Catalytic Steam-Reforming of Glycerol over LDHs-Derived Ni–Al Nanosheet Array Catalysts for Stable Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
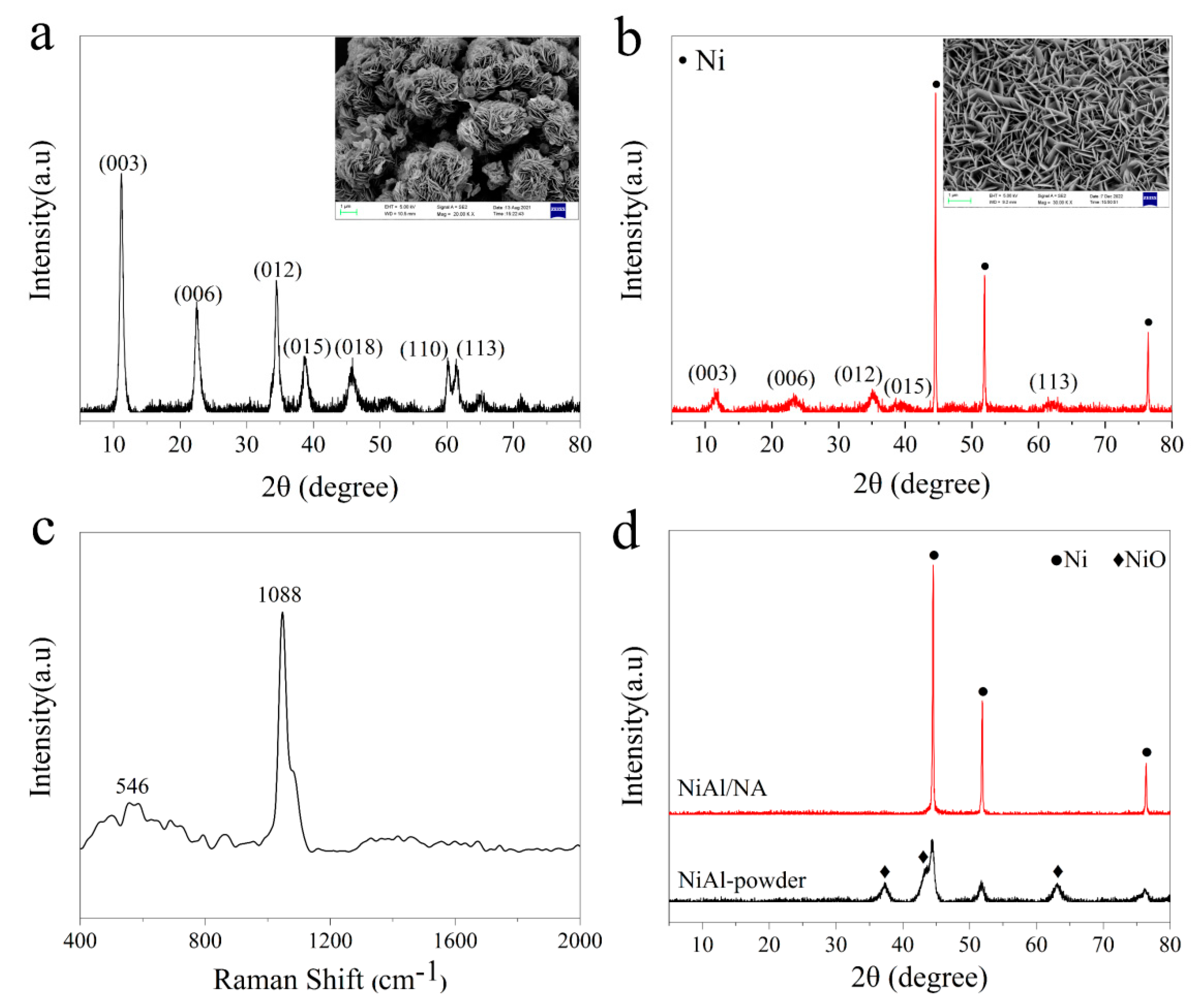
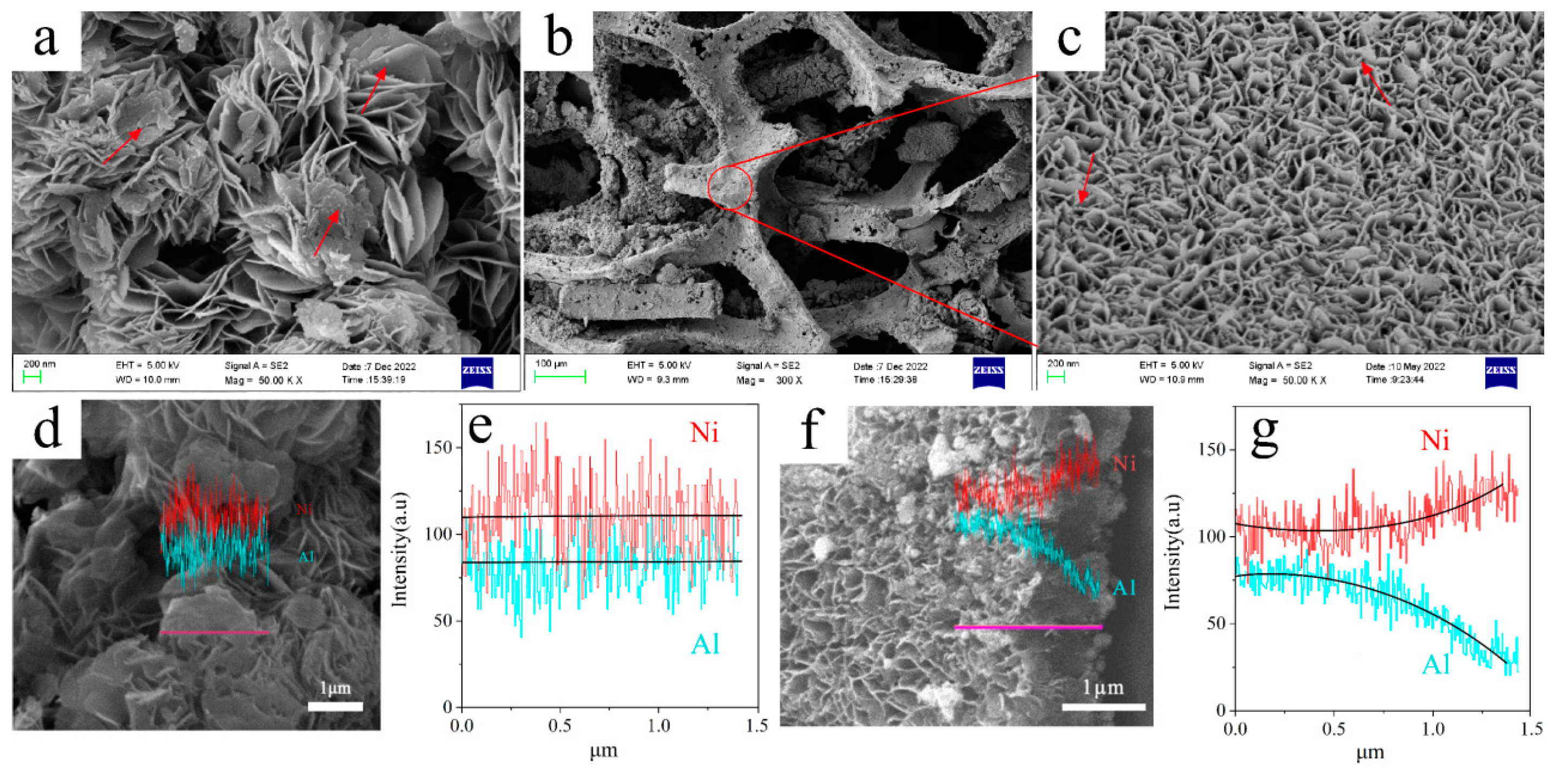
2.1. Physiochemical Properties of the Fresh Catalysts
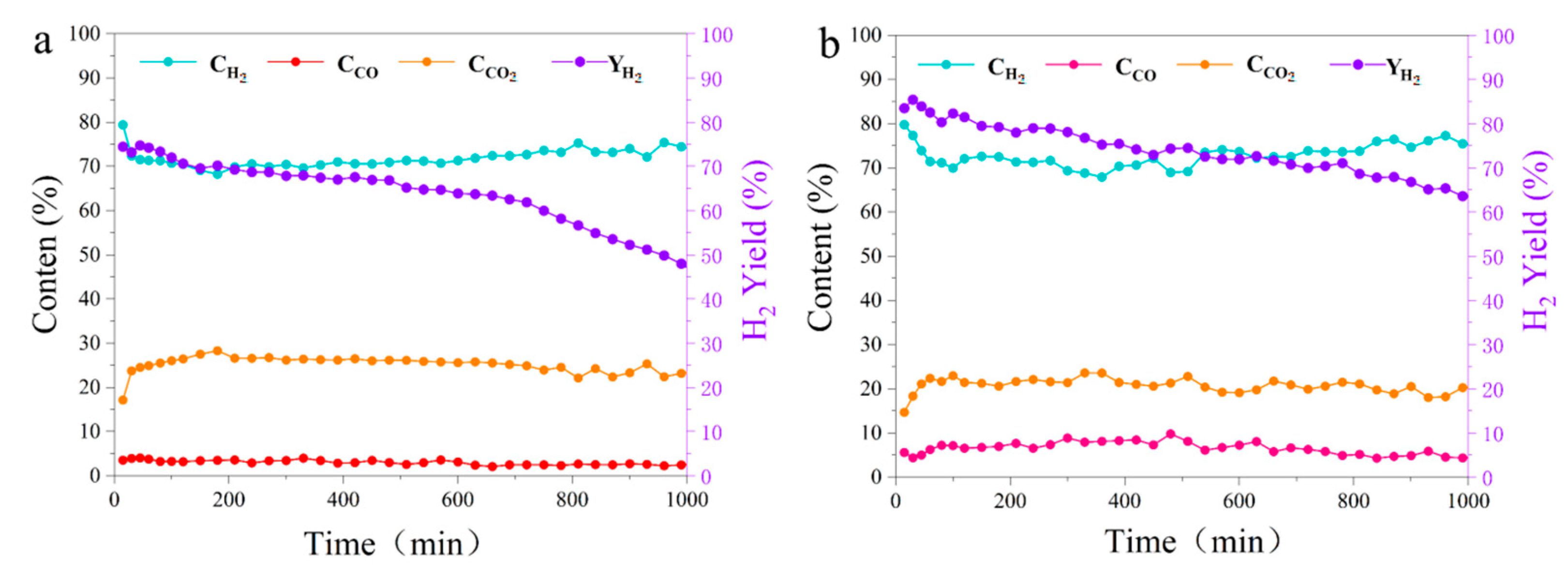
2.2. Evaluating the Catalytic Performance for Glycerol Steam-Reforming
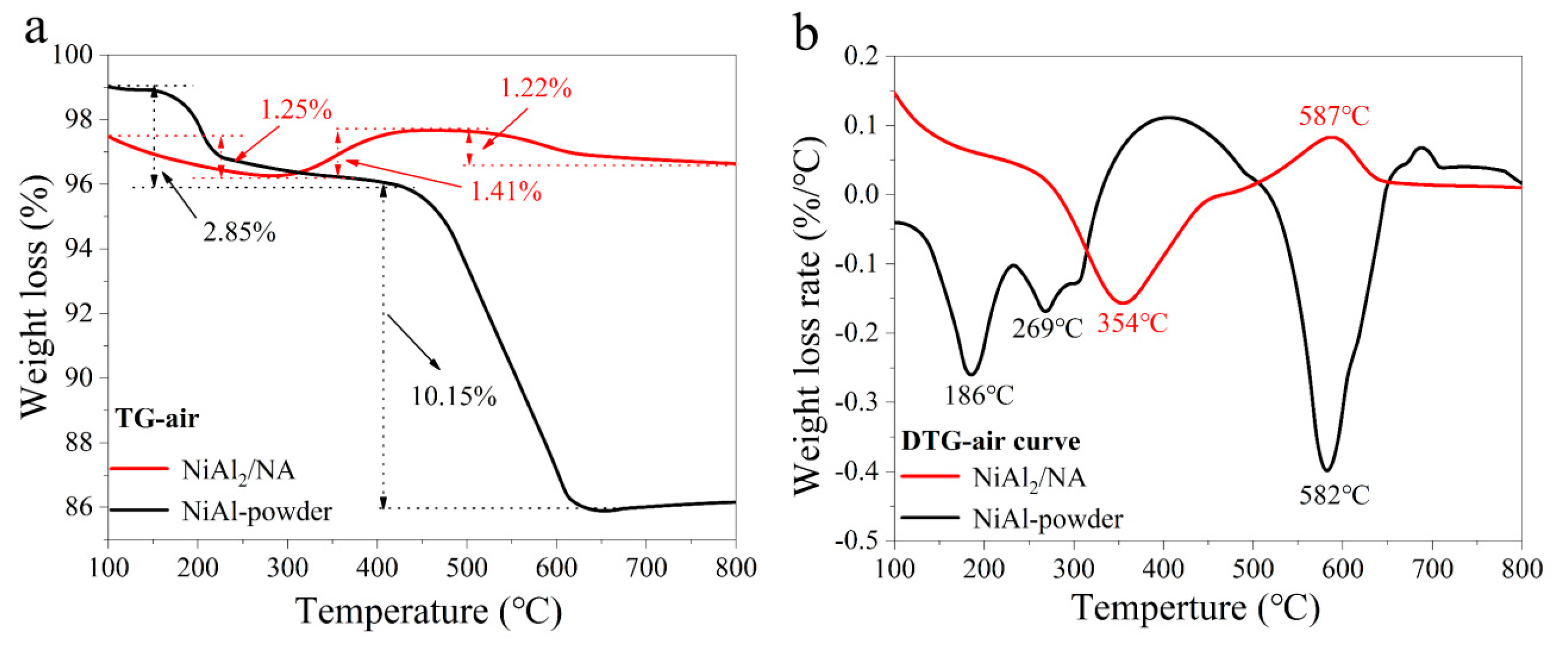
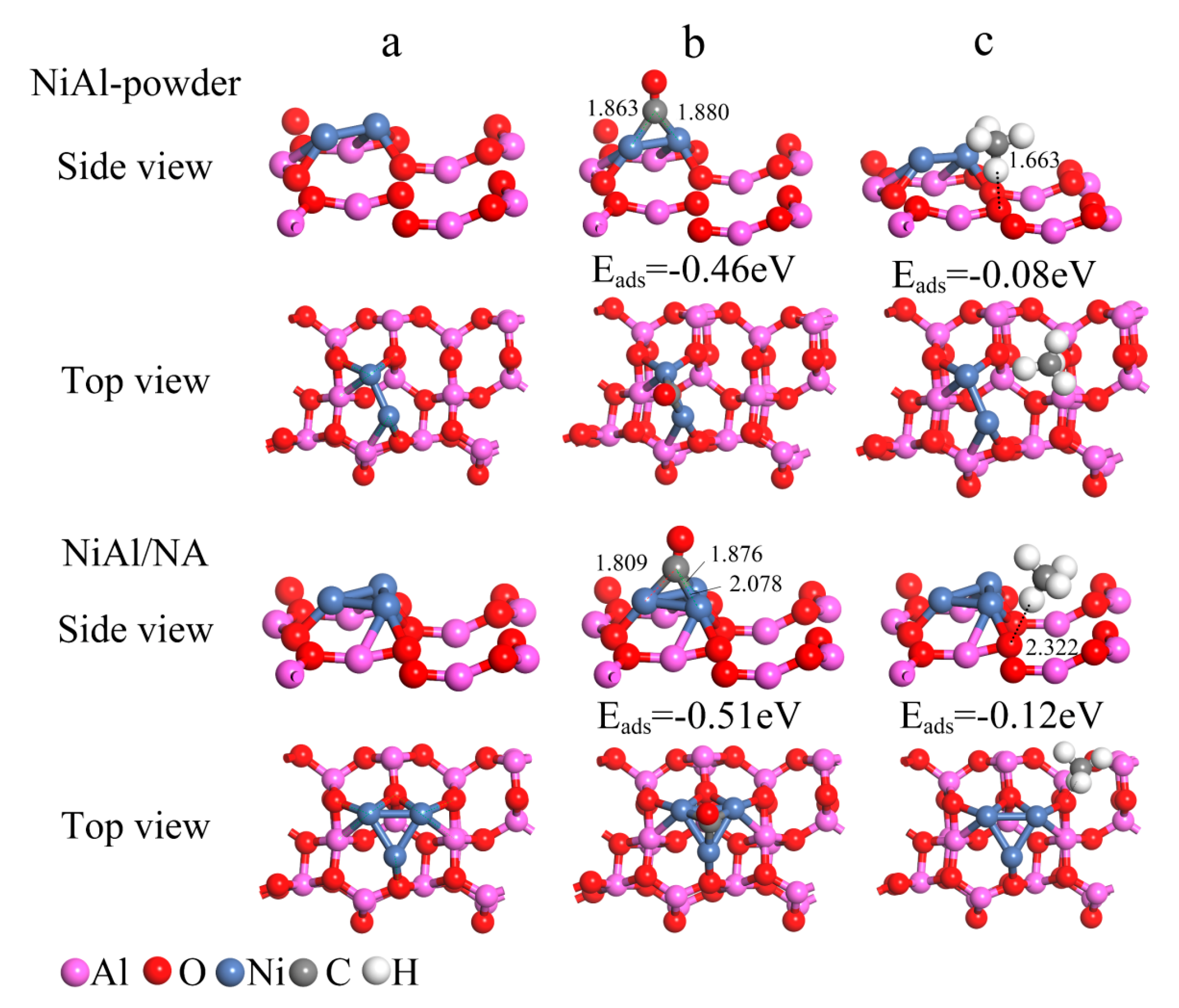
2.3. Characterization of the Coke Species in the Spent Catalysts
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Computational Detail
3.4. Experimental Apparatus and Procedure
3.5. Products Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlHumaidan, F.S.; Halabi, M.A.; Rana, M.S.; Vinoba, M. Blue hydrogen: Current status and future technologies. Energy Convers. Manag. 2023, 283, 116840. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, turquoise, blue, or grey? Environmentally friendly hydrogen production in transforming energy systems. Prog. Energy Combust. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abidin, S.Z.; Ideris, A.; Vo, D.V. A review on glycerol reforming processes over Ni-based catalyst for hydrogen and syngas productions. Int. J. Hydrogen Energy 2020, 45, 18466–18489. [Google Scholar] [CrossRef]
- Barreto, R.D.; Ramos, L.P.; Jorge, R.M.; Jorge, L.M. Turning glycerol surplus into renewable syngas through glycerol steam reforming over a sol-gel Ni–Mo2C–Al2O3 catalyst. Int. J. Hydrogen Energy 2023, 48, 16614–16629. [Google Scholar] [CrossRef]
