High-Performance Sr0.95Fe0.8Mo0.1Ni0.1O3−δ Electrode for Reversible Symmetrical Solid Oxide Cells
Abstract
:1. Introduction
2. Experiment
2.1. Materials Synthesis
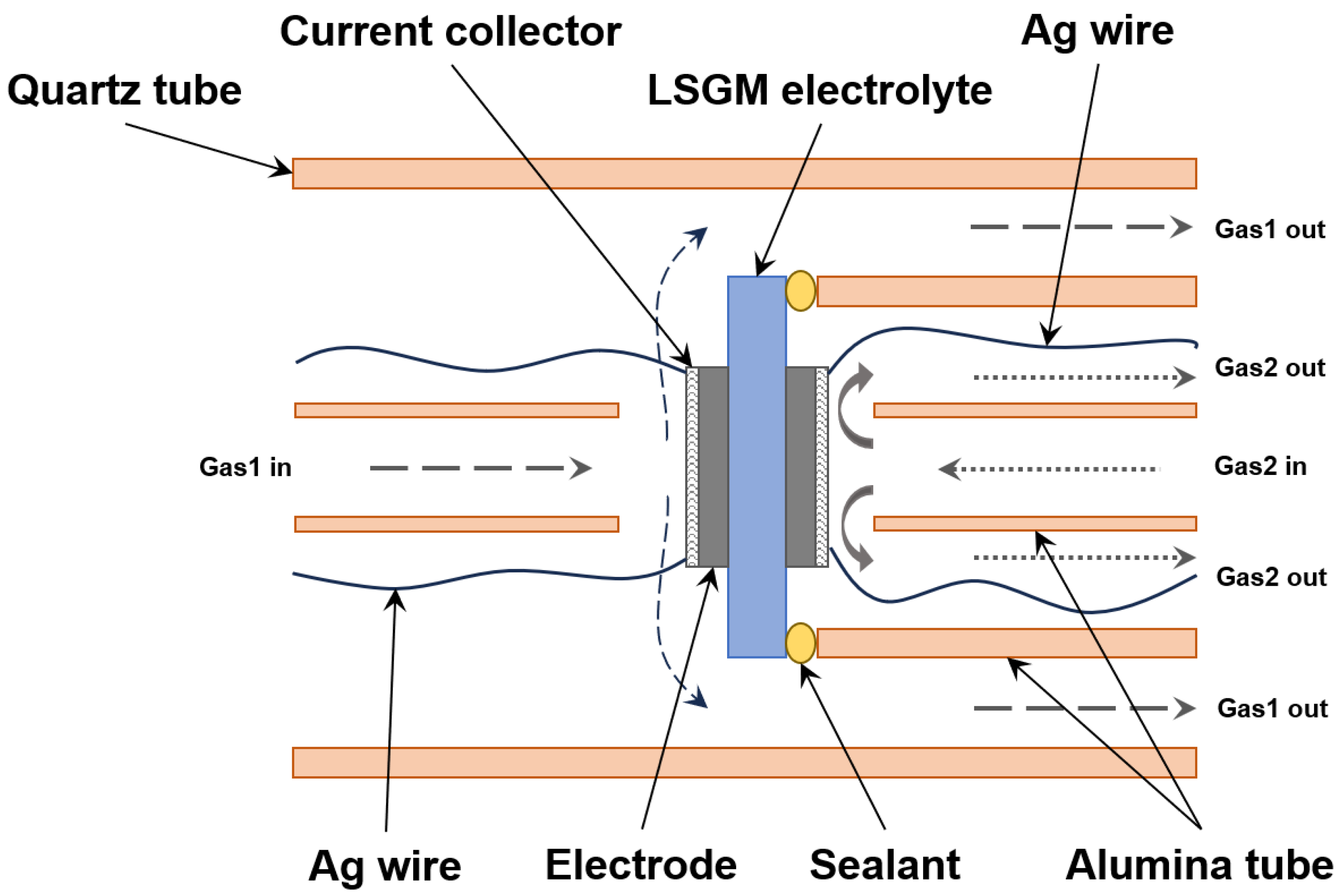
2.2. Cell Fabrication and Test
2.3. Characterization Methods
2.4. Theoretical Calculations
3. Results and Discussions
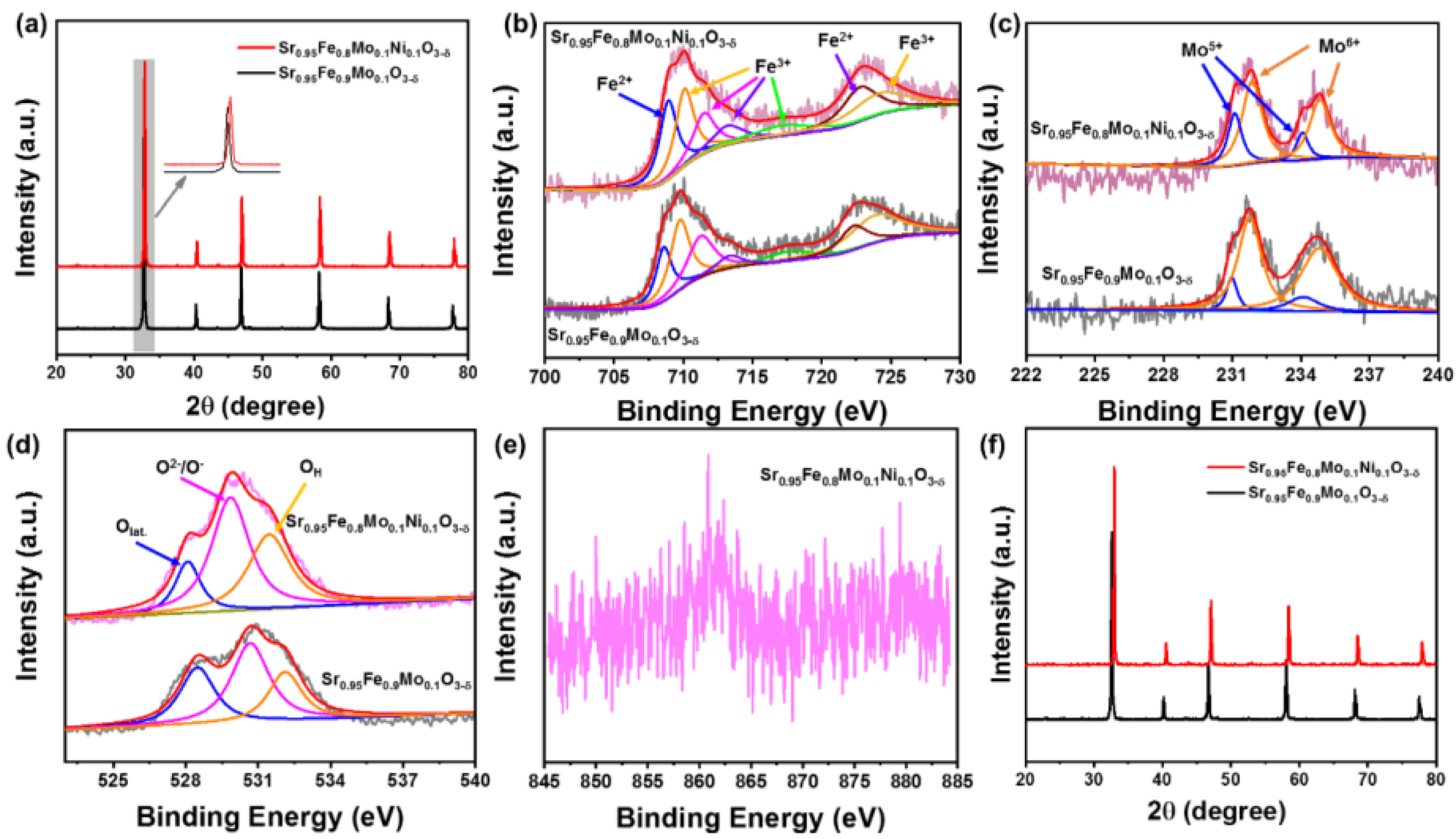
3.1. Structural Characterizations
3.2. Morphological Characterization
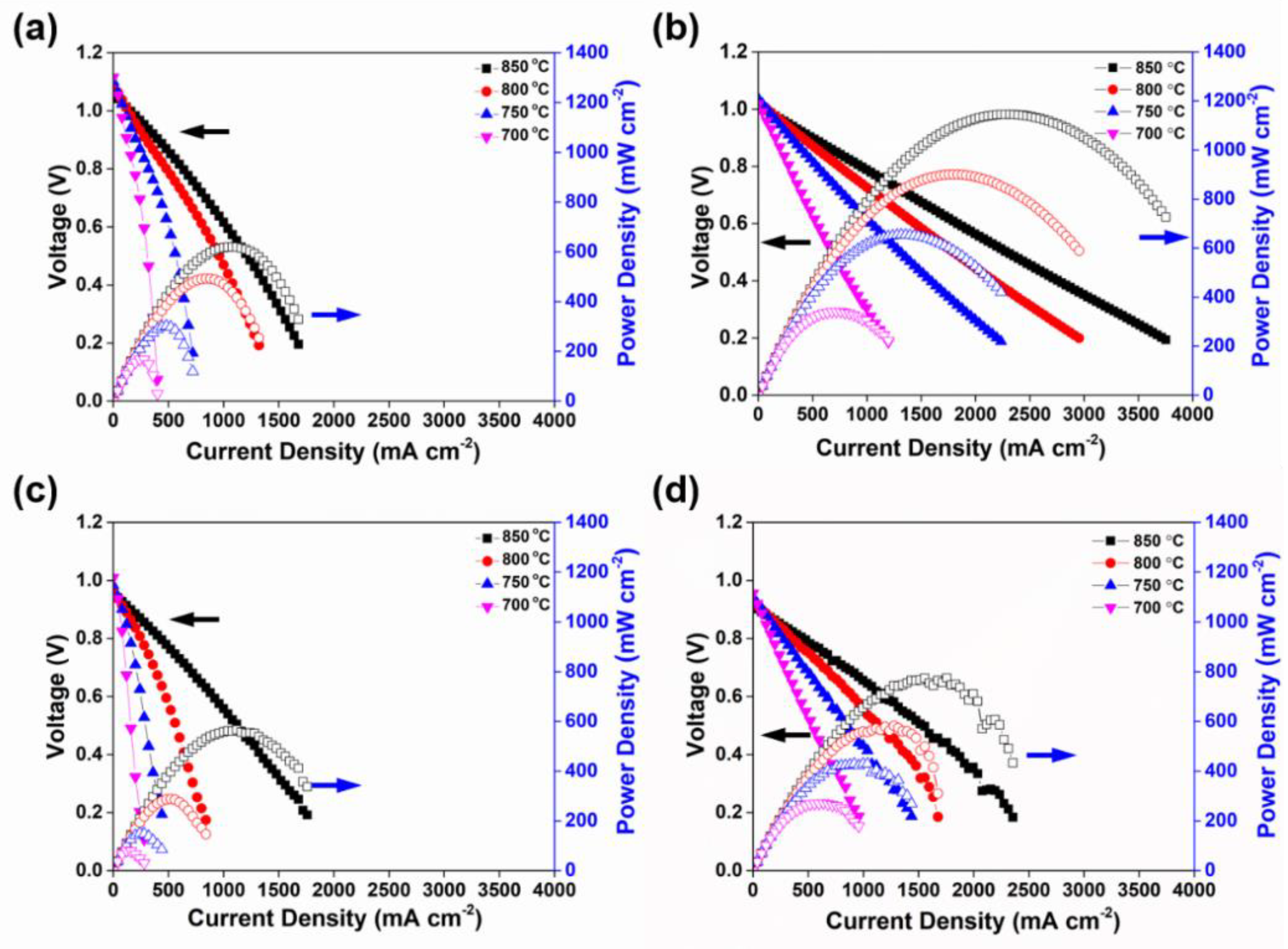
3.3. Electrochemical Performance
3.4. H2O Reduction Pathway on the SFM/SFMN (111) Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang:, L.; Xu, C.; Sun, W.; Ren, R.; Yang, X.; Luo, Y.; Qiao, J.; Wang, Z.; Zhen, S.; Sun, K. Constructing perovskite/alkaline-earth metal composite heterostructure by infiltration to revitalize CO2 electrolysis. Sep. Purif. Technol. 2022, 298, 121475. [Google Scholar] [CrossRef]
- Xi, X.; Fan, Y.; Zhang, J.; Luo, J.-L.; Fu, X.-Z. In situ construction of hetero-structured perovskite composites with exsolved Fe and Cu metallic nanoparticles as efficient CO2 reduction electrocatalysts for high performance solid oxide electrolysis cells. J. Mater. Chem. A 2022, 10, 2509–2518. [Google Scholar] [CrossRef]
- Sun, C.; Bian, L.; Qi, J.; Yu, W.; Li, S.; Hou, Y.; Wang, L.; Peng, J.; An, S. Boosting CO2 directly electrolysis by electron doping in Sr2Fe1.5Mo0.5O6-δ double perovskite cathode. J. Power Sources 2022, 521, 230984. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Shen, J.K.; Wu, H.H.; Chen, H.P.; Yuan, C.Z.; Wu, N.T.; Liu, G.L.; Guo, D.L.; Liu, X.M. Self-supported electrocatalysts for the hydrogen evolution reaction. Mater. Chem. Front. 2023, 7, 567–606. [Google Scholar] [CrossRef]
