Size Effect on Diffusion and Catalytic Performance of Mordenite in Dimethyl Ether Carbonylation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of MOR Zeolite Crystals with Different Size
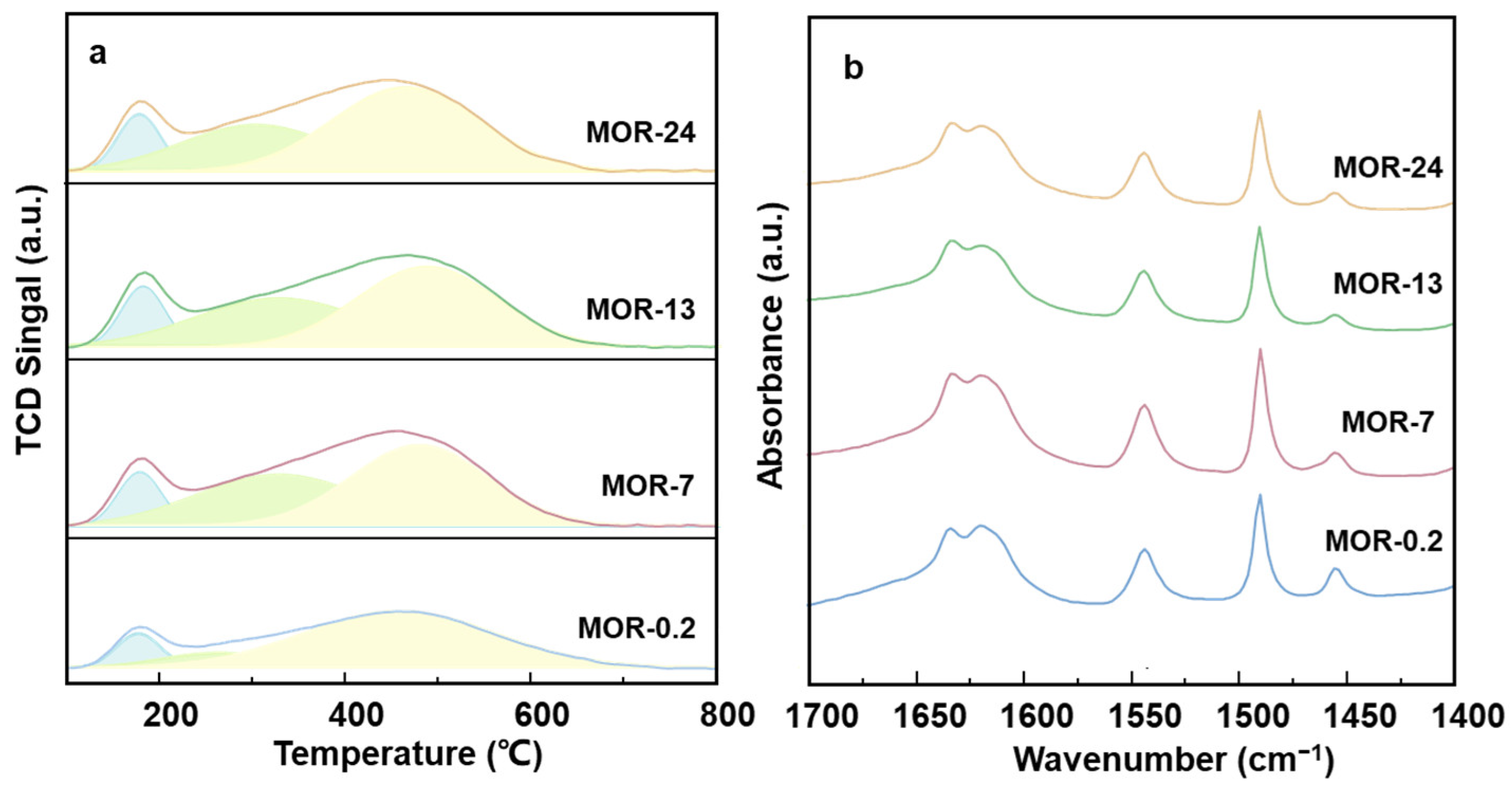
2.2. Acidity Characterizations
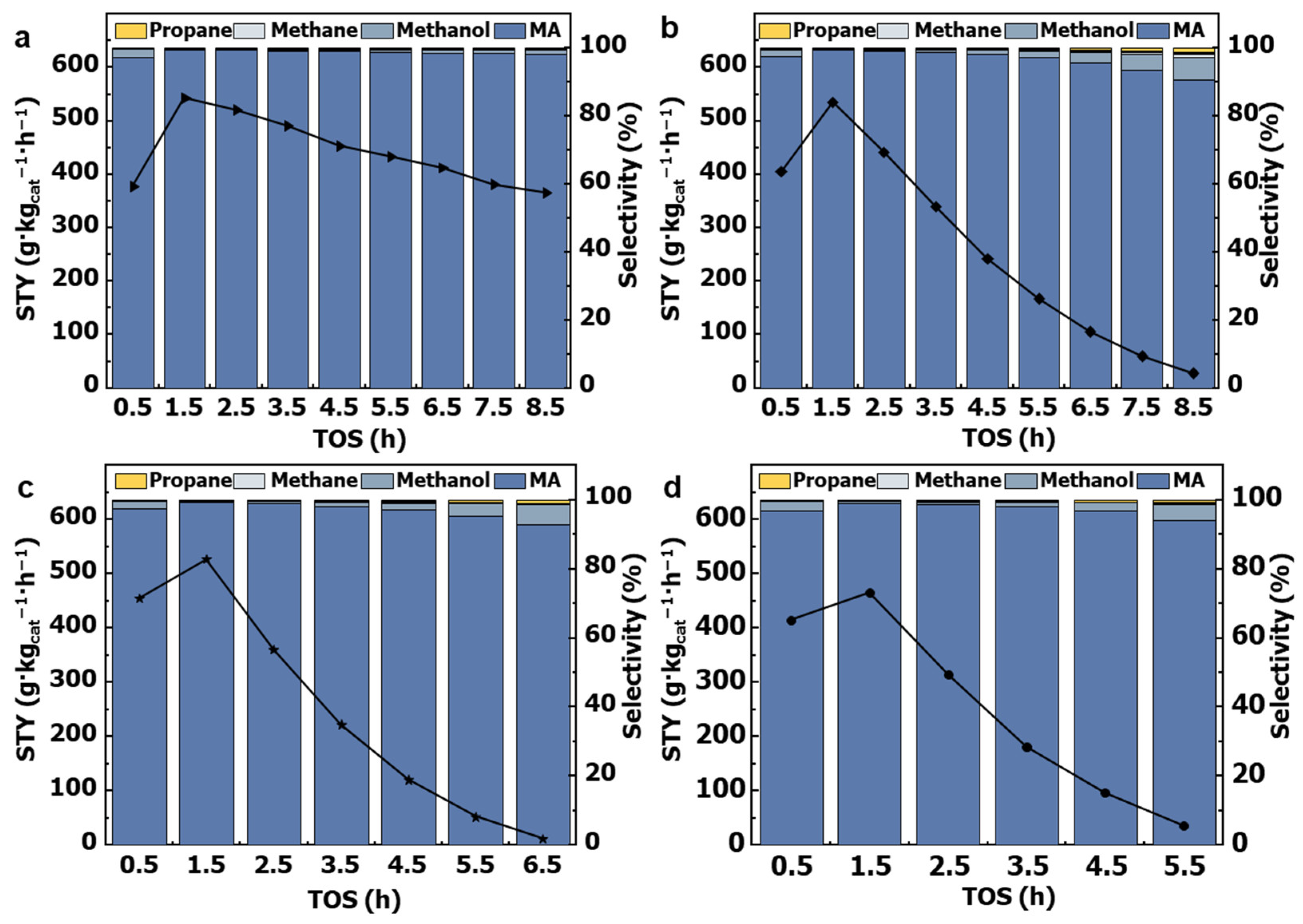
2.3. Catalytic Performance
2.4. Effect of Mass Transfer on DME Carbonylation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Characterization
3.3. Zero Length Column for Diffusion Measurement
3.4. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Yang, G.; Peng, X.; Wu, J.; Tsubaki, N. Recent advances in the routes and catalysts for ethanol synthesis from syngas. Chem. Soc. Rev. 2022, 51, 5606–5659. [Google Scholar] [CrossRef] [PubMed]
- Zhan, E.S.; Xiong, Z.P.; Shen, W.J. Dimethyl ether carbonylation over zeolites. J. Energy Chem. 2019, 36, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.B.; Li, Y.; Huang, S.Y.; Cai, K.; Cheng, Z.Z.; Lv, J.; Ma, X.B. The carbonylation of dimethyl ether catalyzed by supported heteropoly acids: The role of Bronsted acid properties. Catal. Today 2019, 330, 117–123. [Google Scholar] [CrossRef]
- Cheung, P.; Bhan, A.; Sunley, G.J.; Iglesia, E. Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites. Angew. Chem. Int. Ed. 2006, 45, 1617–1620. [Google Scholar] [CrossRef]
- Bhan, A.; Allian, A.D.; Sunley, G.J.; Law, D.J.; Iglesia, E. Specificity of sites within eight-membered ring zeolite channels for carbonylation of methyls to acetyls. J. Am. Chem. Soc. 2007, 129, 4919–4924. [Google Scholar] [CrossRef]
- Boronat, M.; Martinez-Sanchez, C.; Law, D.; Corma, A. Enzyme-like specificity in zeolites: A unique site position in mordenite for selective carbonylation of methanol and dimethyl ether with CO. J. Am. Chem. Soc. 2008, 130, 16316–16323. [Google Scholar] [CrossRef]