- Umar, A.; Neagu, D.; Irvine, J.T. Renewable syngas & hydrogen synthesis via steam reforming of glycerol over ceria-mediated exsolved metal nano catalysts. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Schwengber, C.A.; Alves, H.J.; Schaffner, R.A.; Da Silva, F.A.; Sequinel, R.; Bach, V.R.; Ferracin, R.J. Overview of glycerol reforming for hydrogen production. Renew. Sustain. Energy Rev. 2016, 58, 259–266. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Qingli, X.; Zhengdong, Z.; Kai, H.; Shanzhi, X.; Chuang, M.; Chenge, C.; Huan, Y.; Yang, Y.; Yongjie, Y. Ni supported on MgO modified attapulgite as catalysts for hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2021, 46, 27380–27393. [Google Scholar] [CrossRef]
- Dieuzeide, M.L.; Iannibelli, V.; Jobbagy, M.; Amadeo, N. Steam reforming of glycerol over Ni/Mg/γ–Al2O3 catalysts. Effect of calcination temperatures. Int. J. Hydrogen Energy 2012, 37, 14926–14930. [Google Scholar] [CrossRef]
- Sahraei, O.A.; Desgagnés, A.; Larachi, F.; Iliuta, M.C. A comparative study on the performance of M (Rh, Ru, Ni)–promoted metallurgical waste driven catalysts for H2 production by glycerol steam reforming. Int. J. Hydrogen Energy 2021, 46, 32017–32035. [Google Scholar] [CrossRef]