- Wu, H.H.; Zhuo, F.; Qiao, H.; Venkataraman, L.K.; Zheng, M.; Wang, S.; Huang, H.; Li, B.; Mao, X.; Zhang, Q. Polymer-/Ceramic-based Dielectric Composites for Energy Storage and Conversion. Energy Environ. Mater. 2022, 5, 486–514. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.M.; Zhang, J.; Wu, N.T.; Liu, G.L.; Chen, H.P.; Yuan, C.Z.; Liu, X.M. Optimizing electronic structure of porous Ni/MoO2 heterostructure to boost alkaline hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 627, 862–871. [Google Scholar] [CrossRef]
- Shimada, H.; Yamaguchi, T.; Kishimoto, H.; Sumi, H.; Yamaguchi, Y.; Nomura, K.; Fujishiro, Y. Nanocomposite electrodes for high current density over 3 A cm(-2) in solid oxide electrolysis cells. Nat. Commun. 2019, 10, 5432. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tong, Y.; Liu, T.; Zhang, D.; Yu, N.; Zhou, J.; Li, Y.; Gu, X.K.; Wang, Y. Robust Ruddlesden-Popper phase Sr3Fe1.3Mo0.5Ni0.2O7-δ decorated with in-situ exsolved Ni nanoparticles as an efficient anode for hydrocarbon fueled solid oxide fuel cells. SusMat 2022, 2, 487–501. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Cai, C.; Deng, H.; Yang, F.; Lu, Y.; Song, X.; An, S.; Zhao, H. High performance oxygen permeation membrane: Sr and Ti co-doped BaFeO3-δ ceramics. Sep. Purif. Technol. 2022, 289, 120742. [Google Scholar] [CrossRef]
- Peng, L.; Li, Q.; Sun, L.; Zhao, H. An Effective Strategy to Enhance the Electrocatalytic Activity of Ruddlesden−Popper Oxides Sr3Fe2O7−δ Electrodes for Solid Oxide Fuel Cells. Catalysts 2021, 11, 1400. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Li, J.; Pu, J.; Chi, B. Sr-free orthorhombic perovskite Pr0.8Ca0.2Fe0.8Co0.2O3-δ as a high-performance air electrode for reversible solid oxide cell. J. Power Sources 2022, 528, 231202. [Google Scholar] [CrossRef]