- Li, B.J.; Xu, J.; Han, B.; Wang, X.M.; Qi, G.D.; Zhang, Z.F.; Wang, C.; Deng, F. Insight into dimethyl ether carbonylation reaction over mordenite zeolite from in-situ solid-state NMR spectroscopy. J. Phys. Chem. C. 2013, 117, 5840–5847. [Google Scholar] [CrossRef]
- Wang, M.X.; Huang, S.Y.; Lu, J.; Cheng, Z.Z.; Li, Y.; Wang, S.P.; Ma, X.B. Modifying the acidity of H-MOR and its catalytic carbonylation of dimethyl ether. Chin. J. Catal. 2016, 37, 1530–1538. [Google Scholar] [CrossRef]
- Xia, Y.M.; Li, Z.H.; Li, Y.; Cai, K.; Liu, Y.D.; Lv, J.; Huang, S.Y.; Ma, X.B. Promotion effect and mechanism of Ga modification on dimethyl ether carbonylation catalyzed by mordenite. Catal. Today 2022, 405–406, 152–158. [Google Scholar] [CrossRef]
- Li, Y.; Yu, M.; Cai, K.; Wang, M.Y.; Lv, J.; Howe, R.F.; Huang, S.Y.; Ma, X.B. Template-induced Al distribution in MOR and enhanced activity in dimethyl ether carbonylation. Phys. Chem. Chem. Phys. 2020, 22, 11374–11381. [Google Scholar] [CrossRef]
- Cai, K.; Li, Y.; Shen, H.B.; Cheng, Z.Z.; Huang, S.Y.; Wang, Y.; Ma, X.B. A density functional theory study on the mechanism of Dimethyl ether carbonylation over heteropolyacids catalyst. Front. Chem. Sci. Eng. 2021, 15, 319–329. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, J.; Gu, Y.T.; Zhang, W.N.; Cao, K.P.; Cui, W.H.; Xu, S.T.; Fan, D.; Tian, P.; Liu, Z.M. Designed synthesis of MOR zeolites using gemini-type bis(methylpyrrolidinium) dications as structure directing agents and their DME carbonylation performance. J. Mater. Chem. A 2022, 10, 8334–8343. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Feng, X.B.; He, Z.M.; Chen, F.; Su, C.; Zhao, X.Y.; Cao, J.P.; He, Y.R. Enhancing the stability of dimethyl ether carbonylation over Fe-doped MOR zeolites with tunable 8-MR acidity. Chem. Eng. Sci. 2022, 256, 10. [Google Scholar] [CrossRef]
