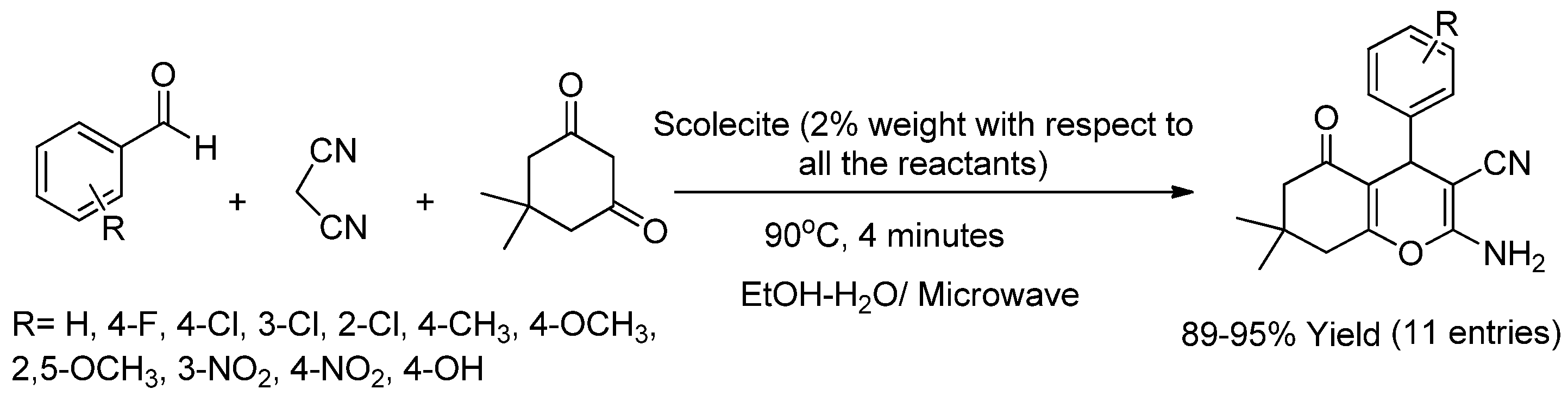
2.1.1. Preparation of Chromenes
2.1.2. Preparation of Pyridines
2.1.3. Preparation of Pyrroles
2.1.4. Preparation of Triazoles
2.1.5. Preparation of Pyrazoles
2.1.6. Preparation of Tetrazoles
2.1.7. Synthesis of Trans and Cis Julolidines
2.1.8. Synthesis of Xanthenes
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, R.W.; Combs, A.P.; Tempest, P.A.; Brown, S.D.; Keating, T.A. Multiple-Component Condensation Strategies for Combinatorial Library Synthesis. Acc. Chem. Res. 1996, 29, 123–131. [Google Scholar] [CrossRef]
- Graziano, G.; Stefanachi, A.; Contino, M.; Prieto-Díaz, R.; Ligresti, A.; Kumar, P.; Scilimati, A.; Sotelo, E.; Leonetti, F. Multicomponent Reaction-Assisted Drug Discovery: A Time- and Cost-Effective Green Approach Speeding Up Identification and Optimization of Anticancer Drugs. Int. J. Mol. Sci. 2023, 24, 6581. [Google Scholar] [CrossRef]
- Nandi, S.; Jamatia, R.; Sarkar, R.; Sarkar, F.K.; Alam, S.; Pal, A.K. One-Pot Multicomponent Reaction: A Highly Versatile Strategy for the Construction of Valuable Nitrogen-Containing Heterocycles. ChemistrySelect 2022, 7, e202201901. [Google Scholar] [CrossRef]
- Pagadala, R.; Kasi, V.; Shabalala, N.G.; Jonnalagadda, S.B. Ultrasound-assisted multicomponent synthesis of heterocycles in water—A review. Arab. J. Chem. 2022, 15, 103544–103564. [Google Scholar] [CrossRef]
- John, S.E.; Gulati, S.; Shankaraiah, N. Recent advances in multi-component reactions and their mechanistic insights: A triennium review. Org. Chem. Front. 2021, 8, 4237–4287. [Google Scholar] [CrossRef]
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef]
- Damera, T.; Pagadala, R. A New and an Eco-Friendly Approach for the Construction of Multi-Functionalized Benzenes with Computational Studies. Chem. Biodivers. 2023, 20, e202201224. [Google Scholar] [CrossRef]
- Pagadala, R.; Maddila, S.; Moodley, V.; van Zyl, W.E.; Jonnalagadda, S.B. An efficient method for the multicomponent synthesis of multisubstituted pyridines, a rapid procedure using Au/MgO as the catalyst. Tetrahedron Lett. 2014, 55, 4006–4010. [Google Scholar] [CrossRef]
- Pagadala, R.; Maddila, S.; Rana, S.; Jonnalagadda, S.B. Ce-Zr/SiO2: A versatile reusable heterogeneous catalyst for three-component synthesis and solvent free oxidation of benzyl alcohol. RSC Adv. 2014, 4, 6602–6607. [Google Scholar] [CrossRef]
- Pagadala, R.; Maddila, S.; Jonnalagadda, S.B. Eco-efficient ultrasonic responsive synthesis of pyrimidines/pyridines. Ultrason. Sonochemistry 2014, 21, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Bálint, E.; Keglevich, G. The Spread of the Application of the Microwave Technique in Organic synthesis. In Milestones in Microwave Chemistry; Springer International Publishing Switzerland: Zurich, Switzerland, 2016. [Google Scholar] [CrossRef]
- Vialkova, E.; Obukhova, M.; Belova, L. Microwave Irradiation in Technologies of Wastewater and Wastewater Sludge Treatment: A Review. Water 2021, 13, 1784. [Google Scholar] [CrossRef]
- Rinaldi, L.; Carnaroglio, D.; Rotolo, L.; Cravotto, G. A Microwave-Based Chemical Factory in the Lab: From Milligram to Multigram Preparations. J. Chem. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Bassyouni, F.A.; Abu-Bakr, S.M.; Rehim, M.A. Evolution of microwave irradiation and its application in green chemistry and biosciences. Res. Chem. Intermed. 2011, 38, 283–322. [Google Scholar] [CrossRef]
- Talreja, S.; Tiwari, S. Green Chemistry and Microwave Irradiation Technique: A Review. J. Pharm. Res. Int. 2022, 74–79. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Pang, C.; Li, X.; Gao, X. The Advances in the Special Microwave Effects of the Heterogeneous Catalytic Reactions. Front Chem. 2020, 8, 355. [Google Scholar] [CrossRef]
- Okada, Y.; Maeda, R. Effect of Microwave Irradiation on Oximation of Acetylferrocene. Green Sustain. Chem. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Albuquerque, H.M.T.; Pinto, D.; Silva, A.M.S. Microwave Irradiation: Alternative Heating Process for the Synthesis of Biologically Applicable Chromones, Quinolones, and Their Precursors. Molecules 2021, 26, 6293. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Treadwell, L.J.; Wiley, J.B. Microwave Synthetic Routes for Shape-Controlled Catalyst Nanoparticles and Nanocomposites. Molecules 2021, 26, 3647. [Google Scholar] [CrossRef]
- Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owens, E.A.; Cravotto, G. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv. 2020, 10, 14170–14197. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, W.; You, Z.; Wang, Z.; Luo, Y.; Gao, L.; Yin, C.; Peng, R.; Lan, L. A new type of power energy for accelerating chemical reactions: The nature of a microwave-driving force for accelerating chemical reactions. Sci. Rep. 2016, 6, 25149. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Prieto, P.; de la Hoz, A.; Diaz-Ortiz, A.; Martin, D.R.; Garcia, J.I. Influence of Polarity and Activation Energy in Microwave-Assisted Organic Synthesis (MAOS). ChemistryOpen 2015, 4, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: A review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Dudley, G.B.; Richert, R.; Stiegman, A.E. On the existence of and mechanism for microwave-specific reaction rate enhancement. Chem. Sci. 2015, 6, 2144–2152. [Google Scholar] [CrossRef]
- Wada, Y.; Fujii, S.; Suzuki, E.; Maitani, M.M.; Tsubaki, S.; Chonan, S.; Fukui, M.; Inazu, N. Smelting Magnesium Metal using a Microwave Pidgeon Method. Sci. Rep. 2017, 7, 46512. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Heakal, F.E.; Sarhan, Y.B.; Maamoun, M.A.; Bakry, A.M.; Abdel-Monem, Y.K.; Ghayad, I.M. Hydrothermal Microwave-Assisted Fabrication of Nanohydroxyapatite Powder and Optimization of Its Nanocomposite Coatings on Magnesium Alloy for Orthopedic Applications. ACS Omega 2022, 7, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Zawar, L.; Bari, S. Microwave Induced Solid Dispersion as a Novel Technique for Enhancing Dissolution Rate of Repaglinide. Adv. Pharmacol. Pharm. 2013, 1, 95–101. [Google Scholar] [CrossRef]
- Martina, K.; Cravotto, G.; Varma, R.S. Impact of Microwaves on Organic Synthesis and Strategies toward Flow Processes and Scaling Up. J. Org. Chem. 2021, 86, 13857–13872. [Google Scholar] [CrossRef]
- Cuéllar-Herrera, L.; Arce-Estrada, E.; Romero-Serrano, A.; Ortiz-Landeros, J.; Cabrera-Sierra, R.; Tirado-López, C.; Hernández-Ramírez, A.; López-Rodríguez, J. Microwave-Assisted Synthesis and Characterization of γ-MnO2 for High-Performance Supercapacitors. J. Electron. Mater. 2021, 50, 5577–5589. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Harbuzaru, A.; Donoso, B.; Prieto, P.; Ponce Ortiz, R.; Diaz-Ortiz, A. Microwave Irradiation as a Powerful Tool for the Preparation of n-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors. Molecules 2022, 27, 4340. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Fei, C.; Lv, L.; Liu, X.; Zhao, Z.; Cao, G. Microwave-Assisted Synthesis of SnO2 Nanosheets Photo anodes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 25931–25938. [Google Scholar] [CrossRef]
- Lastovina, T.A.; Budnyk, A.P.; Soldatov, M.A.; Rusalev, Y.V.; Guda, A.A.; Bogdan, A.S.; Soldatov, A.V. Microwave-assisted synthesis of magnetic iron oxide nanoparticles in oleylamine–oleic acid solutions. Mendeleev Commun. 2017, 27, 487–489. [Google Scholar] [CrossRef]
- Gartshore, A.; Kidd, M.; Joshi, L.T. Applications of Microwave Energy in Medicine. Biosensors 2021, 11, 96. [Google Scholar] [CrossRef]
- Shruthi, B.S.; Vinodhkumar, P.; Kashyap, B.; Reddy, P.S. Use of microwave in diagnostic pathology. J. Cancer Res. Ther. 2013, 9, 351–355. [Google Scholar] [CrossRef]
- Bandici, L.; Vicaş, S.I.; Teuşdea, A.C.; Bandici, G.E.; Popa, D. Microwave-assisted extraction as a method of improving the quality of wines. J. Microw. Power Electromagn. Energy 2017, 51, 161–177. [Google Scholar] [CrossRef]
- Alcazar, J.; Oehlrich, D. Recent applications of microwave irradiation to medicinal chemistry. Future Med. Chem. 2010, 2, 169–176. [Google Scholar] [CrossRef]
- Santagada, V.; Frecentese, F.; Perissutti, E.; Fiorino, F.; Severino, B.; Caliendo, G. Microwave assisted synthesis: A new technology in drug discovery. Mini Rev. Med. Chem. 2009, 9, 340–358. [Google Scholar] [CrossRef] [PubMed]
- Berrino, E.; Supuran, C.T. Advances in microwave-assisted synthesis and the impact of novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 861–873. [Google Scholar] [CrossRef]
- Mavandadi, F.; Pilotti, A. The impact of microwave-assisted organic synthesis in drug discovery. Drug Discov. Today 2006, 11, 165–174. [Google Scholar] [CrossRef]
- Vasudev, H.; Singh, G.; Bansal, A.; Vardhan, S.; Thakur, L. Microwave heating and its applications in surface engineering: A review. Mater. Res. Express 2019, 6, 102001. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, D.; Jain, V.; Sharma, A.K. Microwave Processing of Materials and Applications in Manufacturing Industries: A Review. Mater. Manuf. Process. 2014, 30, 1–29. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Poli, G.; Leonelli, C.; Corradi, A.B.; Casagrande, A.; Boromei, I. Ni–Al–Ti coatings obtained by microwave assisted combustion synthesis. Surf. Eng. 2013, 28, 91–95. [Google Scholar] [CrossRef]
- Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Muhamad, I.I.; Khudzari, A.Z. Microwave-assisted fibrous decoration of mPE surface utilising Aloe vera extract for tissue engineering applications. Int. J. Nanomed. 2015, 10, 5909–5923. [Google Scholar] [CrossRef]
- Cho, H.; Török, F.; Török, B. Energy efficiency of heterogeneous catalytic microwave-assisted organic reactions. Green Chem. 2014, 16, 3623–3634. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S. Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilisation and surface functionalisation. Chem. Sci. 2011, 2, 837–846. [Google Scholar] [CrossRef]
- Gopi, C.; Krupamai, G.; Dhanaraju, M.D. A Recent Progress in Microwave-assisted Synthesis of Heterocyclic Compounds Containing Nitrogen, Sulphur and Oxygen. Rev. J. Chem. 2020, 9, 255–289. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, R.; Jain, A. Microwave-assisted synthesis of nitrogen-containing heterocycles. Green Chem. Lett. Rev. 2013, 6, 151–182. [Google Scholar] [CrossRef]
- Gulati, S.; John, S.E.; Shankaraiah, N. Microwave-assisted multi-component reactions in heterocyclic chemistry and mechanistic aspects. Beilstein J. Org. Chem. 2021, 17, 819–865. [Google Scholar] [CrossRef]
- Bougrin, K.; Loupy, A.; Soufiaoui, M. Microwave-assisted solvent-free heterocyclic synthesis. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 139–167. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew. Chem. Int. Ed. Engl. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Driowya, M.; Saber, A.; Marzag, H.; Demange, L.; Benhida, R.; Bougrin, K. Microwave-Assisted Synthesis of Bioactive Six-Membered Heterocycles and Their Fused Analogues. Molecules 2016, 21, 492. [Google Scholar] [CrossRef]
- Meera, G.; Rohit, K.R.; Saranya, S.; Anilkumar, G. Microwave assisted synthesis of five membered nitrogen heterocycles. RSC Adv. 2020, 10, 36031–36041. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Z.; Xu, L. Knoevenagel condensation of aldehydes with active methylene compounds catalysed by MgC2O4/SiO2 under microwave irradiation and solvent-free conditions. Res. Chem. Intermed. 2011, 38, 393–402. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Miller, D.D. Microwave-assisted synthesis of medicinally relevant indoles. Curr. Med. Chem. 2011, 18, 615–637. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.-F.E.; Aly, A.A.; Farag, H.H.; Beshr, E.A. Microwave assisted synthesis of triazoloquinazolinones and benzimidazoquinazolinones. Beilstein J. Org. Chem. 2007, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Robustelli Della Cuna, F.S.; Russo, E.; Ibrahim, M.F.; Grignani, E.; Preda, S. Microwave-Assisted and Conventional Extractions of Volatile Compounds from Rosa x damascena Mill. Fresh Petals for Cosmetic Applications. Molecules 2022, 27, 3963. [Google Scholar] [CrossRef] [PubMed]
- Mukku, N.; Madivalappa Davanagere, P.; Chanda, K.; Maiti, B. A Facile Microwave-Assisted Synthesis of Oxazoles and Diastereoselective Oxazolines Using Aryl-Aldehydes, p-Toluenesulfonylmethyl Isocyanide under Controlled Basic Conditions. ACS Omega 2020, 5, 28239–28248. [Google Scholar] [CrossRef]
- Mohammadkhani, L.; Heravi, M.M. Microwave-Assisted Synthesis of Quinazolines and Quinazolinones: An Overview. Front. Chem. 2020, 8, 580086. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schubert, U.S. Microwave-Assisted Polymer Synthesis: Recent Developments in a Rapidly Expanding Field of Research. Macromol. Rapid Commun. 2007, 28, 368–386. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kaushik, P.; Pankaj; Rana, V.S.; Kamil, D.; Khatri, D.; Shakil, N.A. Microwave Assisted Synthesis, Characterization and Biological Activities of Ferrocenyl Chalcones and Their QSAR Analysis. Front. Chem. 2019, 7, 814. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Khan, M.Z.H.; Khatun, K.; Sattar, M.A. Microwave-Assisted Synthesis of Arylidene Acetophenones. J. Eng. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Muranaka, A.; Murayama, T.; Uchiyama, M.; Takaya, H.; Yamada, Y.M.A. Microwave-assisted photooxidation of sulfoxides. Sci. Rep. 2021, 11, 20505. [Google Scholar] [CrossRef]
- Ambasana, P.A.; Vachhani, D.D.; Van der Eycken, E.V. Microwave-assisted synthesis of natural products (analogs) with potential biological applications. In Microwaves in Drug Discovery and Development: Recent Advances; Future Science Ltd.: London, UK, 2014; pp. 134–153. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. 14. Microwave-assisted synthesis of nanoparticles. In Microwave Chemistry; Walter de Gruyter: Berlin, Germany, 2017; pp. 248–269. [Google Scholar] [CrossRef]