- Krammer, M.; Schmid, A.; Siebenhofer, M.; Bumberger, A.E.; Herzig, C.; Limbeck, A.; Kubicek, M.; Fleig, J. Formation and Detection of High-Pressure Oxygen in Closed Pores of La(0.6)Sr(0.4)CoO(3-delta) Solid Oxide Electrolysis Anodes. ACS Appl. Energy Mater. 2022, 5, 8324–8335. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Pang, J.; Chen, L.; Zhang, H.; Lei, Z.; Yang, Z. Structure, synthesis, properties and solid oxide electrolysis cells application of Ba(Ce, Zr)O3 based proton conducting materials. Chem. Eng. J. 2022, 429, 132314. [Google Scholar] [CrossRef]
- Ling, Y.; Wu, Y.; Tian, Y.; Wang, X.; Shen, S.; Ou, X.; Wang, S. Stable solid oxide electrolysis cells with SSF-based symmetrical electrode for direct high-temperature steam electrolysis. Ceram. Int. 2022, 48, 981–991. [Google Scholar] [CrossRef]
- Bernadet, L.; Moncasi, C.; Torrell, M.; Tarancón, A. High-performing electrolyte-supported symmetrical solid oxide electrolysis cells operating under steam electrolysis and co-electrolysis modes. Int. J. Hydrogen Energy 2020, 45, 14208–14217. [Google Scholar] [CrossRef]
- Zhou, J.; Shin, T.-H.; Ni, C.; Chen, G.; Wu, K.; Cheng, Y.; Irvine, J.T.S. In Situ Growth of Nanoparticles in Layered Perovskite La0.8Sr1.2Fe0.9Co0.1O4−δ as an Active and Stable Electrode for Symmetrical Solid Oxide Fuel Cells. Chem. Mater. 2016, 28, 2981–2993. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, W.; Liu, Y.; Naden, A.; Xu, M.; Wu, S.; Chi, B.; Pu, J.; Irvine, J.T.S. Achieving Strong Coherency for a Composite Electrode via One-Pot Method with Enhanced Electrochemical Performance in Reversible Solid Oxide Cells. ACS Catal. 2021, 11, 3704–3714. [Google Scholar] [CrossRef]