- Cai, K.; Huang, S.Y.; Li, Y.; Cheng, Z.Z.; Lv, J.; Ma, X.B. Influence of acid strength on the reactivity of dimethyl ether carbonylation over H-MOR. ACS Sustain. Chem. Eng. 2018, 7, 2027–2034. [Google Scholar] [CrossRef]
- Chen, W.; Li, G.; Yi, X.; Day, S.J.; Tarach, K.A.; Liu, Z.; Liu, S.B.; Edman Tsang, S.C.; Gora-Marek, K.; Zheng, A. Molecular understanding of the catalytic consequence of ketene intermediates under confinement. J. Am. Chem. Soc. 2021, 143, 15440–15452. [Google Scholar] [CrossRef]
- Rasmussen, D.B.; Christensen, J.M.; Temel, B.; Studt, F.; Moses, P.G.; Rossmeisl, J.; Riisager, A.; Jensen, A.D. Ketene as a reaction intermediate in the carbonylation of dimethyl ether to methyl acetate over mordenite. Angew. Chem. Int. Ed. 2015, 54, 7261–7264. [Google Scholar] [CrossRef]
- Cheng, Z.Z.; Huang, S.Y.; Li, Y.; Lv, J.; Cai, K.; Ma, X.B. Deactivation kinetics for the carbonylation of dimethyl ether to methyl acetate on H-MOR. Ind. Eng. Chem. Res. 2017, 56, 13618–13627. [Google Scholar] [CrossRef]
- Liu, S.P.; Fang, X.D.; Liu, Y.; Liu, H.C.; Ma, X.G.; Zhu, W.L.; Liu, Z.M. Dimethyl ether carbonylation over mordenite zeolite modified by alkyimidazolium ions. Catal. Commun. 2020, 147. [Google Scholar] [CrossRef]
- He, P.; Li, Y.; Cai, K.; Xiong, X.; Lv, J.; Wang, Y.; Huang, S.Y.; Ma, X.B. Nano-assembled mordenite zeolite with tunable morphology for carbonylation of dimethyl ether. ACS Appl. Nano Mater. 2020, 3, 6460–6468. [Google Scholar] [CrossRef]