- Liu, G.; Yang, S.; Song, B.; Xue, W.; Hu, D.; Jin, L.; Lu, P. Microwave assisted synthesis of N-arylheterocyclic substituted-4-aminoquinazoline derivatives. Molecules 2006, 11, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Mangasuli, S.N.; Hosamani, K.M.; Managutti, P.B. Microwave assisted synthesis of coumarin-purine derivatives: An approach to in vitro antioxidant, DNA cleavage, crystal structure, DFT studies and Hirshfeld surface analysis. Heliyon 2019, 5, e01131. [Google Scholar] [CrossRef]
- Kempe, K.; Becer, C.R.; Schubert, U.S. Microwave-Assisted Polymerisations: Recent Status and Future Perspectives. Macromolecules 2011, 44, 5825–5842. [Google Scholar] [CrossRef]
- Bordoni, C.; Cima, C.M.; Azzali, E.; Costantino, G.; Brancale, A. Microwave-assisted organic synthesis of nucleoside ProTide analogues. RSC Adv. 2019, 9, 20113–20117. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Varma, R.S. Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef]
- Shore, G.; Tsimerman, M.; Organ, M.G. Gold film-catalysed benzannulation by microwave-assisted, continuous flow organic synthesis (MACOS). Beilstein J. Org. Chem. 2009, 5, 35. [Google Scholar] [CrossRef]
- Bálint, E.; Popovics-Tóth, N.; Tajti, Á.; Rávai, B.; Szabó, K.E.; Perdih, F. Microwave-Assisted Multicomponent Syntheses of Heterocyclic Phosphonates. In Proceedings of the 24th International Electronic Conference on Synthetic Organic Chemistry, Santiago de Compostela, Galicia, Spain, 15 November–15 December 2020. [Google Scholar] [CrossRef]
- Amariucai-Mantu, D.; Mangalagiu, V.; Danac, R.; Mangalagiu, I.I. Microwave Assisted Reactions of Azaheterocycles Formedicinal Chemistry Applications. Molecules 2020, 25, 716. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Edrees, M.M.; Faty, R.A.M.; Muhammad, Z.A.; Mabkhot, Y.N. Microwave-assisted one pot three-component synthesis of some novel pyrazole scaffolds as potent anticancer agents. Chem. Cent. J. 2017, 11, 37. [Google Scholar] [CrossRef]
- Xue, C.; Mao, Y.; Wang, W.; Song, Z.; Zhao, X.; Sun, J.; Wang, Y. Current status of applying microwave-associated catalysis for the degradation of organics in aqueous phase—A review. J. Environ. Sci. 2019, 81, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and Heterogeneous Catalysis: A Review on Selected Catalytic Processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef]
- Ni, B.; Lee, C.; Sun, R.-C.; Zhang, X. Microwave assisted heterogeneous catalysis: Effects of varying oxygen concentrations on the oxidative coupling of methane. React. Kinet. Catal. Lett. 2009, 98, 287–302. [Google Scholar] [CrossRef]
- Mazur, M.O.; Zhelavskyi, O.S.; Zviagin, E.M.; Shishkina, S.V.; Musatov, V.I.; Kolosov, M.A.; Shvets, E.H.; Andryushchenko, A.Y.; Chebanov, V.A. Effective microwave-assisted approach to 1,2,3-triazolobenzodiazepinones via tandem Ugi reaction/catalyst-free intramolecular azide-alkyne cycloaddition. Beilstein J. Org. Chem. 2021, 17, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.I.; Serrano, E.; Luque, R.; García-Martínez, J. Microwave-assisted catalysis by iron oxide nanoparticles on MCM-41: Effect of the support morphology. Appl. Catal. A Gen. 2013, 453, 383–390. [Google Scholar] [CrossRef]
- Khormi, A.Y.; Farghaly, T.A.; Shaaban, M.R. Microwave-Assisted Synthesis of 2-Aryl and 2,5-Diarylthiophene Derivatives via Suzuki-Miyaura Cross-Coupling Using Novel Palladium Complex as a Catalyst. Polycycl. Aromat. Compd. 2021, 42, 3893–3907. [Google Scholar] [CrossRef]
- Cabrera-Rivera, F.A.; Hernández-Vázquez, L.G.; Flores-Sánchez, P.; Durán-Galván, M.; Escalante, J. Solvent- and Catalyst-Free Microwave-Assisted Decarboxylation of Malonic Acid Derivatives. Green Sustain. Chem. 2017, 7, 270–280. [Google Scholar] [CrossRef]
- Petricci, E.; Risi, C.; Ferlin, F.; Lanari, D.; Vaccaro, L. Avoiding hot-spots in Microwave-assisted Pd/C catalysed reactions by using the biomass derived solvent gamma-Valerolactone. Sci. Rep. 2018, 8, 10571. [Google Scholar] [CrossRef]
- Yoshii, T.; Nakatsuka, K.; Kuwahara, Y.; Mori, K.; Yamashita, H. Specific Enhancement of Activity of Carbon-supported Single-site Co Catalyst in the Microwave-assisted Solvent-free Aerobic Oxidation. Chem. Lett. 2017, 46, 789–791. [Google Scholar] [CrossRef]
- Daştan, A.; Kulkarni, A.; Török, B. Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches†. Green Chem. 2012, 14, 17–37. [Google Scholar] [CrossRef]