- Li, Q.; Kuang, K.; Sun, Y.; Zheng, Y.; Liu, Q.; Chan, S.H.; Zhang, H.; Wang, W.; Li, T.; Wang, J. Deficiency of hydrogen production in commercialized planar Ni-YSZ/YSZ/LSM-YSZ steam electrolysis cells. Int. J. Hydrogen Energy 2022, 47, 23514–23519. [Google Scholar] [CrossRef]
- Heng, Z.; Wan, Y.; Xia, C. Calcium stabilized La0.6Sr0.4Fe0.8Mn0.2O3-δ perovskite as ceramic fuel electrode for solid oxide cell. J. Power Sources 2022, 537, 231535. [Google Scholar] [CrossRef]
- Wang, W.; Tian, Y.; Liu, Y.; Abhishek, N.; Li, Y.; Chi, B.; Pu, J. Tailored Sr-Co-free perovskite oxide as an air electrode for high-performance reversible solid oxide cells. Sci. China Mater. 2021, 64, 1621–1631. [Google Scholar] [CrossRef]
- Duan, N.; Yang, J.; Gao, M.; Zhang, B.; Luo, J.-L.; Du, Y.; Xu, M.; Jia, L.; Chi, B.; Li, J. Multi-functionalities enabled fivefold applications of LaCo0.6Ni0.4O3−δ in intermediate temperature symmetrical solid oxide fuel/electrolysis cells. Nano Energy 2020, 77, 105207. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Zhang, X.; Zhang, H.; Jiang, G.; Zhao, W.; Guo, J.; Chen, F.; Yan, M.; Zhang, Y.; et al. Robust redox-reversible perovskite type steam electrolyser electrode decorated with in situ exsolved metallic nanoparticles. J. Mater. Chem. A 2020, 8, 582–591. [Google Scholar] [CrossRef]