- Ma, M.; Huang, X.M.; Zhan, E.S.; Zhou, Y.; Xue, H.F.; Shen, W.J. Synthesis of mordenite nanosheets with shortened channel lengths and enhanced catalytic activity. J. Mater. Chem. A 2017, 5, 8887–8891. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zhao, N.; Xian, H.; Cheng, Q.P.; Tan, Y.S.; Tsubaki, N.; Li, X.G. Facilely synthesized H-mordenite nanosheet assembly for carbonylation of dimethyl ether. ACS Appl. Mater. Interfaces 2015, 7, 8398–8403. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wu, Q.M.; Fan, J.Q.; Komiyama, S.; Yong, X.J.; Zhang, W.; Zhao, T.J.; Guo, Z.S.; Yang, G.H.; Tsubaki, N. A Carbonylation Zeolite with Specific Nanosheet Structure for Efficient Catalysis. ACS Nano 2021, 15, 13568–13578. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Cheng, Z.Z.; Li, Y.; Sun, J.H.; Cai, K.; Huang, S.Y.; Lv, J.; Wang, S.P.; Ma, X.B. Improved catalytic performance in dimethyl ether carbonylation over hierarchical mordenite by enhancing mass transfer. Ind. Eng. Chem. Res. 2020, 59, 13861–13869. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, R.J.; Yu, C.C.; Liu, Y.X.; Zhang, L.Y.; Xu, C.M.; Zhou, H.J. Enhancing the dimethyl ether carbonylation performance over mordenite catalysts by simple alkaline treatment. Fuel 2019, 239, 794–803. [Google Scholar] [CrossRef]
- Xue, H.F.; Huang, X.M.; Ditzel, E.; Zhan, E.S.; Ma, M.; Shen, W.J. Dimethyl ether carbonylation to methyl acetate over nanosized mordenites. Ind. Eng. Chem. Res. 2013, 52, 11510–11515. [Google Scholar] [CrossRef]