- Sanchez, E.A.; Comelli, R.A. Hydrogen by glycerol steam reforming on a nickel–alumina catalyst: Deactivation processes and regeneration. Int. J. Hydrogen Energy 2012, 37, 14740–14746. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, S.; Lu, J.; Liu, J.; Zhao, Y.; He, S.; Zhao, Y.; Lu, H.; Luo, Y. Boosting hydrogen production from steam reforming of glycerol via constructing moderate metal-support interaction in Ni@Al2O3 catalyst. Fuel 2022, 324, 124583. [Google Scholar] [CrossRef]
- Macedo, M.S.; Kraleva, E.; Ehrich, H.; Soria, M.A.; Madeira, L.M. Hydrogen production from glycerol steam reforming over Co-based catalysts supported on La2O3, AlZnOx and AlLaOx. Int. J. Hydrogen Energy 2022, 47, 33239–33258. [Google Scholar] [CrossRef]
- Macedo, M.S.; Soria, M.A.; Madeira, L.M. Process intensification for hydrogen production through glycerol steam reforming. Renew. Sustain. Energy Rev. 2021, 146, 111151. [Google Scholar] [CrossRef]
- Moogi, S.; Lee, I.G.; Hwang, K.R. Catalytic steam reforming of glycerol over Ni–La2O3–CeO2/SBA−15 catalyst for stable hydrogen-rich gas production. Int. J. Hydrogen Energy 2020, 45, 28462–28475. [Google Scholar] [CrossRef]
- Jing, F.; Liu, S.; Wang, R.; Li, X.; Yan, Z.; Luo, S.; Chu, W. Hydrogen production through glycerol steam reforming over the NiCexAl catalysts. Renew. Energy 2020, 158, 192–201. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, S.; Lu, J.; He, S.; Lu, H.; Song, D.; Chen, D.; Luo, Y. Novel nanowire self-assembled hierarchical CeO2 microspheres loaded with nickel-based catalysts for hydrogen production from steam reforming of glycerol. Fuel Process. Technol. 2023, 243, 107677. [Google Scholar] [CrossRef]