- Li, M.; Dong, J.; Chen, Z.; Huang, K.; Xiong, K.; Li, R.; Rao, M.; Chen, C.; Ling, Y.; Lin, B. Excessive Na-Doped La0.75Sr0.25Cr0.5Fe0.4Cu0.1O3-δ Perovskite as an Additional Internal Reforming Catalyst for Direct Carbon Dioxide-Ethanol Solid Oxide Fuel Cells. Catalysts 2022, 12, 1600. [Google Scholar]
- Wang, S.; Jiang, H.; Gu, Y.; Yin, B.; Chen, S.; Shen, M.; Zheng, Y.; Ge, L.; Chen, H.; Guo, L. Mo-doped La0.6Sr0.4FeO3-δ as an efficient fuel electrode for direct electrolysis of CO2 in solid oxide electrolysis cells. Electrochim. Acta 2020, 337, 135794. [Google Scholar] [CrossRef]
- Lv, H.; Lin, L.; Zhang, X.; Gao, D.; Song, Y.; Zhou, Y.; Liu, Q.; Wang, G.; Bao, X. In situ exsolved FeNi3 nanoparticles on nickel doped Sr2Fe1.5Mo0.5O6−δ perovskite for efficient electrochemical CO2 reduction reaction. J. Mater. Chem. A 2019, 7, 11967–11975. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Y.; Wang, L.; Zhang, Q.; Chou, K.-C. Electrochemical properties of La0.5Sr0.5Fe0.95Mo0.05O3−δ as cathode materials for IT-SOEC. RSC Adv. 2021, 11, 32077–32084. [Google Scholar] [CrossRef]
- Xu, C.; Zhen, S.; Ren, R.; Chen, H.; Song, W.; Wang, Z.; Sun, W.; Sun, K. Cu-Doped Sr2Fe1.5Mo0.5O6-δ as a highly active cathode for solid oxide electrolytic cells. Chem. Commun. 2019, 55, 8009–8012. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, H.; Yi, S.; Xia, Q.; Gong, Y.; Zhang, Y.; Cheng, X.; Li, Y.; Gu, L.; Swierczek, K. High-Performance Anode Material Sr2FeMo0.65Ni0.35O6-delta with In Situ Exsolved Nanoparticle Catalyst. ACS Nano 2016, 10, 8660–8669. [Google Scholar] [CrossRef]