- Sheng, H.B.; Qian, W.X.; Zhang, H.T.; Zhao, P.; Ma, H.F.; Ying, W.Y. Synthesis of hierarchical porous H-mordenite zeolite for carbonylation of dimethyl ether. Microporous Mesoporous Mater. 2020, 295. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Huang, S.Y.; Cai, K.; Qu, Z.; Zhang, J.F.; Wang, Y.; Ma, X.B. Morphology-dependent catalytic performance of mordenite in carbonylation of dimethyl ether: Enhanced activity with high c/b ratio. ACS Appl. Mater. Interfaces 2019, 11, 24000–24005. [Google Scholar] [CrossRef]
- Xu, C.; Guo, Y.; Xiao, Q.; Zhong, Y.; Zhu, W. Synthesis and characterization of large, pure mordenite crystals. J. Porous Mater. 2011, 19, 847–852. [Google Scholar] [CrossRef]
- Ghanbari, B.; Zangeneh, F.K.; Sastre, G.; Moeinian, M.; Marhabaie, S.; Rizi, Z.T. Computational elucidation of the aging time effect on zeolite synthesis selectivity in the presence of water and diquaternary ammonium iodide. Phys. Chem. Chem. Phys. 2021, 23, 21240–21248. [Google Scholar] [CrossRef]
- Ogura, M.; Kawazu, Y.; Takahashi, H.; Okubo, T. Aluminosilicate species in the hydrogel phase formed during the aging process for the crystallization of FAU zeolite. Chem. Mater. 2003, 15, 2661–2667. [Google Scholar] [CrossRef]
- Keoh, S.H.; Chaikittisilp, W.; Muraoka, K.; Mukti, R.R.; Shimojima, A.; Kumar, P.; Tsapatsis, M.; Okubo, T. Factors governing the formation of hierarchically and sequentially intergrown MFI zeolites by using simple diquaternary ammonium structure-directing agents. Chem. Mat. 2016, 28, 8997–9007. [Google Scholar] [CrossRef]
- Lu, B.; Oumi, Y.; Sano, T. Convenient synthesis of large mordenite crystals. J. Cryst. Growth 2006, 291, 521–526. [Google Scholar] [CrossRef]
- Bajpai, P.K.; Rao, M.S.; Gokhale, K.V.G.K. Synthesis of mordenite type zeolites. Ind. Eng. Chem. Prod. Res. Dev. 1978, 17, 223–227. [Google Scholar] [CrossRef]