- Baral, N.; Mishra, D.R.; Mishra, N.P.; Mohapatra, S.; Raiguru, B.P.; Panda, P.; Nayak, S.; Nayak, M.; Kumar, P.S. Microwave-assisted rapid and efficient synthesis of chromene-fused pyrrole derivatives through multi-component reaction and evaluation of antibacterial activity with molecular docking investigation. J. Heterocycl. Chem. 2019, 57, 575–589. [Google Scholar] [CrossRef]
- El Azab, I.H.; Youssef, M.M.; Amin, M.A. Microwave-assisted synthesis of novel 2H-chromene derivatives bearing phenylthiazolidinones and their biological activity assessment. Molecules 2014, 19, 19648–19664. [Google Scholar] [CrossRef]
- Lambat, T.L. Microwave assisted scolecite as heterogeneous catalyst for multi-component one-pot synthesis of novel chromene scaffolds with quantitative yields. J. Chin. Adv. Mater. Soc. 2018, 6, 134–144. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, S.A.; Patil, R. Microwave-assisted synthesis of chromenes: Biological and chemical importance. Future Med. Chem. 2015, 7, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri Adreani, L.; Lapi, E. On some new esters of coumarin-3-carboxylic acid wit balsamic and bronchodilator action. Boll. Chim. Farm. 1960, 99, 583–586. [Google Scholar]
- Gaikwad, P.; Kamble, S. Microwave enhanced green and convenient synthesis of 2-amino-4H-chromenes in aqueous hydrotropic medium. Curr. Res. Green Sustain. Chem. 2020, 3, 100014. [Google Scholar] [CrossRef]
- Molaei, H.R.; Sadeghi, B. Microwave assisted multicomponent synthesis of 4H-chromene derivatives by nano-coconut shell-BF3 as a new heterogeneous catalyst. J. Appl. Chem. Res. 2019, 1, 85–96. Available online: https://dorl.net/dor/20.1001.1.20083815.2019.13.1.7.2 (accessed on 1 January 2019).
- Hiremath, P.B.; Kamanna, K. A Microwave Accelerated Sustainable Approach for the synthesis of 2-amino-4H-chromenes Catalysed by WEPPA: A Green Strategy. Curr. Microw. Chem. 2019, 6, 30–43. [Google Scholar] [CrossRef]
- Nope, E.; Sathicq, Á.G.; Martínez, J.J.; Rojas, H.; Macías, M.A.; Castillo, J.-C.; Romanelli, G. Solvent-Free Microwave-Assisted Multicomponent Synthesis of 4H-Chromenes Using Fe3O4-Based Hydrotalcites as Bifunctional Catalysts. ChemistrySelect 2022, 7, e202104360. [Google Scholar] [CrossRef]
- Poursattar Marjani, A.; Khalafy, J.; Eslamipour, P.; Ahmadi Sabegh, M. Synthesis of a New Series of 4H-benzo [h] chromenes by a Multi-component Reaction under Solvent-Free Microwave Conditions. Iran. J. Chem. Chem. Eng. Res. Artic. 2019, 38, 51–57. [Google Scholar] [CrossRef]
- Dwi, F.; Antonius, H.C.; Rika, T.Y. A microwave assisted, Fe3O4/Camphor-catalysed three component synthesis of 2-amino-4H-chromenes and their antibacterial and antioxidant activity. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012036. [Google Scholar] [CrossRef]
- Kusy, D.; Maniukiewicz, W.; Błażewska, K.M. Microwave-assisted synthesis of 3-formyl substituted imidazo[1,2-a]pyridines. Tetrahedron Lett. 2019, 60. [Google Scholar] [CrossRef]
- Denora, N.; Lee, C.; Iacobazzi, R.M.; Choi, J.Y.; Song, I.H.; Yoo, J.S.; Piao, Y.; Lopalco, A.; Leonetti, F.; Lee, B.C.; et al. TSPO-targeted NIR-fluorescent ultra-small iron oxide nanoparticles for glioblastoma imaging. Eur. J. Pharm. Sci. 2019, 139, 105047. [Google Scholar] [CrossRef]
- Priyanga, S.; Khamrang, T.; Velusamy, M.; Karthi, S.; Ashokkumar, B.; Mayilmurugan, R. Coordination geometry-induced optical imaging of L-cysteine in cancer cells using imidazopyridine-based copper(ii) complexes. Dalton Trans. 2019, 48, 1489–1503. [Google Scholar] [CrossRef]
- Vanda, D.; Zajdel, P.; Soural, M. Imidazopyridine-based selective and multifunctional ligands of biological targets associated with psychiatric and neurodegenerative diseases. Eur. J. Med. Chem. 2019, 181, 111569. [Google Scholar] [CrossRef] [PubMed]
- Abignente, E.; Arena, F.; De Caprariis, P.; Nuzzetti, R.; Marmo, E.; Lampa, E.; Rosatti, E.; Ottavo, R. Research on heterocyclic compounds. X.-Imidazo[1,2-a]pyrazine derivatives: Synthesis and anti-inflammatory activity. Farmaco Sci. 1981, 36, 61–80. [Google Scholar] [CrossRef]
- Commeiras, L.; Woodcock, S.C.; Baldwin, J.E.; Adlington, R.M.; Cowley, A.R.; Wilkinson, P.J. New access to the 1H-pyrazolo[4,3-c]pyridine core from bis-acetylenic-N-benzoylhydrazones. Tetrahedron 2004, 60, 933–938. [Google Scholar] [CrossRef]
- Samar, C.; Fayçel, J.; Jameleddine, K. Convenient synthesis of pyrazolo[3,4-b]pyridin-3-ones and pyrazolo[3,4-b]pyridine-5-carbaldehyde using vinamidinium salts. Tetrahedron Lett. 2011, 52, 3648–3650. [Google Scholar] [CrossRef]