- Jayaprakash, S.; Dewangan, N.; Jangam, A.; Das, S.; Kawi, S. LDH-derived Ni–MgO–Al2O3 catalysts for hydrogen-rich syngas production via steam reforming of biomass tar model: Effect of catalyst synthesis methods. Int. J. Hydrogen Energy 2021, 46, 18338–18352. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Sun, L.; Xie, X.; Zhao, B.; Si, H.; Zhang, X.; Hua, D. Novel Ni–Al nanosheet catalyst with homogeneously embedded nickel nanoparticles for hydrogen-rich syngas production from biomass pyrolysis. Int. J. Hydrogen Energy 2021, 46, 1762–1776. [Google Scholar] [CrossRef]
- Zhou, J.; Dou, Y.; Wu, X.Q.; Zhou, A.; Shu, L.; Li, J.R. Alkali-Etched Ni (II)-Based Metal–Organic Framework Nanosheet Arrays for Electrocatalytic Overall Water Splitting. Small 2020, 16, 1906564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dou, Y.; Zhou, A.; Shu, L.; Chen, Y.; Li, J.R. Layered metal–organic framework-derived metal oxide/carbon nanosheet arrays for catalyzing the oxygen evolution reaction. ACS Energy Lett. 2018, 3, 1655–1661. [Google Scholar] [CrossRef]
- Xie, W.; Li, H.; Cui, G.; Li, J.; Song, Y.; Li, S.; Zhang, X.; Lee, J.Y.; Shao, M.; Wei, M. NiSn atomic pair on an integrated electrode for synergistic electrocatalytic CO2 reduction. Angew. Chem. 2021, 133, 7458–7464. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Zhang, X.; Yang, W.; Song, G. Enhanced adsorption of Congo red dye by functionalized carbon nanotube/mixed metal oxides nanocomposites derived from layered double hydroxide precursor. Chem. Eng. J. 2015, 275, 315–321. [Google Scholar] [CrossRef]
- Zhou, F.; Pan, N.; Chen, H.; Xu, X.; Wang, C.; Du, Y.; Guo, Y.; Zeng, Z.; Li, L. Hydrogen production through steam reforming of toluene over Ce, Zr or Fe promoted Ni–Mg–Al hydrotalcite-derived catalysts at low temperature. Energy Convers. Manag. 2019, 196, 677–687. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, G.; Nie, Q.; Meng, C.; Sun, W.; Si, J.; Liu, Y.; Lu, Y. Ni–foam-structured Ni–Al2O3 ensemble as an efficient catalyst for gas-phase acetone hydrogenation to isopropanol. ACS Appl. Mater. Interfaces 2021, 13, 28334–28347. [Google Scholar] [CrossRef]
- Palmer, S.J.; Frost, R.L.; Ayoko, G.; Nguyen, T. Synthesis and Raman spectroscopic characterisation of hydrotalcite with CO32− and (MoO4)2− anions in the interlayer. J. Raman Spectrosc. 2008, 39, 395–401. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Chen, L.; Sun, L.; Zhao, B.; Si, H.; Xie, X.; Meng, F. Pyrolysis of sawdust with various Fe-based catalysts: Influence of support characteristics on hydrogen-rich gas production. J. Anal. Appl. Pyrolysis 2019, 137, 29–36. [Google Scholar] [CrossRef]