- Bordiga, S.; Lamberti, C.; Bonino, F.; Travert, A.; Thibault-Starzyk, F. Probing zeolites by vibrational spectroscopies. Chem. Soc. Rev. 2015, 44, 7262–7341. [Google Scholar] [CrossRef] [PubMed]
- Lonyi, F.; Valyon, J. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite. Microporous Mesoporous Mater. 2001, 47, 293–301. [Google Scholar] [CrossRef]
- He, P.; Wang, J.H.; Li, Y.; Huang, S.Y.; Wang, M.Y.; Wang, Y.; Lv, J.; Ma, X.B. Efficient synthesis of mordenite zeolite for dimethyl ether carbonylation. Ind. Eng. Chem. Res. 2021, 60, 8085–8093. [Google Scholar] [CrossRef]
- Jiao, F.; Pan, X.L.; Gong, K.; Chen, Y.X.; Li, G.; Bao, X.H. Shape-selective zeolites promote ethylene formation from syngas via a ketene intermediate. Angew. Chem. Int. Ed. 2018, 57, 4692–4696. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Cheng, Z.Z.; Huang, S.Y.; Li, Y.; Cai, K.; Yao, D.W.; Lv, J.; Wang, S.P.; Ma, X.B. Carbonylation of dimethyl ether over MOR and Cu/H-MOR catalysts: Comparative investigation of deactivation behavior. Appl. Catal. A-Gen. 2019, 576, 1–10. [Google Scholar] [CrossRef]
- Karger, J.; Valiullin, R. Mass transfer in mesoporous materials: The benefit of microscopic diffusion measurement. Chem. Soc. Rev. 2013, 42, 4172–4197. [Google Scholar] [CrossRef]
- Eic, M.; Ruthven, D.M. A new experimental technique for measurement of intracrystalline diffusivity. Zeolites 1988, 8, 40–45. [Google Scholar] [CrossRef]
- Vattipalli, V.; Qi, X.; Dauenhauer, P.J.; Fan, W. Long Walks in Hierarchical Porous Materials due to Combined Surface and Configurational Diffusion. Chem. Mat. 2016, 28, 7852–7863. [Google Scholar] [CrossRef]
- Han, M.H.; Yin, X.Y.; Jin, Y.; Chen, S. Diffusion of aromatic hydrocarbon in ZSM-5 studied by the improved zero length column method. Ind. Eng. Chem. Res. 1999, 38, 3172–3175. [Google Scholar] [CrossRef]