- Allais, C.; Grassot, J.M.; Rodriguez, J.; Constantieux, T. Metal-free multi-component syntheses of pyridines. Chem. Rev. 2014, 114, 10829–10868. [Google Scholar] [CrossRef]
- Yang, X.; Shang, Q.; Bo, C.; Hu, L.; Zhou, Y. Facile synthesis and antioxidant activity of lignin-related imidazo[1,2-a]pyridine derivatives. Arkivoc 2018, 2018, 184–193. [Google Scholar] [CrossRef]
- Rao, R.N.; Mm, B.; Maiti, B.; Thakuria, R.; Chanda, K. Efficient Access to Imidazo[1,2-a]pyridines/pyrazines/pyrimidines via Catalyst-Free Annulation Reaction under Microwave Irradiation in Green Solvent. ACS Comb. Sci. 2018, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.C.; Maldonado, R.A.; Ramírez-García, G.; Díaz Cervantes, E.; Cruz, F.N. Microwave-assisted synthesis and luminescent activity of imidazo[1,2-a]pyridine derivatives. J. Heterocycl. Chem. 2020, 57, 2279–2287. [Google Scholar] [CrossRef]
- Sarma, J.; Singh, G.; Gupta, M.; Gupta, R.; Kapoor, B. Synthesis, Characterisation and in Vitro Antimicrobial Evaluation of Some Novel Benzimidazole Derivatives Bearing Hydrazone Moiety. Asian J. Pharm. Clin. Res. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Ma, T.; Pai, S.B.; Zhu, Y.L.; Lin, J.S.; Shanmuganathan, K.; Du, J.; Wang, C.; Kim, H.; Newton, M.G.; Cheng, Y.C.; et al. Structure--activity relationships of 1-(2-Deoxy-2-fluoro-beta-L-arabinofuranosyl)pyrimidine nucleosides as anti-hepatitis B virus agents. J. Med. Chem. 1996, 39, 2835–2843. [Google Scholar] [CrossRef]
- Klumpp, K.; Leveque, V.; Le Pogam, S.; Ma, H.; Jiang, W.R.; Kang, H.; Granycome, C.; Singer, M.; Laxton, C.; Hang, J.Q.; et al. The novel nucleoside analog R1479 (4′-azidocytidine) is a potent inhibitor of NS5B-dependent RNA synthesis and hepatitis C virus replication in cell culture. J. Biol. Chem. 2006, 281, 3793–3799. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Hsia, C.W.; Jayakumar, T.; Sheu, J.R.; Hsia, C.H.; Khamrang, T.; Chen, Y.J.; Manubolu, M.; Chang, Y. Structure(-)Activity Relationship Study of Newly Synthesized Iridium-III Complexes as Potential Series for Treating Thrombotic Diseases. Int. J. Mol. Sci. 2018, 19, 3641. [Google Scholar] [CrossRef]
- Nirogi, R.; Mohammed, A.R.; Shinde, A.K.; Bogaraju, N.; Gagginapalli, S.R.; Ravella, S.R.; Kota, L.; Bhyrapuneni, G.; Muddana, N.R.; Benade, V.; et al. Synthesis and SAR of Imidazo[1,5-a]pyridine derivatives as 5-HT4 receptor partial agonists for the treatment of cognitive disorders associated with Alzheimer’s disease. Eur. J. Med. Chem. 2015, 103, 289–301. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Y.; Li, L.; Pei, H.; Chen, Y.; Hu, J.; Qin, Y.; Li, Y.; Li, W.; Liu, W. Titanium complexes supported by imidazo[1,5-a]pyridine-containing pyrrolyl ligand as catalysts for hydroamination and polymerisation reactions, and as an antitumor reagent. RSC Adv. 2015, 5, 10318–10325. [Google Scholar] [CrossRef]
- Gujar, J.B.; Chaudhari, M.A.; Kawade, D.S.; Shingare, M.S. Sodium chloride: A proficient additive for the synthesis of pyridine derivatives in aqueous medium. Tetrahedron Lett. 2014, 55, 6939–6942. [Google Scholar] [CrossRef]
- Eldeab, H.A. Ecofriendly microwave assisted synthesis of some new pyridine glycosides. Nucleosides Nucleotides Nucleic Acids 2019, 38, 509–520. [Google Scholar] [CrossRef]
- Pooja, K.; Rahul, Y.; Ruchi, B.; Tasneem, P. Regioselective synthesis of pyrimidine-fused tetrahydropyridines and pyridines by microwave-assisted one-pot reaction Synthesis of xanthenes. Mol. Divers. 2020, 24, 107–117. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Microwave-assisted MgO NP catalysed one-pot multi-component synthesis of polysubstituted steroidal pyridines. New J. Chem. 2018, 42, 184–197. [Google Scholar] [CrossRef]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Yurttas, L.; Ozkay, Y.; Kaplancikli, Z.A.; Tunali, Y.; Karaca, H. Synthesis and antimicrobial activity of some new hydrazone-bridged thiazole-pyrrole derivatives. J. Enzyme Inhib. Med. Chem. 2013, 28, 830–835. [Google Scholar] [CrossRef] [PubMed]
- La Regina, G.; Bai, R.; Coluccia, A.; Famiglini, V.; Pelliccia, S.; Passacantilli, S.; Mazzoccoli, C.; Ruggieri, V.; Sisinni, L.; Bolognesi, A.; et al. New pyrrole derivatives with potent tubulin polymerisation inhibiting activity as anticancer agents including hedgehog-dependent cancer. J. Med. Chem. 2014, 57, 6531–6552. [Google Scholar] [CrossRef]