- Peng, L.; Xiong, P.; Ma, L.; Yuan, Y.; Zhu, Y.; Chen, D.; Luo, X.; Lu, J.; Amine, K.; Yu, G. Holey two-dimensional transition metal oxide nanosheets for efficient energy storage. Nat. Commun. 2017, 8, 15139. [Google Scholar] [CrossRef]
- Zhou, D.; Jia, Y.; Duan, X.; Tang, J.; Xu, J.; Liu, D.; Xiong, X.; Zhang, J.; Luo, J.; Zheng, L.; et al. Breaking the symmetry: Gradient in NiFe layered double hydroxide nanoarrays for efficient oxygen evolution. Nano Energy 2019, 60, 661–666. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, W.; Yang, L.; Lu, J.; Cheng, S.; Mai, W.; Tang, Z.; Li, L.; Chen, S. Ultrahigh-performance pseudocapacitor electrodes based on transition metal phosphide nanosheets array via phosphorization: A general and effective approach. Adv. Funct. Mater. 2015, 25, 7530–7538. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Wang, H.; Li, X.; Huang, H.; Liu, Y.; Li, H. Durable and high performing Ti supported Ni0.4Cu0.6Co2O4 nanoleaf-like array catalysts for hydrogen production. Renew. Energy 2021, 169, 660–669. [Google Scholar] [CrossRef]
- Yan, W.; Bao, W.; Tang, Y.; Li, Y.; Zhang, J.; Zhao, H.; Li, J.; Yu, F. Enhanced CO hydrogenation performance via two-dimensional NiAl–layered double oxide decorated by SiO2 nanoparticles. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. 2016, 128, 5363–5367. [Google Scholar] [CrossRef]
- Tao, H.B.; Fang, L.; Chen, J.; Yang, H.B.; Gao, J.; Miao, J.; Chen, S.; Liu, B. Identification of surface reactivity descriptor for transition metal oxides in oxygen evolution reaction. J. Am. Chem. Soc. 2016, 138, 9978–9985. [Google Scholar] [CrossRef]