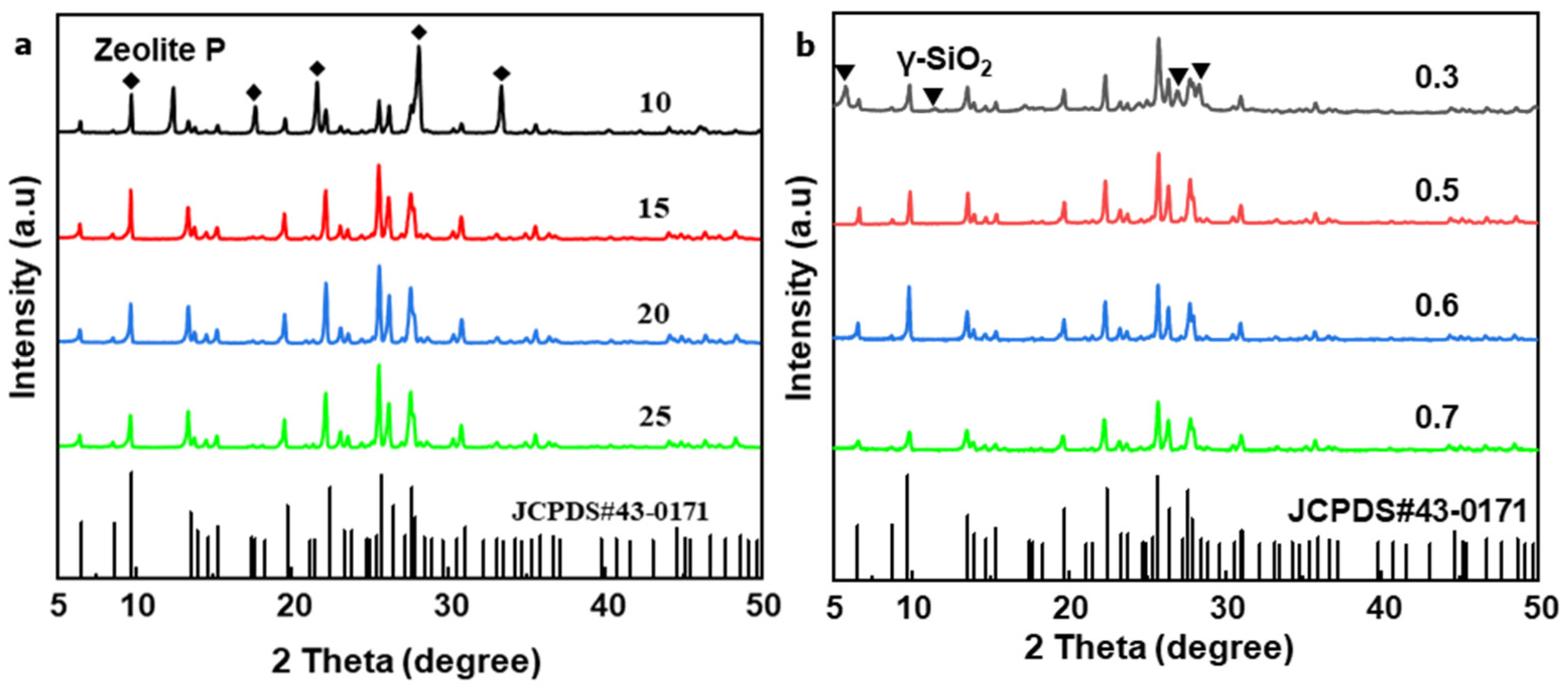
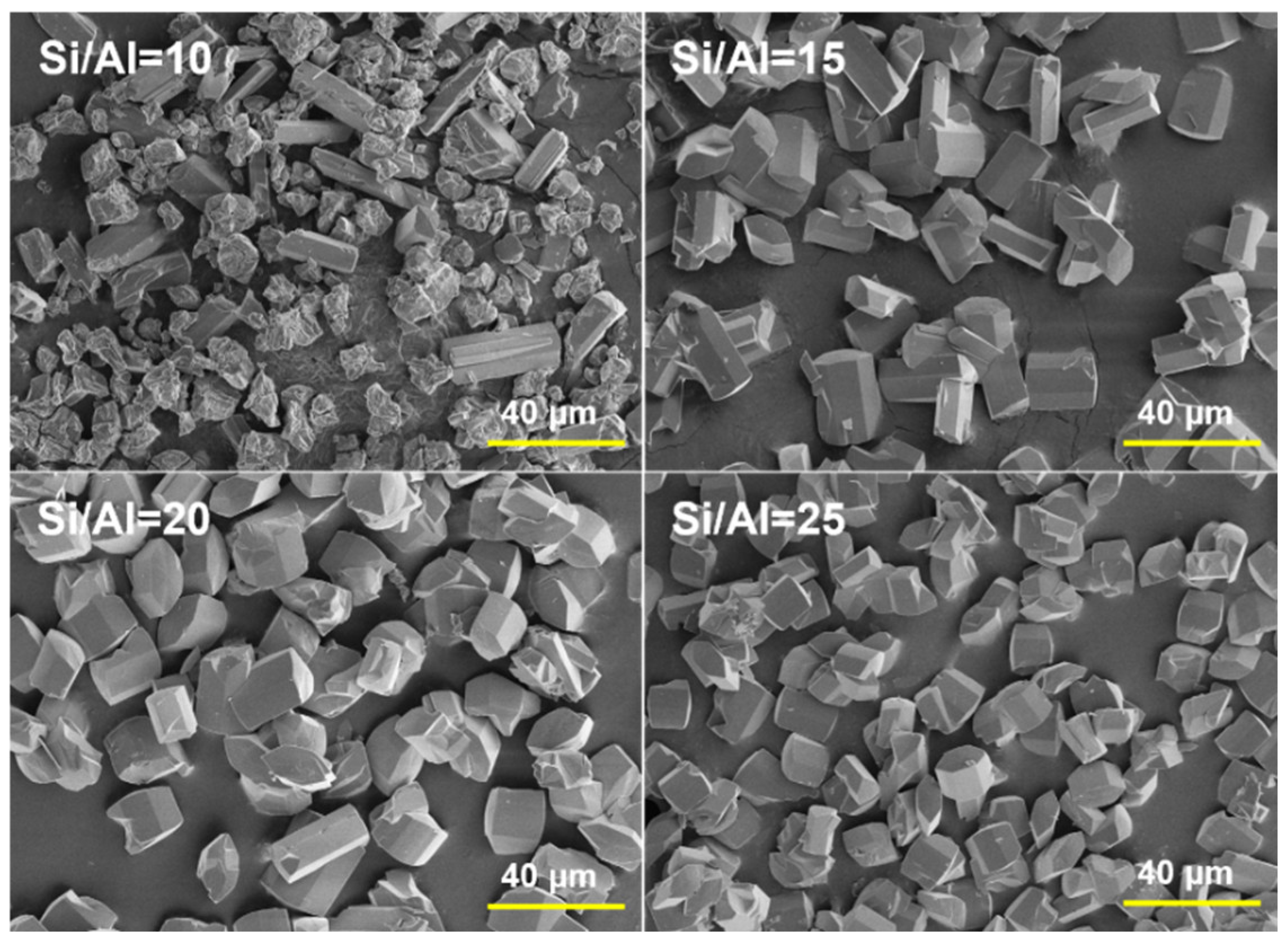
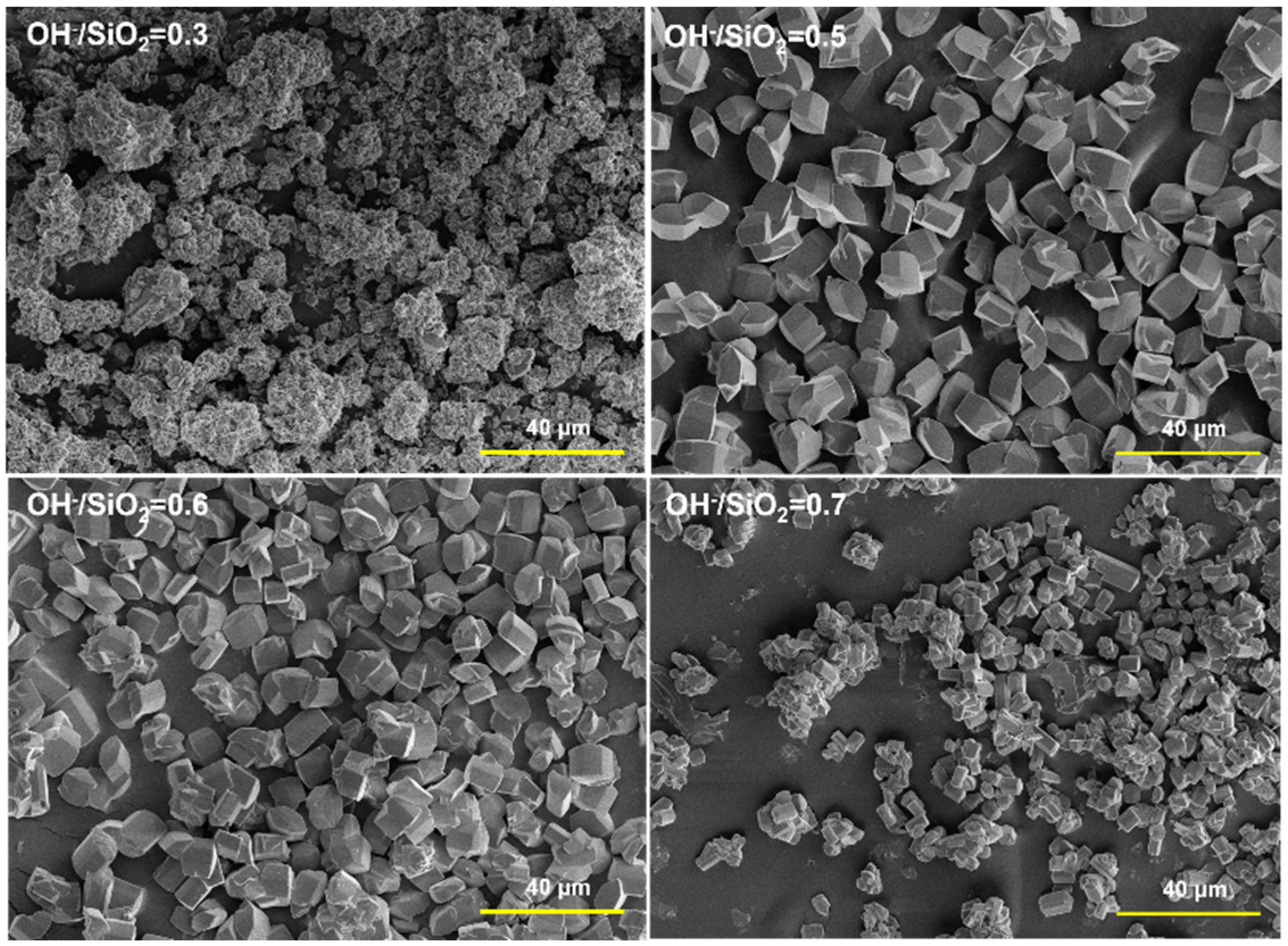
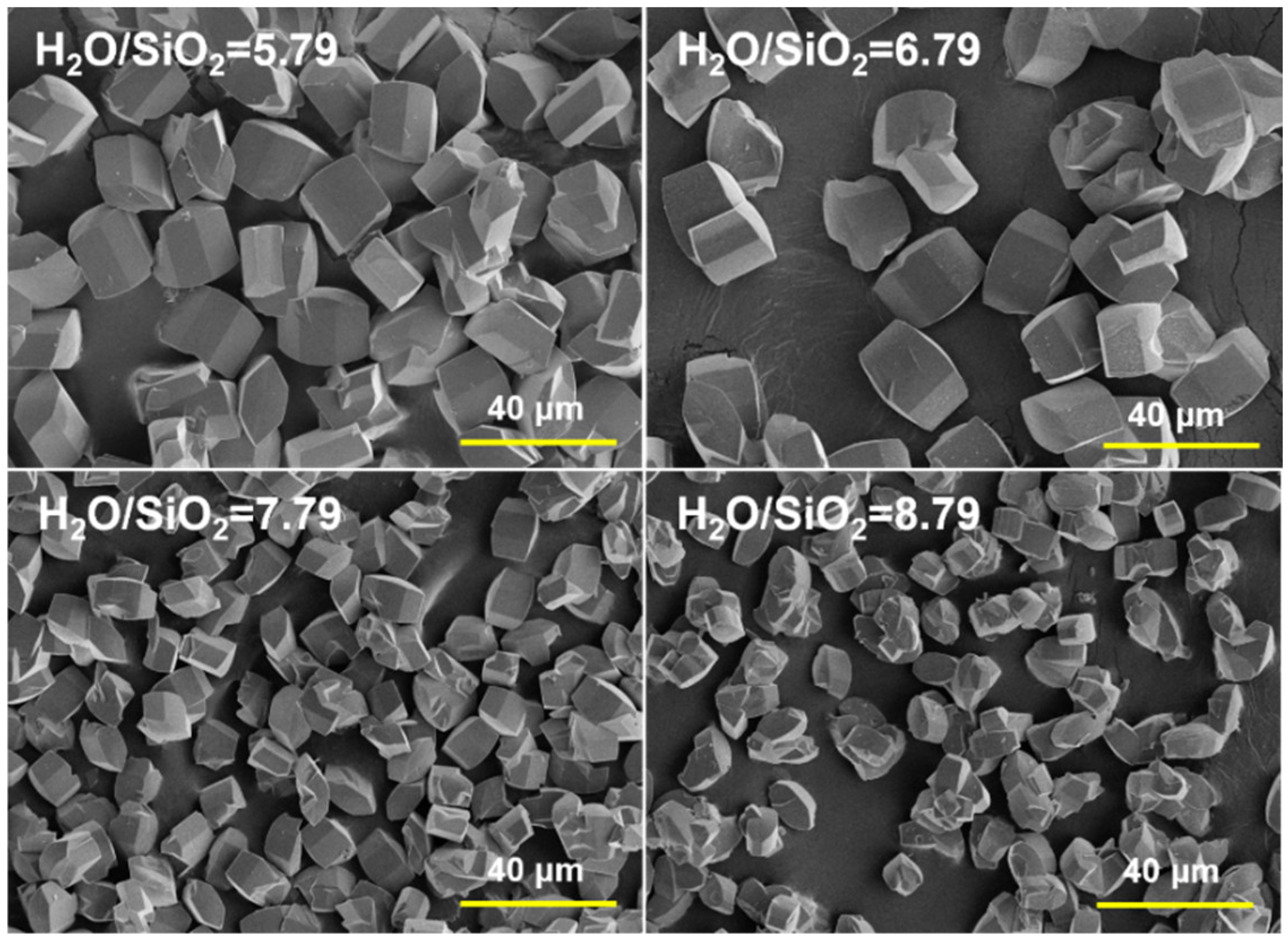



| Sample | Si/Al in Gel | H2O/SiO2 | OH−/SiO2 | Aging Time/h | Size /μm | Si/Al in Product |
|---|---|---|---|---|---|---|
| 1 | 20 | 7.79 | 0.5 | 4 | 10 × 16 | 5.3 |
| 2 | 20 | 7.79 | 0.5 | 8 | 7 × 15 | - |
| 3 | 20 | 7.79 | 0.5 | 1 | 4 × 7 | 5.1 |
| 4 | 20 | 7.79 | 0.6 | 4 | 12 × 17 | 6.2 |
| 5 | 20 | 7.79 | 0.7 | 4 | 7 × 18 | - |
| 6 | 20 | 5.79 | 0.5 | 4 | 17 × 24 | 7.0 |
| 7 | 20 | 6.79 | 0.5 | 4 | 11 × 22 | - |
| 8 | 20 | 8.79 | 0.5 | 4 | 8 × 14 | 5.1 |
| 9 | 15 | 7.79 | 0.5 | 4 | 16 × 17 | - |
| 10 | 25 | 7.79 | 0.5 | 4 | 6 × 11 | 8.0 |
| 11 | 25 | 7.79 | 0.5 | 1 | 8 × 13 | 6.1 |
| 12 | 25 | 8.79 | 0.5 | 4 | 3 × 7 | 6.5 |
| Sample | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | ||||
|---|---|---|---|---|---|---|
| BET | Micropore | Mesopore | Total | Micropore | Mesopore | |
| MOR-0.2 | 548 | 526 | 22 | 0.254 | 0.203 | 0.051 |
| MOR-7 | 560 | 557 | 3 | 0.214 | 0.210 | 0.004 |
| MOR-13 | 559 | 559 | 0 | 0.210 | 0.210 | 0 |
| MOR-24 | 550 | 549 | 1 | 0.208 | 0.210 | 0.002 |
| Sample | BASTotal a (μmol·g−1) | LASTotal a (μmol·g−1) | BAS12-MR b (μmol·g−1) | LAS12-MR b (μmol·g−1) | BAS8-MR c (μmol·g−1) | TOFMA (h−1, 1.5 h) |
|---|---|---|---|---|---|---|
| MOR-0.2 | 1016 | 681 | 237 | 67 | 779 | 9.4 |
| MOR-7 | 1380 | 1218 | 321 | 58 | 1059 | 6.8 |
| MOR-13 | 1421 | 1033 | 304 | 40 | 1117 | 6.4 |
| MOR-24 | 1593 | 876 | 248 | 39 | 1345 | 4.7 |
| Sample | T (K) | L | D/r2 (s−1) | Ea (kJ/mol) |
|---|---|---|---|---|