- Zheng, K.; Świerczek, K.; Polfus, J.M.; Sunding, M.F.; Pishahang, M.; Norby, T. Carbon Deposition and Sulfur Poisoning in SrFe0.75Mo0.25O3-δ and SrFe0.5Mn0.25Mo0.25O3-δ Electrode Materials for Symmetrical SOFCs. J. Electrochem. Soc. 2015, 162, F1078. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, H.; Zhang, L.; Chi, B.; Pu, J.; Li, J. Direct Electrolysis of CO2 in Symmetrical Solid Oxide Electrolysis Cell Based on La0.6Sr0.4Fe0.8Ni0.2O3-δ Electrode. J. Electrochem. Soc. 2018, 165, F17. [Google Scholar] [CrossRef]
- Irshad, M.; Ain, Q.u.; Siraj, K.; Raza, R.; Tabish, A.N.; Rafique, M.; Idrees, R.; Khan, F.; Majeed, S.; Ahsan, M. Evaluation of BaZr0.8X0.2 (X = Y, Gd, Sm) proton conducting electrolytes sintered at low temperature for IT-SOFC synthesized by cost effective combustion method. J. Alloys Compd. 2020, 815, 152389. [Google Scholar] [CrossRef]
- Irshad, M.; Khalid, M.; Rafique, M.; Tabish, A.N.; Shakeel, A.; Siraj, K.; Ghaffar, A.; Raza, R.; Ahsan, M.; Ain, Q.T.; et al. Electrochemical Investigations of BaCe0.7-xSmxZr0.2Y0.1O3-δ Sintered at a Low Sintering Temperature as a Perovskite Electrolyte for IT-SOFCs. Sustainability 2021, 13, 12595. [Google Scholar] [CrossRef]
- Irshad, M.; Rafique, M.; Tabish, A.N.; Ghaffar, A.; Shakeel, A.; Siraj, K.; Ain, Q.U.; Raza, R.; Assiri, M.A.; Imran, M. Influence of Sintering Temperature on the Structural, Morphological, and Electrochemical Properties of NiO-YSZ Anode Synthesized by the Autocombustion Route. Metals 2022, 12, 219. [Google Scholar] [CrossRef]
- Peng, X.; Tian, Y.; Liu, Y.; Wang, W.; Jia, L.; Pu, J.; Chi, B.; Li, J. An efficient symmetrical solid oxide electrolysis cell with LSFM-based electrodes for direct electrolysis of pure CO2. J. CO2 Util. 2020, 36, 18–24. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Guo, S.; Liu, G.; Han, T.; Zhang, Z.; Liu, Y. K-Modulated Co Nanoparticles Trapped in La-Ga-O as Superior Catalysts for Higher Alcohols Synthesis from Syngas. Catalysts 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wan, S.; Wang, Y.; Huang, S.; Yuan, Z.; Chen, F.; Zhang, Y.; Liu, T. Enhancing performance of molybdenum doped strontium ferrite electrode by surface modification through Ni infiltration. Int. J. Hydrogen Energy 2021, 46, 10876–10891. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Hou, Z.; Shi, C.; Zhang, G.; Zeng, F.; Zhou, J.; Wang, S. La1-xCaxFeO3-δ air electrode fabricated by glycine-nitrate combustion method for solid oxide electrolysis cell. Ceram. Int. 2021, 47, 32318–32323. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, T.; Li, X.; Li, J.; Wu, N.; Cao, A.; Yuan, W.; Pan, K.M.; Guo, D.; Liu, X. MoS2@C with S vacancies vertically anchored on V2C-MXene for efficient lithium and sodium storage. Inorg. Chem. Front. 2023, 10, 1587–1602. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, F.; Zhang, T.; Gu, Y.; Li, J.; Guo, D.; Xu, M.; Wu, N.; Cao, A.; Liu, X. In-situ growth of MoO2@N doped carbon on Mo2C-MXene for superior lithium storage. Appl. Surf. Sci. 2022, 597, 153688. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Jia, L.; Naden, A.; Chen, J.; Chi, B.; Pu, J.; Irvine, J.T.S.; Li, J. A novel electrode with multifunction and regeneration for highly efficient and stable symmetrical solid oxide cell. J. Power Sources 2020, 475, 228620. [Google Scholar] [CrossRef]
- Sun, X.; Ye, Y.; Zhou, M.; Chen, H.; Li, Y.; Chen, P.; Dong, D.; Ling, Y.; Khan, M.; Chen, Y. Layered-perovskite oxides with in situ exsolved Co–Fe alloy nanoparticles as highly efficient electrodes for high-temperature carbon dioxide electrolysis. J. Mater. Chem. A 2022, 10, 2327–2335. [Google Scholar] [CrossRef]