- Battilocchio, C.; Poce, G.; Alfonso, S.; Porretta, G.C.; Consalvi, S.; Sautebin, L.; Pace, S.; Rossi, A.; Ghelardini, C.; Di Cesare Mannelli, L.; et al. A class of pyrrole derivatives endowed with analgesic/anti-inflammatory activity. Bioorg. Med. Chem. 2013, 21, 3695–3701. [Google Scholar] [CrossRef]
- Venkatesan, K.; Ramakanth, P.; Anjaneyulu, C. Microwave Assisted One Pot Synthesis of Functionalized Pyrrole Derivatives Catalyzed by Uranyl Nitrate Hexa Hydrate. CVR J. Sci. Technol. 2020, 18, 156–159. [Google Scholar] [CrossRef]
- Sumit, K.; Nishant, V.; Naseem, A. Microwave assisted highly efficient one-pot multi-component synthesis of novel 2-(tetrasubstituted-1H-pyrrol-3-yl)-4H-chroman-4-ones catalyzed by heterogeneous reusable silica gel supported polyphosphoric acid (PPA/SiO2). J. Saudi Chem. Soc. 2018, 22, 136–145. [Google Scholar] [CrossRef]
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzyme Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chai, X.; Wang, Y.; Cao, Y.; Zhang, J.; Wu, Q.; Zhang, D.; Jiang, Y.; Yan, T.; Sun, Q. Triazole derivatives with improved in vitro antifungal activity over azole drugs. Drug Des. Devel. Ther. 2014, 8, 383–390. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Meldal, M.; Tornoe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, P.; Bhorali, P.; Islam, I.; Bhuyan, A.J.; Saikia, L. Magnetically recoverable copper ferrite catalysed cascade synthesis of 4-Aryl-1H-1, 2, 3-triazoles under microwave irradiation. Tetrahedron Lett. 2018, 59, 1587–1591. [Google Scholar] [CrossRef]
- Saikia, A.A.; Rao, R.N.; Das, S.; Jena, S.; Rej, S.; Maiti, B.K. Chanda, B. Sequencing [3 + 2]-Cycloaddition and Multi-component Reactions: A Regioselective Microwave-Assisted Synthesis of 1,4-Disubstituted 1,2,3-Triazoles Using Ionic Liquid Supported Cu(II) Precatalysts in Methanol. Tetrahedron Lett. 2020, 61, 152273. [Google Scholar] [CrossRef]
- Narsimha, S.; Battula, K.S.; Reddy, Y.N.; Nagavelli, V.R. Microwave-assisted Cu-catalyzed C–C bond formation: One-pot synthesis of fully substituted 1, 2, 3-triazoles using nonsymmetrical iodoalkynes and their biological evaluation. Chem. Heterocycl. Compd. 2018, 54, 1161–1167. [Google Scholar] [CrossRef]
- Arias-Gomez, A.; Godoy, A.; Portilla, J. Functional Pyrazolo[1,5-a]pyrimidines: Current Approaches in Synthetic Transformations and Uses As an Antitumor Scaffold. Molecules 2021, 26, 2708. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Fustero, S.; Sanchez-Rosello, M.; Barrio, P.; Simon-Fuentes, A. From 2000 to mid-2010: A fruitful decade for the synthesis of pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Ali, A.; Asif, M. Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Recent progress in chemosensors based on pyrazole derivatives. RSC Adv. 2020, 10, 19693–19712. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, O.; Nawaz, F.; Alam, M.J.; Alam, P. Current status of pyrazole and its biological activities. J. Pharm. Bioallied Sci. 2016, 8, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M.; Pearson, C.M.; Kennedy, J.M. A Clinical Trial of Indomethacin in Rheumatoid Arthritis. Clin. Pharmacol. Ther. 1965, 6, 25–30. [Google Scholar] [CrossRef]
- Gomez, L.; Hack, M.D.; McClure, K.; Sehon, C.; Huang, L.; Morton, M.; Li, L.; Barrett, T.D.; Shankley, N.; Breitenbucher, J.G. SAR studies of 1,5-diarylpyrazole-based CCK1 receptor antagonists. Bioorganic Med. Chem. Lett. 2007, 17, 6493–6498. [Google Scholar] [CrossRef]
- Keter, F.K.; Darkwa, J. Perspective: The potential of pyrazole-based compounds in medicine. Biometals 2011, 25, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.H.; Timaniya, J.B.; Patel, M.J.; Patel, K.P. Microwave-assisted synthesis of pyrano[2,3-c]-pyrazole derivatives and their anti-microbial, anti-malarial, anti-tubercular, and anti-cancer activities. J. Mol. Struct. 2021, 1249, 131605. [Google Scholar] [CrossRef]
- One-Pot Multi Component Microwave Assisted Synthesis of 4H-Pyrano [2,3-c] Pyrazoles in Methanol and their Antibacterial Study. Lett. Appl. NanoBioScience 2021, 11, 3441–3448. [CrossRef]
- Berghmans, S.; Hunt, J.; Roach, A.; Goldsmith, P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res. 2007, 75, 18–28. [Google Scholar] [CrossRef]
- Montagner, C.; Nigen, M.; Jacquin, O.; Willet, N.; Dumoulin, M.; Karsisiotis, A.I.; Roberts, G.C.; Damblon, C.; Redfield, C.; Matagne, A. The Role of Active Site Flexible Loops in Catalysis and of Zinc in Conformational Stability of Bacillus cereus 569/H/9 β-Lactamase. J. Biol. Chem. 2016, 291, 16124–16137. [Google Scholar] [CrossRef]