| MOR-0.2 | 313 | 123.0 | 3.2 × 10−4 | 4.7 |
| 333 | 117.0 | 3.6 × 10−4 | ||
| 353 | 116.0 | 3.9 × 10−4 | ||
| MOR-7 | 313 | 99.0 | 2.2 × 10−4 | 9.6 |
| 333 | 91.7 | 2.7 × 10−4 | ||
| 353 | 94.3 | 3.4 × 10−4 | ||
| MOR-13 | 313 | 70.6 | 2.1 × 10−4 | 10.4 |
| 333 | 60.3 | 2.7 × 10−4 | ||
| 353 | 54.2 | 3.2 × 10−4 | ||
| MOR-24 | 313 | 54.4 | 1.8 × 10−4 | 12.3 |
| 333 | 45.7 | 2.2 × 10−4 | ||
| 353 | 54.5 | 3.1 × 10−4 |
| Coke Content (wt.%) Obtained via TG | C Content (%) Obtained via XPS | C Content (%) Obtained via XPS with Etching | BET Surface Area (m2·g−1) | Micropore Volume (cm3·g−1) | |
|---|---|---|---|---|---|
| Spent MOR-0.2 | 4 | 16 | 76 | 296 | 0.108 |
| Spent MOR-24 | 11 | 36 | 80 | 4 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, C.; Liu, Y.; Xia, Y.; Liu, H.; Zhang, Y.; Huang, S.; Ma, X. Size Effect on Diffusion and Catalytic Performance of Mordenite in Dimethyl Ether Carbonylation. Catalysts 2023, 13, 1049. https://doi.org/10.3390/catal13071049
Dong C, Liu Y, Xia Y, Liu H, Zhang Y, Huang S, Ma X. Size Effect on Diffusion and Catalytic Performance of Mordenite in Dimethyl Ether Carbonylation. Catalysts. 2023; 13(7):1049. https://doi.org/10.3390/catal13071049
Chicago/Turabian StyleDong, Chenchen, Yunduo Liu, Yumeng Xia, Hu Liu, Yan Zhang, Shouying Huang, and Xinbin Ma. 2023. "Size Effect on Diffusion and Catalytic Performance of Mordenite in Dimethyl Ether Carbonylation" Catalysts 13, no. 7: 1049. https://doi.org/10.3390/catal13071049
APA StyleDong, C., Liu, Y., Xia, Y., Liu, H., Zhang, Y., Huang, S., & Ma, X. (2023). Size Effect on Diffusion and Catalytic Performance of Mordenite in Dimethyl Ether Carbonylation. Catalysts, 13(7), 1049. https://doi.org/10.3390/catal13071049