- Li, F.L.; Wang, P.; Huang, X.; Young, D.J.; Wang, H.F.; Braunstein, P.; Lang, J.P. Large-scale, bottom-up synthesis of binary metal–organic framework nanosheets for efficient water oxidation. Angew. Chem. 2019, 131, 7125–7130. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Liu, J.; Chen, D. Catalytic characteristics of innovative Ni/slag catalysts for syngas production and tar removal from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 11848–11860. [Google Scholar] [CrossRef]
- Dang, C.; Yang, W.; Zhou, J.; Cai, W. Porous Ni–Ca–Al–O bi-functional catalyst derived from layered double hydroxide intercalated with citrate anion for sorption-enhanced steam reforming of glycerol. Appl. Catal. B Environ. 2021, 298, 120547. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Koc, S.; Avci, A.K. Reforming of glycerol to hydrogen over Ni-based catalysts in a microchannel reactor. Fuel Process. Technol. 2017, 156, 357–365. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533. [Google Scholar] [CrossRef]
- Sheppard, D.; Xiao, P.; Chemelewski, W.; Johnson, D.D.; Henkelman, G. A generalized solid-state nudged elastic band method. J. Chem. Phys. 2012, 136, 74103. [Google Scholar] [CrossRef]
- Liu, J.C.; Ma, X.L.; Li, Y.; Wang, Y.G.; Xiao, H.; Li, J. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 2018, 9, 1610. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Liu, Z.; Wang, C.; Li, J.; Zeng, J.; Liu, Y.; Zhang, M.; Li, J.; Yu, F. Three-dimensional porous Mn–Ni/Al2O3 microspheres for enhanced low temperature CO hydrogenation to produce methane. Int. J. Hydrogen Energy 2021, 46, 7912–7925. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Li, Y.; Chen, L.; Xu, G.; Hou, J.; Sun, L.; Li, T.; Xie, X.; Yi, X.; Zhao, B.; et al. Catalytic Steam-Reforming of Glycerol over LDHs-Derived Ni–Al Nanosheet Array Catalysts for Stable Hydrogen Production. Catalysts 2023, 13, 1047. https://doi.org/10.3390/catal13071047
Yang S, Li Y, Chen L, Xu G, Hou J, Sun L, Li T, Xie X, Yi X, Zhao B, et al. Catalytic Steam-Reforming of Glycerol over LDHs-Derived Ni–Al Nanosheet Array Catalysts for Stable Hydrogen Production. Catalysts. 2023; 13(7):1047. https://doi.org/10.3390/catal13071047
Chicago/Turabian StyleYang, Shuangxia, Yu Li, Lei Chen, Guiying Xu, Jianjun Hou, Laizhi Sun, Tianjin Li, Xinping Xie, Xiaolu Yi, Baofeng Zhao, and et al. 2023. "Catalytic Steam-Reforming of Glycerol over LDHs-Derived Ni–Al Nanosheet Array Catalysts for Stable Hydrogen Production" Catalysts 13, no. 7: 1047. https://doi.org/10.3390/catal13071047
APA StyleYang, S., Li, Y., Chen, L., Xu, G., Hou, J., Sun, L., Li, T., Xie, X., Yi, X., Zhao, B., Si, H., & Hua, D. (2023). Catalytic Steam-Reforming of Glycerol over LDHs-Derived Ni–Al Nanosheet Array Catalysts for Stable Hydrogen Production. Catalysts, 13(7), 1047. https://doi.org/10.3390/catal13071047