- Dippold, A.A.; Izsák, D.; Klapötke, T.M.; Pflüger, C. Combining the Advantages of Tetrazoles and 1,2,3-Triazoles: 4,5-Bis(tetrazol-5-yl)-1,2,3-triazole, 4,5-Bis(1-hydroxytetrazol-5-yl)-1,2,3-triazole, and their Energetic Derivatives. Chem.—A Eur. J. 2016, 22, 1768–1778. [Google Scholar] [CrossRef]
- Fischer, D.; Klapötke, T.M.; Stierstorfer, J. 1,5-Di(nitramino)tetrazole: High Sensitivity and Superior Explosive Performance. Angew. Chem. Int. Ed. 2015, 54, 10299–10302. [Google Scholar] [CrossRef]
- Hammerl, A.; Klapötke, T.M.; Nöth, H.; Warchhold, M.; Holl, G. Synthesis, Structure, Molecular Orbital and Valence Bond Calculations for Tetrazole Azide, CHN7. Propellants Explos. Pyrotech. 2003, 28, 165–173. [Google Scholar] [CrossRef]
- Gálvez-Ruiz, J.C.; Holl, G.; Karaghiosoff, K.; Klapötke, T.M.; Löhnwitz, K.; Mayer, P.; Nöth, H.; Polborn, K.; Rohbogner, C.J.; Suter, M. Derivatives of 1, 5-diamino-1H-tetrazole: A new family of energetic heterocyclic-based salts. Inorg. Chem. 2005, 44, 4237–4253. [Google Scholar] [CrossRef]
- Akbarzadeh, P.; Koukabi, N.; Kolvari, E. Three-component solvent-free synthesis of 5-substituted-1H-tetrazoles catalyzed by unmodified nanomagnetite with microwave irradiation or conventional heating. Res. Chem. Intermed. 2018, 45, 1009–1024. [Google Scholar] [CrossRef]
- Naeimi, H.; Kiani, F.; Moradian, M. Rapid microwave promoted heterocyclization of primary amines with triethyl orthoformate and sodium azide using zinc sulfide nanoparticles as recyclable catalyst. Green Chem. Lett. Rev. 2018, 11, 361–369. [Google Scholar] [CrossRef]
- de Paiva, W.F.; Braga, I.B.; de Assis, J.V.; Castañeda, S.M.B.; Sathicq, G.; Palermo, V.; Romanelli, G.P.; Natalino, R.; da Silva, M.J.; Martins, F.T.; et al. Microwave-assisted multicomponent synthesis of julolidines using silica-supported calix[4]arene as heterogeneous catalyst. Tetrahedron 2019, 75, 3740–3750. [Google Scholar] [CrossRef]
- Kahandal, S.S.; Burange, A.S.; Kale, S.R.; Prinsen, P.; Luque, R.; Jayaram, R.V. An efficient route to 1,8-dioxo-octahydroxanthenes and -decahydroacridines using a sulfated zirconia catalyst. Catal. Commun. 2017, 97, 138–145. [Google Scholar] [CrossRef]
- Shchekotikhin, Y.M.; Nikolaeva, T.G. Transformations of sym-octahydroxanthene-1,8-diones and 1,8-dioxo-sym-octahydroxanthylium salts in recyclization under the influence of amines. Chem. Heterocycl. Compd. 2006, 42, 28–33. [Google Scholar] [CrossRef]
- Rewcastle, G.W.; Atwell, G.J.; Zhuang, L.; Baguley, B.C.; Denny, W.A. Potential antitumor agents. 61. Structure-activity relationships for in vivo colon 38 activity among disubstituted 9-oxo-9H-xanthene-4-acetic acids. J. Med. Chem. 1991, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Chibale, K.; Visser, M.; van Schalkwyk, D.; Smith, P.J.; Saravanamuthu, A.; Fairlamb, A.H. Exploring the potential of xanthene derivatives as trypanothione reductase inhibitors and chloroquine potentiating agents. Tetrahedron 2003, 59, 2289–2296. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Rana, K.C.; Mujahid, M.; Sehar, I.; Saxena, A.K. Synthesis and in vitro study of 14-aryl-14H-dibenzo[a.j]xanthenes as cytotoxic agents. Bioorganic Med. Chem. Lett. 2009, 19, 5590–5593. [Google Scholar] [CrossRef]
- Pagadala, R.; Kusampally, U. BTADCI Promoted One-pot Synthesis of 14-aryl-14H-dibenzo [a, j] Xanthenes. J. Heterocycl. Chem. 2018, 55, 1499–1503. [Google Scholar] [CrossRef]
- Pagadala, R.; Kusampally, U. BTMA-Br3 Promoted One Pot Synthesis of 1, 8-Dioxo-octahydroxanthenes under Eco-friendly Conditions. Org. Prep. Proced. Int. 2020, 52, 496–502. [Google Scholar] [CrossRef]






















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damera, T.; Pagadala, R.; Rana, S.; Jonnalagadda, S.B. A Concise Review of Multicomponent Reactions Using Novel Heterogeneous Catalysts under Microwave Irradiation. Catalysts 2023, 13, 1034. https://doi.org/10.3390/catal13071034
Damera T, Pagadala R, Rana S, Jonnalagadda SB. A Concise Review of Multicomponent Reactions Using Novel Heterogeneous Catalysts under Microwave Irradiation. Catalysts. 2023; 13(7):1034. https://doi.org/10.3390/catal13071034
Chicago/Turabian StyleDamera, Thirupathi, Ramakanth Pagadala, Surjyakanta Rana, and Sreekantha Babu Jonnalagadda. 2023. "A Concise Review of Multicomponent Reactions Using Novel Heterogeneous Catalysts under Microwave Irradiation" Catalysts 13, no. 7: 1034. https://doi.org/10.3390/catal13071034
APA StyleDamera, T., Pagadala, R., Rana, S., & Jonnalagadda, S. B. (2023). A Concise Review of Multicomponent Reactions Using Novel Heterogeneous Catalysts under Microwave Irradiation. Catalysts, 13(7), 1034. https://doi.org/10.3390/catal13071034