- Hernández, E.; Baiutti, F.; Morata, A.; Torrell, M.; Tarancón, A. Infiltrated mesoporous oxygen electrodes for high temperature co-electrolysis of H2O and CO2 in solid oxide electrolysis cells. J. Mater. Chem. A 2018, 6, 9699–9707. [Google Scholar] [CrossRef] [Green Version]

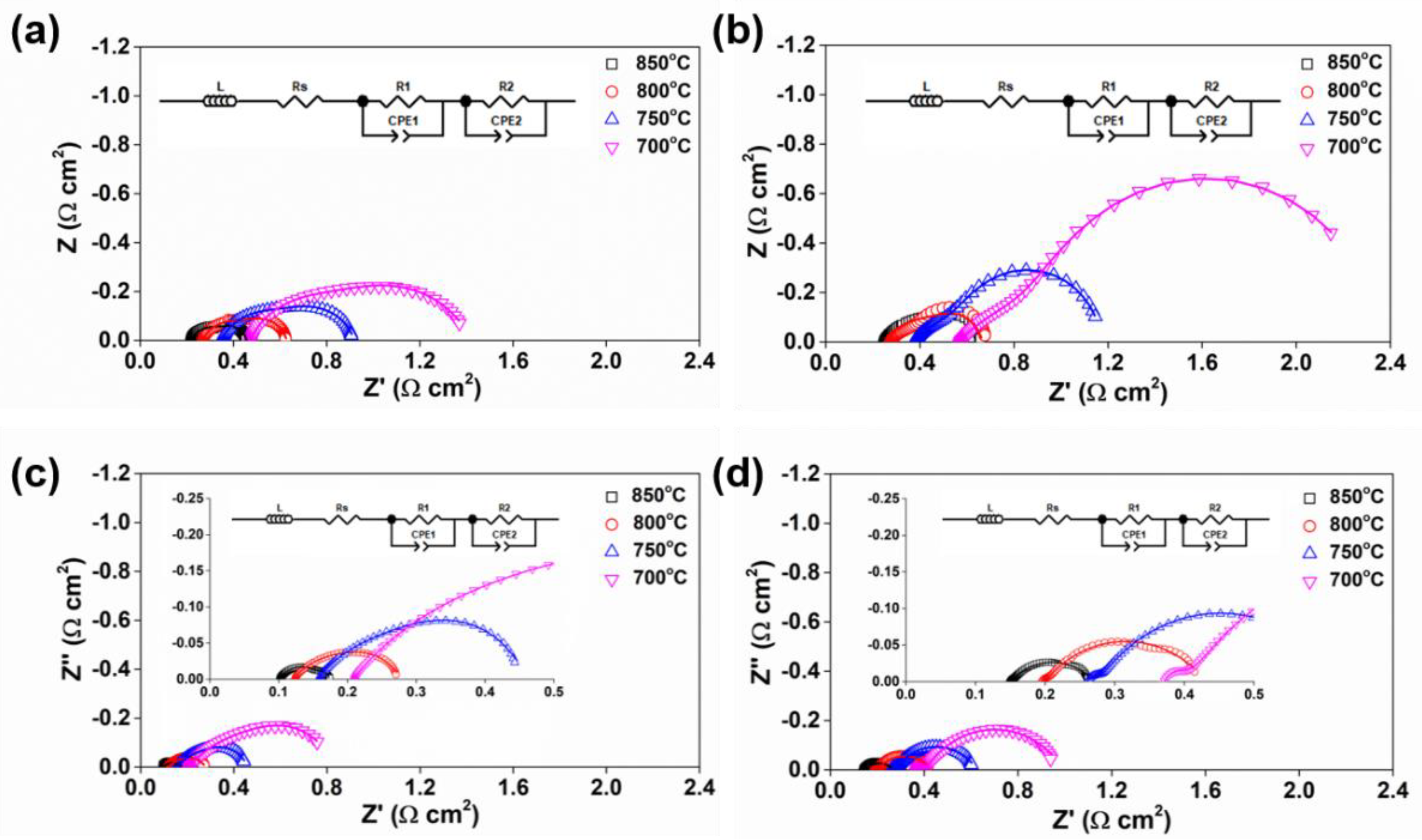
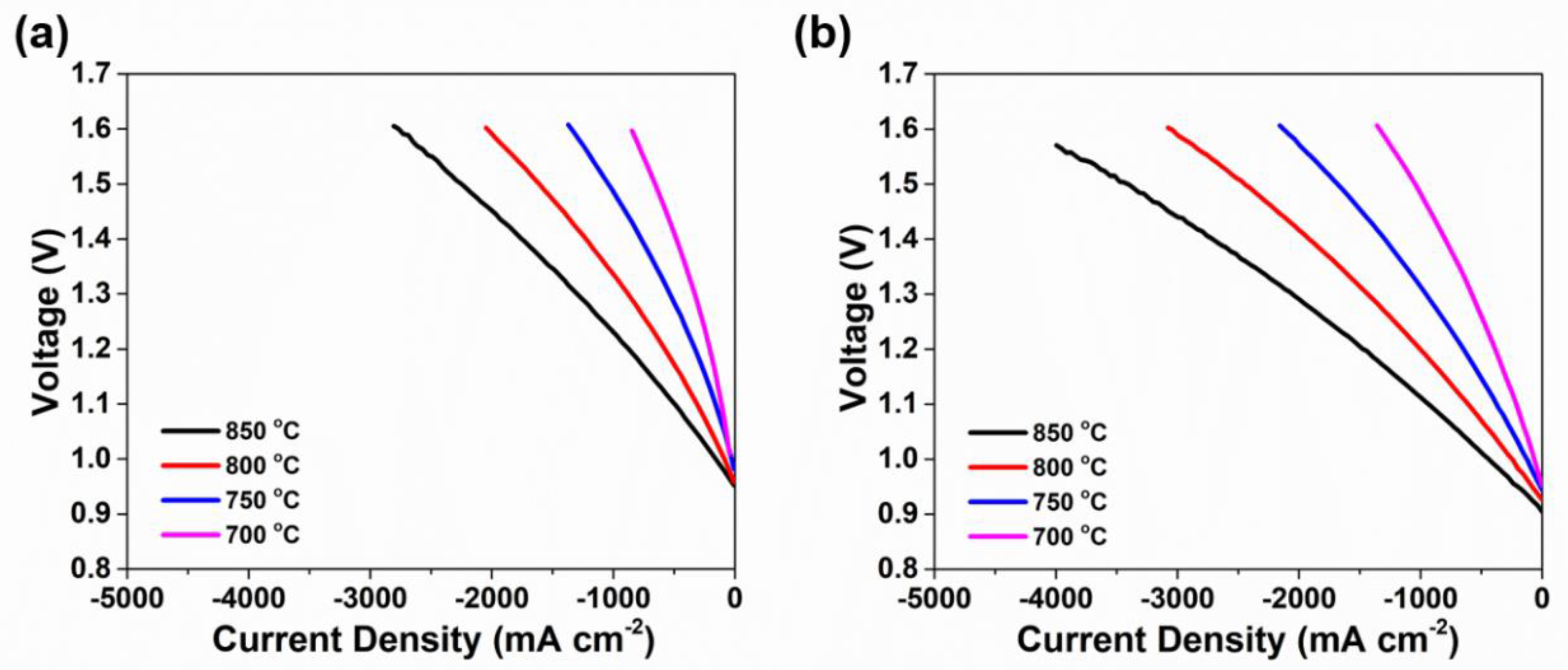

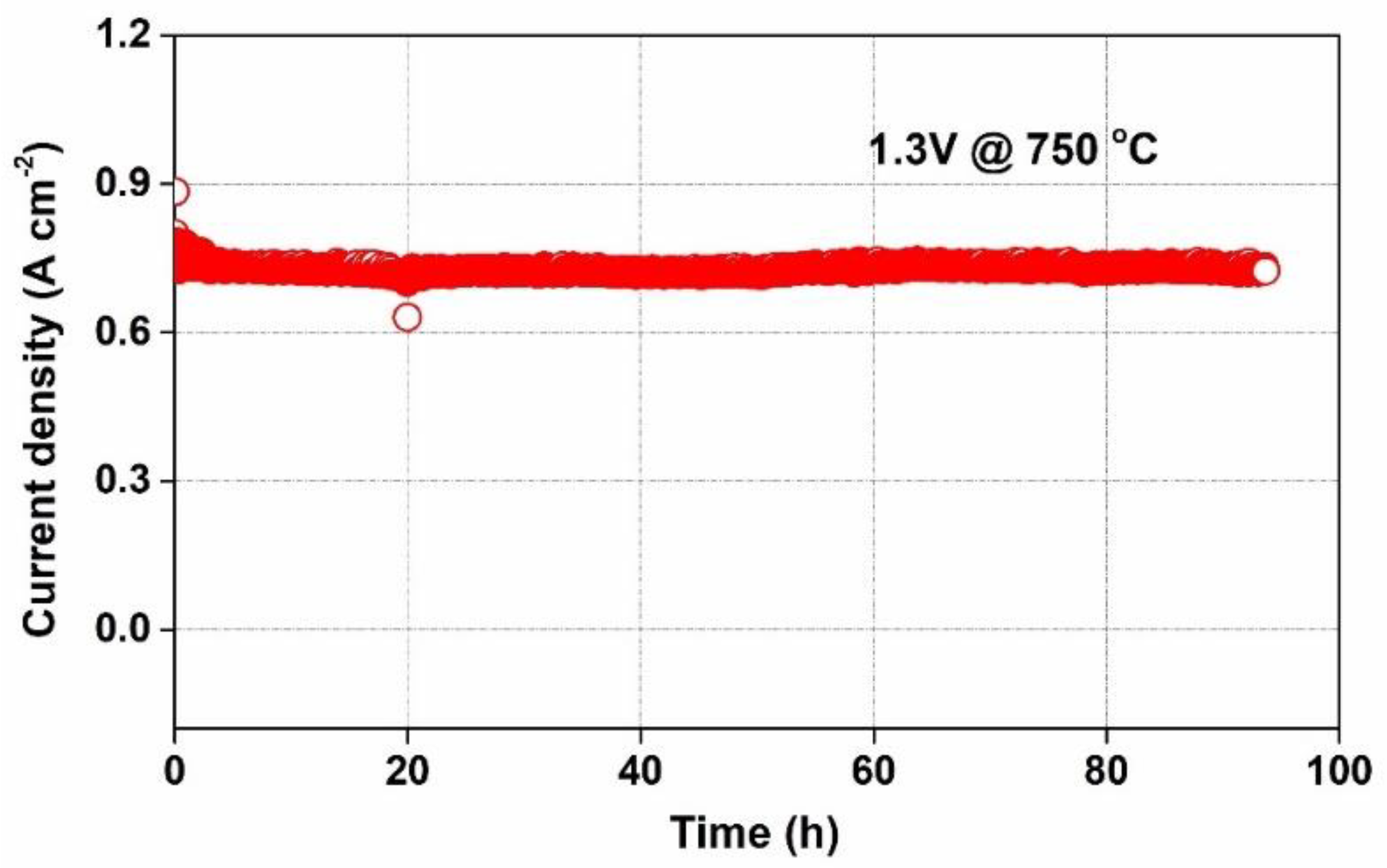


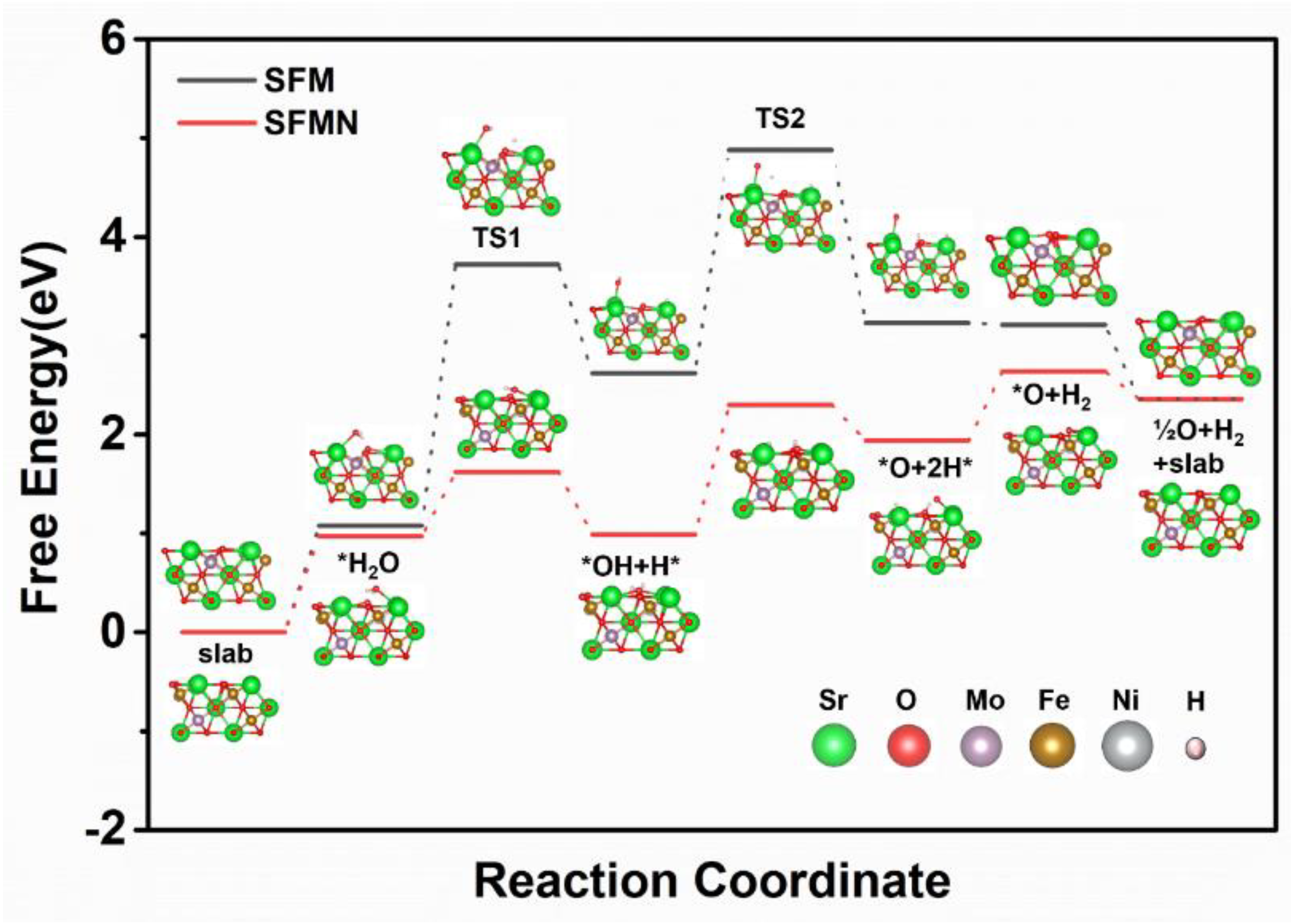
| Temperatures | SFM-SDC/LSGM/SFM-SDC | SFMN-SDC/LSGM/SFMN-SDC | ||
|---|---|---|---|---|
| Rs/Ω | Rp/Ω | Rs/Ω | Rp/Ω | |
| 700 °C | 0.404 | 1.541 | 0.280 | 0.479 |
| 750 °C | 0.284 | 0.796 | 0.196 | 0.243 |
| 800 °C | 0.213 | 0.464 | 0.147 | 0.175 |
| 850 °C | 0.158 | 0.200 | 0.115 | 0.131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Wang, J.; Bian, L.; Wang, Y.; Wang, F.; Guo, W.; Wu, Z. High-Performance Sr0.95Fe0.8Mo0.1Ni0.1O3−δ Electrode for Reversible Symmetrical Solid Oxide Cells. Catalysts 2023, 13, 1043. https://doi.org/10.3390/catal13071043
Gong Y, Wang J, Bian L, Wang Y, Wang F, Guo W, Wu Z. High-Performance Sr0.95Fe0.8Mo0.1Ni0.1O3−δ Electrode for Reversible Symmetrical Solid Oxide Cells. Catalysts. 2023; 13(7):1043. https://doi.org/10.3390/catal13071043
Chicago/Turabian StyleGong, Yudong, Jinyi Wang, Liuzhen Bian, Yige Wang, Fan Wang, Weiqi Guo, and Zhan Wu. 2023. "High-Performance Sr0.95Fe0.8Mo0.1Ni0.1O3−δ Electrode for Reversible Symmetrical Solid Oxide Cells" Catalysts 13, no. 7: 1043. https://doi.org/10.3390/catal13071043




